Abstract
Simple Summary
Cryptosporidiosis is a global, zoonotic disease of concern. Cryptosporidium spp. can infect susceptible hosts via a main fecal–oral route due to cross-contamination of raw food and surface water from reservoir animals in the neighborhood, farms, or slaughter houses, besides some mechanical vectors, such as cockroaches and flies. Cryptosporidium parvum alleles are the most common species infecting children, and its potential reservoir is cattle. Hence, understanding the epidemiology of Cryptosporidium spp. in preweaned calves, along with the diagnosis of the predominant species and subtypes infecting them, can play a role in preventing Cryptosporidium spp. spread in the environment. In this study, Cryptosporidium parvum subtype IIaA15G2R1 was the most dominant Cryptosporidium spp. detected in preweaned calves in Kuwait. This subtype was recorded previously in Kuwaiti children suffering from diarrhea. Maintaining good personal hygiene in humans and reducing, controlling, or eliminating the causal risk factors in preweaned calves is a superb strategy for preventing and narrowing the spread of this disease.
Abstract
Cryptosporidium is a worldwide enteric protozoan parasite that causes gastrointestinal infection in animals, including humans. The most notable species is Cryptosporidium parvum because of its zoonotic importance; it is also the leading cause of cryptosporidiosis in preweaned calves. A cross-sectional study was conducted to determine the prevalence of Cryptosporidium infection, investigate the potential risk factors, and use molecular diagnosis to identify the predominant Cryptosporidium spp. in preweaned calves in Kuwait. Of 175 preweaned calves, Cryptosporidium antigens were detected in 58 (33.1%) using rapid lateral immunochromatography assay (IC). Calves less than one month of age (OR = 4.32, p = 0.0001) and poor hygiene (OR = 2.85, p = 0.0075) were identified as significant risk factors associated with Cryptosporidium infection. Molecular identification revealed that C. parvum (62.8%) was the dominant species infecting preweaned calves in Kuwait. In contrast, C. bovis and C. andersoni were recorded at 5.7% and 2.9%, respectively. All C. parvum gp60 nucleotide sequences were subtype IIaA15G2R1. Calves could be a source of C. parvum infection due to the similarity of the subtypes recorded previously in Kuwaiti children and preweaned calves in this study. Therefore, more research is needed to understand the Cryptosporidium transmission cycle in Kuwait.
Keywords: Cryptosporidium spp., C. parvum IIaA15G2R1, preweaned calves, risk factors, Kuwait
1. Introduction
Cryptosporidium is a globally distributed protozoan parasite. It accounts for most cryptosporidiosis cases in newborn farm animals, and is a significant causative agent of diarrhea in children in many parts of the world [1,2,3].
C. parvum is one of the main enteropathogens of neonatal calf diarrhea; it infects preweaned calves ≤ six weeks of age [4]. However, calves ranging from one to three weeks old appear to be the most susceptible age. Infected calves suffer from acute profuse diarrhea, high morbidity, possible mortality, and reduced growth rate as long-term effects of cryptosporidiosis [3]. This species is also predominant in humans in the Middle East, notably its IIa allele, implying that cattle may be involved in zoonotic infections [5,6,7].
Kuwait is a small country in a desert region. Vegetation is highly scarce given the type of climate and soil. The climate is continental, with a dry, hot season (April–November) and a mild, cold, wet season (December–March). Dust storms are expected during the hot season, and temperatures can reach 50 °C. Due to this harsh climate and limited vegetation, dairy farming presents unique challenges. The 2020 census recorded 31,484 cattle in the country [8]. Cattle are reared for milk production under an intensive farming system with zero grazing. The breed of most dairy cattle is Friesian, and their farms are confined to the Sulaibiya area. Most dairy cattle were imported from Germany and Holland to rebuild the dairy sector after the destruction of livestock during the Iraq invasion.
No studies have been conducted in Kuwait to determine the prevalence of Cryptosporidium, identify Cryptosporidium spp. and subtypes in cattle, or investigate their public health significance. In contrast, many research papers have been published on the molecular characterization of bovine Cryptosporidium spp. and their global prevalence [1,6,7,9,10,11,12,13,14,15]. Consequently, the objectives of this study were to estimate the prevalence and determine the risk factors of Cryptosporidium infection in preweaned calves (≤3 months of age) and identify the genotypes and subtypes of Cryptosporidium in this animal species, furthermore to assess their public health importance in Kuwait.
2. Materials and Methods
2.1. Study Design, Data, and Sample Collection
Between October 2014 and September 2015, a cross-sectional study was conducted. A single visit was made to each farm that took part in the study to collect samples and data. For data collection from each farm, a structured questionnaire (open-ended, closed-ended, dichotomous, or multiple choice) was created. The host factors (breed, age, sex), the environmental factors (location, season) were the subjects of the data collection. In addition, management factors, such as management system, herd size, frequency of cleaning and bedding change, presence of feed and water troughs, source of water, separation of age groups, etc., were collected
All cattle farms in Kuwait are private dairy farms of Friesian breed, located in Sulaibiya (29°28′56.0″ N, 47°81′80.0″ E), and reared under an intensive farming system with zero grazing. The farms were supplied with desalinated potable water from a municipal source. The presence of feed and water troughs, maternity facilities, and the separation of age groups were almost identical among all the farms. Data on the frequency of cleaning and bedding changes were consistent with on-farm cleanliness visual monitoring during the visit to determine the hygienic farm status as poor or good. The farms were chosen without knowing whether or not they were infected with Cryptosporidium.
