Abstract
Simple Summary
The activity of brain-derived neurotrophic factor (BDF) in the central nervous system has been well-studied, but its physiological role in other organs has not been clearly defined. This review summarizes the current findings on the functionality of BDNF in various peripheral tissues and discusses several unresolved questions in the field.
Abstract
Brain-derived neurotrophic factor (BDNF) is an important growth factor in the central nervous system. In addition to its well-known activities in promoting neuronal survival, neuron differentiation, and synaptic plasticity, neuronal BDNF also regulates energy homeostasis by modulating the hypothalamus’s hormonal signals. In the past decades, several peripheral tissues, including liver, skeletal muscle, and white adipose tissue, were demonstrated as the active sources of BDNF synthesis in response to different metabolic challenges. Nevertheless, the functions of BDNF in these tissues remain obscure. With the use of tissue-specific Bdnf knockout animals and the availability of non-peptidyl BDNF mimetic, increasing evidence has reported that peripheral tissues-derived BDNF might play a significant role in maintaining systemic metabolism, possibly through the regulation of mitochondrial dynamics in the various tissues. This article reviews the autocrine/paracrine/endocrine functions of BDNF in non-neuronal tissues and discusses the unresolved questions about BDNF’s function.
Keywords: BDNF, metabolism, mitochondria, peripheral tissues
1. Introduction
Together with nerve growth factor (NGF), Neurotrophin-3 (NT-3), and Neurotrophin-4 (NT-4), brain-derived neurotrophic factor (BDNF) is a member of the structurally related neurotrophin family that plays crucial roles in neurological activities, such as neurogenesis during the tissue development, differentiation and survival of neurons, regulation of synaptic plasticity for memory formation, and guidance of tissue–neuron interaction. Mature BDNF (designated as mBDNF in this review) exists as a dimer of two non-covalently linked peptides, which is formed after the intracellular endopeptidase cleavage of pro-BDNF in the endoplasmic reticulum or via the membrane-bound protease like matrix metalloproteases extracellularly (Figure 1) [1]. In addition to serving as the precursor for mBDNF synthesis, pro-BDNF might work as a functional protein via interacting with the p75 neurotrophin receptor (p75NTR)-sortilin complex [2]. In contrast, mBDNF exerts biological functions via binding to the single transmembrane receptor tyrosine kinase, tropomyosin receptor kinase B (TrkB). The binding of mBDNF to TrkB initiates three major signaling cascades: phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK), and phospholipase C γ (PLCγ)/cAMP response element-binding protein (CREB) pathways, which upregulate the transcription of pro-survival genes in the brain [3]. The Ca2+ influx that follows PLCγ activation also increases the activity of the N-methyl-D-aspartate (NMDA) receptor [3], which contributes to synaptic maturation and memory formation [4]. Because neuronal BDNF plays an important role in long-term potentiation, synaptic plasticity, and neurogenesis, reduced BDNF expression is associated with neuronal diseases such as bipolar disorder, Huntington’s disease, Alzheimer’s disease, and Parkinson’s disease [5]. The neurotrophic functions of BDNF have been extensively described, and readers who are interested in this topic are referred to other excellent reviews [6,7,8].
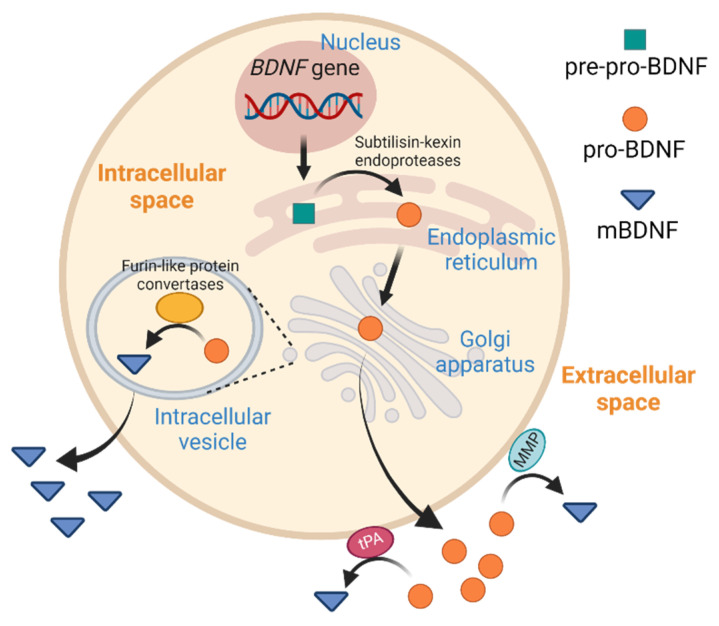
Figure 1.
Synthesis of pro-BDNF and mature BDNF (mBDNF). BDNF mRNA is translated in the endoplasmic reticulum to form pre-pro-BDNF, which is subsequently cleaved to form pro-BDNF. After being transported into the Golgi apparatus, the pro-BDNF is further converted into mBDNF by furin-like protein convertases. Alternatively, pro-BDNF is exported as a functional hormone or is further processed by the tissue type plasminogen activator (tPA) or matrix metalloproteinase (MMP) to form mBDNF extracellularly.
Studies in Bdnf and Ntrk2 (the TrkB gene) knockout mice revealed that mBDNF participates in the regulation of body weight through controlling food intake [9,10]. In the fed state, high serum glucose and leptin levels activate the neurons that express cocaine- and amphetamine-regulated transcript/pro-opiomelanocortin (POMC) in the arcuate nucleus in the hypothalamus to prevent over-eating [11,12]. The anorexigenic activities of POMC-positive neurons depend on the production of 𝛼-melanocyte stimulating hormone (𝛼-MSH) as the neurotransmitter, which activates melanocortin 4 receptor activity of other feeding-control neurons in multiple brain regions, such as the ventromedial hypothalamus (VMH) [13]. Xu et al. have reported that Bdnf is an 𝛼-MSH-responsive gene in the neuron of the VMH and ablation of Bdnf or TrkB in these neurons causes hyperphagia and excessive weight gain in mice [10,14]. Corroborating these findings, mutation of BDNF or NTRK2 in human beings leads to the development of obesity and other related metabolic disorders [15,16]. Interestingly, subcutaneous infusion of mBDNF effectively mitigated glucose homeostasis in obese db/db mice after pair-feeding, suggesting that mBDNF is able to manage systemic metabolism independently of its anorexigenic functions [17]. Although it has been proposed that subcutaneously administrated mBDNF regulates the integral metabolism via the neuronal output to various tissues, mBDNF might also act directly on peripheral tissues to orchestrate their metabolic functions. Indeed, mBDNF and TrkB proteins can be found in key organs for metabolic controls, including the liver, pancreas, skeletal muscles (SkM), and white adipose tissues (WAT), but their authentic functions in these peripheral tissues are still ambiguous [18,19]. With the help of genetic engineering that confines the overexpression or ablation of Bdnf or Ntrk2 in a single tissue, it is now clear that BDNF is involved in numerous tissue-specific and systemic metabolic activities. This review will outline the actions of tissue-specific BDNF in local and systemic metabolism regulation and discuss several key issues to fully elucidate the functional spectrum of peripheral tissue-derived BDNF.
