Abstract
Irreversible blindness resulting from retinal or optic nerve diseases continues to devastate patients. The use of stem cell technology to reverse blindness promises great individual and societal benefit. Stem cell therapies seek to replace or enhance the function of damaged tissues. Currently, there are no FDA approved and commercially available products in this arena. Numerous clinical trials are underway, but as of today, we are still awaiting rigorous study data. Meanwhile, physicians and patients are desperate to restore vision and are lured to clinics claiming success. It remains our duty to vet these claims and guide our patients with their best interests in mind.
Introduction
In the future, stem cell therapies may become a mainstay for degenerative diseases of the eye. These therapies are currently being designed, developed, and evaluated in animal models and in some cases, early clinical trials. The promise of such interventions has encouraged the development of “ocular regenerative medicine”, a field which takes advantage of both the relative accessibility and immune privilege of the eye. As any developing field grows, often there are advances and setbacks. This is certainly true in the area of ocular regenerative therapies. As we learn more about the benefits and risks of stem cell-based treatments for ocular disease, regulatory institutions will need to ensure both efficacy and safety of the proposed therapies while still encouraging innovation. This article reviews the current status of intraocular stem cell interventions, as well as some critical precautionary advice. Why? Because we need to be very critical of the state of the field as we attempt to help our patients understand the landscape and prospects for the use of stem cell therapy in the management of ocular disease. As most of the public is aware there already have been instances of misguided physicians who have attempted to side step FDA guidelines on stem cell therapies and put patient safety at risk.
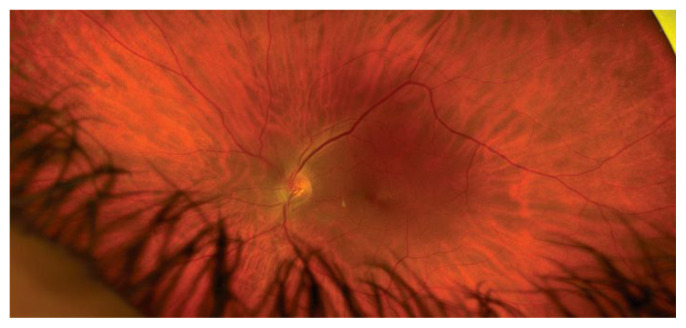
The promise of ocular regenerative medicine derives from the ability of stem cells to differentiate into function-specific cells. In the eye, this means using stem cells to replace tissues damaged by degeneration, trauma or ischemia. In the posterior pole of the eye this means replacing damaged cells in the retina, retinal pigment epithelium, and optic nerve. Less obvious, but of possible great value, is the regenerative potential for stem cells to replace supportive tissues, such as glial cells, which are critical for the normal structure and function of the retina and optic nerve (Figure 1.)
Figure 1.