Twenty-two dairy cattle farms were visited in the Sulaibiya area. The overall number of dairy cattle on farms visited was 9365 (882 preweaned calves). Herd size of cattle ranged from 12 to 2400 animals (median 300). The Epi Info 7-Stat Cal tool was used to determine the sample size, and systematic random sampling was used to select animals from each visited farm. One hundred seventy-five preweaned calves (≤3 months of age) were randomly selected to examine their fecal samples. Using a sterile screw-capped bottle labelled with the animal’s data (such as sex, age, and health status) and the date of the sample, 5–10 gm of feces were obtained directly from the rectum or shortly after defecation. The specimens were stored at 4 °C or processed within 48 h after being placed in an icebox and transported to the lab.
2.2. Processing of Samples
Each sample was divided into two portions in the laboratory: the first part for detecting Cryptosporidium antigen by IC. The second portion was used for storage either in 2.5% potassium dichromate or at −20 °C and was sent to Prof. Dr. Lihua Xiao (College of Veterinary Medicine, South China Agricultural University, Guangdong Province, China) for molecular diagnosis and typing of Cryptosporidium spp. if the sample was diagnosed as positive for Cryptosporidium antigen by rapid lateral immunochromatographic assay (IC).
2.3. Detection of Cryptosporidium in Fecal Samples
A commercial rapid lateral immunochromatographic assay (Anigen Rapid BoviD-4 Ag Test Kit; BioNote Inc., Gyeonggi-do, Korea) was used to detect Cryptosporidium, rotavirus, coronavirus, and E. coli K99 antigens. The procedures and results’ interpretation were performed following the manufacturer’s prescripts.
2.4. DNA Extraction, PCR Amplification, and Subtyping
For the typing and subtyping of Cryptosporidium spp., 35 IC specimens, that tested positive for the parasite, were chosen. Prior to DNA isolation, the specimens, stored in potassium dichromate, were centrifuged double in DH2O. Using the FastDNA SPIN kit for soil (MP Biomedicals, Santa Ana, CA, USA), DNA was pulled from all samples. Afterward, nested PCR, targeting an approximately 830-bp region of the small subunit (SSU) rRNA gene, was used to check the recovered DNA for Cryptosporidium species [16,17].
By performing a restriction fragment length polymorphism (RFLP) analysis on the secondary PCR products with SspI and MboII, as previously published [17], Cryptosporidium spp. were distinguished. Using the negative control (reagent water) and the positive control (Cryptosporidium baileyi DNA), each sample was examined at least twice. The secondary PCR results from typical specimens were sequence analyzed to validate the identification of Cryptosporidium species.
Using an ABI 3130 genetic analyzer, all gp60 gene PCR products and representative SSU rRNA gene PCR products from C. parvum were sequenced (Applied Biosystems, Foster City, CA, USA). In order to identify Cryptosporidium spp. (based on SSU rRNA sequences) and subtypes, for C. parvum subtypes, bi-directional sequences were obtained and assembled using the ChromasPro (version 1.5) software (Technelysium Pty Ltd, South Brisbane, Australia. Webpage: http://technelysium.com.au/?page_id=27 accessed on 25 April 2018). These sequences were then aligned with each other, and referenced sequences of each gene were downloaded from Gen Bank using ClustalX (Conway Institute UCD, Dublin, Republic of Ireland. Webpage: http://www.Clustal.Org/, accessed on 30 April 2018). The existing Cryptosporidium subtype nomenclature approach was used to name the C. parvum subtypes [5].
2.5. Statistical Analysis
The Microsoft Office program’s MS Access® database information system (Microsoft Corporation, Redmond, WA, USA) was used to store test results and questionnaires. The data was exported to SXW® for statistical analysis (Statistix 10, Analytical software, Tallahassee, FL, USA). The independent variables under study were age group, sex, season, herd size, and farm hygiene status. The correlation between variables and the prevalence of Cryptosporidium-infection in calves was examined using univariate analysis (Chi-square test, χ2) at a 95% Confidence Interval. The significant variables (p ≤ 0.05) were analyzed using multivariate stepwise logistic regression. The Hosmer–Lemeshow test was applied to determine the goodness of fit for the logistic regression model, and p > 0.05 indicated a good fit model.
3. Results
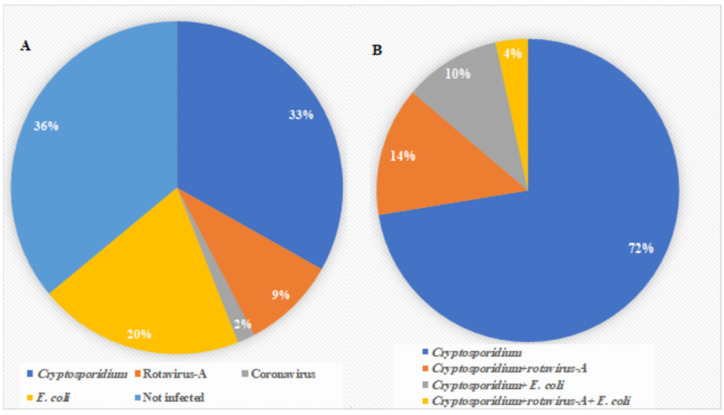
The prevalence of Cryptosporidium among the examined calves was 33% (58/175). Rotavirus, coronavirus, and E. coli were detected in 16 (9%), 3 (2%), and 35 (20%) of the samples, respectively (Figure 1). Cryptosporidium spp. mono-infection was discovered in 42 preweaned calves (72%). Co-infections with Cryptosporidium were 14% (8/58) for rotavirus, 10% (6/58) for E. coli, and 4% (2/58) for rotavirus and E. coli (Figure 1).
