Abstract
Purpose:
We aim to systematically review and summarize the demographics, clinical features, management strategies, and clinical outcomes of primary and radiation-induced skull-base osteosarcoma (SBO).
Methods:
PubMed, Scopus, and Cochrane databases were used to identify relevant articles. Papers including SBO cases and sufficient clinical outcome data were included. A comprehensive clinical characteristic review and survival analysis were also conducted.
Results:
Forty-one studies describing 67 patients were included. The median age was 31 years (male = 59.7%). The middle skull-base was most commonly involved (52.7%), followed by anterior (34.5%) and posterior (12.7%) skull-base. Headache (27%), exophthalmos (18%), and diplopia (10%) were common presenting symptoms. Sixty-eight percent of patients had primary SBO, while 25% had radiation-induced SBO. Surgery was the main treatment modality in 89% of cases. Chemotherapy was administered in 65.7% and radiotherapy in 50%. Median progression-free survival (PFS) was 12 months, and the overall 5-year survival was 22%. The five-year survival rates of radiation-induced SBO and primary SBO were 39% and 16%, respectively (P < 0.05).
Conclusion:
SBO is a malignant disease with poor survival outcomes. Surgical resection is the primary management modality, in conjunction with chemotherapy and radiotherapy. Complete surgical resection showed better survival rates compared to partial resection. Radiation-induced SBO has a superior survival outcome as compared to its primary counterpart.
Keywords: Skull-base osteosarcoma, primary osteosarcoma, radiation induced osteosarcoma, systematic review
Introduction
Osteosarcoma is a rare, debilitating neoplasm that may arise from de novo mutation, metastasis from another location, or radiation exposure. It is the most common type of bone cancer in children and adolescents, exhibiting an incidence of 3.4 per million per year.[1] Although it primarily affects long bones, nearly 10% of osteosarcomas present in the head and neck, often manifesting in the mandible and maxilla.[2][3] Primary skull-base osteosarcoma (SBO) or head and neck osteosarcoma (HNO) with skull-base invasion is a considerably rare presentation.[4] In contrast to long bone osteosarcoma, which usually presents in the first and second decades of life, head and neck osteosarcoma (including SBO) often presents in the third and fourth decades.[5] Predisposing factors include prior radiation and underlying conditions such as Paget disease.[6–9]
Regardless of the involved site, the mainstay of treatment consists of gross total resection, with adjuvant chemotherapy and radiotherapy. However, anatomical constraints are often a barrier to complete resection. Given the rare incidence of disease, most conclusions about SBO are derived from single institution reports and registries. With the paucity of data in the literature and discrepancy among the reported cases regarding SBO, the clinical features remain indistinct and a consensus on a standardized treatment protocol has not yet been reached.[4, 10–12] In this literature review, we aim to summarize the demographics, clinical features, management strategies, and clinical outcomes of SBO to inform where additional research is needed.
Methods
Literature Search
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[13] PubMed, Scopus, and Cochrane databases were searched from inception to June 2020. A medical subject headings (MeSH) term and keyword search of each database were conducted using the Boolean operators OR and AND. Terms used were as follows: “skull,” “base,” and “osteosarcoma.” Identified papers were uploaded into Mendeley, and duplicates were eliminated.
Study Selection
Pre-established inclusion and exclusion criteria were deductively defined. Studies were included if they met the following criteria: 1) English language, 2) prospective or retrospective studies involving at least one patient, 3) patients with histologically confirmed SBO in any age group, 4) available data on clinical features and treatment outcomes. Studies were excluded if they: 1) did not adequately identify and report on clinical outcomes or management of SBO or 2) were meta-analyses, reviews, editorials, letters, or books.
Two authors (O.B.A. and N.S.S.) independently assessed the titles and abstracts of all extracted papers based on the inclusion and exclusion criteria. Studies that met inclusion criteria were then further evaluated independently with full text review by the same two authors. Eligible studies were selected based on the pre-specified criteria, and disagreements between the two authors were resolved via a third author (A.S.H). References of the included articles were also screened to retrieve any relevant papers.
