Abstract
Background
Understanding mosquito biting behaviours is important for designing and evaluating protection methods against nuisance biting and mosquito-borne diseases (e.g. dengue, malaria and zika). We investigated the preferred biting sites by Aedes aegypti and Anopheles arabiensis on adult volunteers in standing or sleeping positions; and estimated the theoretical protection limits affordable from protective clothing or repellent-treated footwear.
Methods
Adult volunteers dressed in shorts and t-shirts were exposed to infection-free laboratory-reared mosquitoes inside screened chambers from 6am to noon (for day-biting Ae. aegypti) or 6pm to midnight (night-biting An. arabiensis). Attempted bites on different body parts were recorded. Comparative observations were made on same volunteers while wearing sandals treated with transfluthrin, a vapour-phase pyrethroid that kills and repels mosquitoes.
Results
An. arabiensis bites were mainly on the lower limbs of standing volunteers (95.9% of bites below the knees) but evenly-distributed over all exposed body surfaces when the volunteers were on sleeping positions (only 28.8% bites below knees). Ae. aegypti bites were slightly concentrated on lower limbs of standing volunteers (47.7% below knees), but evenly-distributed on sleeping volunteers (23.3% below knees). Wearing protective clothing that leave only hands and head uncovered (e.g. socks + trousers + long-sleeved shirts) could theoretically prevent 78–83% of bites during sleeping, and at least 90% of bites during non-sleeping hours. If the feet are also exposed, protection declines to as low as 36.3% against Anopheles. The experiments showed that transfluthrin-treated sandals reduced An. arabiensis by 54–86% and Ae. aegypti by 32–39%, but did not change overall distributions of bites.
Conclusion
Biting by An. arabiensis and Ae. aegypti occur mainly on the lower limbs, though this proclivity is less pronounced in the Aedes species. However, when hosts are on sleeping positions, biting by both species is more evenly-distributed over the exposed body surfaces. High personal protection might be achieved by simply wearing long-sleeved clothing, though protection against Anopheles particularly requires covering of feet and lower legs. The transfluthrin-treated footwear can reduce biting risk, especially by An. arabiensis. These findings could inform the design and use of personal protection tools (both insecticidal and non-insecticidal) against mosquitoes and mosquito-borne diseases.
Background
Vector-borne diseases are widespread across the globe and are a major cause of public health and economic failures affecting millions. The most prevalent of these diseases are malaria and dengue fever, which are transmitted by Anopheles and Aedes mosquitoes, respectively [1] While vector control has contributed significantly to malaria control in Africa [2], both dengue fever and other Aedes-borne diseases remain highly neglected in the continent. Despite recent successes with the use of Wolbachia endosymbionts [3], the control of Aedes-borne viruses still relies mostly on personal protection measures [4, 5].
Successful transmission of mosquito-borne pathogens is mediated by the blood-feeding habits of female mosquitoes, which may express preferences for specific blood hosts [6]. To acquire a blood meal, the host-seeking females must successfully locate and bite their hosts. They identify human hosts by detecting specific cues in the environment before biting at selected sites [7]. The mosquitoes rely on a variety of environmental and host-derived stimuli such as visual cues, moisture, heat, carbon dioxide and odours from skin emanations [8, 9]. Since female mosquitoes depend on blood meals for eggs development [10], these man-vector contacts are a vital component of the disease transmission process. Efficient vectors of human pathogens therefore tend to live near humans and can develop high degrees of anthropophily and anthropophagy.
Once mosquitoes have reached humans, their actual landing sites and the resulting distribution of biting are evidently non-random, as some body parts receive more bites than others [11]. It has been shown that malaria vectors, such as Anopheles arabiensis, An. funestus, An. gambiae often bite mostly on the feet and ankles of people sitting upright but this preference diminishes when people lie down [9, 11, 12]. On the other hand, Aedes aegypti, Ae. simpsoni and Ae. atroparvus prefer biting around the head and shoulders [8], while Ae. albopictus prefers biting around the feet [13]. Whilst scale up of the core vector control tools, long-lasting insecticidal nets (LLINs) and indoor residual sprays (IRS) have reduced the burden of mosquito-borne diseases such as malaria [2, 14] further progress is hampered by several factors, among them, the rise of physiological [15] and behavioural resistance [16, 17]. This calls for additional protection, including those suitable for use outdoors and when people are outside bed nets [18]. Passive spatial repellents are being developed to address these gaps and have the advantage of simultaneously protecting multiple people by deterring, inhibiting feeding, and at times killing mosquito vectors [19–21].
In a recent study by Braack et al [11], who demonstrated the differential bite distribution of mosquito species over volunteer bodies, the authors recommended that certain forms of protection such as protective clothing and insecticide-treated footwear may reduce biting risk. Since then, prototype repellent sandals have been demonstrated to reduce overall biting risk under experimental conditions [22] though no studies have been done to illustrate whether the sandals influence mosquito behaviours. Such sandals, if used alongside ITNs, have the potential of conferring round-the-clock protection since footwear are already commonplace and are used most times.
This current study therefore investigated the preferred biting sites by both the dengue vector, Ae. aegypti and the malaria vector, An. arabiensis on adult male volunteers in standing or sleeping positions, and further estimated protection limits affordable by either protective clothing or the repellent-treated footwear.