Figure 1.
Results of rapid IC assay: (A) Prevalence of the four pathogens (Cryptosporidium, rotavirus-A, coronavirus, and E. coli) detected in 175 preweaned calves. (B) Prevalence of Cryptosporidium mono-infection and co-infections with other pathogens detected by IC.
The results of the univariate analysis showed that four variables were distinguished as putative risk variables for Cryptosporidium infection in the examined calves (Table 1). There was a significant distinction in Cryptosporidium prevalence rates among the various age group of the examined calves (p = 0.0000), with the highest prevalence in calves less than one month of age (50.6%; CI 39.4–61.7). The Cryptosporidium infection rate was higher in the wet than in the dry season (37.6%; CI 29.1–46.7; p = 0.0476). Similarly, herd size significantly influenced the Cryptosporidium infection rate (p = 0.0062). The prevalence rate in calves reared in large herd sizes (42.4%; CI 32.2–53.1) was higher than those in small (22.9%; CI 14.4–33.4). Furthermore, farm hygiene status significantly affected the rate of infection (p = 0.0005). Calves reared on farms with poor farm hygiene (42.9%; CI 33.5–53.0) had more infections than those with good hygiene (17.6%; CI 9.5–28.8). On the other hand, the sex of the examined calves did not significantly affect the Cryptosporidium prevalence rate (p = 0.7412); hence, this factor was excluded from the multivariable analysis. The four variables (age group, season, herd size, and farm hygiene) were subjected to multivariate logistic regression, which revealed that calves less than one month of age (OR = 4.32; CI 2.09–8.57; p = 0.0001) and poor hygiene (OR = 2.85; CI 1.32–6.13; p = 0.0075) were the significant risk factors identified in this study, as shown in Table 2.
Table 1.
Univariate analysis results of variables associated with Cryptosporidium-infected calves (No. 175) in Kuwait.
| Risk Factors | No. of Samples | Prevalence of Cryptosporidium | p-Value | ||
|---|---|---|---|---|---|
| No. | % (95% CI) * | ||||
|
Age group
(in months) |
<1 | 83 | 42 | 50.6 (39.4–61.7) | 0.0000 |
| ≥1– <2 | 43 | 9 | 20.9 (10.0–36.0) | ||
| ≥2– ≤3 | 49 | 7 | 14.3 (5.9–27.2) | ||
| Sex | Male | 95 | 33 | 34.7 (25.2–45.2) | 0.7412 |
| Female | 80 | 25 | 31.3 (21.4–42.6) | ||
| Season | Wet | 125 | 47 | 37.6 (29.1–46.7) | 0.0476 |
| Dry | 50 | 11 | 22.0 (11.5–36.0) | ||
|
Herd Size
(heads) |
Large > 300 | 92 | 39 | 42.4 (32.2–53.1) | 0.0062 |
| Small ≤ 300 | 83 | 19 | 22.9 (14.4–33.4) | ||
| Farm hygiene | Good | 68 | 12 | 17.7 (9.5–28.8) | 0.0005 |
| Poor | 107 | 46 | 42.9 (33.5–53.0) | ||
| Overall prevalence | 175 | 58 | 33.1 (26.2–40.6) | ||
* CI Confidence Interval.
Table 2.
Multivariate stepwise logistic regression analysis * of risk factors for Cryptosporidium infection that were significant using univariate analysis.
| Variables | Coefficient β | Std. Error | p-Value | OR ** | 95% CI *** | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age group (in months) < 1 | 1.442 | 0.360 | 0.0001 | 4.23 | 2.09 | 8.57 |
| Poor hygiene | 1.046 | 0.391 | 0.0075 | 2.85 | 1.32 | 6.13 |
* p value (Hosmer–Lemeshow goodness of fit test) = 0.1315, ** OR odds ratio, *** CI Confidence Interval.
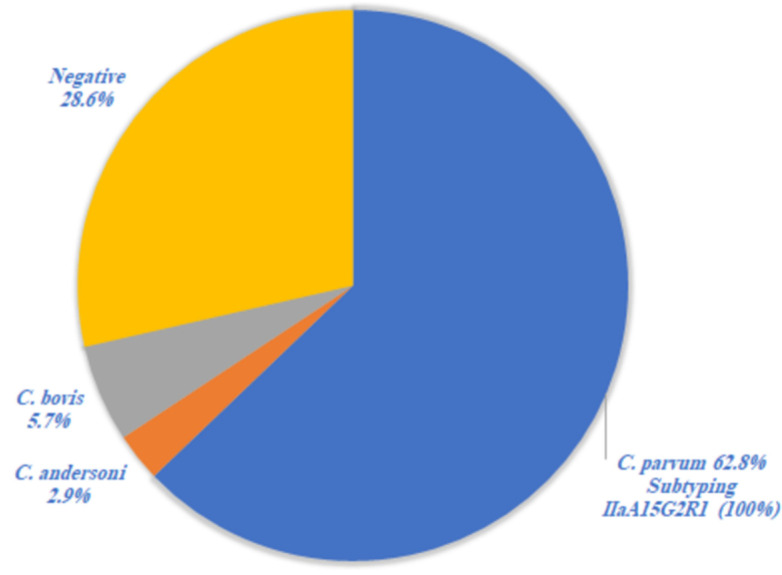
Molecular identification of the positive fecal samples showed that Cryptosporidium was detected in 71.4% (25/35). The Cryptosporidium species identified by RFLP analysis of the SSU rRNA PCR product included C. parvum in 22 (62.8%), C. bovis in 2 (5.7%), and C. andersoni in 1 (2.9%) of the examined samples (Figure 2). Subtyping of C. parvum at the gp60 locus revealed that all 22 samples (100%) belonged to one subtype IIaA15G2R1 (Figure 2).
