Abstract
Thyroid eye disease (TED) is an immune mediated infiltration and inflammation of the orbital and periorbital soft tissues leading to facial disfigurement. Classically broken into two “phases,” active inflammatory and quiescent, disease modifying therapy and surgical intervention are used to improve a number of clinical aspects of TED. Many medical modalities have been utilized to halt the inflammatory phase of the disease including steroids, orbital radiation, and targeted steroid-sparing chemotherapy. Teprotumumab is currently the only Federal Drug Administration approved therapy for the treatment of TED. Significant improvements in proptosis, diplopia and quality of life are noted following its 24-week course of therapy.
Introduction
Thyroid eye disease (TED) is the most common orbital disorder in adults, predominately occurring during mid-adulthood.1 This female-predominate disease occurs in up to 50% of patients with autoimmune thyroid disease, especially Graves’ Disease, with a prevalence of up to 1/10,000.2–6 Known to be more common and profoundly worse in smokers, this autoimmune mediated disease is propagated by autoantibodies targeting the thyrotropin receptor.6,7 TED is characterized by an orbital inflammatory cascade leading to muscle and fat expansion, ultimately ending, in some cases, with progressive fibrosis. Infiltration of inflammatory cells and activated fibroblasts within the bony orbit can lead to proptosis, strabismus, eyelid swelling and eyelid retraction. Clinical findings include periorbital edema, conjunctival redness and swelling, eyelid retraction, strabismus, exophthalmos, and in rare cases, compressive optic neuropathy. Debilitating diplopia and facial disfigurement have profound psychosocial and functional effects on afflicted patients.8,9
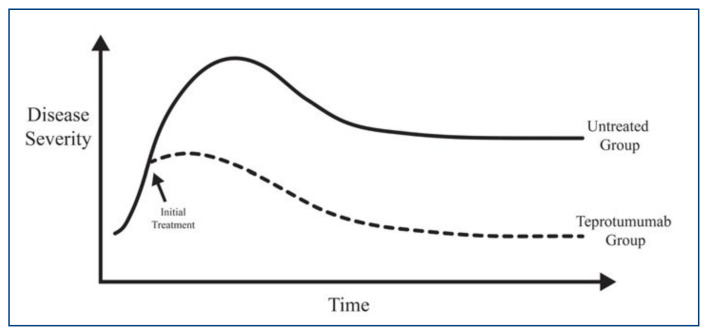
Classically, the disease course is described by Rundle’s curve (Figure 1), which illustrates an active inflammatory phase that reaches a peak of disease severity and plateaus into a static or quiescent phase. This biphasic, self-limited course is seen in most patients, with active phases lasting 6–18 months and with rare patients suffering from chronic inflammation for years.9–12 Recurrences of disease can be seen in 15% of patients.9,13 Most research in medical therapy for disease modification has been focused in the active phase of the disease with the goal of reducing inflammation, fibrosis and overall disease burden. Surgical rehabilitation involving orbital decompression, strabismus surgery, and eyelid surgery is typically offered in the quiescent phase of the disease, unless required for significant corneal disease (usually due to exposure) or in active disease-inducing optic neuropathy.
Figure 1.
Rundle’s Curve illustrating a progressive active disease early in the course followed by a peak in severity and plateau to stability. With intervention, such as Teprotumumab, during the active phase, overall disease burden may be reduced.