Methods
Semi-field system
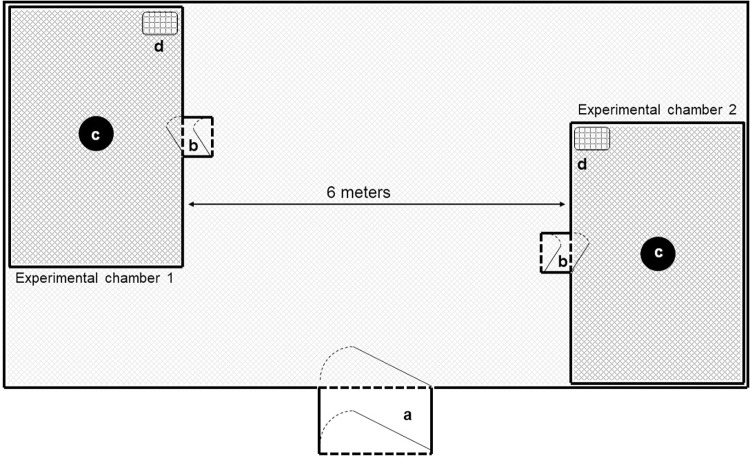
The study was conducted at the Ifakara Health Institute’s semi-field facility located in Ifakara, Tanzania. The facility has three chambers each measuring 9.6m wide × 21m long) [23], and one of which was used for this study. Two large experimental cages (6m wide × 6m long × 2.8m high), made of fiberglass netting and PVC flooring were erected 6m apart inside the semi-field chamber (Fig 1). It is inside these fibreglass netting cages that the actual experiments were conducted.
Fig 1.
Illustration of the experimental chambers inside the semi-field facility, showing: a) Entry into the main chamber; b) Entries into the experimental cages; c) Volunteer stations and d) Mosquito release points.
Mosquitoes
Laboratory-reared nulliparous 4–9 days old An. arabiensis, and Ae. aegypti mosquitoes, starved for six hours prior to experimentation, were used. These infection-free mosquito colonies were maintained using standard procedures as previously described [23, 24].
Study volunteers
Adult male volunteers (25–36 years old) were involved in the study. The volunteers were recruited upon providing a written informed consent once the purpose, benefits and potential risks of the study had been explained to them. They were instructed not to use any fragranced soap or perfume, tobacco or alcohol throughout the experiment period.
Transfluthrin-treated sandals
We used the modified design of repellent-treated sandals from previously described by Sangoro et al [22]. The sandals had an active surface area of 395cm2 each and were made of hessian (Fig 2). They were treated using a 10% transfluthrin solution to achieve 0.04 g/cm2. The treated sandals were dried and wrapped in aluminium foil, and were stored after every experiment. The sandals were stored under the shade to minimize the wear out of the insecticide.
Fig 2. A pair of transfluthrin-treated sandals.
Study design
Four volunteers working in two pairs were involved in the experiment. Two of the volunteers were the actual test subjects, while the other two collected mosquitoes landing on the test subjects. These volunteers were recruited upon consent and trained on the specific procedures for this study. The volunteer pairs occupied separate large cages (Fig 1) and worked together throughout the experiment. In each pair of volunteers, the test subject wore only short trousers and a short-sleeved t-shirt, while the second volunteer, i.e. the mosquito collector, wore closed shoes, long-sleeved shirt, long-trousers and gloves to prevent mosquito bites. This fully-clothed volunteer monitored and collected the mosquitoes landing on his colleague, the test subject. Using this approach, we observed the distribution of landing sites of the released mosquitoes on the bodies of the test volunteers when they were either lying down horizontally on a flat bed or standing upright. The experiments were completed with the test volunteers either wearing or not-wearing a transfluthrin treated sandal.
Observations were done for six hours starting either early morning (for day-biting Ae. aegypti; 06:00am to 12:00 noon) or early evening (for night-biting An. arabiensis; 06:00pm to 12:00 mid-night). During each experimental replicate, 100 sugar-starved female mosquitoes (4–9 day old nulliparous) were released in each of the experimental cages (Fig 1), 50 mosquitoes at the beginning and another 50 after three hours. The first releases were done just before the volunteers entered the chambers and were left for 10 minutes to acclimatize with the environment. The volunteers collected mosquitoes for 45 minutes and rested for 15 minutes of each hour of the experiment. The collector tallied all observed mosquito landings by body part. The landing mosquitoes were captured using mouth aspirators and their locations considered a proxy for actual biting sites.
The observations were replicated for at least 20 days (Ae. aegypti) and 20 nights (An. arabiensis) for each of the following set-ups: a) tests involving volunteers in sleeping position and wearing untreated sandals, b) tests involving the volunteers in standing position and wearing untreated sandals, c) tests involving the volunteers in sleeping position and wearing treated sandals, and d) tests involving the volunteers in standing position and wearing treated sandals. The tests without treated sandals were completed before the tests with treated sandals.
Data analysis
Data were analysed using Stata® 15.1 (College Station, TX, USA). Frequencies, percentages, means and 95% confidence intervals were estimated to describe the distribution of mosquito landings on the volunteers for the two species, when the volunteer was either on a sleeping position or upright, as well as when the volunteer, in either of the two positions, was wearing untreated sandals or transfluthrin-treated sandals. A paired t-test was performed to compare bites occurring on different human body parts when the volunteer was lying down and standing upright with untreated sandals and wearing transfluthrin-treated sandals. In this analysis, we counted only the mosquito that were landing on the volunteers who were the test subject of the experiment. The collectors remained fully clothed during the experiments and did not experience any bites, thus they were not included in the analysis.