Figure 2.
Cryptosporidium spp. identified by RFLP analysis in positive IC samples (No. 35) and subtyping of C. parvum at gp60 locus.
4. Discussion
Bovine cryptosporidiosis is globally distributed and has been reported as a considerable risk factor for calf enteritis [3,4,18,19]. This study was the first attempt to identify the risk factors and molecular typing of species involved in Cryptosporidium infection among preweaned calves in Kuwait. Many epidemiological studies have been conducted to detect cryptosporidiosis in calves worldwide [10,15,20,21,22,23,24].
In the present study, the overall infection rate of Cryptosporidium in the examined calves was 33.1% (95 % CI 26.2–40.6%). In the Middle East, previous studies of Cryptosporidium infection in preweaned calves reported prevalence rates of 47.9% in Iraq [25], 18.7% in Jordan [10], 14.7% in Iran [20], 84% in Algeria [13], 58.3% in Sudan [14], 9.7% in Egypt [15], and 53.6% in Turkey [23]. These variations in infection rates could be due to geographical differences in the prevalence of Cryptosporidium infections, besides other factors related to differences in diagnostic methods, sampling strategies, farm management, and hygiene [3]. For instance, the rate of infection may be elevated if specimens were only collected from diarrheic preweaned calves. Additionally, prevalence rate could be underestimated in a cross-sectional study compared with a longitudinal study, because the shedding profile of Cryptosporidium oocysts is intermittent and could be missed [26]. However, cross-sectional studies can provide more consistent evaluations of disease risk factors than longitudinal studies [27].
The present study used the rapid IC assay to diagnose cryptosporidiosis in preweaned calves. The IC assay’s advantages are accurate, rapid, cost-effective, and easy to conduct, as it does not require other specialized instruments compared with microscopy, ELISA, and PCR techniques. Many investigators study the diagnostic performance of these assays. Sensitivity rates for different commercially available IC detecting Cryptosporidium copro-antigen have previously been reported to vary from 75% to 100%, whereas their specificity rates ranged from 92% to 100% [28,29,30].
The present study observed a significant correlation between Cryptosporidium infection and calves’ age. Calves less than one month of age were more likely to harbor Cryptosporidium, and infection rates diminished progressively with age. These results were consistent with most existing studies, showing that this protozoan is most common in neonatal calves [3,4,22,23]. The highest peak of Cryptosporidium infection rate was reported in two-week-old calves [23,31]. This could be due to their immature immune systems [32].
In the present study, multivariate analysis identified farm hygiene status as a risk factor for Cryptosporidium infection in preweaned calves. The prevalence of Cryptosporidium is approximately three times higher in farms with poor hygienic status (OR = 2.85; 95% CI = 1.32–6.13) compared to those with good hygiene. Similar results were previously observed [24,33,34]. This result could be explained by the fact that a dirty and muddy farm could create appropriate climatic conditions for the presence or survival of Cryptosporidium oocysts in the farms or animal houses. Consequently, it raises the risk of calves contracting Cryptosporidium infection by contaminating their feed and water [35].
In this study, sex, season, and herd size was not considered risk factors for Cryptosporidium infection in preweaned calves in Kuwait. Similar results have been observed. The absence of association between season and the presence of the infection has been formerly described, as season is not considered a risk factor for cryptosporidiosis in countries where the temperature never drops below the freezing point [36,37,38]. At the same time, previous studies found that the sex of the animal and the herd size did not affect the prevalence of Cryptosporidium infection [24,34,39].
Cryptosporidium parvum was the predominant species, representing 62.9% of the identified species by RFLP, followed by C. bovis at 5.7% and C. andersoni at 2.9%. Previous studies reported the predominance of C. parvum in preweaned calves globally [11,15,40,41,42,43,44]. However, C. andersoni is mainly reported in postweaned and adults, more than in preweaned calves. It infects the abomasum and causes maldigestion, weight loss, and a decrease in milk yield [45,46,47]. In this study, C. andersoni was identified in one preweaned calf. A similar result was obtained previously; C. andersoni was detected in 1% (one 4-week-old calf/161) of Cryptosporidium spp. isolated from preweaned calves in the USA [40]. Kváč et al. [41] reported that C. andersoni was identified in 13% (21/161) of dairy calves of up to two months of age, suggesting that direct contact with adult animals could be a risk factor for C. andersoni infection. Although most researchers declared that C. bovis are found predominantly in postweaned calves and never reported clinical disease in different age groups of infected cattle [3], C. bovis oocyst shedding was observed in a five-day old calf [38].