2. BDNF in Liver
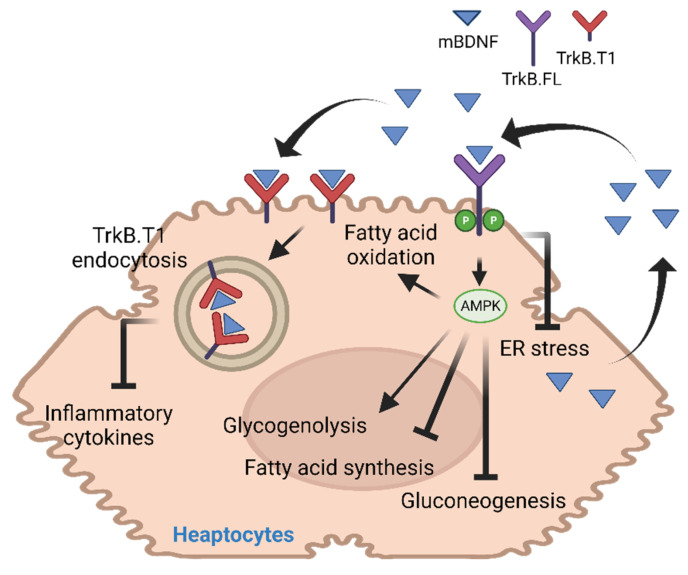
The liver is a vital organ for maintaining metabolic homeostasis. During the fasting state, high glucagon and low insulin levels increase hepatic glucose production to stabilize the blood glucose concentration [20]. On the other hand, excessive glucose in dietary sources is converted into triacylglycerides (TAG) via de novo lipogenesis in the liver, which is delivered to other tissues via very-low-density lipoproteins. Disruption of these regulatory processes leads to the development of metabolic diseases, including hyperlipidemia, hyperglycemia, and hepatic steatosis [21]. Although regulation of hepatic metabolism depends mainly on the signal from the pancreas via the production of insulin and glucagon, subcutaneous injection of mBDNF also potentiates insulin-induced PI3K activity in the liver [22]. However, it is not clear if mBDNF directly activates its receptor in hepatocytes to potentiate insulin sensitivity, because Ntrk2 expression in hepatocytes is very low [23]. Moreover, the observation that intracerebroventricular infusion of mBDNF suppresses hepatic glucose production, possibly acting via the vagus nerve, further questions the direct action of mBDNF on hepatocytes [24]. When Bdnf was overexpressed in the mouse liver with non-alcoholic steatohepatitis by AAV-mediated gene delivery, however, the animals displayed lower hepatic damage, reduced inflammatory gene expression, and improved fibrosis and steatosis, indicating that the hepatocyte-synthesized BDNF has a local protective function to the liver against metabolic challenges [25]. Studies in cultured mouse hepatocyte cell lines AML12 provided further proof that mBDNF is a direct stimulator of fatty acid oxidation (FAO) and glycogenesis but a suppressor of the hepatic fatty acid (FA) synthesis and gluconeogenesis [26]. Furthermore, BDNF deficiency in the heterozygous Bdnf knockout mice (BDNF+/−) sensitizes their hepatocytes to ER stress-induced cell death, which is a common consequence seen in the obese tissues [27]. Hence, the FA-induced Bdnf expression in the livers of mice after high fat diet (HFD) feeding might prevent the ectopic lipid accumulation and the related adverse metabolic consequences [26,28]. Mechanistically, mBDNF destabilizes the TrkB isoform, TrkB.T1, in hepatocytes to protect the cells from lipotoxicity [25]. Generated by alternative splicing of the Ntrk2 gene, TrkB.T1 is a truncated isoform of the full-length TrkB (Trkb.FL) without the kinase and C-terminal domains [29]. Because TrkB.T1 expression was increased in the mouse liver after HFD feeding, which potentiated the TNFα-induced cell damage and inflammatory response through a ligand-independent mechanism, the high mBDNF production in the HFD-fed mice prevents further damage by reducing the cellular content of TrkB.T1 [25]. Nevertheless, an opposing view on BDNF’s protective action on the liver has also been proposed. For instance, higher hepatic content of mBDNF is detected in patients with major depressive disorder, schizophrenia, and bipolar disorder, and the authors proposed that hepatic mBDNF might contribute to the high incidence of liver disease in these psychiatric diseases [30]. In support of this notion, hepatocyte-specific Bdnf knockout mice displayed reduced liver damage, alleviated hepatic steatosis, and augmented FAO in the liver when the knockout animals were fed with HFD, suggesting Bdnf expression might not be beneficial to the liver metabolism [31]. It remains to be determined why opposite functions are observed in mice after HFD feeding, and more functional characterization of liver-specific Bdnf knockout mice is definitely needed to clarify the metabolic role of BDNF in the liver (Figure 2).
Figure 2.
Functional activities of mBDNF in the liver.
3. BDNF in Adipose Tissue
WAT is the chief energy reservoir of mammals that catabolizes the stored TAG via lipolysis to fuel other peripheral tissues during energy scarcity. Since the discovery of leptin and its activities in the central nervous system (CNS) to control the systemic energy metabolism, however, the functionality of WAT has been changed from a simple and inert energy depot to an organ that actively shapes whole-body metabolism. It is now widely accepted that WAT is a metabolic tissue for lipid storage, adipokines secretion, and insulin sensitivity maintenance [32]. A significant amount of BDNF could be detected in WAT [19,33], whose expression was induced by streptozotocin injection [34] or HFD feeding [35]. Because WAT is a heterogeneous tissue that contains multiple cell types, including mature adipocytes, hematopoietic lineage of immune cells, preadipocytes, vascular endothelial cells, and pericytes [36], the elevated Bdnf expression in WAT might not necessarily occur in adipocytes. Studies in various cell type-specific Bdnf knockout mice have revealed a complex response of BDNF in WAT. Firstly, Bdnf expression in WAT was not abolished in adipocyte-specific knockout mice, suggesting most Bdnf expression occurs in the cells of the heterogeneous stromal vascular fraction (SVF) of WAT [35]. In another study performed in the myeloid lineage-specific Bdnf knockout mice, only a mild reduction of BDNF in the SVF was found, which excludes the possibility that adipose immune cells are the primary source of BDNF production in the tissue [37]. Presumably, the adipose progenitor cells might be the major cell types of BDNF synthesis in WAT. Indeed, human preadipocytes have a high expression of Bdnf, which is significantly reduced during adipogenesis [33]. Because inhibiting Bdnf expression in pre-adipocytes led to a mild reduction in adipocyte differentiation, it is suggested that BDNF is only critical to the commitment of progenitor cells to form adipogenic cell lineage but not the maturation of preadipocytes [33]. However, later studies showed that treatment of 3T3-L1 preadipocyte with 7,8-dihydroxyflavone (7,8-DHF), a bioavailable non-peptidyl BDNF mimetic [38], reduced adipocyte differentiation via inhibiting the expression of key adipogenic transcription factors such as CCAAT/enhancer binding protein α and peroxisome proliferator-activated receptor γ [39,40], suggesting BDNF is an inhibitory factor to the formation of new adipocytes.
Recently, it was proposed that the BDNF production in progenitor cells of WAT is detrimental to the tissue’s metabolic health during aging. Using the inducible adipocyte progenitor-specific Bdnf knockout (BDNFPdgfra KO) mice, Song et al. demonstrated that abolishing the production of BDNF in adipocyte progenitor cells prevents the aging-induced inflammation and glucose intolerance [41]. Because in vitro assays showed that pro-BDNF promoted adipocyte apoptosis and reduced the cellular mitochondria content, the authors concluded that an excessive pro-BDNF production in the adipose progenitor cells of aged animals triggered the death of adipocytes, causing the infiltration of immune cells and deterioration of the metabolic fitness. Nevertheless, the study did not include an assessment of the production and functional activity of mBDNF in the aged WAT, and it remains unknown if the elevated pro-BDNF production is an isoform-specific event or an overall increase of BDNF transcription that enhances the mBDNF synthesis as well.
Although the above studies suggest that mature adipocytes might not produce mBDNF, they do respond to both pro-BDNF and mBDNF stimulations as a significant amount of TrkB and p75NTR could be found in WAT [35,42]. Moreover, more mitochondrial fission and browning were detected in the differentiated 3T3-L1 adipocytes after BDNF stimulation [43]. It is possible that the non-adipose cells in WAT produce BDNF paracrine to modulate the metabolic activities of their adjacent adipocytes. While there are no direct studies on BDNF’s metabolic modulation in adipocytes, the TrkB-BDNF signaling in the adipocyte is important to food intake regulation as adipocyte-specific TrkB knockout (Adipoq-TrkB CKO) mice fed a high-fat/high-sucrose (HFHS) diet displayed hypophagia [35], indicating that the TrkB in adipocyte is responsible for generating a stimulatory afferent input to the CNS for initiating feeding behavior. Because Ntrk2 expression in WAT is diminished in diet-induced obese mice [35], the TrkB in WAT might represent an adiposity signal that suppresses further food intake when the animals consume energy-dense food.
Macrophages and their monocyte precursors comprise the highest fraction of immune cells in adipose tissues [44]. During obesity development, the number of pro-inflammatory M1 adipose tissue macrophages (ATM), but not the resolving M2 ATM, is significantly increased in WAT, contributing to a chronic state of tissue inflammation [45]. These macrophages express bioactive BDNFs (both mature and pro-BDNF) and TrkB [46,47,48,49]. Several studies have demonstrated that mBDNF stimulation promoted the activation of M1 to M2 macrophage transformation, suppressed inflammatory cytokine secretion, and triggered the migration of macrophages towards the damage site [50,51,52,53]. Since the hypertrophic adipocytes in obese animals produce tumor necrosis factor α (TNFα) and interleukin 6 (IL-6), which are stimulators of BDNF synthesis in monocytes [54,55], it is tempting to hypothesize that BDNF production in ATM is a protective mechanism to promote the formation of M2 macrophages in response to the HFD feeding. In line with this hypothesis, myeloid-specific Bdnf knockout mice, which have no Bdnf expression in their monocytes, mature macrophages, and granulocytes, displayed lower energy expenditure and exacerbated adiposity when they were fed with HFD [37]. Because the authors have not examined the concentration of type 1 cytokines that cause metabolic dysfunctions in the HFD-fed KO mice, whether the immune cells-derived BDNF contributes to the inflammatory responses that are commonly seen in WAT of obese animals remains unanswered. Instead, Blaszkiewicz et al. showed that the myeloid-derived BDNF is essential for maintaining sympathetic innervation in WAT [37], which resembles the axon guidance role of BDNF in the CNS [56]. In contrast to the stimulatory activities of mBDNF, pro-BDNF has an inhibitory effect on macrophage activation and migration, but the metabolic outcomes of enhanced pro-BDNF production on ATM’s behavior have not been explored [49]. Instead, the studies on the pro-BDNF receptor, p75NTR, in adipocytes provide hints on the metabolic functions of pro-BDNF in adipose tissue. When the p75NTR gene (Ngfr) was ablated in adipocytes, lipolysis and membrane translocation of glucose transporter (GLUT4) were enhanced [42,57]. Hence, the adipocyte-specific Ngfr knockout mice are resistant to HFD-induced adiposity, hepatic steatosis, and insulin resistance. These protective effects were not observed in the skeletal muscle Ngfr knockout mice, suggesting that WAT is the primary site of metabolic action for p75NTR [42]. On the other hand, Ngfr overexpression in cultured adipocytes attenuated lipolysis and lipid oxidation by suppressing the activity of protein kinase A. Hence, the elevated Ngfr expression exclusively in WAT of HFD-fed mice provides an additional mechanism to account for the dysregulated lipid metabolism in the tissue [58] (Figure 3).
Figure 3.