In early studies, long-term oral steroids were utilized to reduce active inflammation; however multiple studies have shown a high rate of recurrence and significant adverse effects from this approach to therapy.14,15 Orbital radiation is thought to induce terminal differentiation in inflamed orbital fibroblasts and is still commonly utilized by some TED specialists. Studies on radiotherapies role in the treatment of TED is conflicting.16–18 Intravenous (IV) pulse dose steroids are another commonly utilized therapy, especially utilized in by European TED specialists.19–22 In fact, in 2016 a consensus statement from the European Group on Graves’ Orbitopathy suggests high dose IV pulse dose steroids as the first-line treatment for active TED.23 Studies comparing pulse dose IV and chronic oral steroid favored IV steroids with lower adverse effects and improved efficacy.15 Other off-label immunomodulatory medications including methotrexate, rituximab and infliximab have been utilized for active TED with variable success.24–31
Research involving the molecular process of inflammation in the orbit with TED has highlighted an interaction between the insulin-like growth factor receptor (IGF-R) and the thyroid stimulating hormone receptor (TSH-R).32,33 The IGF-R is a toll-like receptor that is intimately associated with the TSH-R and acts as a “gate keeper” in activating orbital fibroblasts. During the disease, an upregulation of IGF-R and TSH-R is noted, and initial in vitro studies showed an inhibition of TSH-R stimulation with blockade of IGF-R.34 In vitro studies also showed blockade of the IGF-R led to decreased production of extracellular matrix and cytokines in activated fibroblasts.35–37 Progressive research in the understanding of the synergistic roles that TSH-R and IGF-R play in the development of TED38–42 led to the development of Teprotumumab, a fully humanized monoclonal antibody targeted against IGF-R1. Originally, studied and found to be unsuccessful in the treatment of patients with metastatic solid organ tumors,43,44 Teprotumumab was shown to decrease inflammatory factors in TSH-dependent fibrocytes.45,46 Following randomized, placebo-controlled clinical trials, Teprotumumab (Tepezza™) became the first Food and Drug Administration (FDA)-approved therapy for TED.
Studies on Efficacy
In 2017, results of the Phase 2, multicentered, double-masked, randomized, placebo-controlled clinical trial were published in the New England Journal of Medicine.46 Washington University was one of the study sites and the author was one of the investigators. Eighty-seven patients (42 Teprotumumab and 45 placebo) were randomly assigned to Teprotumumab infusion once every three weeks for eight doses or placebo. Teprotumumab infusions are weight-based dosing with first dose being 10mg/kg and all other infusions being 20mg/kg. Inclusion criteria included patients with active, moderate TED, clinical activity score (CAS) of four or more, diagnosed within nine months of the trial’s initiation.47 The primary outcome measurement was a decrease in clinical activity score (CAS) of two or more points and proptosis reduction of 2mm or more. At 24 weeks, 29 patients (69%) in the treatment arm of the study and nine patients (20%) in the placebo arm achieved the primary outcome response. Furthermore, onset of improvement in the primary outcome was achieved much earlier in the treatment group. In the treatment group, an average reduction in CAS by nearly 3.5 points was achieved as well as an average reduction in proptosis by nearly 2.5mm, with 17 patients (40%) achieving greater than a 4mm reduction in proptosis. Additionally, secondary outcomes of validated subjective diplopia scores and validated Graves’ quality of life (QOL) analysis improved significantly more in the treatment group.46