In the present study, all C. parvum isolates were subtyped as IIaA15G2R1. Similarly, in some Middle Eastern countries, subtype IIa was the only reported subtype family with different alleles identified from cattle [10,13]. Subtypes IIa and IId were found in other countries, with the subtype IIa being more dominant [6,12,43], whereas in Egypt, IId was the predominant subtype over IIa [48,49]. Subtype IId alleles were the only reported subtype from diarrheic calves in Sudan [14].
In Kuwait, a previous study reported that the subtype IId alleles were the most commonly identified in small ruminants [50]. Similar results were recorded in Romania, where most of the examined cattle (86.7%) were infected with subtype IIa, while the ovine isolates belonged to subtype IId [51]. Furthermore, a previous study in Spain recorded that 98% of C. parvum subtypes from small ruminants belonged to subtype IId, suggesting that this subtype is adapted to small ruminants [52]. In contrast, subtype IIa has been reported in cattle worldwide [1]. The distribution of the two subtypes is most likely related to animal management, rather than host adaptation. Cattle and small ruminants are kept in separate areas in Kuwait; thus, cross-transmission is difficult due to this separation. In Turkey, cattle herd mingling during grazing increased the opportunity for Cryptosporidium transmission among herds, resulting in higher genetic diversity and the appearance of many genotypically mixed isolates in animal populations [12]. Additionally, animal movement, such as transportation or importation, may form a parasite population structure in an infected animal population in a given area [53,54].
Cryptosporidium parvum subtype IIaA15G2R1, reported in cattle in this study, is also the predominant allele in cattle in European countries [55]. Thus, the source of Cryptosporidium genotypes and C. parvum subtypes in Kuwait may be Germany and Netherlands, from which the cattle were imported to rebuild livestock after destruction during the Iraqi invasion.
Regarding zoonotic importance, only two studies previously reported C. parvum subtypes in children in Kuwait. Iqbal et al. [56] identified C. parvum IIa as the dominant subtype of Cryptosporidium in diarrheic children. Simultaneously, Sulaiman et al. [5] recorded the C. parvum subtype IIaA15G2R1 as the predominant Cryptosporidium spp. in children in Kuwait. Cryptosporidium parvum infects a broad variety of mammals, and is a significant zoonotic diseases issue [57]. More research is required to fully understand the C. parvum transmission cycle in Kuwait, because its predominance in Kuwaiti children suggests an animal to human transfer, particularly when subtyping findings are taken into account.
5. Conclusions
In conclusion, calf age and farm hygiene were the most significant risk factors for Cryptosporidiosis in preweaned calves in Kuwait. Calves less than one month of age were more likely to harbor Cryptosporidium, and infection rates reduced progressively with age. Cryptosporidium parvum was the most commonly identified species from preweaned calves less than three months of age, preceded by C. bovis and C. andersoni. All C. parvum gp60 nucleotide sequences were subtype IIaA15G2R1. Since the distribution of Cryptosporidium parvum subtypes in children and cattle is comparable, these animals could be a source of Cryptosporidium infection in Kuwaiti humans. Further study is needed to clarify the Cryptosporidium transmission cycle in Kuwait.
Acknowledgments
The authors would like to thank the Public Authority of Agriculture Affairs and Fish Resources (PAAFR) for supporting the project team throughout the work.
Author Contributions
Conceptualization, N.-E.M.I.A., Q.A.H.M., M.T.A.-S. and M.S.A.; methodology, N.-E.M.I.A. and Q.A.H.M.; formal analysis, N.-E.M.I.A.; funding acquisition, Q.A.H.M.; project administration, M.S.A.; resources, M.T.A.-S.; writing—original draft, N.-E.M.I.A.; writing—review editing, N.-E.M.I.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ethics Committee of the Kuwait Foundation for Advancement of Sciences approved this field study (KFAS-Award Number 2012-1207-04). All methods were carried out per relevant institutional, national, and international guidelines and regulations and the manuscript conform to the journal’s policies.
Informed Consent Statement
Written informed consent was obtained from farm owners prior to sample collection.