Ethical consideration
Ethical approval was provided by Institutional Review Board (IRB) of Ifakara Health Institute approval number IHI/IRB/NO: 10–2017; and the Medical Research Coordinating Committee of the National Institute for Medical Research, in Tanzania with approval number NIMR/HQ/R.8a/VOL1X/2555. The permission to publish this study was granted by director general of National Institute of Medical Research in Tanzania (Ref: NIMR/HQ/P.12 VOL XXXIV/18)
Results
General distribution of biting sites by Ae. aegypti
Densities of the observed Ae. aegypti landings (as a proxy for biting) are summarised in Table 1. A total of 4,065 mosquitoes were recaptured in tests where the volunteers were in a sleeping position, and 5,848 mosquitoes recaptured in tests with volunteers in standing position. When in a sleeping position, most of the host-seeking Ae. aegypti mosquitoes landed on the forearms (26.8%) and the rest were distributed generally evenly over the rest of the exposed body surfaces. Cumulative estimates from foot to head show that only 23% of the landings were below the knee (Table 1). On the other hand, when the volunteers were on a standing position, most landings were on the legs followed by forearms, with 47.7% of landings below the knees.
Table 1. Distribution of Aedes aegypti landing sites on bodies of volunteers in sleeping and standing positions.
| Volunteers in sleeping position | Volunteers in standing position | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body part | N | Recaptured | Mean [95% CI] | Distribution (Percentage) | Cumulative Distribution (%) | Recaptured | Mean [95% CI] | Distribution (Percentage) | Cumulative Distribution (%) | Paired t-test p-value |
| Head | 80 | 522 | 6.5 [5.5–7.6] | 12.8 | 100.0 | 291 | 3.6 [3.0–4.3] | 5.0 | 100.0 | <0.001 |
| Hand | 80 | 361 | 4.5 [3.8–5.2] | 8.9 | 87.2 | 290 | 3.6 [2.9–4.3] | 5.0 | 95.0 | 0.0769 |
| Fore arm | 80 | 1,089 | 13.6 [12.0–15.2] | 26.8 | 78.3 | 995 | 12.4 [11.3–13.6] | 17.0 | 90.1 | 0.2143 |
| Upper arm | 80 | 467 | 5.8 [4.7–7.0] | 11.5 | 51.5 | 332 | 4.2 [3.4–4.9] | 5.7 | 73.1 | 0.0115 |
| Torso | 80 | 155 | 1.9 [1.4–2.5] | 3.8 | 40.0 | 329 | 4.1 [3.3–4.9] | 5.6 | 67.4 | <0.001 |
| Upper leg | 80 | 534 | 6.7 [5.4–7.9] | 13.1 | 36.2 | 821 | 10.3 [9.0–11.5] | 14.0 | 61.7 | <0.001 |
| Lower leg | 80 | 613 | 7.7 [6.3–9.1] | 15.1 | 23.1 | 1,713 | 21.4 [18.8–24.0] | 29.3 | 47.7 | <0.001 |
| Foot | 80 | 324 | 4.1 [2.8–5.3] | 8.0 | 8.0 | 1,077 | 13.5 [10.9–16.1] | 18.4 | 18.4 | <0.001 |
| 4065 | 5848 | -43.9% | ||||||||
Generally, there were 44% fewer mosquitoes observed on volunteers while on standing compared to sleeping positions. The paired t-tests revealed significantly fewer landings on standing volunteers for all exposed body parts except hand (p = 0.078), forearms (p = 0.2143) and upper arm (p = 0.0115).
General distribution of biting sites by An. arabiensis
Results for biting distribution by An. arabiensis are summarized in Table 2. A total of 2,754 mosquitoes were recaptured in tests where the volunteers were in a standing position, and 3,057 when the volunteers were in sleeping position. In the sleeping position, most of the host-seeking An. arabiensis landed on the forearms (26.7%) and the rest were distributed evenly over other exposed body surfaces. Only 28.8% of the landings were below the knee (Table 2). On the other hand, when the volunteers were standing, nearly all the landings occurred on the legs (95.9% on the foot and lower leg). There was a 10% increase in mosquitoes landing on standing volunteers compared to sleeping volunteers. The paired t-tests revealed significantly more landings on standing volunteers for all exposed body parts (p ≤ 0.01).
Table 2. Distribution of An. arabiensis landing sites on bodies of volunteers in sleeping and standing positions.