Functional activities of mBDNF and pro-BDNF in the white adipose tissue. The mBDNF and pro-BDNF are mainly synthesized by the progenitor cells and macrophages, which act on the mature adipocyte to modulate its metabolism. The BDNFs might also act as an autocrine to modulate the differentiation of pre-adipocytes and the transformation of macrophages.
In short, BDNF in WAT is mostly synthesized by the non-adipose cells to modulate the cellular activity of mature adipocytes.
4. BDNF in Skeletal Muscle
Bdnf mRNA can be detected in the soleus, tibialis anterior (TA), extensor digitorum longus (EDL), gastrocnemius, and diagram muscles [59,60,61]. Within the soleus muscle, both slow- and fast-type myofibers express Bdnf [62], but type II glycolytic myofibers contain higher expression of Bdnf than type I oxidative myofibers [63]. In humans and rats, increased BDNF expression and protein content in Skm are observed following a single running session or regular treadmill trainings [64,65]. Because electrical stimulation is a potent inducer of BDNF secretion in cultured muscle, it is believed that the myofiber contraction is the primary driving factor of BDNF production during exercise, but the functional significance of elevated mBDNF content in Skm after exercise remains obscure [66,67,68]. In pioneer studies to determine the mBDNF’s function in cultured muscle cells, it has been shown that mBDNF was able to stimulate FAO via AMP-activated protein kinase (AMPK) activation [60,66]. A similar observation of AMPK-induced FAO was found in C2C12 after TrkB stimulation by 7,8-DHF [69]. Our recent report further demonstrated that BDNF activated AMPK in the muscle cells via triggering an intracellular Ca2+ surge, which acted through the calmodulin K kinase 2 (CamKK2) to induce AMPK phosphorylation [28]. AMPK is an imperative metabolic sensor that balances the energy metabolism, whose activity is provoked under an energy-deficient state. In the cells that are experiencing energy deficit, AMPK activation inhibits the anabolic process in order to reduce ATP consumption and promotes the catabolic process, which generates ATP [70]. Hence, it is suggested that BDNF in muscle is mainly responsible for the elevation of FAO to meet the energy demand of Skm during exercise by provoking the activity of AMPK [71]. However, studies in transgenic mice that express a kinase-dead AMPKα2 in Skm [72] or in inducible muscle-specific Prkaa1 and Prkaa2 (AMPK α1 and α2 subunit genes) double-knockout mice demonstrated that the contraction-induced FAO was not impaired in their muscle [73], suggesting AMPK activation is dispensable for FAO during exercise. Instead, O’Neill et al. reported that AMPK in Skm is needed for the glucose uptake stimulated by contraction [74]. The role of BDNF in muscle performance during exercise is also unascertained as contradictory results have been reported in studies using muscle-specific Bdnf knockout (MBKO) mice. While we found that the total daily locomotion, exercise endurance, and muscle strength were weakened in mice without BDNF in their muscle, Delezie et al. observed that MBKO mice had greater resistance to contraction-induced fatigue, although they also found the mice displayed lower daily locomotion [60,63]. Hence, the relationship between BDNF-AMPK signaling and exercise-provoked muscle responses needs further verification.
FAO in muscle is also increased during fasting [75]. While glucose uptake, glycolysis, and pyruvate oxidation are favored in the postprandial period when glucose consumption is high, FAO is suppressed in Skm, WAT, and liver to ensure that tissue exposure to hyperglycemia is minimized. On the other hand, the inhibited FAO is unlocked by the action of AMPK via phosphorylating acetyl Co-A carboxylase (ACC) directly, making FA the primary energy source during fasting [76,77]. This “fuel selection (or metabolic flexibility)” is a pivotal response to spare glucose for organs, such as the brain, that utilize glucose as their sole energy source [78,79]. At the molecular level, metabolic flexibility relies on the configuration of signaling pathways that manage nutrient sensing, uptake, transport, storage, and utilization. In cultured C2C12 myotubes, BDNF secretion was provoked by the biochemical factors of fasting, including glucose depletion, amino acid restriction, and β-hydroxybutyrate (βHB) stimulation [28,60,80,81]. We and others have also shown that Bdnf expression in Skm is increased during fasting, suggesting mBDNF might be involved in regulating metabolism to move through the fed-fast cycle [60,81]. Giacco et al. further demonstrated that fasting-induced Bdnf expression is associated with elevated phosphorylation of cAMP-responsive element-binding protein (CREB), TrkB, and AMPK in the skeletal muscle [81]. Despite the elevated Bdnf expression in Skm, the activity of Akt, a major downstream effector of BDNF-TrkB signaling in neurons [82], is downregulated during fasting [81]. Possibly, BDNF might provoke tissue-specific cascades or other fasting-induced responses overwhelm the stimulatory effect of BDNF in muscle during fasting, which requires further exploration. In any case, MBKO mice could not handle the increased FA influx to Skm during fasting because of the impaired FAO ability, leading to the ectopic accumulation of lipids. Eventually, the animals develop lipotoxicity-induced insulin resistance [60]. On the other hand, excessive energy supply such as HFD feeding suppresses Bdnf expression in the Skm, leading to insufficient AMPK phosphorylation, exaggerated accumulation of lipids, and severe diet-induced insulin resistance [28]. These findings suggest that the BDNF-AMPK cascade in Skm is a homeostatic signaling to cope with nutrient availability.
In addition to lipid metabolism, AMPK is crucial to mitochondrial remodeling and homeostasis via controlling the mitochondrial biogenesis, regulating the shape of the mitochondrial network, and clearing the defective mitochondria [83].
Mitochondrial biogenesis occurs when a cell experiences a high energy demand. The increase of mitochondrial content requires the transcription of genes encoded in the nuclear and mitochondrial genomes. Gain- or loss-of-function studies show that AMPK activity positively correlates with the mitochondrial number in Skm [84,85]. Mechanistically, AMPK promotes mitochondrial biogenesis via modulating the activity of peroxisome proliferator-activated receptor γ co-activator 1 α (PGC-1α), which is an inducer of transcriptional coactivation of TRF-1 (Nuclear Respiratory Factor 1) and TFAM (Transcription Factor A, Mitochondrial) [86]. Through direct phosphorylation and expression control, AMPK promotes the activity of PGC-1α to increase the number of mitochondria in Skm [87]. Several studies have shown that BDNF or 7,8-DHF stimulation increased the mitochondrial content and cellular respiration in Skm via the AMPK-PGC-1α pathway in mice [28,60,88,89]. Consequently, BDNF administration or 7,8-DHF consumption effectively reduces the body weight gain of mice under HFD feeding and ameliorates the locomotion after myocardial infarction [88,89]. Stimulation of mitochondrial biogenesis is possibly a universal function of BDNF, as this activity could also be detected during neuronal dendritogenesis [90]. Nevertheless, ERK and CREB, but not AMPK, are responsible for BDNF-promoted PGC-1α and mitochondrial biogenesis in neurons [90]. Because BDNF is able to induce CREB phosphorylation, it is possible that the BDNF-elevated PGC-1α in myotubes is also CREB-dependent [60]. It is also interesting to find that AMPK-PGC-1α signaling and mitochondrial content were only reduced in the MBKO mice during fasting but not in the fed status, which reinforces the physiological role of BDNF as a stress-induced myokine to cope with the energy demand [60]. Presumably, the drop of extracellular glucose and the increase of βHB content in muscle [81] during fasting promote the BDNF production in muscle fibers, which activates AMPK-PGC-1α pathways to facilitate mitochondrial biogenesis and the glycolysis-to-FAO shift.
Defective AMPK signaling in the MBKO muscle not only decreases the synthesis of new mitochondria but also hinders the clearance of the faulty mitochondria. In energy-deficient events, such as exercise and fasting, a large amount of reactive oxygen species (ROS) is generated by the electron transport chain (ETC) complex I and III, which imposes significant damage to the mitochondrial proteins [91]. Interestingly, increased glucose and FA intake in obesity also elevates ROS production, contributing to mitochondrial dysfunction [92]. Mitochondrial fragmentation (mitofission) is facilitated in these conditions to segregate the damaged organelle portion for selective degradation by mitophagy [93]. AMPK is an upstream regulator of the process, as ablating Prkaa1 and Prkaa2 expression results in suppressed mitofission in a variety of cell types [94,95]. While activated AMPK triggers mitofission through phosphorylating the mitochondrial fission factor (MFF) directly, which is the pre-requisite for dynamin-related protein 1 (DRP1) recruitment to induce mitochondrial division [94], it also phosphorylates Unc-5 like activating kinase 1 (ULK1) to promote phagophore formation for non-selective autophagy [96]. The activated ULK1 then induces rapid phosphorylation on the ubiquitin ligase Parkin to prepare its mitochondrial retention [97]. Moreover, AMPK phosphorylates and promotes the accumulation of PTEN-induced kinase 1 (PINK1), a critical inducer of mitophagy [98], at the mitochondrial membrane surface. PINK1 at the mitochondrial membrane phosphorylates the ubiquitin ligase Parkin, which is a critical post-translational modification for the mitochondrial localization and ligase activity of Parkin towards mitochondrial membrane proteins such as voltage-dependent anion channel (VDAC) [99]. Adaptors, including p62, optineurin (OPTN), and nuclear dot protein 52 kDa (NDP52), then recognize the polyubiquitinated mitochondrial proteins and bridge them with the LC3 on phagophore for autophagosome formation [100]. When AMPK is inhibited genetically or pharmacologically, the mitofission and removal of stressed mitochondrial are hampered [101,102]. As an upstream activator of AMPK, stimulation of C2C12 myotubes with mBDNF promotes mitophagy in an AMPK-dependent manner [28]. Interestingly, Skm-derived mBDNF is dispensable for basal mitochondrial dynamics, but is critical to mitophagy initiation when the cells are under metabolic stress, such as the palmitic acid challenge [28]. Hence, the clearance of damaged mitochondria is diminished in the muscle of MBKO mice, leading to the accumulation of dysfunctional mitochondria for efficient FAO, severe insulin resistance, and obesity [28]. It is noteworthy that BDNF- or 7,8-DHF-regulated mitophagy could also be detected in cultured cardiomyocytes, adipocytes, retinal ganglion, and vascular endothelial cells, but whether AMPK in these cells is involved in the process remains to be determined [43,103,104,105]
In addition to muscle [88,106], BDNF has a significant role in regulating the mitochondrial respiration in other tissues. For instance, mBDNF or 7,8-DHF augments the mitochondrial respiration in neuron preparation [107], injured cortical neurons, exfoliated deciduous stem cells-differentiated dopaminergic neurons [108], retinal ganglion cells [105], neuroblastoma [109], cultured cardiomyocytes [110], and placenta trophoblasts [111]. The detailed mechanism of how BDNF modulates mitochondrial respiration remains largely unknown, but the localization of TrkB.FL and TrkB.T1 on mitochondrial membranes provides a possibility that BDNF might act directly on the organelle to regulate its activity [112].