Data Availability Statement
The datasets used and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Kuwait Foundation for Advancement of Sciences (KFAS) [grant number 2012-1207-04] to study the epidemiology of cryptosporidiosis and rotavirus infection in livestock in Kuwait.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Chalmers R., Giles M. Zoonotic cryptosporidiosis in the UK—Challenges for control. J. Appl. Microbiol. 2010;109:1487–1497. doi: 10.1111/j.1365-2672.2010.04764.x. [DOI] [PubMed] [Google Scholar]
- 3.Thomson S., Hamilton C., Hope J., Katzer F., Morrison L., Innes E. Bovine cryptosporidiosis: Impact, host-parasite interaction and control strategies. Vet. Res. 2017;48:42. doi: 10.1186/s13567-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson S., Innes E., Jonsson N., Katzer F. Shedding of Cryptosporidium in calves and dams: Evidence of re-infection and shedding of different gp60 subtypes. Parasitology. 2019;146:1404–1413. doi: 10.1017/S0031182019000829. [DOI] [PubMed] [Google Scholar]
- 5.Sulaiman I.M., Hira P.R., Zhou L., Al-Ali F.M., Al-Shelahi F.A., Shweiki H.M., Iqbal J., Khalid N., Xiao L. Unique endemicity of cryptosporidiosis in children in Kuwait. J. Clin. Microbiol. 2005;43:2805–2809. doi: 10.1128/JCM.43.6.2805-2809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazemalhosseini-Mojarad E., Haghighi A., Taghipour N., Keshavarz A., Mohebi S.R., Zali M.R., Xiao L. Subtype analysis of Cryptosporidium parvum and Cryptosporidium hominis isolates from humans and cattle in Iran. Vet. Parasitol. 2011;179:250–252. doi: 10.1016/j.vetpar.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 7.Rahmouni I., Essid R., Aoun K., Bouratbine A. Glycoprotein 60 diversity in Cryptosporidium parvum causing human and cattle cryptosporidiosis in the rural region of Northern Tunisia. Am. J. Trop. Med. Hyg. 2014;90:346–350. doi: 10.4269/ajtmh.13-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FAOSTAT Food and Agriculture Organization of the United Nations (FAO) Statistics Division. 2020. [(accessed on 22 February 2022)]. Available online: https://www.fao.org/faostat/en/#data/QCL.
- 9.Tanriverdi S., Markovics A., Arslan M.O., Itik A., Shkap V., Widmer G. Emergence of distinct genotypes of Cryptosporidium parvum in structured host populations. Appl. Environ. Microbiol. 2006;72:2507–2513. doi: 10.1128/AEM.72.4.2507-2513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hijjawi N., Mukbel R., Yang R., Ryan U. Genetic characterization of Cryptosporidium in animal and human isolates from Jordan. Vet. Parasitol. 2016;228:116–120. doi: 10.1016/j.vetpar.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Kassouha M., Soukkarieh C., Alkhaled A. First genotyping of Cryptosporidium spp. in pre-weaned calves, broiler chickens and children in Syria by PCR-RFLP analysis. Vet. Parasitol. 2016;225:86–90. doi: 10.1016/j.vetpar.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Taylan-Ozkan A., Yasa-Duru S., Usluca S., Lysen C., Ye J., Roellig D.M., Feng Y., Xiao L. Cryptosporidium species and Cryptosporidium parvum subtypes in dairy calves and goat kids reared under traditional farming systems in Turkey. Exp. Parasitol. 2016;170:16–20. doi: 10.1016/j.exppara.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Benhouda D., Hakem A., Sannella A.R., Benhouda A., Cacciò S.M. First molecular investigation of Cryptosporidium spp. in young calves in Algeria. Parasite. 2017;24:15. doi: 10.1051/parasite/2017014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taha S., Elmalik K., Bangoura B., Lendner M., Mossaad E., Daugschies A. Molecular characterization of bovine Cryptosporidium isolated from diarrheic calves in the Sudan. Parasitol. Res. 2017;116:2971–2979. doi: 10.1007/s00436-017-5606-8. [DOI] [PubMed] [Google Scholar]
- 15.Naguib D., El-Gohary A.H., Mohamed A.A., Roellig D.M., Arafat N., Xiao L. Age patterns of Cryptosporidium species and Giardia duodenalis in dairy calves in Egypt. Parasitol. Int. 2018;67:736–741. doi: 10.1016/j.parint.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Xiao L., Singh A., Limor J., Graczyk T.K., Gradus S., Lal A. Molecular characterization of Cryptosporidium oocysts in samples of raw surface water and wastewater. Appl. Environ. Microbiol. 2001;67:1097–1101. doi: 10.1128/AEM.67.3.1097-1101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y., Ortega Y., He G., Das P., Xu M., Zhang X., Fayer R., Gatei W., Cama V., Xiao L. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Vet. Parasitol. 2007;144:1–9. doi: 10.1016/j.vetpar.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Mosier D.A., Oberst R.D. Cryptosporidiosis: A global challenge. Ann. N. Y. Acad. Sci. 2000;916:102–111. doi: 10.1111/j.1749-6632.2000.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 19.Cho Y.I., Yoon K.J. An overview of calf diarrhea-infectious etiology. J. Vet. Sci. 2014;15:1–17. doi: 10.4142/jvs.2014.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shafieyan H., Alborzi A., Hamidinejat H., Tabandeh M.R., Hajikolaei M.R. Prevalence of Cryptosporidium spp. in ruminants of Lorestan province, Iran. J. Parasit. Dis. 2016;40:1165–1169. doi: 10.1007/s12639-014-0642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alali F., Jawad M.K., Al-Khayat K. Direct Detection of Cryptosporidium spp. in Cattle in Karbala Province and its Environs. Int. J. Vet. Anim. Res. IJVAR. 2021;4:87–91. [Google Scholar]