| Volunteers in sleeping position | Volunteers in standing position | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body part | Recaptured | Mean [95% CI] | Distribution (Percentage) | Cumulative Distribution (%) | Recaptured | Mean [95% CI] | Distribution (Percentage) | Cumulative Distribution (%) | Paired t-test | |
| p-value | ||||||||||
| Head | 80 | 169 | 2.1 [1.6–2.6] | 5.5 | 100.0 | 2 | 0.0 [0.0–0.1] | 0.1 | 100.0 | <0.001 |
| Hand | 80 | 359 | 4.5 [3.5–5.4] | 11.7 | 94.5 | 2 | 0.0 [0.0–0.1] | 0.1 | 99.9 | <0.001 |
| Fore arm | 80 | 817 | 10.2 [8.9–11.5] | 26.7 | 82.7 | 21 | 0.3 [0.0–0.5] | 0.8 | 99.9 | <0.001 |
| Upper arm | 80 | 302 | 3.8 [2.9–4.6] | 9.9 | 56.0 | 0 | 0 | 0.0 | 99.1 | <0.001 |
| Torso | 80 | 111 | 1.4 [1.0–1.8] | 3.6 | 46.1 | 1 | 0 | 0.0 | 99.1 | <0.001 |
| Upper leg | 80 | 418 | 5.2 [4.1–6.4] | 13.7 | 42.5 | 88 | 1.1 [0.5–1.7] | 3.2 | 99.1 | <0.001 |
| Lower leg | 80 | 594 | 7.4 [6.0–8.9] | 19.4 | 28.8 | 891 | 11.1 [8.3–14.0] | 32.4 | 95.9 | 0.0087 |
| Foot | 80 | 287 | 3.6 [2.6–4.6] | 9.4 | 9.4 | 1,749 | 21.9 [16.9–26.8] | 63.5 | 63.5 | <0.001 |
| 3057 | 2754 | 9.9% | ||||||||
Theoretical estimates of protection by protective clothing
We have estimated these theoretical protection measures from Tables 1 and 2, assuming that clothing, even if non-insecticidal will directly prevent bites on the covered surfaces. An. arabiensis bites were mainly on the lower limbs of standing volunteers. In this case, up to 95.9% were below the knees suggesting that protective clothing on these regions would greatly reduce biting. However, biting by the same species were evenly-distributed over all exposed body surfaces when the volunteers were on sleeping positions, as only 28.8% of the bites were below knees. For Ae. aegypti, the bites were marginally concentrated below the knees of standing volunteer, but evenly-distributed on sleeping volunteers.
Based on these estimates and the assumptions of physical barrier protection, wearing protective clothing that leave only hands and head uncovered, for example socks + trousers + long-sleeved shirts, can prevent 78–83% of all bites during sleeping, and 90–99.9% of all bites during non-sleeping hours. However, if the feet are also exposed (e.g. when people are wearing sandals and no socks, then the protection declines to as low as 36.3% against Anopheles during non-sleeping hours and to 70–73% in all other tested situations with either Anopheles in sleeping position or Aedes in both sleeping and non-sleeping positions.
Effect of transfluthrin-treated sandals on biting distribution and densities
For Ae. aegypti approaching a sleeping volunteer, transfluthrin-treated sandals reduced potential bites over the whole body by 38.8%, without markedly changing the actual distribution of the landing sites relative to sleeping volunteers with untreated sandals. Statistically-significant reductions were observed on the feet (by 98%; p<0.001), lower leg (74%; p<0.001), upper leg (58%; p<0.001) and hands (36%; p = 0.0065), but not head or upper arms (Table 3). We also observed the change in biting pattern by Ae aegypti when the volunteer wore sandals, as most of the bites shifted from the lower limb to the upper legs and torso. Similarly, the transfluthrin-treated sandals reduced overall Ae. aegypti landings on standing volunteers by 32.2%, though statistically significant reductions occurred only on the lower legs (38%; p<0.001) and feet (78%; p<0.001) (Table 3).
Table 3. Mean number of Aedes aegypti caught at different body parts of volunteers with and without the transfluthrin-treated sandals.
| Body part | N | Volunteers on a sleeping position | Paired t-test p-value | ||||||
| Without Sandals | With Sandals | % Reduction | |||||||
| Recaptured | Mean [95% CI] | Recaptured | Mean [95% CI] | ||||||
| Head | 40 | 221 | 5.5 [4.1–6.9] | 301 | 7.5 [5.9–9.1] | 0.0602 | -36 | ||
| Hand ++ | 40 | 220 | 5.5 [4.3–6.6] | 141 | 3.5 [2.8–4.2] | 0.0065 | 36 | ||
| Fore arm | 40 | 600 | 15 [12.9–17.1] | 489 | 12.2 [9.9–14.6] | 0.0775 | 19 | ||
| Upper arm | 40 | 226 | 5.7 [3.8–7.5] | 241 | 6.0 [4.6–7.4] | 0.7523 | -7 | ||
| Torso | 40 | 76 | 1.9 [1.0–2.8] | 79 | 2.0 [1.3–2.6] | 0.9039 | -4 | ||
| Upper leg * | 40 | 375 | 9.4 [7.5–11.2] | 159 | 4.0 [2.7–5.2] | <0.001 | 58 | ||
| Lower leg * | 40 | 485 | 12.1 [10.3–13.9] | 128 | 3.2 [2.3–4.1] | <0.001 | 74 | ||
| Foot * | 40 | 319 | 8.0 [6.3–9.7] | 5 | 0.1 [0.0–0.2] | <0.001 | 98 | ||
| Volunteers on standing position | |||||||||
| N | Without Sandals | With Sandals | Paired t-test p-value | % Reduction | |||||
| Recaptured | Mean [95% CI] | Recaptured | Mean [95% CI] | ||||||
| Head | 40 | 147 | 3. 7 [2.7–4.6] | 144 | 3.6 [2.8–4.4] | 0.8758 | 2 | ||
| Hand | 40 | 167 | 4.2 [3.0–5.3] | 123 | 3.1 [2.3–3.8] | 0.0645 | 26 | ||
| Fore arm | 40 | 521 | 13.0 [11.4–14.7] | 474 | 11.9 [10.2–13.5] | 0.2462 | 9 | ||
| Upper arm | 40 | 175 | 4.3 [3.2–5.5] | 157 | 3.9 [3.0–4.8] | 0.5026 | 10 | ||
| Torso | 40 | 160 | 4.0 [3.0–5.0] | 169 | 4.2 [2.9–5.5] | 0.7555 | -6 | ||
| Upper leg | 40 | 375 | 9.4 [7.8–11.0] | 446 | 11.2 [9.1–13.2] | 0.156 | -19 | ||
| Lower leg * | 40 | 1,058 | 26.5 [23.3–29.6] | 655 | 16.4 [12.8–19.9] | <0.001 | 38 | ||
| Foot * | 40 | 882 | 22.1 [18.8–25.3] | 195 | 4.9 [3.4–6.3] | <0.001 | 78 | ||
# p≤ 0.05
++ p≤0.01
* p≤0.001. Reporting the two-sided p-value of paired T-test.