BDNF is also involved in muscle development (myogenesis) and regeneration. These processes require a series of clonal expansion, differentiation, and fusion of muscle cells. During myogenesis, the small multipotent satellite cells (SCs) will be activated and exit the cell cycle to form myoblasts, which align spatially into chains and fuse into the multinucleated myotubes. Muscle regeneration also requires the recruitment SCs to the site of injury, where they are differentiated and fused to form multinucleated myotubes [113]. A high expression of Bdnf was detected in cultured myoblasts, which was downregulated during myogenic differentiation and fusion [61,114,115]. Reduced Bdnf expression can also be detected in the developing muscle of mice [61]. Seidl et al. hypothesized that the presence of myoblast-derived neurotrophin is essential to support the myogenic cell migration. When these cells have reached their ultimate position, terminal differentiation is initiated by the downregulation of neurotrophin synthesis. The cessation of neurotrophin production from the mature muscle cells also provides a cue to proper innervation by eliminating the unnecessary motoneuron synapse [114]. This hypothesis is supported by the observation that chronic mBDNF stimulation decreased the synaptic maturation of the neuromuscular junction (NMJ) in the Xenopus neuron-muscle co-culture [116]. However, contradictory findings have also been reported that mBDNF promotes the structural and functional maturation of neuromuscular synapses via TrkB.FL activation [117]. Garcia et al. further proposed that a minimal synthesis of BDNF from neonatal muscle is indispensable, as it serves as a retrograde modulator to upregulate neurotransmission in all synaptic contacts, regardless of the level of axonal maturation [118]. In alignment with this hypothesis, disrupting the activity of TrkB.FL or overexpressing the TrkB.T1 on the postsynaptic muscle fiber resulted in the disassembly of acetylcholine receptor cluster at the motor endplate [119]. Because the functional ability, apposition, and integrity of the motor endplate were not altered in the MBKO mice [63], the BDNF that is necessary for maintaining the postsynaptic functions might possibly come from the motor neuron or other cell types, such as Schwann cells or satellite cells. Indeed, Bdnf expression was found in “active (Pax7+/MyoD+)” SCs during early differentiation [120], and BDNF stimulation induced a significant increase in myoblast proliferation [120]. Because BDNF content in Skm increases after exercise-induced injury, it is believed that Bdnf expression is important to myogenesis initiation during muscle regeneration [120,121]. Although Bdnf ablation in SCs did not compromise their differentiation into the myogenic lineage in mice, the expression of differentiation markers of later steps of myotube differentiation was significantly reduced, resulting in a delay in the early regeneration of Skm after cardiotoxin-induced injury [122] (Figure 4).
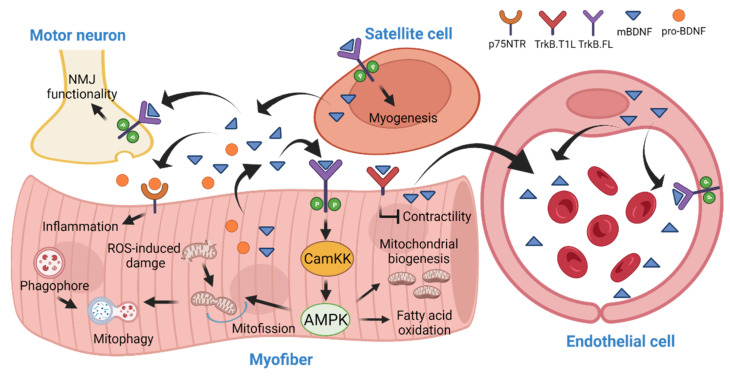
Figure 4.
Functional activities of BDNF in skeletal muscle (Skm). In Skm, mBDNF is produced by the myofiber, satellite cells, and blood vessel endothelial cells. It acts in an autocrine and paracrine manner to modulate the mitochondrial dynamics and lipid metabolism in myofibers, control the myogenesis, and maintain the functions of the neuromuscular junction. The myofiber-generated BDNF is also secreted into circulation to regulate the functions of the pancreas.
Ultimately, BDNF in muscle is not only synthesized by myofibers, but other cells, such as satellite cells and blood vessel endothelial cells, also play a significant role. In addition to the autocrine/paracrine activities to regulate the metabolism of myofibers and muscle regeneration, muscle-derived BDNF serves as a hormone to communicate with other tissues such as the pancreas for maintaining systemic glucose and lipid homeostasis (to be discussed in the next section).
5. Unresolved Questions
In comparison with the studies performed in CNS, our understanding of BDNF’s activity in peripheral tissues is still rudimentary. Studies on BDNF’s function and expression regulation in various non-CNS tissues are fragmented, and inconsistent results are frequently reported, possibly due to the distinct and opposite functions of BDNF isoforms, especially in gene knockout studies where both pro-BDNF and mBDNF are depleted. For instance, ablating the Bdnf gene in the Skm mitigates the denervation-induced muscle atrophy but overexpressing Bdnf also has an ameliorating effect on the defective muscle function in neuromuscular disease [123,124]. Moreover, the lack of precise tools significantly impedes the result interpretation and conclusion to be made. An obvious example is the non-specificity of antibodies that recognize BDNF and TrkB. Because most commercially available antibodies show a certain degree of cross-reactivity with other proteins, it is difficult to accurately determine the cell types that express BDNF and TrkB in tissue or serum. This technical issue can be solved using the knock-in animals with a highly specific tag fused to the BDNF protein or immunoprecipitation as demonstrated by Fulgenzi et al. [68]. Nevertheless, the pathological features in the muscle- or liver-specific Bdnf knockout mice have proved that peripheral tissue-generated BDNF (either mBDNF or pro-BDNF) is equally important to the CNS-derived BDNF in maintaining metabolic homeostasis. Thus, enhancing the BDNF signaling has a beneficial effect on the overall metabolism, particularly in preventing or treating metabolic and neurological disorders [69,88,125].
To better understand the functions of BDNF outside the CNS, a foremost important question to be solved is the source that contributes to the change of circulating BDNF in various physiological and pathological conditions [121,126,127,128]. Because the concentration of BDNF in platelet-poor plasma is low, it is proposed that megakaryocyte/platelet is the origin of BDNF in blood. However, the increased BDNF level in plasma after exercise suggests that circulating BDNF can be originated from other tissues [129]. By comparing the circulating BDNF concentrations between the radial artery and jugular vein, it is concluded that the brain contributes ~70% of BDNF in blood during resting and exercise [130,131]. Nevertheless, there are arguments against the contribution of brain to the circulating BDNF level because in conditions such as stroke and exercise, where the concentration of BDNF in the brain is increased, there is no change in the blood BDNF content [132]. Although Mathew et al. demonstrated that BDNF is an autocrine or paracrine in muscle to regulate the local tissue function because over-expressing Bdnf transiently in skeletal muscle did not change the BDNF concentration in the blood [66], a study using the knock-in mice with a V5 tag in the Bdnf locus showed that muscle-derived BDNF could be secreted into the circulation [68]. Our findings that the amount of circulation BDNF is significantly reduced in the MBKO mice during fasting support this notion [60]. Presumably, the electroporation-mediated Bdnf overexpression in hindlimb muscle as performed by Mathew et al. might not produce a sufficient amount of BDNF to be detected in the circulation. A recent report further argues that the endothelial cells but not the myofibers are the major production site of BDNF in Skm, which may account for the elevation of blood BDNF levels in response to physical exercise [62]. It is also possible that the BDNF in blood is a combinatory secretion from megakaryocytes, endothelial cells, lymphocytes, monocytes, and interstitial fluid from different peripheral tissues, and CNS. In spite of these arguments on the source of BDNF in muscle, the release of Skm-derived BDNF into circulation is undoubtful, which implies that BDNF might function as a hormone to communicate with other tissues. Indeed, Fulgenzi et al. have demonstrated that the BDNF from Skm is responsible for inducing insulin secretion from the pancreatic β-cells, which might represent an inter-organ communication for normalizing hyperglycemia following exercise [68,133]. It would be interesting to investigate in future if BDNF production in other peripheral tissues, such as the liver, would also serve as an endocrine to orchestrate the metabolism or functions in different tissues.