- 22.Díaz P., Navarro E., Remesar S., García-Dios D., Martínez-Calabuig N., Prieto A., López-Lorenzo G., López C.M., Panadero R., Fernández G., et al. The age-related Cryptosporidium species distribution in asymptomatic cattle from north-western Spain. Animals. 2021;11:256. doi: 10.3390/ani11020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yildirim A., Sevinc F., Önder Z., Düzlü Ö., Ekici O.D., Isik N., Çiloğlu A., Şimşek E., Yetişmiş G., Inci A. Comparison of three diagnostic methods in the diagnosis of cryptosporidiosis and 60 subtyping of in diarrheic calves in Central Anatolia Region of Turkey. EuroBiotech. J. 2021;5:63–69. doi: 10.2478/ebtj-2021-0010. [DOI] [Google Scholar]
- 24.Ebiyo A., Haile G. Prevalence and Factors Associated with Cryptosporidium Infection in Calves in and around. Vet. Med. Int. 2022;2022:1468242. doi: 10.1155/2022/1468242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Zubaidi M.T.S. Prevalence of some Cryptosporidium species in cattle in Baghdad, Iraq. AL-Qadisiyah J. Vet. Med. Sci. 2012;11:177–182. doi: 10.29079/vol11iss2art207. [DOI] [Google Scholar]
- 26.Shaw H.J., Armstrong C., Uttley K., Morrison L.J., Innes E.A., Katzer F. Genetic diversity and shedding profiles for Cryptosporidium parvum in adult cattle and their calves. Curr. Res. Parasitol. Vector-Borne Dis. 2021;1:100027. doi: 10.1016/j.crpvbd.2021.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markovitz A.R., Goldstick J.E., Levy K., Cevallos W., Mukherjee B., Trostle J.A., Eisenberg J.N. Where science meets policy: Comparing longitudinal and cross-sectional designs to address diarrhoeal disease burden in the developing world. Int. J. Epidemiol. 2012;41:504–513. doi: 10.1093/ije/dyr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weitzel T., Dittrich S., Möhl I., Adusu E., Jelinek T. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin. Microbiol. Infect. 2006;12:656–659. doi: 10.1111/j.1469-0691.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- 29.Chalmers R.M., Campbell B.M., Crouch N., Charlett A., Davies A.P. Comparison of diagnostic sensitivity and specificity of seven Cryptosporidium assays used in the UK. J. Med. Microbiol. 2011;60:1598–1604. doi: 10.1099/jmm.0.034181-0. [DOI] [PubMed] [Google Scholar]
- 30.Papini R., Bonelli F., Montagnani M., Sgorbini M. Evaluation of three commercial rapid kits to detect Cryptosporidium parvum in diarrhoeic calf stool. Ital. J. Anim. Sci. 2018;17:1059–1064. doi: 10.1080/1828051X.2018.1452055. [DOI] [Google Scholar]
- 31.Soltane R., Guyot K., Dei-Cas E., Ayadi A. Prevalence of Cryptosporidium spp. (Eucoccidiorida: Cryptosporiidae) in seven species of farm animals in Tunisia. Parasite. 2007;14:335–338. doi: 10.1051/parasite/2007144335. [DOI] [PubMed] [Google Scholar]
- 32.Fayer R., Santín M., Trout J. Prevalence of Cryptosporidium species and genotypes in mature dairy cattle on farms in eastern United States compared with younger cattle from the same locations. Vet. Parasitol. 2007;145:260–266. doi: 10.1016/j.vetpar.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Abebe R., Wossene A., Kumsa B. An epidemiological study of Cryptosporidium infection in dairy calves on selected dairy farms of central Ethiopia. Revue Méd. Vét. 2008;159:107–111. [Google Scholar]
- 34.Ayele A., Seyoum Z., Leta S. Cryptosporidium infection in bovine calves: Prevalence and potential risk factors in northwest Ethiopia. BMC Res. Notes. 2018;11:105. doi: 10.1186/s13104-018-3219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Khodery S.A., Osman S.A. Cryptosporidiosis in buffalo calves (Bubalus bubalis): Prevalence and potential risk factors. Trop. Anim. Health Prod. 2008;40:419–426. doi: 10.1007/s11250-007-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wade S., Mohammed H., Schaaf S. Prevalence of Giardia sp., Cryptosporidium parvum and Cryptosporidium muris (C. andersoni) in 109 dairy herds in five counties of southeastern New York. Vet. Parasitol. 2000;93:1–11. doi: 10.1016/S0304-4017(00)00337-X. [DOI] [PubMed] [Google Scholar]
- 37.Castro-Hermida J.A., González-Losada Y.A., Ares-Mazás E. Prevalence of and risk factors involved in the spread of neonatal bovine cryptosporidiosis in Galicia (NW Spain) Vet. Parasitol. 2002;106:1–10. doi: 10.1016/S0304-4017(02)00036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Åberg M., Emanuelson U., Troell K., Björkman C. Infection dynamics of Cryptosporidium bovis and Cryptosporidium ryanae in a Swedish dairy herd. Vet. Parasitol. 2019;276:100010. doi: 10.1016/j.vpoa.2019.100010. [DOI] [PubMed] [Google Scholar]