Data Extraction
Data from included studies were extracted by one author (O.B.A.) and confirmed independently by two other authors (A.S.H. and N.S.S.) to ensure accuracy. Extraction variables included: 1) author’s name, 2) date of publication, 3) study design, 4) sample size, 5) gender, 6) prior interventions (radiotherapy or chemotherapy), 7) management course and treatment modalities used (radiotherapy, chemotherapy, surgical approach), 8) complication, and 9) survival. Terms “gross-total resection” or “complete surgical resection” were considered equivalent to “complete resection.” Likewise, the terms “sub-total resection,” “near-total resection,” and “debulking” were considered equivalent to ‘partial resection.’
Data Synthesis
The primary outcomes of interest were the clinical features, management course, and survival analysis of both primary SBO and HNO with skull-base invasion. The secondary outcomes of interest were complications with a comparison between primary SBO and radiation- induced SBO. Meta-analysis was precluded due to heterogeneity in outcome measures and the limited number of studies. Moreover, evaluation of the risk of bias across the papers was not conducted given that all included studies were observational studies for which there is no validated tool to assess for risk of bias.[14]
Statistical Analysis
Means and ranges were used to summarize continuous variables, while frequencies and percentages were used to summarize categorical variables. Overall and progression-free survivals were illustrated using Kaplan–Meier curves. The log-rank test was used to compare survival curves of primary, radiation-induced, and metastatic SBO and compare survival curves of complete and partial resection in patients who received adjuvant therapy. A P-value <0.05 was considered significant for all analyses. Analyses were performed using the statistical software SPSS V.25 (IBM Corp, Armonk, New York).