N–total number of replicates, we had two volunteers in two different chambers thus each volunteer had 20 replicates per treatment per position. Recaptured–Total number of mosquitoes caught landing on the body part.
For An. arabiensis approaching a sleeping volunteer, transfluthrin-treated sandals reduced potential bites over the whole body by 54.1%, without changing the actual distribution of the landing sites (Table 4). Statistically-significant reductions were observed on all exposed body parts except head, torso and upper arms. The transfluthrin-treated sandals had a much greater effect on when the volunteers were standing, as overall reduction of An. arabiensis landings reached 85.7%. Here, statistically significant reductions occurred everywhere except head, hands and upper arms (Table 4).
Table 4. Mean number of Anopheles arabiensis caught at different body parts of volunteers with and without the transfluthrin-treated sandals.
| Volunteers on a sleeping position | |||||||||
| Body part | N | Without sandals | With Sandals | Paired t-test p-value | % Reduction | ||||
| Recaptured | Mean [95% CI] | Recaptured | Mean [95% CI] | ||||||
| Head # | 40 | 64 | 1.6 [1.0–2.2] | 105 | 2.6 [1.8–3.4] | 0.0452 | -64 | ||
| Hand * | 40 | 252 | 6.3 [4.8–7.8] | 107 | 2.7 [1.8–3.6] | <0.001 | 57 | ||
| Fore arm # | 40 | 471 | 11.8 [10.1–13.5] | 346 | 8.7 [6.8–10.5] | 0.026 | 27 | ||
| Upper arm | 40 | 180 | 4.5 [3.1–5.9] | 122 | 3.1 [2.0–4.1] | 0.1353 | 32 | ||
| Torso | 40 | 63 | 1.6 [0.9–2.3] | 48 | 1.2 [0.7–1.7] | 0.381 | 24 | ||
| Upper leg * | 40 | 317 | 7.9 [6.1–9.7] | 101 | 2.5 [1.7–3.3] | <0.001 | 68 | ||
| Lower leg * | 40 | 467 | 11.7 [9.6–13.8] | 127 | 3.2 [2.3–4.1] | <0.001 | 73 | ||
| Foot * | 40 | 280 | 7.0 [5.6–8.4] | 7 | 0.2 [0.0–0.3] | <0.001 | 98 | ||
| N | Volunteers on standing position | Paired t-test p-value | % Reduction | ||||||
| Without sandals | With Sandals | ||||||||
| Recaptured | Mean [95% CI] | Recaptured | Mean [95% CI] | ||||||
| Head | 40 | 0 | 0 | 2 | 0.1 [0.0–0.1] | 0.1599 | _ | ||
| Hand | 40 | 0 | 0 | 2 | 0.1 [0.0–0.1] | 0.1599 | _ | ||
| Fore arm | 40 | 12 | 0.3 [-0.1–0.7] | 9 | 0.2 [0.0–0.5] | 0.7575 | 25 | ||
| Upper arm | 40 | 0 | 0 | 0 | 0 | _ | _ | ||
| Torso | 40 | 1 | 0 | 0 | 0 | _ | 100 | ||
| Upper leg ++ | 40 | 73 | 1.8 [0.6–3.1] | 15 | 0.4 [0.1–0.6] | 0.0217 | 79 | ||
| Lower leg * | 40 | 718 | 18.0 [13.3–22.6] | 173 | 4.3 [2.9–5.8] | <0.001 | 76 | ||
| Foot * | 40 | 1,605 | 40.1 [34.4–45.8] | 144 | 3.6 [2.4–4.8] | <0.001 | 91 | ||
# p≤ 0.05
++ p≤0.01
* p≤0.001. Reporting the two-sided p-value of paired T-test.
N–total number of replicates, we had two volunteers in two different chambers thus each volunteer had 40 replicates per position. Recaptured–Total number of mosquitoes caught landing on the body part.
Discussion
Insecticide treated nets and indoor residual sprays can significantly suppress vector populations and the diseases they transmit [2]. However, gaps have been identified in the personal protection they currently confer. For example, these interventions are not effective against exophagic mosquitoes and cannot protect people during waking and active hours [25]. Moreover, their continuous use can cause changes in the feeding patterns and host preferences of mosquitoes [26–30]. These challenges, indicate the need to continue studying the behaviours of vectors and humans, so as to develop complementary measures to curb any persistent transmission. This current study investigated the preferred biting sites by the dengue vector, Ae. aegypti and the malaria vector, An. arabiensis on adult volunteers in standing or sleeping positions; and estimated protection limits affordable from protective repellent-treated footwear.