- 39.Abdullah D.A., Ola-Fadunsin S.D., Ruviniyia K., Gimba F.I., Chandrawathani P., Lim Y., Jesse F., Sharma R. Molecular detection and epidemiological risk factors associated with Cryptosporidium infection among cattle in Peninsular Malaysia. Food Waterborne Parasitol. 2019;14:e00035. doi: 10.1016/j.fawpar.2019.e00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santín M., Trout J.M., Xiao L., Zhou L., Greiner E., Fayer R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 2004;122:103–117. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Kváč M., Hromadová N., Květoňová D., Rost M., Sak B. Molecular characterization of Cryptosporidium spp. in pre-weaned dairy calves in the Czech Republic: Absence of C. ryanae and management-associated distribution of C. andersoni, C. bovis and C. parvum subtypes. Vet. Parasitol. 2011;177:378–382. doi: 10.1016/j.vetpar.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 42.Yildirim A., Adanir R., Inci A., Yukari B.A., Duzlu O., Onder Z., Ciloglu A., Simsek E. Prevalence and genotyping of bovine Cryptosporidium species in the Mediterranean and Central Anatolia Region of Turkey. Comp. Immunol. Microbiol. Infect. Dis. 2020;69:101425. doi: 10.1016/j.cimid.2020.101425. [DOI] [PubMed] [Google Scholar]
- 43.Yasur-Landau D., Zilberberg M., Perry Markovich M., Behar A., Fleiderovitz L., Leszkowicz Mazuz M. Cryptosporidium parvum subtypes from diarrheic dairy calves in Israel. Vet. Parasitol. Reg. Stud. Rep. 2021;25:100608. doi: 10.1016/j.vprsr.2021.100608. [DOI] [PubMed] [Google Scholar]
- 44.Hoque S., Mavrides D.E., Pinto P., Costas S., Begum N., Azevedo-Ribeiro C., Liapi M., Kváč M., Malas S., Gentekaki E., et al. High occurrence of zoonotic subtypes of Cryptosporidium parvum in Cypriot dairy farms. Microorganisms. 2022;10:531. doi: 10.3390/microorganisms10030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindsay D.S., Upton S.J., Owens D.S., Morgan U.M., Mead J.R., Blagburn B.L. Cryptosporidium andersoni n. sp. (Apicomplexa: Cryptosporiidae) from cattle, Bos taurus. J. Eukaryot. Microbiol. 2000;47:91–95. doi: 10.1111/j.1550-7408.2000.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 46.Kváč M., Vitovec J. Prevalence and pathogenicity of Cryptosporidium andersoni in one herd of beef cattle. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2003;50:451–457. doi: 10.1046/j.0931-1793.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- 47.Ralston B., Thompson R.C., Pethick D., McAllister T.A., Olson M.E. Cryptosporidium andersoni in Western Australian feedlot cattle. Aust. Vet. J. 2010;88:458–460. doi: 10.1111/j.1751-0813.2010.00631.x. [DOI] [PubMed] [Google Scholar]
- 48.Amer S., Honma H., Ikarashi M., Tada C., Fukuda Y., Suyama Y., Nakai Y. Cryptosporidium genotypes and subtypes in dairy calves in Egypt. Vet. Parasitol. 2010;169:382–386. doi: 10.1016/j.vetpar.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Helmy Y.A., Krücken J., Nöckler K., von Samson-Himmelstjerna G., Zessin K.H. Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet. Parasit. 2013;193:15–24. doi: 10.1016/j.vetpar.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Majeed Q.A.H., El-Azazy O.M.E., Abdou N.M.I., Al-Aal Z.A., El-Kabbany A.I., Tahrani L., AlAzemi M.S., Wang Y., Feng Y., Xiao L. Epidemiological observations on cryptosporidiosis and molecular characterization of Cryptosporidium spp. in sheep and goats in Kuwait. Parasitol. Res. 2018;117:1631–1636. doi: 10.1007/s00436-018-5847-1. [DOI] [PubMed] [Google Scholar]
- 51.Imre K., Dărăbuş G., Mederle N., Oprescu I., Morariu S., Ilie M., Hotea I., Imre M., Indre D., Balint A., et al. Intraspecific characterization of some Cryptosporidium parvum isolates from calves and lambs in Western Romania using molecular techniques. Sci. Parasitol. 2010;11:47–50. [Google Scholar]
- 52.Quílez J., Torres E., Chalmers R.M., Hadfield S.J., Del Cacho E., Sánchez-Acedo C. Cryptosporidium genotypes and subtypes in lambs and goat kids in Spain. Appl. Environ. Microbiol. 2008;74:6026–6031. doi: 10.1128/AEM.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng M., Wilson M., Holland R., Meshick S., Lal A., Xiao L. Genetic diversity of Cryptosporidium spp. in cattle in Michigan; implications for understanding the transmission dynamics. Parasitol. Res. 2003;90:175–180. doi: 10.1007/s00436-003-0834-5. [DOI] [PubMed] [Google Scholar]
- 54.De Waele V., Van den Broeck F., Huyse T., McGrath G., Higgins I., Speybroeck N., Berzano M., Raleigh P., Mulcahy G.M., Murphy T.M. Panmictic structure of the Cryptosporidium parvum population in Irish calves: Influence of prevalence and host movement. Appl. Environ. Microbiol. 2013;79:2534–2541. doi: 10.1128/AEM.03613-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imre K., Dărăbuş G. Distribution of Cryptosporidium species, genotypes and C. parvum subtypes in cattle in European countries. Sci. Parasitol. 2011;12:1–9. [Google Scholar]
- 56.Iqbal J., Khalid N., Hira P. Cryptosporidiosis in kuwaiti children: Association of clinical characteristics with Cryptosporidium species and subtypes. J. Med. Microbiol. 2011;60:647–652. doi: 10.1099/jmm.0.028001-0. [DOI] [PubMed] [Google Scholar]
- 57.Pumipuntu N., Piratae S. Cryptosporidiosis: A zoonotic disease concern. Vet. World. 2018;11:681–686. doi: 10.14202/vetworld.2018.681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.