A Phase 3 multicenter clinical trial for Treatment of Graves’ Orbitopathy to Reduce Proptosis with Teprotumumab Infusions (OPTIC) was conducted with an emphasis on the treatment of proptosis.48 Washington University was one of the study sites and the author was one of the investigators. Identical inclusion criteria and treatment protocol to the Phase 2 clinical trial were utilized and patients were randomized to treatment with teprotumumab or placebo. A total of 83 patients (41 Teprotumumab and 42 placebo) were enrolled. The primary outcome of proptosis reduction (2mm of reduction or more) and secondary outcomes, including “overall response” (reduction of CAS by two or more plus reduction in proptosis of 2mm or more), subjective diplopia scores, and Graves’ QOL scores were evaluated at 24 weeks. Some patients underwent follow-up at 48 weeks. At 24 weeks, the reduction of proptosis was statistically significant with 34 patients (83%) in the treatment group and four patients (10%) in the placebo group achieving a reduction in proptosis of 2 mm or more. The average reduction in proptosis was 3.32mm, and all patients treated with Teprotumumab exhibited improvement regardless of baseline severity. Again, early response was noted in the treatment group with all secondary end points statistically significantly favoring the Teprotumumab group over placebo. Six patients in this study had pre- and post-treatment imaging which demonstrated reduction in proptosis with radiographically confirmed reduction in extraocular muscle volume, orbital fat volume or both. The average reduction in extraocular muscle volume was 35%.48
Combined data from Phase 2 and Phase 3 trials was evaluated by Kahaly et al.49 to analyze treatment efficacy, subgroup analysis, adverse effects and long-term off-treatment durability. This pooled dataset included 171 patients, 84 received teprotumumab and 87 received placebo. In this study 65 (77%) patients in the Teprotumumab group as compared to 13 (15%) in the placebo group achieved a reduction in proptosis of 2mm or more. The mean reduction in proptosis was 3.14mm in the treatment group as compared to 0.37mm in the placebo group. Reduction of both proptosis and diplopia was further evaluated in a sub-group analysis that took into account gender, age, tobacco use, time of diagnosis of TED, time of diagnosis of Graves’ disease, baseline CAS and baseline levels of anti-thyrotropin antibodies. In the analysis, patients exhibiting a reduction in proptosis had statistically significant similar effects amongst all sub-groups. Similarly, for those patients with a reduction in diplopia, all sub-groups achieved statistically significant improvement except active tobacco users.49
An open label extension study (OPTIC-X, NCT03298867) was created following the Phase 3 trial. Patients from the trial were offered entrance into the study if they did not have a therapeutic response in proptosis or had recurrence of proptosis during the follow-up period. The purpose of this extension trial was to examine the efficacy and safety of a second course (24 week, eight infusions) of Teprotumumab (Figure 2). Results of this study were intended to indicate the efficacy of treatment regardless of disease duration, along with the effect of additional treatment in “non-responders” and patients with a relapse. These results have not been published in peer-reviewed literature to date, but Horizon Pharmaceuticals released data on July 31, 2020, and in a press release on November 13, 2020.50,51 Fifty-one of 83 original OPTIC trial participants were included, 37 from the placebo group and 14 from the treatment group. Of the 14 from the treatment group in the original OPTIC trial, five had no initial response Teprotumumab and nine experienced a relapse of proptosis. At 24 weeks, 33 of 37 patients (89%) who had previously received placebo experienced improvement in proptosis of 2mm or more. Fourteen of 23 (61%) patients who started with diplopia experienced improvement at 24 weeks. Two of five patients who did not have a clinical response during OPTIC, experienced a significant response following a second course of therapy with Teprotumumab. Sixty percent of patients who experienced relapse after OPTIC achieved a response at 24 weeks after a second round of Teprotumumab.
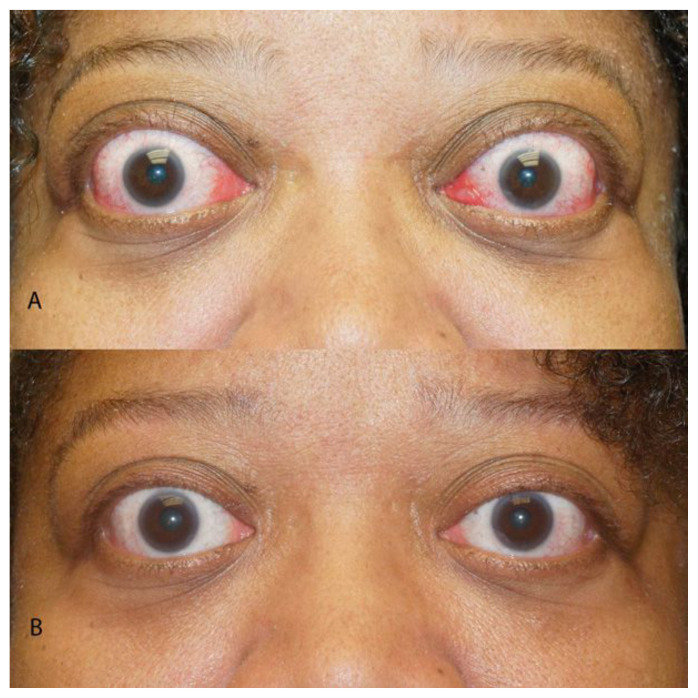
Figure 2a.