Earlier studies on the biting behaviour of vectors have described the preference of different mosquito species to bite on different parts of human body [11, 31]. For example, Culex pipiens mosquitoes were observed to preferentially bite on the lower parts of the body when the host was in sitting position [32] while Ae. aegypti preferred biting all over the exposed body parts [8]. On the other hand, Anopheles mosquitoes, such as those that transmit malaria, typically bite lower parts of the body [11, 12]. Braack et al demonstrated that Anopheles species appear to bite more on the lower limbs when individuals are standing upright but more evenly distributed over exposed body parts whenever the people are lying down [11]. Sangoro et al provides initial evidence that repellent impregnate footwear [22] can provide personal protection against disease transmitting mosquitoes. However this current study found that with transfuthrin treated sandals, the density of An. Arabiensis in the low limbs reduced by 91% when standing upright and 98% when sleeping. This findings gives important evidence that repellent impregnate footwear [22] can provide personal protection against malaria transmitting mosquitoes (An. Arabiensis), even if the sandals do not change the overall distribution of mosquito bites.
Thus, this data is important for advocating the inventions of new interventions that provide personal protections for day biting as well as the night biting mosquitoes. The direct observations of the distribution of bites allows for theoretical estimations of the potential impact of protective clothing, especially those that cover preferred biting areas, such as feet and lower legs. The comparative observations done on both waking and sleeping volunteers also confirms that while the differential proclivity of bites may be useful for designing interventions during waking hours, it is not as useful for sleeping hours.
This study observed that transfluthrin-treated sandals were more effective against malaria vector, An. arabiensis than against the dengue vector, Ae. aegypti. They reduced An. arabiensis bites by 54–86% and Ae. aegypti bites by 32–39%, but did not change overall distributions of bites. Given that current personal protection against mosquito biting is still challenging especially for outdoors and before bedtime [33], such an intervention could contribute effectively to providing complementary protection at times before people go under their bed nets. Previous study suggested the use of tropical repellent [34] for personal protection, despite the occasional limitations such as requiring multiple applications. The spatial repellent products carrying transfluthrin as the main active ingredient so circumvent this challenge by not requiring regular retreatment. This can be especially useful in locations such as rural Tanzania, where for multiple reasons such as lack of electricity and the types of housing, residents spend significant periods of their time in the evening outdoors doing various activities such as cooking, eating, telling stories and other household activities [35–37]. Such outdoor activities put communities at high risk of getting malaria and other mosquito borne diseases [38], and may be addressed at least in part by interventions such as the repellent-treated sandals.
Perhaps the more direct indication of this study was the potential of personal protection. Based on the distribution of bites, the study theoretical calculations suggest that wearing protective clothing that leave only hands and head uncovered (e.g. socks + trousers + long-sleeved shirts) could prevent 78–83% of all bites during sleeping, and at between 90% and 99.9% of the bites during non-sleeping hours. The evidence also suggests that if the feet are also exposed, the actual levels of protection will decline to as low as 36% against Anopheles. It is important therefore that personal protection measures are primarily targeted at the areas where mosquitoes are most likely to bite, especially during waking hours. The advantage of transfluthrin treated sandals is they can offer round the clock protection against the bites. In this case, simple footwear and socks, and where possible the addition of long sleeved clothing and long trousers, can provide significant protection in communities where access to other forms of personal protection is limited. Insecticide-impregnated clothing are already demonstrated to prevent bites, and often require no daily reapplication [39–41]. However, it is likely that even simple physical protection would provide substantial protection already.
It is clear that the repellent sandals were less effective against Aedes mosquitoes than against Anopheles. However, the distribution of bites suggest that it would still be possible to achieve upwards of 70% protection with just protective clothing. To control the day biting Aedes, although topical repellents could be a suitable alternative, these are limited by the fact they need to be reapplied often thus user compliance is attenuated [34, 42, 43]. By combining transfluthrin treated sandals and impregnated clothing will provided a suitable personal protection against Ae. aegypti which shown to bite at both upper and lower parts of the body [44]. As the malaria and other mosquito borne diseases keep remain a challenge, it is the important to consider these innovations for fighting against mosquito borne diseases.
Stakeholders should consider advocating the scaling up of these innovations to reduce the burden of mosquito borne diseases. Social and behaviour change communication (SBCC) is important tool in advocating malaria control and elimination [45]. Previous studies reported that SBCC has been used to informing malaria surveillance, seasonal variation of malaria cases, treatment management of malaria cases and mosquito borne diseases preventions [46]. Since this study has shown the potential of fighting against mosquito biting by targeting their behaviours, SBCC can also be used to communicate these behaviours and the new mechanisms like the use of transfluthrin treated sandals for personal protection against malaria transmission. However protecting footwear and clothes should consider the climatic conditions of all malaria endemic countries and should not be uniform, because the climatic condition differ from one county to another, the countries with hot climatic condition need to wear open shoes as well as light material clothing compared to cold climate. Also the comfortability of the communities that will be using those interventions should be assessed and communicated earlier before the launching the interventions. However SBCC can be used to assess the need, acceptability and opinions of the communities who are the end users of these interventions. Nevertheless SBCC can be a useful mechanism since it can be used even in hard to reach communities to advocate the new interventions.
Conclusions
This study adds to the body of evidence on species differences in distribution of landing and biting sites over human bodies when individuals are in the upright position. And shows that when hosts are sleeping, bites from different species might be evenly-distributed over the exposed body surfaces. This study highlights the importance of insecticide treated and untreated personal protection measures in preventing mosquito bites and pathogen transmission from Anopheline and Aedes mosquitoes. Moreover, that transfluthrin-treated footwear can confer protection against different mosquito species but more against species like An. arabiensis that prefer to feed on the lower limbs of the human body. Since previous studies also demonstrated An. gambiae and An. funestus prefer to bite on the lower parts of the body, especially lower limbs and lower legs, targeted personal protection measures as well as transfluthrin-treated sandals may be potentially effective against these major malaria vectors as well. However, resistance tests should be conducted regularly to ascertain efficacy against the candidate insecticides. Contrarily, simply wearing long-sleeved clothing might suffice in offering the personal protection required. Lastly, using treated personal protection measures on targeted body might shift the biting preferences in some of the host seeking mosquitos as seen for Ae. Aegypti in this study. Thus one should consider using multiple personal protective measures where possible to ensure the entire body is protected.