Results
Study Selection
The initial database search identified 325 articles (Medline: 148, Scopus: 174, Cochrane: 3). After duplicate removal, a total of 245 studies were screened by abstract and title, of which 199 were excluded, leaving 46 full-text studies. Of these, 25 articles failed to meet the inclusion criteria and subsequently excluded. References were additionally screened and identified 20 articles. A total of 41 studies (2 case series and 39 case reports) were included in this systematic review (Fig. 1).[4, 7, 12, 15–51]
Figure 1.
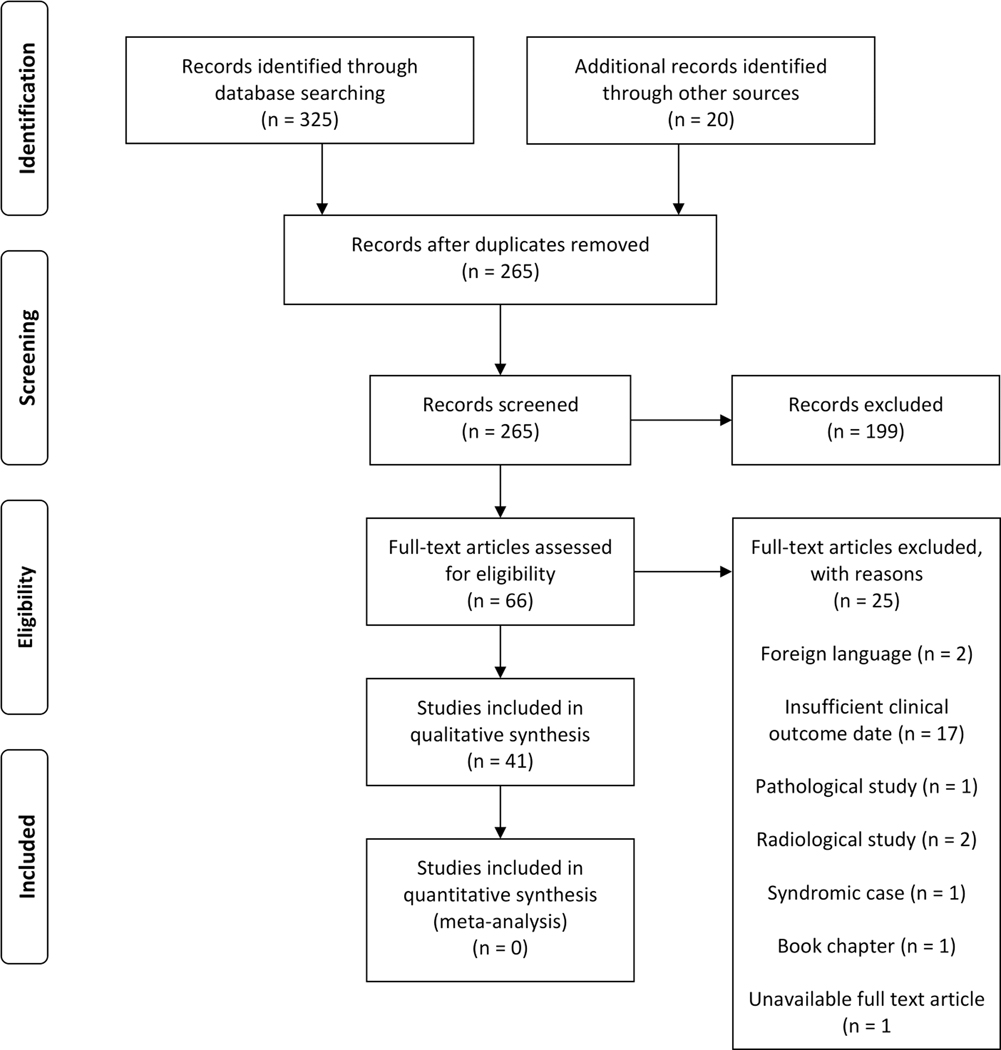
PRISMA flowchart illustrating the search strategy and data selection based on the inclusion and exclusion criteria.
Patient Demographics and Clinical Characteristics
A total of 67 patients with histologically confirmed SBO were analyzed. Patient demographics and clinical characteristics are collectively and individually presented in Tables 1 and 2, respectively. There were 40 males (60%) and 27 females (40%), and the median age at diagnosis was 31 years (range 9–78). Of 55 patients with reported tumor locations, 29 (52.7%) tumors involved the middle skull-base, 19 (34.5%) involved the anterior skull-base and 7 (12.7%) involved the posterior skull-base. Additionally, the most common involved structure was the sphenoid bone (n=15; 22.4%), followed by the sphenoid sinus (n=8; 21%) and the temporal bone (n=6; 9%). The most commonly reported symptoms included headache (27%), exophthalmos (18%), and lastly, diplopia (10.4%).
Table 1.
Individual patient outcome and overview of all the included articles.