Patient with active moderate thyroid eye disease causing orbital inflammation with proptosis and eyelid retraction. 2b. Same patient following 8 treatments using Teprotumumab.
Durability of Treatment
While the Phase 2 trial described above had some patients followed out to 48 weeks, this information was not presented in the representative manuscript. Full follow-up data was presented to the FDA in a briefing document used to encourage approval of the drug.52 When followed out to 72 weeks, 18 of 29 (62%) patients treated with Teprotumumab had a long lasting therapeutic effect with 11 of 29 (38%) having relapse of TED defined by an increase in proptosis (2mm or more) or increase in CAS. Phase 3 trial durability data was released at a presentation at the American Academy of Ophthalmology and with public release of the 48 week off-treatment follow-up in the OPTIC-X data.50,51,53 At 72 weeks following start of treatment, 19/34 (56%) of the proptosis responding group maintained a reduction in proptosis, and of the 15 that qualified as non-maintained response, 8/15 (53%) still had 2mm or more reduction in proptosis as compared to the amount of proptosis prior to treatment. Eleven of 19 patients (58%) who demonstrated a reduction in diplopia at 24 weeks continued to have reduction at 72 weeks. Composite data from patients treated in Phase 2 and Phase 3 data showed durability of proptosis treatment in 67% of patients and diplopia in 69% of patients.49
Safety
Teprotumumab therapy is generally well tolerated with most adverse effects resolving after completion or cessation of infusions. Rates for completion of infusion were similar for Teprotumumab and placebo in both Phase 2 and Phase 3 trials and similar adverse event profiles were seen.46,47,49 Muscle spasms, typically of the lower extremities, occurred in 25% of patients treated with Teprotumumab. Hyperglycemia occurred in 10% of patients, of which nearly two-thirds had previous impaired glucose tolerance or diabetes mellitus. Hearing impairment is of concern because of IGF receptors located in the inner ear.54–56 Hearing issues, including tinnitus and or hypoacusis, were noted in up to 10% of patients. Diarrhea occurred in 12% (8% in the placebo group) but generally was mild and reversible. Inflammatory bowel disease (IBD) is a relative contraindication to treatment with Teprotumumab after one patient in the Phase 2 trial experienced exacerbation of IBD and another patient developed new-onset colitis requiring surgical intervention.46,57–59 Additional notable adverse effects of Teprotumumab treatment included nausea (14%), alopecia (11%), dysgeusia (8%), headache (8%), and dry skin (8%).
Future Studies
While Teprotumumab has been evaluated for the treatment of moderate to severe active TED, we do not know is efficacy in the treatment of patients with longer active phase disease courses, chronic TED, patients in the quiescent phase of TED, or those with dysthyroid optic neuropathy (DON).
Chronic TED
While the data that was released from the OPTIC-X trial is suggestive of efficacy in patients with TED disease with a duration of over one year, data on the effectiveness in patients with chronic TED is lacking. Case reports have suggested a role for Teprotumumab in the treatment of chronic TED,60,61 however, the results of randomized controlled trials for this patient group are not yet available. A clinical trial of Teprotumumab for chronic TED in patients with stable measurements for three or more years is planned.
Optic Neuropathy
It is known that DON primarily occurs because of compression of the optic nerve in the orbital apex from enlarged muscles and less commonly from stretch of the optic nerve secondary to proptosis.6,13 Because some studies have shown a reduction in the volume of EOMs,48,62 it is conceivable that patients with mild compressive optic neuropathy will experience a benefit of treatment with Teprotumumab. Several case reports have suggested improvement in patients with DON with rapid resolution of compression following initiation of treatment.63–65 Due to the limited data, this may be an option for poor surgical candidates experiencing DON, but trials are needed to compare Teprotumumab efficacy with the current gold standard of treatment, orbital decompression surgery.