Acknowledgments
We would like to express our sincere gratitude to our volunteer Moses Mlagani, Bonifas Magwila, Baraka Kidwanga and Ibrahim Mkesa for making this study possible during data collection. The OMC-team for their logistical and technical support during the study; Rukiyah Mohammed, Neema Zephania, Elihaika Minja, Alex Limwagu, Dickson Msaky, Pinda Polianus, Issa Mshani, Emmanuel Happe, Emmanuel Mwanga and Paliga Masalu. Also thanks to our shoes maker at Mozzie house Daniel Mathias and Paulina Mshingo for designing the sandals.
Abbreviations
- LLINs
Long- lasting insecticide-treated bed net
- IRS
indoor residual spray
- ITN
Insecticide treated bed net
- PVC
Polyvinyl chloride
- SBCC
Social and behaviour change communicatios
Data Availability
The relevant data are within the paper.
Funding Statement
This study was funded by United states Agency for International development (USAID) (Grant number AID-OAA-F-16-00093) awarded to FO. Also this study was supported by Wellcome Trust Intermediate Fellowship in Public Health and Tropical medicine (Grant Number: WT 102350/Z/13) and Howard Hughes Medical Institute (HHMI) and Gates foundation (Grant: 0PP1099295) awarded to FOO. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Golding N, Wilson AL, Moyes CL, Cano J, Pigott DM, Velayudhan R, et al. Integrating vector control across diseases. BMC Med. 2015. doi: 10.1186/s12916-015-0491-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015. doi: 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med. Mass Medical Soc; 2021;384: 2177–2186. doi: 10.1056/NEJMoa2030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waewwab P, Sungvornyothin S, Potiwat R, Okanurak K. Impact of dengue-preventive behaviors on Aedes immature production in Bang Kachao, Samut Prakan Province, Thailand: A cross-sectional study. BMC Public Health. 2020;20: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sim S, Ng LC, Lindsay SW, Wilson AL. A greener vision for vector control: The example of the singapore dengue control programme. PLoS Negl Trop Dis. 2020;14: 1–20. doi: 10.1371/journal.pntd.0008428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takken W, Verhulst NO. Host Preferences of Blood-Feeding Mosquitoes. Annu Rev Entomol. 2013;58: 433–453. doi: 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- 7.Dia I, Diallo D, Duchemin J, Ba Y, Konate L, Costantini C, et al. Comparisons of Human-Landing Catches and Odor-Baited Entry Traps for Sampling Malaria Vectors in Senegal. 2005; 104–109. doi: 10.1093/jmedent/42.2.104 [DOI] [PubMed] [Google Scholar]
- 8.De Jong R, Knols BGJ. Selection of biting sites on man by two malaria mosquito species. Entomol Exp Appl. 1995;51: 80–84. [DOI] [PubMed] [Google Scholar]
- 9.Verhulst NO, Weldegergis BT, Menger D, Takken W. Attractiveness of volatiles from different body parts to the malaria mosquito Anopheles coluzzii is affected by deodorant compounds. Nature. 2016; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol.2012;28: 114–121. doi: 10.1016/j.pt.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 11.Braack L, Hunt R, Koekemoer LL, Gericke A, Munhenga G, Haddow AD, et al. Biting behaviour of African malaria vectors: 1. where do the main vector species bite on the human body?. Paracite and Vectors. 2015; 1–10. doi: 10.1186/s13071-015-0677-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dekker T, Takken W, Knols BGJ, Bouman E, Van De Laak S, De Bever A, et al. Selection of biting sites on a human host by Anopheles gambiae s.s., An. arabiensis and An. quadriannulatus. Entomol Exp Appl. 1998. [Google Scholar]
- 13.Shirai Y, Funada H, Kamimura K, Seki T, Morohashi M. Landing sites on the human body preferred by Aedes albopictus. J Am Mosq Control Assoc. 2002;18: 97–99. [PubMed] [Google Scholar]
- 14.World Health Organization. World Malaria Report 2020. World Health. 2020. [Google Scholar]
- 15.Ranson H, Lissenden N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016;32: 187–196. doi: 10.1016/j.pt.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 16.The malERA Consultative Group on Vector Control. A research agenda for malaria eradication: Vector control. PLoS Med. 2011;8: 1–8. doi: 10.1371/journal.pmed.1000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durnez L, Coosemans M. Residual Transmission of Malaria: An Old Issue for New Approaches. INTECH Open. 2011. [Google Scholar]
- 18.Williams YA, Tusting LS, Hocini S, Graves PM, Killeen GF, Kleinschmidt I, et al. Expanding the Vector Control Toolbox for Malaria Elimination: A Systematic Review of the Evidence. Advances in Paracitology.2018. [DOI] [PubMed] [Google Scholar]
- 19.Wouters W, van den Bercken J. Action of pyrethroids. Gen Pharmacol. 1978/01/01. 1978;9: 387–398. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy JS. The excitant and repellent Effects on Mosquitos of sub-lethal Contacts with DDT. Bull Entomol Res. 1947. doi: 10.1017/s0007485300030091 [DOI] [PubMed] [Google Scholar]
- 21.Adams ME, Miller TA. Neural and Behavioral Correlates of Pyrethroid and DDT-Type Poisoning in the House Fly, Musca domestica L. Pesticide Biochemistry and Physiology.1980;147: 137–147. [Google Scholar]