| Case | Author | Age/Gender | Location | Primary/Mets/Radiation Induced/Recurrence | CPS | Histopathology | Tumor size (cm) | Primary treatment | Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IF radiation induced | Grade | Type | Surgery | Chemotherapy | Radiation | Recurrence | PFS | OS | status | ||||||||||
| Site of Initial Radiation Therapy | Dosage | Time before diagnosis | Yes/No | Type of surgery | |||||||||||||||
| 1 | Alleyne et al., 200015 | 16/F | TF | Primary | -- | -- | Headache /confusion/Intracranial hemorrhage | -- | Fibroblastic | 2×2 | Yes | -- | MTX, LCVN, BLMN, VCR | -- | No | 11 | 11 | D | |
| 2 | Ashkan et al., 199816 | 32 / M | PTF | primary | -- | -- | Headache, diplopia CN3 Palsy | -- | Osteoblastic | -- | Yes | GTR | CPN, DXCN, IFD, ETD | No | No | 11 | 11 | A | |
| 3 | Chennupati et al., 200812 | 14/F | Rt Hypoglossal canal, SS | primary | -- | -- | tongue deviation, dysphagia, hoarseness, headache, Rt CNX/ XI/ XII Psy | High | -- | -- | No | -- | CPN, DXCN, IFD, ETD, MTX, Intrathecal(MTX) | Yes, Total 7000 cGy | No | 11 | 12 | A | |
| 4 | Echchikhi et al., 201617 | 29/M | SB, TB, MX, TL | RI | Undifferentiated carcinoma of the nasopharynx. | 70 Gy | 11 years | headache, homolateral facial pain, numbness, diplopia, exophthalmia | -- | -- | -- | No | -- | IFD, CPN, DXCN | No | No | 1 | D | |
| 5 | Ellison et al., 199618 | 11/F | PF, ST, CS, SS, SB, left MSB | Primary | -- | -- | left ear pain, loss of vision, proptosis and pain of the left eye, VI CN palsy. | -- | Chondroblastic | -- | Yes | STR | MTX, LCVN, CPN, ADMN | Yes | No | -- | 2 | A | |
| 6 | Guo et al., 20174 | 28/M | Asb, ms, es, orbit | primary | -- | -- | Epistaxis | -- | -- | -- | Yes | GTR | No | No | Yes | -- | 22 | D | |
| 7 | Guo et al., 20174 | 37/M | Msb, mn, ppf | primary | -- | -- | Facial lumps | -- | Osteoblastic | -- | Yes | NTR | No | Yes | Yes | -- | 38 | D | |
| 8 | Guo et al., 20174 | 52/M | ASB, MX | primary | -- | -- | Facial lumps | -- | -- | 15 | Yes | GTR | Yes | Yes | Yes | -- | 20 | D | |
| 9 | Guo et al., 20174 | 25/F | ASB, MX, MS | primary | -- | -- | Epistaxis | -- | -- | -- | Yes | GTR | No | Yes | yes | -- | 40 | D | |
| 10 | Guo et al., 20174 | 22/F | MSB, IF | primary | -- | -- | Facial lumps | -- | -- | -- | Yes | GTR | Yes | Yes | Yes | -- | 132 | A | |
| 11 | Guo et al., 20174 | 52/F | MSB, MN, IF | primary | -- | -- | Facial lumps | -- | -- | -- | Yes | GTR | No | No | -- | -- | -- | -- | |
| 12 | Guo et al., 20174 | 48/M | ASB, ES | primary | -- | -- | Headache | -- | Chondroblastic | -- | Yes | GTR | Yes | Yes | Yes | -- | 28 | D | |
| 13 | Guo et al., 20174 | 14/F | ASB, MS, IF | primary | -- | -- | Facial lumps | -- | osteoblastic | -- | Yes | GTR | No | No | Yes | -- | 18 | D | |
| 14 | Guo et al., 20174 | 45/M | PSB, Clivus region | primary | -- | -- | Headache | -- | osteoblastic | -- | Yes | NTR | Yes | Yes | Yes | -- | 50 | D | |