Conclusion
Never before has a targeted treatment been available in our armamentarium for the management of TED. The use of Teprotumumab for the effective and safe management of TED through inhibition of the IGF-R is now supported by both laboratory and clinical studies. Significant improvement in proptosis, disease activity, diplopia, and QOL suggests a major role for the use of Teprotumumab in the treatment of many of our TED patients. Adverse effects of the drug including muscle cramping, hyperglycemia and hypoacusis need to be monitored and managed, and in some cases, therapy may need to be halted. However, this cost-prohibitive drug must be evaluated in the entire spectrum of TED including chronic disease, recurrent disease, and DON. Furthermore, its use should be directly compared with other available treatments including medical and surgical therapy. Teprotumumab is currently the only FDA-approved medical therapy for TED and is an effective and relatively safe treatment for active moderate-severe TED.
Funding Statement
This work was supported by an unrestricted grant to the John F. Hardesty MD Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
Footnotes
Steven M. Couch, MD, FACS, is in the John F. Hardesty MD Department of Ophthalmology and Visual Sciences, Washington University School of Medicine, St. Louis, Missouri.
Disclosure
This work was supported by an unrestricted grant to the John F. Hardesty MD Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
References
- 1. Smith TJ, Hegedus L. Graves’ disease. N Engl J Med. 2016;375:1552–1565. doi: 10.1056/NEJMra1510030. [DOI] [PubMed] [Google Scholar]
- 2. Furszyfer J, Kurland LT, McConahey WM, et al. Epidemiologic aspects of Hashimoto’s thyroiditis and Graves’ disease in Rochester, Minnesota 1935–1967 with special reference to temporal trends. Metabolism. 1972;21:197–204. doi: 10.1016/0026-0495(72)90041-8. [DOI] [PubMed] [Google Scholar]
- 3. Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf ) 1977;7:481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 4. Lazarus JH. Epidemiology of Graves’ orbitopathy (GO) and relationship with thyroid disease. Best Pract Res Clin Endocrinol Metab. 2012;26:273–279. doi: 10.1016/j.beem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 5. Bartley GB, Fatourechi V, Kadrmas EF, et al. The incidence of Graves’ ophthalmopathy in Olmsted County. Minnesota Am J Ophthalmol, 1995;120:511–517. doi: 10.1016/s0002-9394(14)72666-2. [DOI] [PubMed] [Google Scholar]
- 6. Bartley GB, Fatourechi V, Kadrmas EF, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol, 1996;121:284–290. doi: 10.1016/s0002-9394(14)70276-4. [DOI] [PubMed] [Google Scholar]
- 7. Nunery WR, Martin RT, Heinz GW, Gavin TJ. The Association of Cigarette Smoking with Clinical Subtypes of Ophthalmic Graves’ Disease Ophthalmic Plast Reconstr Surg. 1993;9:77–82. doi: 10.1097/00002341-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 8. Tanda ML, Piantanida E, Liparulo L, et al. Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab, 2013;98:1443–1449. doi: 10.1210/jc.2012-3873. [DOI] [PubMed] [Google Scholar]
- 9. Naik VM, Naik MN, Goldberg RA, et al. Immunopathogenesis of Thyroid Eye Disease: Emerging Paradigms Surv Ophthalmol. 2010;55:215–226. doi: 10.1016/j.survophthal.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartley GB. Rundle and his curve. Arch Ophthalmol, 2011;129:356–358. doi: 10.1001/archophthalmol.2011.29. [DOI] [PubMed] [Google Scholar]
- 11. Menconi F, Profilo MA, Leo M, et al. Spontaneous improvement of untreated mild Graves’ ophthalmopathy: Rundle’s curve revisited. Thyroid. 2014;24:60–66. doi: 10.1089/thy.2013.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teng CS, Yeo PPB. Occasional ReviewOphthalmic Graves’s disease: natural history and detailed thyroid function studies. Br Med J. 1977:273–275. doi: 10.1136/bmj.1.6056.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uddin JM, Rubinstein R, Hamed-Azzam S. Phenotypes of Thyroid Eye Disease. Ophthal Plast Reconstr Surg, 2018;34:S28–S33. doi: 10.1097/IOP.0000000000001147. [DOI] [PubMed] [Google Scholar]