Normal Retinal Appearance
Types of Stem Cells
One type of stem cell, the pluripotent stem cell, has possibly the greatest potential for the field of ocular regenerative medicine. Pluripotent stem cells are dividing and differentiating most actively during embryonic development, but also in tissues that continually replenish, such as the corneal epithelium. Leveraging the ability of embryonic pluripotent stem cells to develop into numerous tissues would be a natural start when considering tissue replacement therapy. One can think of the use of pluripotent stem cells as using a forward-moving development process to induce cell proliferation into the tissue of interest. Another method for ocular regenerative medicine is to coax partially differentiated cells backward in their developmental lineages and then redirect them onto a different pathway to the desired end tissue. De-differentiation is frequently accomplished through the use of Yamanaka factors, the transcription factors Oct-3/4, Sox2, KLF4 and cMyc. In contrast to embryonic stem cells (ESC), induced pluripotent stem cells (IPS) are derived from mature tissues such as skin or bone marrow that have been treated with the Yamanaka factors. If IPS are harvested from a patient, manipulated for transplant, and re-injected into the donor in an autologous manner, the donor is unlikely to need immunosuppression. However, there is the theoretical risk that these manipulated cells may demonstrate unchecked growth or tumor formation.1, 2
Trials, Methods and Targets
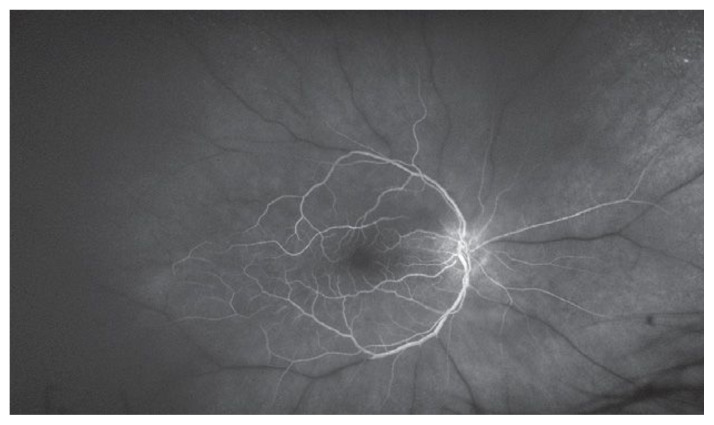
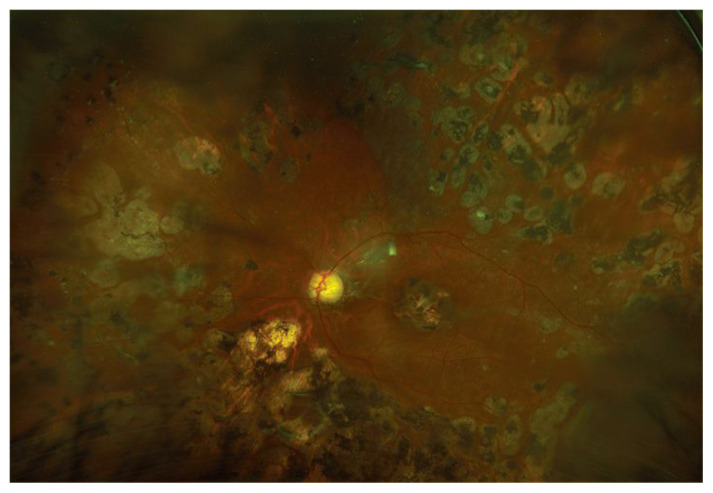
Researchers are testing multiple methods of stem cell delivery for patients with sight threatening diseases. A search of clinicaltrials.gov revealed 150 clinical trials of stem cell-based therapies for eye diseases ranging from dry eye to immune-related retinopathy. Twenty-eight of these trials are currently recruiting patients, 18 in the United States. The remainder of the trials are in various states such as completed, paused, terminated, withdrawn, or suspended. Trials for stem cell treatment of posterior eye disease include approaches to treatment of Age-Related Macular Degeneration, Ischemic Optic Neuropathy, Glaucoma, and Diabetic Retinopathy.3 (Figure 2 and 3) Stem cells have been delivered to study eyes by multiple routes, including intravenous and intraocular injections.
Figure 2.
Massive retinal destruction from laser therapy for proliferative diabetic retinopathy.
Figure 3.
Fluorescein angiography demonstrating massive non-perfusion of retinal vessels due to central retinal artery occlusion. White dye is seen only in patent, perfused area of the retina.