| 15 | Guo et al., 20174 | 11/M | MSB, PSB, IF, PS | primary | -- | -- | Exophthalmos | -- | osteoblastic | -- | Yes | NTR | No | No | No | -- | 3 | D | |
| 16 | Guo et al., 20174 | 55/m | ASB-MSB, Orbit, IF, PS | primary | -- | -- | Toothache, loose teeth | -- | -- | -- | Yes | GTR | Yes | No | -- | -- | -- | -- | |
| 17 | Guo et al., 20174 | 15/M | MSB-PSB, PS | primary | -- | -- | Dysphagia | -- | chondroblastic | 5.5 | Yes | NTE | No | Yes | Yes | -- | 10 | D | |
| 18 | Guo et al., 20174 | 41/M | Asb-msb, mx, if, ps | primary | -- | -- | Toothache, loose teeth | -- | -- | 10 | Yes | GTR | Yes | Yes | Yes | -- | 25 | D | |
| 19 | Guo et al., 20174 | 24/M | Asb, ms | primary | -- | -- | Neoplasm in gums | -- | -- | 3.7 | Yes | GTR | No | Yes | Yes | 59 | 59 | A | |
| 20 | Guo et al., 20174 | 31/M | Asb, es | primary | -- | -- | Exophthalmos | -- | chondroblastic | 4.5 | Yes | GTR | Yes | Yes | Yes | 54 | 54 | A | |
| 21 | Guo et al., 20174 | 41/F | Msb, if | primary | -- | -- | Facial lumps | -- | chondroblastic | 7 | Yes | GTR | Yes | Yes | Yes | 44 | 44 | A | |
| 22 | Guo et al., 20174 | 36/M | Asb, es, ms | primary | -- | -- | Epistaxis | -- | -- | 6.5 | Yes | GTR | Yes | Yes | Yes | -- | 52 | A | |
| 23 | Guo et al., 20174 | 17/M | Asb-msb, mn, if | primary | -- | -- | Toothache, loose teeth | -- | -- | 15 | Yes | GTR | No | No | Yes | -- | 12 | D | |
| 24 | Guo et al., 20174 | 55/F | Msb-psb, ss, clival | primary | -- | -- | Diplopia | -- | -- | 6 | Yes | STR | Yes | Yes | Yes | 5 | 5 | A | |
| 25 | Hadley et al., 201419 | 14/M | MS, ES, SS, ASB, clivus | Primary | -- | -- | Epistaxis, Eye pain | -- | chondroblastic | 4.9 | Yes | GTR | Yes | Yes | No | 12 | 12 | A | |
| 26 | Hettmer et al., 200220 | 31/F | ASB, NC, ES, FS, orbit | primary | -- | -- | Headache, exophthalmos | -- | -- | -- | yes | STR | ADMN, MTX, CPN, IFD (COSS86) | No | Yes | 27 | 27 | LFU | |
| 27 | kachhara et al., 199921 | 38/M | ASB, MSB, SS, clivus | primary | -- | -- | Diplopia/CN VI palsy | -- | -- | 4 | Yes | GTR | ADMN, IFD, CPN | yes (55 Gy) | No | 18 | 18 | A | |
| 28 | Lin et al., 200522 | 15/F | Orbit, MSB, IF | Metastatic | -- | -- | exophthalmos | -- | Osteoblastic | -- | Yes | embolization, STR | MTX | Yes (4000 cGy) | No | 11 | 24 | LFU | |
| 29 | Lee et al., 200123 | 37/F | Sb, TB, FO, ZB | primary | -- | -- | diplopia | High | -- | -- | Yes | GTR | DXCN, VCN, CFD, PDN (CHOP) | No | No | 12 | 12 | A | |
| 30 | Mathkour et al., 201624 | 29/M | Clivus, SS | Primary | -- | -- | Headache | High | -- | 2.7×2.5×3.2 | Yes | GTR | Yes | Yes | No | 24 | 24 | A | |
| 31 | Meel et al., 201225 | 10/M | SB greater wing | Primary | -- | -- | Exophthalmos | -- | -- | -- | Yes | STR | DXCN, CPN | yes (50 Gy) | No | 18 | 18 | A | |
| 32 | Mohadjer et al., 200426 | 23/m | Bilateral orbits, SB | Metastatic | -- | -- | exophthalmos | -- | Telangiectatic | -- | NO | -- | Yes | Yes | No | -- | 46 | D | |