- 14. Wiersinga WA. Immunosuppressive treatment of Graves’ ophthalmopathy. Thyroid. 1992;2:229–233. doi: 10.1089/thy.1992.2.229. [DOI] [PubMed] [Google Scholar]
- 15. Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, Single Blind Trial of Intravenous versus Oral Steroid Monotherapy in Graves’ Orbitopathy. J Clin Endocrinol Metab, 2005;90:5234–5240. doi: 10.1210/jc.2005-0148. [DOI] [PubMed] [Google Scholar]
- 16. Gorman CA, Garrity JA, Fatourechi V, et al. A Prospective, Randomized, Double-blind, Placebo-controlled Study of Orbital Radiotherapy for Graves’ Ophthalmopathy. Ophthalmology. 2001;108:1523–1534. doi: 10.1016/s0161-6420(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 17. Bartalena L, Marcocci C, Pinchera A. Editorial: Orbital Radiotherapy for Graves’ Ophthalmopathy. J Clin Endocrinol Metab, 2004;89:13–14. doi: 10.1210/jc.2003-031769. [DOI] [PubMed] [Google Scholar]
- 18. Prummel MF, Terwee CB, Gerding MN, et al. A Randomized Controlled Trial of Orbital Radiotherapy Versus Sham Irradiation in Patients with Mild Graves’ Ophthalmopathy. J Clin Endocrinol Metab, 2004;89:15–20. doi: 10.1210/jc.2003-030809. [DOI] [PubMed] [Google Scholar]
- 19. Zhao LQ, Yu DY, Cheng JW. Intravenous glucocorticoids therapy in the treatment of Graves’ ophthalmopathy: A systematic review and Metaanalysis. Int J Ophthalmol, 2019;12:1177–1186. doi: 10.18240/ijo.2019.07.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aktaran S, Akarsu E, Erbağci I, et al. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves’ ophthalmopathy. Int J Clin Pract, 2007;61:45–51. doi: 10.1111/j.1742-1241.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 21. Zang S, Ponto KA, Kahaly GJ. Intravenous Glucocorticoids for Graves’ Orbitopathy: Efficacy and Morbidity. J Clin Endocrinol Metab, 2011;96:320–332. doi: 10.1210/jc.2010-1962. [DOI] [PubMed] [Google Scholar]
- 22. Bartalena L, Krassas GE, Wiersinga W, et al. Efficacy and Safety of Three Different Cumulative Doses of Intravenous Methylprednisolone for Moderate to Severe and Active Graves’ Orbitopathy. J Clin Endocrinol Metab, 2012;97:4454–4463. doi: 10.1210/jc.2012-2389. [DOI] [PubMed] [Google Scholar]
- 23. Bartalena L, Baldeschi L, Bororidis K, et al. The 2016 European Thyroid Association/European Group on Graves Orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 2016;5:9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol, 2007;156:33–40. doi: 10.1530/eje.1.02325. [DOI] [PubMed] [Google Scholar]
- 25. Khanna D, Chong KKL, Afifiyan NF, et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology, 2010;117:133–139e2. doi: 10.1016/j.ophtha.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silkiss RZ, Reier A, Coleman A, Lauer SA. Rituximab for thyroid eye disease. Ophthal Plast Reconstr Surg, 2010;26:310–314. doi: 10.1097/IOP.0b013e3181c4dfde. [DOI] [PubMed] [Google Scholar]
- 27. Stan MN, Garrity JA, Carranza Leon BG, et al. Randomized Controlled Trial of Rituximab in Patients With Graves’ Orbitopathy. J Clin Endocrinol Metab, 2015;100:432–441. doi: 10.1210/jc.2014-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perez-Moreiras JV, Alvarez-Lopez A, Gomez EC. Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthal Plast Reconstr Surg, 2014;30:162–167. doi: 10.1097/IOP.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 29. Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, et al. Efficacy of Tocilizumab in Patients With Moderate-to-Severe Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am J Ophthalmol, 2018;195:181–190. doi: 10.1016/j.ajo.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 30. Copperman T, Idowu OO, Kersten RC, Vagefi MR. Subcutaneous Tocilizumab for Thyroid Eye Disease: Simplified Dosing and Delivery. Ophthal Plast Reconstr Surg, 2019;35:E64–E66. doi: 10.1097/IOP.