It is also important to determine the dominant receptor of BDNF to exert its metabolic functions in different tissues. The dogma that BDNF only relies on TrkB.FL to initiate the biological activities has been changed since the discovery that TrkB.T1 could transduce intracellular signals by provoking intracellular calcium ([Ca2+]i) release [134]. This finding also overturns the idea that TkrkB.T1 only acts as a dominant-negative inhibitor of TrkB-T1 or limits the availability of BDNF for TrkB.FL binding [135,136]. However, the function of TrkB.T1 in non-nervous tissues is less studied. In tissues where [Ca2+]i is crucial in their cellular function, such as cardiomyocytes and the pancreatic β-cells, deleting TrkB.T1 abolished the action of BDNF, which impedes heart contraction and insulin secretion [68,137]. Because TrkB.FL is hardly detectable in these tissues, the TrkB.T1 is assumed to be the predominant receptor for BDNF to regulate calcium homeostasis. In contrast, TrkB.T1 seems to work as a negative inhibitor of TrkB.Fl in the Skm as depleting the TrkB.T1 gene results in a greater Ca2+ flux between the cytoplasm and sarcoplasmic reticulum to increase contractility [138]. The differential expression of TrkB.FL and TrkB.T1 as well as their functional interaction in various tissues might represent an additional regulatory mechanism for BDNF response in peripheral tissues. Hence, it would be necessary to consider the role of TrkB.T1 in studying the BDNF’s function in the future.
Last but not least, peripheral tissues-produced mBDNF might act on the CNS to modulate cognitive functions. When considering together that mBDNF is a well-recognized myokine whose expression is increased after exercise, muscle-derived mBDNF is secreted into the circulation [68]. Although Pardridge et al. demonstrated that BDNF in blood was rapidly degraded and no mBDNF transcytosis through the blood–brain barrier (BBB) in rat was observed [139], later studies showed that blood-borne mBDNF can enter the CNS by a rapid, saturable transport system of the BBB [140,141], mBDNF concentration in blood is reduced in psychiatric and neurological disorders [142], and physical exercise is an effective means to alleviate the mental dysfunction in a lot of psychiatric diseases [143]. Thus, it is tempting to hypothesize that peripheral mBDNF is a beneficial “exerkine” to improve the mental health. The muscle BDNF–brain cross-talk has been partially validated, as adeno-associated virus-mediated overexpression of pro-BDNF in skeletal muscle reduced the dendritic length and density in the brain, leading to the development of depressive behavior [144]. Thus, it would be interesting to study if muscle-derived mBDNF would have a beneficial effect on psychological disorders.
Acknowledgments
All figures were created with BioRender.com (accessed on 28 May 2022).
Author Contributions
E.C.Y.I. and C.B.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This researched was funded by the Health and Medical Research Fund (HMRF08193006) and HKU Seed Fund for Basic Research.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kowianski P., Lietzau G., Czuba E., Waskow M., Steliga A., Morys J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teng H.K., Teng K.K., Lee R., Wright S., Tevar S., Almeida R.D., Kermani P., Torkin R., Chen Z.Y., Lee F.S., et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bathina S., Das U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Je H.S., Yang F., Ji Y., Potluri S., Fu X.Q., Luo Z.G., Nagappan G., Chan J.P., Hempstead B., Son Y.J., et al. ProBDNF and mature BDNF as punishment and reward signals for synapse elimination at mouse neuromuscular junctions. J. Neurosci. 2013;33:9957–9962. doi: 10.1523/JNEUROSCI.0163-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marosi K., Mattson M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minichiello L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 7.Lima Giacobbo B., Doorduin J., Klein H.C., Dierckx R., Bromberg E., de Vries E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019;56:3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C.S., Kavalali E.T., Monteggia L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell. 2022;185:62–76. doi: 10.1016/j.cell.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kernie S.G., Liebl D.J., Parada L.F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu B., Goulding E.H., Zang K., Cepoi D., Cone R.D., Jones K.R., Tecott L.H., Reichardt L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Rosa M.C., Zimbone S., Saab M.W., Tomasello M.F. The Pleiotropic Potential of BDNF beyond Neurons: Implication for a Healthy Mind in a Healthy Body. Life. 2021;11:1256. doi: 10.3390/life11111256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bariohay B., Lebrun B., Moyse E., Jean A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–5620. doi: 10.1210/en.2005-0419. [DOI] [PubMed] [Google Scholar]
- 13.Barsh G.S., Schwartz M.W. Genetic approaches to studying energy balance: Perception and integration. Nat. Rev. Genet. 2002;3:589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 14.An J.J., Liao G.Y., Kinney C.E., Sahibzada N., Xu B. Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell Metab. 2015;22:175–188. doi: 10.1016/j.cmet.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo G.S., Connie Hung C.C., Rochford J., Keogh J., Gray J., Sivaramakrishnan S., O’Rahilly S., Farooqi I.S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 16.da Fonseca A.C.P., Abreu G.M., Palhinha L., Zembrzuski V.M., Campos Junior M., Carneiro J.R.I., Nogueira Neto J.F., Magno F., Rosado E.L., Maya-Monteiro C.M., et al. A Rare Potential Pathogenic Variant in the BDNF Gene is Found in a Brazilian Patient with Severe Childhood-Onset Obesity. Diabetes Metab. Syndr. Obes. 2021;14:11–22. doi: 10.2147/DMSO.S267202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa T., Tsuchida A., Itakura Y., Nonomura T., Ono M., Hirota F., Inoue T., Nakayama C., Taiji M., Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- 18.Otani K., Okada M., Yamawaki H. Diverse distribution of tyrosine receptor kinase B isoforms in rat multiple tissues. J. Vet. Med. Sci. 2017;79:1516–1523. doi: 10.1292/jvms.17-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camerino C., Conte E., Cannone M., Caloiero R., Fonzino A., Tricarico D. Nerve Growth Factor, Brain-Derived Neurotrophic Factor and Osteocalcin Gene Relationship in Energy Regulation, Bone Homeostasis and Reproductive Organs Analyzed by mRNA Quantitative Evaluation and Linear Correlation Analysis. Front. Physiol. 2016;7:456. doi: 10.3389/fphys.2016.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen M.C., Vatner D.F., Shulman G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017;13:572–587. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechmann L.P., Hannivoort R.A., Gerken G., Hotamisligil G.S., Trauner M., Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchida A., Nakagawa T., Itakura Y., Ichihara J., Ogawa W., Kasuga M., Taiji M., Noguchi H. The effects of brain-derived neurotrophic factor on insulin signal transduction in the liver of diabetic mice. Diabetologia. 2001;44:555–566. doi: 10.1007/s001250051661. [DOI] [PubMed] [Google Scholar]
- 23.Cassiman D., Denef C., Desmet V.J., Roskams T. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 2001;33:148–158. doi: 10.1053/jhep.2001.20793. [DOI] [PubMed] [Google Scholar]
- 24.Meek T.H., Wisse B.E., Thaler J.P., Guyenet S.J., Matsen M.E., Fischer J.D., Taborsky G.J., Jr., Schwartz M.W., Morton G.J. BDNF action in the brain attenuates diabetic hyperglycemia via insulin-independent inhibition of hepatic glucose production. Diabetes. 2013;62:1512–1518. doi: 10.2337/db12-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong J., Liu T., Mi L., Kuang H., Xiong X., Chen Z., Li S., Lin J.D. hnRNPU/TrkB Defines a Chromatin Accessibility Checkpoint for Liver Injury and Nonalcoholic Steatohepatitis Pathogenesis. Hepatology. 2020;71:1228–1246. doi: 10.1002/hep.30921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genzer Y., Chapnik N., Froy O. Effect of brain-derived neurotrophic factor (BDNF) on hepatocyte metabolism. Int. J. Biochem. Cell Biol. 2017;88:69–74. doi: 10.1016/j.biocel.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Cirrik S., Hacioglu G., Abidin I., Aydin-Abidin S., Noyan T. Endoplasmic reticulum stress in the livers of BDNF heterozygous knockout mice. Arch. Physiol. Biochem. 2019;125:378–386. doi: 10.1080/13813455.2018.1489850. [DOI] [PubMed] [Google Scholar]
- 28.Ahuja P., Ng C.F., Pang B.P.S., Chan W.S., Tse M.C.L., Bi X., Kwan H.R., Brobst D., Herlea-Pana O., Yang X., et al. Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice. Autophagy. 2021;18:1367–1384. doi: 10.1080/15548627.2021.1985257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoilov P., Castren E., Stamm S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem. Biophys. Res. Commun. 2002;290:1054–1065. doi: 10.1006/bbrc.2001.6301. [DOI] [PubMed] [Google Scholar]