- 22.Sangoro OP, Gavana T, Finda M, Mponzi W, Hape E, Limwagu A, et al. Evaluation of personal protection afforded by repellent-treated sandals against mosquito bites in south-eastern Tanzania. Malar J. 2020;19: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siria DJ, Batista EPA, Opiyo MA, Melo EF, Sumaye RD, Ngowo HS, et al. Evaluation of a simple polytetrafluoroethylene (PTFE) -based membrane for blood-feeding of malaria and dengue fever vectors in the laboratory.Paracite and vectors. 2018; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mmbando AS, Ngowo H, Limwagu A, Kilalangongono M, Kifungo K, Okumu FO. Eave ribbons treated with the spatial repellent, transfluthrin, can e ff ectively protect against indoor-biting and outdoor-biting malaria mosquitoes. Malar J. 2018; 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monroe A, Moore S, Okumu F, Kiware S, Lobo NF, Koenker H, et al. Methods and indicators for measuring patterns of human exposure to malaria vectors. Malar J. 2020;19: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killeen GF, Kiware SS, Okumu FO, Sinka ME, Moyes CL, Claire Massey N, et al. Going beyond personal protection against mosquito bites to eliminate malaria transmission: Population suppression of malaria vectors that exploit both human and animal blood. BMJ Glob Heal. 2017;2. doi: 10.1136/bmjgh-2016-000198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley J, Lines J, Fuseini G, Schwabe C, Monti F, Slotman M, et al. Outdoor biting by Anopheles mosquitoes on Bioko Island does not currently impact on malaria control. Malar J. 2015; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moiroux N, Damien GB, Egrot M, Djenontin A, Chandre F. Human Exposure to Early Morning Anopheles funestus Biting Behavior and Personal Protection Provided by Long-Lasting Insecticidal Nets. PloS one. 2014;9: 8–11. doi: 10.1371/journal.pone.0104967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dekker T, Takken W, Knols BGJ, Bouman E, Laak S Van De, Bever A De, et al. arabiensis and An. quadriannulatus. Entomol Exp Appl. 1998;2: 295–300. [Google Scholar]
- 32.Self LS, Abdulcader MH, Tun MM. Preferred biting sites of Culex pipiens fatigans on adult Burmese males. Bull World Health Organ. World Health Organization; 1969;40: 324. [PMC free article] [PubMed] [Google Scholar]
- 33.Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci U S A. 2019;116: 15086–15096. doi: 10.1073/pnas.1820646116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sangoro O, Kelly AH, Mtali S, Moore SJ. Feasibility of repellent use in a context of increasing outdoor transmission: a qualitative study in rural Tanzania. Malar J. 2014; 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS One. 2019;14: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moshi IR, Ngowo H, Dillip A, Msellemu D, Madumla EP, Okumu FO, et al. Community perceptions on outdoor malaria transmission in Kilombero Valley, Southern Tanzania. Malar J. 2017; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monroe A, Asamoah O, Lam Y, Koenker H, Psychas P, Lynch M, et al. Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prevention Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prev. Malar J. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govella NJ, Ferguson H. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta RK, Sweeney AW, Rutledge LC, Cooper RD, Frances SP, Westrom DR. Effectiveness of controlled-release personal-use arthropod repellents and permethrin-impregnated clothing in the field. J Am Mosq Control Assoc. 1987;3: 556–560. [PubMed] [Google Scholar]
- 40.Pennetier C, Chabi J, Martin T, Chandre F, Rogier C, Hougard J-M, et al. New protective battle-dress impregnated against mosquito vector bites. Parasit Vectors. 2010;3: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Specos MMM, García JJ, Tornesello J, Marino P, Vecchia M Della, Tesoriero MVD, et al. Microencapsulated citronella oil for mosquito repellent finishing of cotton textiles. Trans R Soc Trop Med Hyg. 2010;104: 653–658. doi: 10.1016/j.trstmh.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 42.Yap HH, Jahangir K, Zairi J. Field efficacy of four insect repellent products against vector mosquitoes in a tropical environment. J Am Mosq Control Assoc. 2000;16: 241–244. [PubMed] [Google Scholar]
- 43.Frances SP, Van Dung N, Beebe NW, Debboun M. Field evaluation of repellent formulations against daytime and nighttime biting mosquitoes in a tropical rainforest in northern Australia. J Med Entomol. Oxford University Press Oxford, UK; 2002;39: 541–544. doi: 10.1603/0022-2585-39.3.541 [DOI] [PubMed] [Google Scholar]
- 44.Knols BGJ. Odour-mediated host-seeking behaviour of the Afro-tropical malaria vector Anopheles gambiae Giles. Wageningen University and Research; 1996. [Google Scholar]
- 45.To End Malaria RBMP. The Strategic Framework for Malaria Social and Behaviour Change Communication 2018–2030. US Pres Malar Initiat. 2018. [Google Scholar]
- 46.Koenker H, Keating J, Alilio M, Acosta A, Lynch M, Nafo-Traore F. Strategic roles for behaviour change communication in a changing malaria landscape. Malar J.2014;13: 1–4. doi: 10.1186/1475-2875-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The relevant data are within the paper.