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 31. Durrani OM, Reuser TQ, Murray PI. Infliximab: a novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit, 2005;24:117–119. doi: 10.1080/01676830590912562. [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Investig Ophthalmol Vis Sci, 2014;55:1735–1748. doi: 10.1167/iovs.14-14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohyi M, Smith TJ. IGF1 receptor and thyroid-associated ophthalmopathy. J Mol Endocrinol, 2018;61:T29–T43. doi: 10.1530/JME-17-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pritchard J, Han R, Horst N, et al. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol, 2003;170:6348–6354. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 35. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–5080. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 36. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–5080. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 37. Douglas R, Naik V, Hwang CJ, et al. B cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol. 2008;181:5768–5774. doi: 10.4049/jimmunol.181.8.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity, 1993;16:251–257. doi: 10.3109/08916939309014643. [DOI] [PubMed] [Google Scholar]
- 39. Marino M, Rotondo Dottore G, Ionni I, et al. Serum antibodies against the insulin-like growth factor-1 receptor (IGF-1R) in Graves’ disease and Graves’ orbitopathy. J Endocrinol Invest, 2019;42:471–480. doi: 10.1007/s40618-018-0943-8. [DOI] [PubMed] [Google Scholar]
- 40. Smith T. TSHR as a therapeutic target in Graves’ disease. Expert Opin Ther Targets, 2017;21:427–432. doi: 10.1080/14728222.2017.1288215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol, 2008;181:4397–4405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Philippou A, Christopoulos PF, Koutsilieris DM. Clinical studies in humans targeting the various components of the IGF system show lack of efficacy in the treatment of cancer. Mutat Res Rev Mutat Res, 2017;772:105–122. doi: 10.1016/j.mrrev.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 43. Kurzrock R, Patnaik A, Aisner J, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res, 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 44. Xin Y, Xu F, Gao Y, et al. SAT-432 Pharmacokinetics (PK) and Exposure-Response Relationship of Teprotumumab, an Insulin-Like Growth Factor-1 Receptor (IGF-1R) Blocking Antibody. Active Thyroid Eye Disease (TED) J Endocr Soc. 2020;4:276–277. [Google Scholar]
- 45. Smith TJ, Janssen JAMJL. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocr Rev, 2019;40:236–267. doi: 10.1210/er.2018-00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–1761. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017:376. doi: 10.1056/NEJMoa1614949. Supplementary Material: Protocol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N Engl J Med. 2020;382:341–352. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 49. Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomized, double-masked, placebo-controlled, multicentere trials. Lanced Diabetes Endocrinol. 2021 Apr 15; doi: 10.1016/S2213-8587(21)00056-5. S2213-8587(21)00056-5. [DOI] [PubMed] [Google Scholar]
- 50.Horizon Therapeutics. New Topline TEPEZZAR (teprotumumab-trbw) Data Underscore its Efficacy in Longer Disease Duration, Long-Term Durability and Potential for Retreatment in People Living with Thyroid Eye Disease (TED) https://ir.horizontherapeutics.com/news-releases/news-release-details/new-topline-tepezzar-teprotumumab-trbw-data-underscore-its .
- 51.Business Wire. New Data Build on Growing Evidence Supporting TEPEZZAR (teprotumumab-trbw) Efficacy in Thyroid Eye Disease (TED), Including in Patients With Less Severe Disease and Longer Disease Duration. https://www.businesswire.com/news/home/20201113005158/en/New-Data-Build-on-Growing-Evidence-Supporting-TEPEZZAR-teprotumumab-trbw-Efficacy-in-Thyroid-Eye-Disease-TED-Including-in-Patients-With-Less-Severe-Disease-and-Longer-Disease-Duration .