- 30.Yang B., Ren Q., Zhang J.C., Chen Q.X., Hashimoto K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: Rethinking the brain-liver axis. Transl. Psychiatry. 2017;7:e1128. doi: 10.1038/tp.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teillon S., Calderon G.A., Rios M. Diminished diet-induced hyperglycemia and dyslipidemia and enhanced expression of PPARalpha and FGF21 in mice with hepatic ablation of brain-derived neurotropic factor. J. Endocrinol. 2010;205:37–47. doi: 10.1677/JOE-09-0405. [DOI] [PubMed] [Google Scholar]
- 32.Richard A.J., White U., Elks C.M., Stephens J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K., Dungan K., Hershman J.M., Hofland J., Kalra S., et al., editors. Endotext. Endotext [Internet]; South Dartmouth, MA, USA: 2000. [PubMed] [Google Scholar]
- 33.Bernhard F., Landgraf K., Kloting N., Berthold A., Buttner P., Friebe D., Kiess W., Kovacs P., Bluher M., Korner A. Functional relevance of genes implicated by obesity genome-wide association study signals for human adipocyte biology. Diabetologia. 2013;56:311–322. doi: 10.1007/s00125-012-2773-0. [DOI] [PubMed] [Google Scholar]
- 34.Sornelli F., Fiore M., Chaldakov G.N., Aloe L. Adipose tissue-derived nerve growth factor and brain-derived neurotrophic factor: Results from experimental stress and diabetes. Gen. Physiol. Biophys. 2009;28:179–183. [PubMed] [Google Scholar]
- 35.Nakagomi A., Okada S., Yokoyama M., Yoshida Y., Shimizu I., Miki T., Kobayashi Y., Minamino T. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. NPJ Aging Mech. Dis. 2015;1:15009. doi: 10.1038/npjamd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijay J., Gauthier M.F., Biswell R.L., Louiselle D.A., Johnston J.J., Cheung W.A., Belden B., Pramatarova A., Biertho L., Gibson M., et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat. Metab. 2020;2:97–109. doi: 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaszkiewicz M., Wood E., Koizar S., Willows J., Anderson R., Tseng Y.H., Godwin J., Townsend K.L. The involvement of neuroimmune cells in adipose innervation. Mol. Med. 2020;26:126. doi: 10.1186/s10020-020-00254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang S.W., Liu X., Yepes M., Shepherd K.R., Miller G.W., Liu Y., Wilson W.D., Xiao G., Blanchi B., Sun Y.E., et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J.W., Lee C.W., Lee J., Choi D.J., Sohng J.K., Park Y.I. 7,8-Dihydroxyflavone inhibits adipocyte differentiation via antioxidant activity and induces apoptosis in 3T3-L1 preadipocyte cells. Life Sci. 2016;144:103–112. doi: 10.1016/j.lfs.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Jo D., Son Y., Yoon G., Song J., Kim O.Y. Role of Adiponectin and Brain Derived Neurotrophic Factor in Metabolic Regulation Involved in Adiposity and Body Fat Browning. J. Clin. Med. 2020;10:56. doi: 10.3390/jcm10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song H.D., Kim S.N., Saha A., Ahn S.Y., Akindehin S., Son Y., Cho Y.K., Kim M., Park J.H., Jung Y.S., et al. Aging-Induced Brain-Derived Neurotrophic Factor in Adipocyte Progenitors Contributes to Adipose Tissue Dysfunction. Aging Dis. 2020;11:575–587. doi: 10.14336/AD.2019.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baeza-Raja B., Sachs B.D., Li P., Christian F., Vagena E., Davalos D., Le Moan N., Ryu J.K., Sikorski S.L., Chan J.P., et al. p75 Neurotrophin Receptor Regulates Energy Balance in Obesity. Cell Rep. 2016;14:255–268. doi: 10.1016/j.celrep.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colitti M., Montanari T. Brain-derived neurotrophic factor modulates mitochondrial dynamics and thermogenic phenotype on 3T3-L1 adipocytes. Tissue Cell. 2020;66:101388. doi: 10.1016/j.tice.2020.101388. [DOI] [PubMed] [Google Scholar]
- 44.Grant R., Youm Y.H., Ravussin A., Dixit V.D. Quantification of adipose tissue leukocytosis in obesity. Methods Mol. Biol. 2013;1040:195–209. doi: 10.1007/978-1-62703-523-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo L., Lumeng C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barouch R., Appel E., Kazimirsky G., Brodie C. Macrophages express neurotrophins and neurotrophin receptors. Regulation of nitric oxide production by NT-3. J. Neuroimmunol. 2001;112:72–77. doi: 10.1016/S0165-5728(00)00408-2. [DOI] [PubMed] [Google Scholar]
- 47.Kerschensteiner M., Gallmeier E., Behrens L., Leal V.V., Misgeld T., Klinkert W.E., Kolbeck R., Hoppe E., Oropeza-Wekerle R.L., Bartke I., et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J. Exp. Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kruse N., Cetin S., Chan A., Gold R., Luhder F. Differential expression of BDNF mRNA splice variants in mouse brain and immune cells. J. Neuroimmunol. 2007;182:13–21. doi: 10.1016/j.jneuroim.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Wong I., Liao H., Bai X., Zaknic A., Zhong J., Guan Y., Li H.Y., Wang Y.J., Zhou X.F. ProBDNF inhibits infiltration of ED1+ macrophages after spinal cord injury. Brain Behav. Immun. 2010;24:585–597. doi: 10.1016/j.bbi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Ji X.C., Dang Y.Y., Gao H.Y., Wang Z.T., Gao M., Yang Y., Zhang H.T., Xu R.X. Local Injection of Lenti-BDNF at the Lesion Site Promotes M2 Macrophage Polarization and Inhibits Inflammatory Response After Spinal Cord Injury in Mice. Cell Mol. Neurobiol. 2015;35:881–890. doi: 10.1007/s10571-015-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asami T., Ito T., Fukumitsu H., Nomoto H., Furukawa Y., Furukawa S. Autocrine activation of cultured macrophages by brain-derived neurotrophic factor. Biochem. Biophys. Res. Commun. 2006;344:941–947. doi: 10.1016/j.bbrc.2006.03.228. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki S., Takeda K., Ouhara K., Shirawachi S., Kajiya M., Matsuda S., Kono S., Shiba H., Kurihara H., Mizuno N. Involvement of Rac1 in macrophage activation by brain-derived neurotrophic factor. Mol. Biol. Rep. 2021;48:5249–5257. doi: 10.1007/s11033-021-06531-6. [DOI] [PubMed] [Google Scholar]
- 53.Yu H.C., Huang H.B., Huang Tseng H.Y., Lu M.C. Brain-Derived Neurotrophic Factor Suppressed Proinflammatory Cytokines Secretion and Enhanced MicroRNA(miR)-3168 Expression in Macrophages. Int. J. Mol. Sci. 2022;23:570. doi: 10.3390/ijms23010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulte-Herbruggen O., Nassenstein C., Lommatzsch M., Quarcoo D., Renz H., Braun A. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J. Neuroimmunol. 2005;160:204–209. doi: 10.1016/j.jneuroim.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Kern P.A., Ranganathan S., Li C., Wood L., Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 56.Hoshino N., Vatterott P., Egwiekhor A., Rochlin M.W. Brain-derived neurotrophic factor attracts geniculate ganglion neurites during embryonic targeting. Dev. Neurosci. 2010;32:184–196. doi: 10.1159/000313902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baeza-Raja B., Li P., Le Moan N., Sachs B.D., Schachtrup C., Davalos D., Vagena E., Bridges D., Kim C., Saltiel A.R., et al. p75 neurotrophin receptor regulates glucose homeostasis and insulin sensitivity. Proc. Natl. Acad. Sci. USA. 2012;109:5838–5843. doi: 10.1073/pnas.1103638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaidhu M.P., Anthony N.M., Patel P., Hawke T.J., Ceddia R.B. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL, and AMPK. Am. J. Physiol. Cell Physiol. 2010;298:C961–C971. doi: 10.1152/ajpcell.00547.2009. [DOI] [PubMed] [Google Scholar]
- 59.Saka Y., Yoshimura O., Tahara H., Takeda Y., Moriyama H., Maejima H., Tobimatsu Y. The mRNA expression of neurotrophins in different skeletal muscles of young rats. Hiroshima J. Med. Sci. 2007;56:23–28. [PubMed] [Google Scholar]
- 60.Yang X., Brobst D., Chan W.S., Tse M.C.L., Herlea-Pana O., Ahuja P., Bi X., Zaw A.M., Kwong Z.S.W., Jia W.H., et al. Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility. Sci. Signal. 2019;12:eaau1468. doi: 10.1126/scisignal.aau1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mousavi K., Jasmin B.J. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J. Neurosci. 2006;26:5739–5749. doi: 10.1523/JNEUROSCI.5398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cefis M., Chaney R., Quirie A., Santini C., Marie C., Garnier P., Prigent-Tessier A. Endothelial cells are an important source of BDNF in rat skeletal muscle. Sci. Rep. 2022;12:311. doi: 10.1038/s41598-021-03740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delezie J., Weihrauch M., Maier G., Tejero R., Ham D.J., Gill J.F., Karrer-Cardel B., Rüegg M.A., Tabares L., Handschin C. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc. Natl. Acad. Sci. USA. 2019;116:16111–16120. doi: 10.1073/pnas.1900544116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maderova D., Krumpolec P., Slobodova L., Schon M., Tirpakova V., Kovanicova Z., Klepochova R., Vajda M., Sutovsky S., Cvecka J., et al. Acute and regular exercise distinctly modulate serum, plasma and skeletal muscle BDNF in the elderly. Neuropeptides. 2019;78:101961. doi: 10.1016/j.npep.2019.101961. [DOI] [PubMed] [Google Scholar]
- 65.Cuppini R., Sartini S., Agostini D., Guescini M., Ambrogini P., Betti M., Bertini L., Vallasciani M., Stocchi V. Bdnf expression in rat skeletal muscle after acute or repeated exercise. Arch. Ital. Biol. 2007;145:99–110. [PubMed] [Google Scholar]
- 66.Matthews V.B., Astrom M.B., Chan M.H., Bruce C.R., Krabbe K.S., Prelovsek O., Akerstrom T., Yfanti C., Broholm C., Mortensen O.H., et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 67.Kimura T., Kaneko F., Iwamoto E., Saitoh S., Yamada T. Neuromuscular electrical stimulation increases serum brain-derived neurotrophic factor in humans. Exp. Brain Res. 2019;237:47–56. doi: 10.1007/s00221-018-5396-y. [DOI] [PubMed] [Google Scholar]