Most clinical trials for the treatment of ocular disease with stem cell therapies have been small, designed to demonstrate feasibility and safety. Some have used patient derived bone marrow CD34+ and mononuclear cells, extracted and then re-injected in an autologous manner. In trials using autologous cells conducted by Park and Siqueira to treat retinal vascular occlusion, nonexudative age related macular degeneration, retinitis pigmentosa, or rod-cone dystrophy, 11/11 patients did not develop serious complications. However, the patients also failed to have any functional recovery of vision, perhaps due to the advanced course of the disease.4, 5
Intra-ocular therapies in development have been primarily directed at restoring retinal ganglion cells (RGC), retinal pigment epithelial cells (RPE), and photoreceptors. RGCs, the innermost cells of the retina that project to the thalamus through the optic nerve, do not naturally replicate or regenerate. One approach to regenerate these cells has been to induce retinal Mueller cells, a type of glia, to differentiate into RGCs when driven by the proper cytokine stimulus. However, harvesting, differentiating and properly integrating these cells back into the retina has proven to be challenging.
In contrast, work has moved more rapidly on the therapeutic use of mesenchymal stem cells. When injected into the eye these stem cells appear to play a neuroprotective role for existing RGCs, a different approach than trying to replace diseased RGCs. These fibroblast-like stem cells found in blood, adipose, and bone marrow appear to produce factors that protect existing RGCs from immune-mediated inflammation as well as possibly inducing proliferation of retinal stem cells.6–8
The interaction between RPE and photoreceptors is essential for visual function. Damaged RPE occurs in Age-Related Macular Degeneration and RPE dystrophies, such as Stargardt disease. Subretinal transplantation of pluripotent stem cells to treat these diseases may be possible, and several small phase 1 trials have been performed demonstrating safety of this approach.9–11 Most of these studies have looked at delivery of cell suspensions, however, placement of sheets of cells into the sub-retinal space may prove to be more effective in preserving tissue architecture and perhaps allow better integration of the stem cell derived tissue into the retina-RPE complex. 12, 13
Damaged photoreceptors do not regenerate and are affected in Age-Related Macular Degeneration, diabetic retinopathy and degenerative conditions such as Retinitis Pigmentosa. Photoreceptors may be easier to restore than other retinal cells in that the regenerated cells probably need to make fewer functional connections. Several studies demonstrate the feasibility of total thickness pluripotent stem cell retinal sheets or subretinal photoreceptor progenitor cells.14, 15
FDA and Innovation
While a number of small, controlled studies of stem cell therapies for retinal disease have been carried out demonstrating some degree of safety under very tightly regulated conditions, the concept of stem cell therapy has become part of the popularized regenerative medicine industry, with internet communication enabling an explosion of online marketing of unproven treatments.16 As we await more evidence-based therapies, we rely on advice and oversight of the FDA. The FDA states that it is “responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices…FDA is responsible for advancing the public health by helping to speed innovations that make medical products more effective, safer, and more affordable and by helping the public get the accurate, science-based information they need to use medical products and foods to maintain and improve their health.”17
A Cautious Approach Is Needed
There is enthusiasm for the therapeutic potential of stem cell technology, especially for degenerative diseases. While the FDA will regulate the use of stem cell therapy to ensure both safety and efficacy, the use of autologous stem cells to regenerate damaged tissue may not fall under the regulation of this agency.18 For example, the FDA may not have jurisdiction over treatment of autologous materials that are harvested and re-implanted as part of the same procedure. The desire to improve a patient’s vision is understandable, but when approved therapies are not readily available for a given condition, desire may turn to desperation. Several high-profile cases of stem cell therapies for ocular disease have left patients with serious complications. In these cases, stem cells were injected intravitreally, resulting in scar formation, tractional retinal detachments, and vision loss.19 In 2017 The FDA clarified its rules in an effort to help companies comply with Federal regulations. Enforcement of these rules was delayed to allow companies the opportunity to comply. In July of 2020, the FDA extended this enforcement deadline to May of 2021 due to the COVID-19 public health emergency.20 According to this notice, the intention was never to “provide cover to bad actors,” and the FDA continues “to take action against manufacturers and health care providers who are offering unapproved regenerative medicine products that have the potential to put patients at significant risk”.