- 52.United States Food and Drug Administration. FDA Center for Drug Evaluation and Research; Application Number: 761143Orig1s000; Summary Review. [Accessed 04/20/2020.]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/761143Orig1s000SumR.pdf .
- 53. Douglas RS, Holt RJ, Vescio T, et al. Long-Term Assessment of Proptosis and Diplopia From the OPTIC Trial of Teprotumumab in Thyroid Eye Disease. American Academy of Ophthalmology. 2020:PA038. [Google Scholar]
- 54. Fujiwara T, Hato N, Nakagawa T, et al. Insulin-like growth factor I treatment via hydrogels rescues cochlear hair cells from ischemic injury. Neuroreport, 2008;19:1585–1588. doi: 10.1097/WNR.0b013e328311ca4b. [DOI] [PubMed] [Google Scholar]
- 55. Lassale C, David Batty G, Steptoe A, Zaninotto P. Insulin-like Growth Factor 1 in relation to future hearing impairment: Findings from the English Longitudinal Study of Ageing. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-017-04526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gao L, Nakagawa T. Insulin-like growth factor 1: role in the auditory system and therapeutic potential in otology. Curr Opin Otolaryngol Head Neck Surg. 2020;28:286–290. doi: 10.1097/MOO.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 57. Eivindson M, Nielsen JN, Gronbak H, et al. The insulin-like growth factor system and markers of inflammation in adult patients with inflammatory bowel disease. Horm Res, 2005;64:9–15. doi: 10.1159/000087190. [DOI] [PubMed] [Google Scholar]
- 58. Corkins MR, Gohil AD, Fitzgerald JF. The insulin-like growth factor axis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr, 2003;36:228–234. doi: 10.1097/00005176-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 59. Krakowska-Stasiak M, Cibor D, Domagała-Rodacka R, et al. Insulin-like growth factor system in remission and flare of inflammatory bowel diseases. Polish Arch Intern Med, 2017;127:832–839. doi: 10.20452/pamw.4136. [DOI] [PubMed] [Google Scholar]
- 60. Ozzello DJ, Kikkawa DO, Korn BS. Early experience with teprotumumab for chronic thyroid eye disease. Am J Ophthalmol Case Reports. 2020;19:100744. doi: 10.1016/j.ajoc.2020.100744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozzello DJ, Dallalzadeh L, Men C, et al. Initial Results of Teprotumumab for Chronic Thyroid Eye Disease. American Society of Ophthalmic Plastic & Reconstructive Surgery 51st Annual Fall Scientific Symposium. Virtual Meeting; 2020.; [Google Scholar]
- 62. Jain AP, Gellada N, Ugradar S, et al. Teprotumumab reduces extraocular muscle and orbital fat volume in thyroid eye disease. Br J Ophthalmol, i. 2020:1–7. doi: 10.1136/bjophthalmol-2020-317806. [DOI] [PubMed] [Google Scholar]
- 63. Sears CM, Azad AZ, Dosiou C, Kossler AL. Teprotumumab for Dysthyroid Optic Neuropathy. Ophthalmic Plast Reconstr Surg. 2020 doi: 10.1097/IOP.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 64. Slentz DH, Smith TJ, Kim DS, Joseph SS. Teprotumumab for Optic Neuropathy in Thyroid Eye Disease. JAMA Ophthalmol. 2020:3–5. doi: 10.1001/jamaophthalmol.2020.5296. [DOI] [PubMed] [Google Scholar]
- 65.Merritt H, Pfeiffer ML, Merritt J.Improvement of Dysthyroid Optic Neuropathy Following Teprotumumab Infusion. American Society of Ophthalmic Plastic & Reconstructive Surgery 51st Annual Fall Scientific Symposium. Virtual Meeting; 2020.; [Google Scholar]