- 68.Fulgenzi G., Hong Z., Tomassoni-Ardori F., Barella L.F., Becker J., Barrick C., Swing D., Yanpallewar S., Croix B.S., Wess J., et al. Novel metabolic role for BDNF in pancreatic β-cell insulin secretion. Nat. Commun. 2020;11:1950. doi: 10.1038/s41467-020-15833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan C.B., Tse M.C., Liu X., Zhang S., Schmidt R., Otten R., Liu L., Ye K. Activation of muscular TrkB by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem. Biol. 2015;22:355–368. doi: 10.1016/j.chembiol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carbone J.W., McClung J.P., Pasiakos S.M. Skeletal muscle responses to negative energy balance: Effects of dietary protein. Adv. Nutr. 2012;3:119–126. doi: 10.3945/an.111.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pedersen B.K., Pedersen M., Krabbe K.S., Bruunsgaard H., Matthews V.B., Febbraio M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 2009;94:1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- 72.Dzamko N., Schertzer J.D., Ryall J.G., Steel R., Macaulay S.L., Wee S., Chen Z.P., Michell B.J., Oakhill J.S., Watt M.J., et al. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J. Physiol. 2008;586:5819–5831. doi: 10.1113/jphysiol.2008.159814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hingst J.R., Kjobsted R., Birk J.B., Jorgensen N.O., Larsen M.R., Kido K., Larsen J.K., Kjeldsen S.A.S., Fentz J., Frosig C., et al. Inducible deletion of skeletal muscle AMPKalpha reveals that AMPK is required for nucleotide balance but dispensable for muscle glucose uptake and fat oxidation during exercise. Mol. Metab. 2020;40:101028. doi: 10.1016/j.molmet.2020.101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neill H.M., Maarbjerg S.J., Crane J.D., Jeppesen J., Jorgensen S.B., Schertzer J.D., Shyroka O., Kiens B., van Denderen B.J., Tarnopolsky M.A., et al. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc. Natl. Acad. Sci. USA. 2011;108:16092–16097. doi: 10.1073/pnas.1105062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carroll J.E., Villadiego A., Morse D.P. Fatty acid oxidation intermediates and the effect of fasting on oxidation in red and white skeletal muscle. Muscle Nerve. 1983;6:367–373. doi: 10.1002/mus.880060505. [DOI] [PubMed] [Google Scholar]
- 76.de Lange P., Farina P., Moreno M., Ragni M., Lombardi A., Silvestri E., Burrone L., Lanni A., Goglia F. Sequential changes in the signal transduction responses of skeletal muscle following food deprivation. FASEB J. 2006;20:2579–2581. doi: 10.1096/fj.06-6025fje. [DOI] [PubMed] [Google Scholar]
- 77.Brockhoff M., Rion N., Chojnowska K., Wiktorowicz T., Eickhorst C., Erne B., Frank S., Angelini C., Furling D., Ruegg M.A., et al. Targeting deregulated AMPK/mTORC1 pathways improves muscle function in myotonic dystrophy type I. J. Clin. Investig. 2017;127:549–563. doi: 10.1172/JCI89616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith R.L., Soeters M.R., Wust R.C.I., Houtkooper R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018;39:489–517. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Lange P., Moreno M., Silvestri E., Lombardi A., Goglia F., Lanni A. Fuel economy in food-deprived skeletal muscle: Signaling pathways and regulatory mechanisms. FASEB J. 2007;21:3431–3441. doi: 10.1096/fj.07-8527rev. [DOI] [PubMed] [Google Scholar]
- 80.Antony R., Li Y. BDNF secretion from C2C12 cells is enhanced by methionine restriction. Biochem. Biophys. Res. Commun. 2020;533:1347–1351. doi: 10.1016/j.bbrc.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giacco A., Cioffi F., Cuomo A., Simiele R., Senese R., Silvestri E., Amoresano A., Fontanarosa C., Petito G., Moreno M., et al. Mild Endurance Exercise during Fasting Increases Gastrocnemius Muscle and Prefrontal Cortex Thyroid Hormone Levels through Differential BHB and BCAA-Mediated BDNF-mTOR Signaling in Rats. Nutrients. 2022;14:1166. doi: 10.3390/nu14061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dolcet X., Egea J., Soler R.M., Martin-Zanca D., Comella J.X. Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor-induced motoneuron survival. J. Neurochem. 1999;73:521–531. doi: 10.1046/j.1471-4159.1999.0730521.x. [DOI] [PubMed] [Google Scholar]
- 83.Herzig S., Shaw R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jorgensen S.B., Treebak J.T., Viollet B., Schjerling P., Vaulont S., Wojtaszewski J.F., Richter E.A. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am. J. Physiol. Endocrinol. Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- 85.Garcia-Roves P.M., Osler M.E., Holmstrom M.H., Zierath J.R. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J. Biol. Chem. 2008;283:35724–35734. doi: 10.1074/jbc.M805078200. [DOI] [PubMed] [Google Scholar]
- 86.Gleyzer N., Vercauteren K., Scarpulla R.C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jager S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wood J., Tse M.C.L., Yang X., Brobst D., Liu Z., Pang B.P.S., Chan W.S., Zaw A.M., Chow B.K.C., Ye K., et al. BDNF mimetic alleviates body weight gain in obese mice by enhancing mitochondrial biogenesis in skeletal muscle. Metabolism. 2018;87:113–122. doi: 10.1016/j.metabol.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Matsumoto J., Takada S., Furihata T., Nambu H., Kakutani N., Maekawa S., Mizushima W., Nakano I., Fukushima A., Yokota T., et al. Brain-Derived Neurotrophic Factor Improves Impaired Fatty Acid Oxidation Via the Activation of Adenosine Monophosphate-Activated Protein Kinase-a—Proliferator-Activated Receptor-r Coactivator-1a Signaling in Skeletal Muscle of Mice With Heart Failure. Circ. Heart Fail. 2021;14:e005890. doi: 10.1161/CIRCHEARTFAILURE.119.005890. [DOI] [PubMed] [Google Scholar]
- 90.Cheng A., Wan R., Yang J.L., Kamimura N., Son T.G., Ouyang X., Luo Y., Okun E., Mattson M.P. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lejri I., Grimm A., Eckert A. Mitochondria, Estrogen and Female Brain Aging. Front. Aging Neurosci. 2018;10:124. doi: 10.3389/fnagi.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhatti J.S., Bhatti G.K., Reddy P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toyama E.Q., Herzig S., Courchet J., Lewis T.L., Jr., Losón O.C., Hellberg K., Young N.P., Chen H., Polleux F., Chan D.C., et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pedersen B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019;15:383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 96.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell. Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hung C.M., Lombardo P.S., Malik N., Brun S.N., Hellberg K., Van Nostrand J.L., Garcia D., Baumgart J., Diffenderfer K., Asara J.M., et al. AMPK/ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy. Sci. Adv. 2021;7:eabg4544. doi: 10.1126/sciadv.abg4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang B., Nie J., Wu L., Hu Y., Wen Z., Dong L., Zou M.H., Chen C., Wang D.W. AMPKalpha2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ. Res. 2018;122:712–729. doi: 10.1161/CIRCRESAHA.117.312317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geisler S., Holmstrom K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 100.Padman B.S., Nguyen T.N., Uoselis L., Skulsuppaisarn M., Nguyen L.K., Lazarou M. LC3/GABARAPs drive ubiquitin-independent recruitment of Optineurin and NDP52 to amplify mitophagy. Nat. Commun. 2019;10:408. doi: 10.1038/s41467-019-08335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laker R.C., Drake J.C., Wilson R.J., Lira V.A., Lewellen B.M., Ryall K.A., Fisher C.C., Zhang M., Saucerman J.J., Goodyear L.J., et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017;8:548. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H., Liu B., Li T., Zhu Y., Luo G., Jiang Y., Tang F., Jian Z., Xiao Y. AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int. J. Mol. Med. 2018;41:69–76. doi: 10.3892/ijmm.2017.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Z., Wang S.P., Shao Q., Li P.F., Sun Y., Luo L.Z., Yan X.Q., Fan Z.Y., Hu J., Zhao J., et al. Brain-derived neurotrophic factor mimetic, 7,8-dihydroxyflavone, protects against myocardial ischemia by rebalancing optic atrophy 1 processing. Free Radic. Biol. Med. 2019;145:187–197. doi: 10.1016/j.freeradbiomed.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 104.Jin H., Zhu Y., Li Y., Ding X., Ma W., Han X., Wang B. BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis. 2019;24:511–528. doi: 10.1007/s10495-019-01535-x. [DOI] [PubMed] [Google Scholar]
- 105.Shim M.S., Kim K.Y., Noh M., Ko J.Y., Ahn S., An M.A., Iwata T., Perkins G.A., Weinreb R.N., Ju W.K. Optineurin E50K triggers BDNF deficiency-mediated mitochondrial dysfunction in retinal photoreceptor cell line. Biochem. Biophys. Res. Commun. 2018;503:2690–2697. doi: 10.1016/j.bbrc.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsumoto J., Takada S., Kinugawa S., Furihata T., Nambu H., Kakutani N., Tsuda M., Fukushima A., Yokota T., Tanaka S., et al. Brain-Derived Neurotrophic Factor Improves Limited Exercise Capacity in Mice With Heart Failure. Circulation. 2018;138:2064–2066. doi: 10.1161/CIRCULATIONAHA.118.035212. [DOI] [PubMed] [Google Scholar]