Conclusion
The promise of restored eye function based on these new technologies is alluring. To attain this goal, we need to evaluate new therapies in well-run and regulated clinical trials with appropriate ethical and safety reviews. These tenets of modern clinical trials may seem obvious; however, they cannot be stressed enough, especially when caring for people with debilitating diseases for which cures are not readily available. The desperation and fear that these patients experience is a set-up for the unethical profiteer. In this era of exploding knowledge and new technologies, our role as advisors is even more critical. Physicians are entrusted with sifting through the onslaught of new information and distilling the information down to understandable advice. To this end, the doctor must keep the source of information sacred, question all methods and the conclusions derived from published information, and vet any clinical trial open for enrollment before endorsing participation by the patient. Our role in this has not changed, whether it is FDA approved therapies or alluring but as of yet unproven, stem cell therapeutics.
Funding Statement
This work was supported by an unrestricted grant to the John F. Hardesty MD Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
Footnotes
P. Kumar Rao, MD, MBA, is in the Department of Ophthalmology and Visual Sciences, Washington University School of Medicine, St. Louis, Missouri.
Disclosure
This work was supported by an unrestricted grant to the John F. Hardesty MD Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
References
- 1. Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11(4):268–77. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 2. Uy H, Chan P, Cruz F. Stem cell therapy: a novel approach for vision restoration in retinitis pigmentosa. Med Hypothesis Discov Innov Ophthalmol. 2013;2(2):52–5. [PMC free article] [PubMed] [Google Scholar]
- 3. Holan V, Palacka K, Hermankova B. Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials. Cells. 2021;10(3) doi: 10.3390/cells10030588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park SS, Bauer G, Abedi M, et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci. 2014;56(1):81–89. doi: 10.1167/iovs.14-15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina. 2011;31(6):1207–1214. doi: 10.1097/IAE.0b013e3181f9c242. [DOI] [PubMed] [Google Scholar]
- 6. Osborne A, Sanderson J, Martin KR. Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells. Stem Cells. 2018;36(1):65–78. doi: 10.1002/stem.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roozafzoon R, Lashay A, Vasei M, et al. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem Biophys Res Commun. 2015;457(2):154–160. doi: 10.1016/j.bbrc.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 8. Volarevic V, Gazdic M, Simovic Markovic B, Jovicic N, Djonov V, Arsenijevic N. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors. 2017;43(5):633–644. doi: 10.1002/biof.1374. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFc1–9. doi: 10.1167/iovs.15-18681. [DOI] [PubMed] [Google Scholar]
- 10. Song WK, Park KM, Kim HJ, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4(5):860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehat MS, Sundaram V, Ripamonti C, et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology. 2018;125(11):1765–1775. doi: 10.1016/j.ophtha.2018.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamao H, Mandai M, Okamoto S, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2(2):205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu Y, Han L, Wang C, et al. A comparison of autologous transplantation of retinal pigment epithelium (RPE) monolayer sheet graft with RPE-Bruch’s membrane complex graft in neovascular age-related macular degeneration. Acta Ophthalmol. 2017;95(6):e443–e452. doi: 10.1111/aos.13054. [DOI] [PubMed] [Google Scholar]
- 14. Arai S, Thomas BB, Seiler MJ, et al. Restoration of visual responses following transplantation of intact retinal sheets in rd mice. Exp Eye Res. 2004;79(3):331–34. doi: 10.1016/j.exer.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 15. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 16. Berger I, Ahmad A, Bansal A, Kapoor T, Sipp D, Rasko JEJ. Global Distribution of Businesses Marketing Stem Cell-Based Interventions. Cell Stem Cell. 2016;19(2):158–162. doi: 10.1016/j.stem.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 17. https://www.fda.gov/about-fda/what-we-do
- 18. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=1271.15
- 19. Kuriyan A, et al. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N Engl J Med. 2017;376(11):1047–1053. doi: 10.1056/NEJMoa1609583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. https://www.fda.gov/news-events/press-announcements/fda-extends-enforcement-discretion-policy-certain-regenerative-medicine-products