- 107.Markham A., Cameron I., Franklin P., Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur. J. Neurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen H.T.N., Kato H., Masuda K., Yamaza H., Hirofuji Y., Sato H., Pham T.T.M., Takayama F., Sakai Y., Ohga S., et al. Impaired neurite development associated with mitochondrial dysfunction in dopaminergic neurons differentiated from exfoliated deciduous tooth-derived pulp stem cells of children with autism spectrum disorder. Biochem. Biophys. Rep. 2018;16:24–31. doi: 10.1016/j.bbrep.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Agrawal R., Tyagi E., Vergnes L., Reue K., Gomez-Pinilla F. Coupling energy homeostasis with a mechanism to support plasticity in brain trauma. Biochim. Biophys. Acta. 2014;1842:535–546. doi: 10.1016/j.bbadis.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 110.Zhao J., Du J., Pan Y., Chen T., Zhao L., Zhu Y., Chen Y., Zheng Y., Liu Y., Sun L., et al. Activation of cardiac TrkB receptor by its small molecular agonist 7,8-dihydroxyflavone inhibits doxorubicin-induced cardiotoxicity via enhancing mitochondrial oxidative phosphorylation. Free Radic. Biol. Med. 2019;130:557–567. doi: 10.1016/j.freeradbiomed.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 111.Prince C.S., Maloyan A., Myatt L. Tropomyosin Receptor Kinase B Agonist, 7,8-Dihydroxyflavone, Improves Mitochondrial Respiration in Placentas From Obese Women. Reprod. Sci. 2018;25:452–462. doi: 10.1177/1933719117716776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wiedemann F.R., Siemen D., Mawrin C., Horn T.F., Dietzmann K. The neurotrophin receptor TrkB is colocalized to mitochondrial membranes. Int. J. Biochem. Cell Biol. 2006;38:610–620. doi: 10.1016/j.biocel.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 113.Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seidl K., Erck C., Buchberger A. Evidence for the participation of nerve growth factor and its low-affinity receptor (p75NTR) in the regulation of the myogenic program. J. Cell Physiol. 1998;176:10–21. doi: 10.1002/(SICI)1097-4652(199807)176:1<10::AID-JCP2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 115.Miura P., Amirouche A., Clow C., Belanger G., Jasmin B.J. Brain-derived neurotrophic factor expression is repressed during myogenic differentiation by miR-206. J. Neurochem. 2012;120:230–238. doi: 10.1111/j.1471-4159.2011.07583.x. [DOI] [PubMed] [Google Scholar]
- 116.Song W., Jin X.A. Brain-derived neurotrophic factor inhibits neuromuscular junction maturation in a cAMP-PKA-dependent way. Neurosci. Lett. 2015;591:8–12. doi: 10.1016/j.neulet.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 117.Wang T., Xie K., Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J. Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garcia N., Tomas M., Santafe M.M., Besalduch N., Lanuza M.A., Tomas J. The interaction between tropomyosin-related kinase B receptors and presynaptic muscarinic receptors modulates transmitter release in adult rodent motor nerve terminals. J. Neurosci. 2010;30:16514–16522. doi: 10.1523/JNEUROSCI.2676-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gonzalez M., Ruggiero F.P., Chang Q., Shi Y.J., Rich M.M., Kraner S., Balice-Gordon R.J. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron. 1999;24:567–583. doi: 10.1016/S0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 120.McKay B.R., Nederveen J.P., Fortino S.A., Snijders T., Joanisse S., Kumbhare D.A., Parise G. Brain-derived neurotrophic factor is associated with human muscle satellite cell differentiation in response to muscle-damaging exercise. Appl. Physiol. Nutr. Metab. 2020;45:581–590. doi: 10.1139/apnm-2019-0501. [DOI] [PubMed] [Google Scholar]
- 121.Yu T., Chang Y., Gao X.L., Li H., Zhao P. Dynamic Expression and the Role of BDNF in Exercise-induced Skeletal Muscle Regeneration. Int. J. Sports Med. 2017;38:959–966. doi: 10.1055/s-0043-118343. [DOI] [PubMed] [Google Scholar]
- 122.Clow C., Jasmin B.J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell. 2010;21:2182–2190. doi: 10.1091/mbc.e10-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aby K., Antony R., Eichholz M., Srinivasan R., Li Y. Enhanced pro-BDNF-p75NTR pathway activity in denervated skeletal muscle. Life Sci. 2021;286:120067. doi: 10.1016/j.lfs.2021.120067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Halievski K., Xu Y., Haddad Y.W., Tang Y.P., Yamada S., Katsuno M., Adachi H., Sobue G., Breedlove S.M., Jordan C.L. Muscle BDNF improves synaptic and contractile muscle strength in Kennedy’s disease mice in a muscle-type specific manner. J. Physiol. 2020;598:2719–2739. doi: 10.1113/JP279208. [DOI] [PubMed] [Google Scholar]
- 125.Pandey S.N., Kwatra M., Dwivedi D.K., Choubey P., Lahkar M., Jangra A. 7,8-Dihydroxyflavone alleviated the high-fat diet and alcohol-induced memory impairment: Behavioral, biochemical and molecular evidence. Psychopharmacology. 2020;237:1827–1840. doi: 10.1007/s00213-020-05502-2. [DOI] [PubMed] [Google Scholar]
- 126.Krabbe K.S., Nielsen A.R., Krogh-Madsen R., Plomgaard P., Rasmussen P., Erikstrup C., Fischer C.P., Lindegaard B., Petersen A.M., Taudorf S., et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 127.Glud M., Christiansen T., Larsen L.H., Richelsen B., Bruun J.M. Changes in Circulating BDNF in relation to Sex, Diet, and Exercise: A 12-Week Randomized Controlled Study in Overweight and Obese Participants. J. Obes. 2019;2019:4537274. doi: 10.1155/2019/4537274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Polyakova M., Stuke K., Schuemberg K., Mueller K., Schoenknecht P., Schroeter M.L. BDNF as a biomarker for successful treatment of mood disorders: A systematic & quantitative meta-analysis. J. Affect. Disord. 2015;174:432–440. doi: 10.1016/j.jad.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 129.Nilsson J., Ekblom O., Ekblom M., Lebedev A., Tarassova O., Moberg M., Lovden M. Acute increases in brain-derived neurotrophic factor in plasma following physical exercise relates to subsequent learning in older adults. Sci. Rep. 2020;10:4395. doi: 10.1038/s41598-020-60124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rasmussen P., Brassard P., Adser H., Pedersen M.V., Leick L., Hart E., Secher N.H., Pedersen B.K., Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 131.Seifert T., Brassard P., Wissenberg M., Rasmussen P., Nordby P., Stallknecht B., Adser H., Jakobsen A.H., Pilegaard H., Nielsen H.B., et al. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- 132.Bejot Y., Mossiat C., Giroud M., Prigent-Tessier A., Marie C. Circulating and brain BDNF levels in stroke rats. Relevance to clinical studies. PLoS ONE. 2011;6:e29405. doi: 10.1371/journal.pone.0029405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Podyma B., Parekh K., Guler A.D., Deppmann C.D. Metabolic homeostasis via BDNF and its receptors. Trends Endocrinol. Metab. 2021;32:488–499. doi: 10.1016/j.tem.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rose C.R., Blum R., Pichler B., Lepier A., Kafitz K.W., Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 135.Biffo S., Offenhauser N., Carter B.D., Barde Y.A. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Development. 1995;121:2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- 136.Eide F.F., Vining E.R., Eide B.L., Zang K., Wang X.Y., Reichardt L.F. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J. Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fulgenzi G., Tomassoni-Ardori F., Babini L., Becker J., Barrick C., Puverel S., Tessarollo L. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J. Cell Biol. 2015;210:1003–1012. doi: 10.1083/jcb.201502100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dorsey S.G., Lovering R.M., Renn C.L., Leitch C.C., Liu X., Tallon L.J., Sadzewicz L.D., Pratap A., Ott S., Sengamalay N., et al. Genetic deletion of trkB.T1 increases neuromuscular function. Am. J. Physiol. Cell Physiol. 2012;302:C141–C153. doi: 10.1152/ajpcell.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pardridge W.M., Kang Y.S., Buciak J.L. Transport of human recombinant brain-derived neurotrophic factor (BDNF) through the rat blood-brain barrier in vivo using vector-mediated peptide drug delivery. Pharm. Res. 1994;11:738–746. doi: 10.1023/A:1018940732550. [DOI] [PubMed] [Google Scholar]
- 140.Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 141.Alcala-Barraza S.R., Lee M.S., Hanson L.R., McDonald A.A., Frey W.H., 2nd, McLoon L.K. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug. Target. 2010;18:179–190. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sen S., Duman R., Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol. Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang X., Cai Z.D., Jiang W.T., Fang Y.Y., Sun W.X., Wang X. Systematic review and meta-analysis of the effects of exercise on depression in adolescents. Child. Adolesc. Psychiatry Ment. Health. 2022;16:16. doi: 10.1186/s13034-022-00453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin L.Y., Kelliny S., Liu L.C., Al-Hawwas M., Zhou X.F., Bobrovskaya L. Peripheral ProBDNF Delivered by an AAV Vector to the Muscle Triggers Depression-Like Behaviours in Mice. Neurotox. Res. 2020;38:626–639. doi: 10.1007/s12640-020-00256-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.