| 33 | Mohindra et al., 201427 | 55/M | Clivus | primary | -- | -- | Headache, nasal obstruction | High | -- | -- | yes | GTR | ADMN, CFD, VCN | YES (4500 cGy) | No | 12 | 12 | A | |
| 34 | Patel et al., 20117 | 54/M | SB, FB, TB | RI | Lt maxillary and orbital adenoid cystic carcinoma | 60 | 12 | -- | -- | -- | 6×6 | Yes | STR | Yes | No | No | -- | 13 | D |
| 35 | Patel et al., 20117 | 63/M | Palate, MS | RI | Nasal cavity squamous cell carcinoma | -- | 4.5 | -- | -- | -- | -- | Yes | GTR | Yes | No | No | -- | 47 | D |
| 36 | Patel et al., 20117 | 50/M | MX, IF | RI | Lt nasal squamous cell carcinoma | -- | 14 | -- | -- | -- | 2.5×2.5 | Yes | GTR | Yes | No | No | -- | 67 | A |
| 37 | Patel et al., 20117 | 44/F | SS, ES, parasailer region, MSB | RI | Craniopharyngioma | 60 | 9 | -- | -- | -- | -- | NO | -- | Yes | No | No | -- | 16 | D |
| 38 | Patel et al., 20117 | 15/M | zygoma | RI | Bilateral retinoblastoma | -- | 15 | -- | -- | -- | -- | Yes | GTR | Yes | No | No | -- | 62 | D |
| 39 | Patel et al., 20117 | 22/M | MS, ES | RI | Rt retinoblastoma | -- | 20 | -- | -- | -- | 5×5×7 | Yes | GTR | Yes | No | No | -- | 2 | LFU |
| 40 | Patel et al., 20117 | 10/M | mastoid and jugular foramen | RI | Embryonal rhabdomyosarcoma | 50.4 | 6.75 | -- | -- | -- | 4.5×3×4.3 | Yes | STR | Yes | Yes | No | -- | 29 | D |
| 41 | Patel et al., 20117 | 31/M | palate, IF, MSB | RI | Rhabdomyosarcoma | 60 | 18 | -- | -- | -- | 4×4×4 | Yes | GTR | Yes | No | No | -- | 143 | D |
| 42 | Patel et al., 20117 | 26/M | orbit, ASB, ES | RI | Bilateral retinoblastoma | 35 | 25 | -- | -- | -- | 3×2.5×2.5 | Yes | GTR | Yes | No | No | -- | 41 | D |
| 43 | Kohyama et al., 201528 | 43/F | ASB | primary | -- | -- | Supraorbital mass | -- | osteoblastic | 1.8×2.5 | yes | GTR | CPN, IFD, CBPN, ETD | No | No | 24 | 24 | A | |
| 44 | Whitehead et al., 199829 | 48/M | SB, MSB, PF, | Primary | -- | -- | temporal pain, blurred vision | High | Telangiectatic | -- | Yes | -- | CPN, DXCN | Yes | No | 4 | 4 | A | |
| 45 | Yamada et al., 201230 | 75/F | SS, CS | RI | Pituitary adenoma | 50 | 20 | Headache | -- | -- | -- | yes (3 times) | STR | iFD, CPN, ETD | Yes | No | 24 | 24 | A |
| 46 | Hazarika et al., 199531 | 19/F | SB, FB | primary | -- | -- | headache, Exomphalos | -- | Telangiectatic | 4×5 | yes | STR | -- | -- | Prog | 12 | 15 | A | |
| 47 | Yamada et al., 201332 | 78/F | SS, ES, CS | Primary | -- | -- | Headache | -- | -- | -- | Yes | STR | No | Yes | No | 8 | 8 | A | |
| 48 | Amine et al., 197633 | 26/F | Sella | RI | Pituitary adenoma | 51 | 10 | DLC | -- | -- | -- | yes | STR | No | Yes | prog | -- | 1.25 | D |
| 49 | Tanaka et al., 198934 | 57/M | SB | RI | Craniopharyngioma |
110 | 15 | change in memory and mental statuse | -- | -- | -- | Yes | embolization, STR | No | No | prog | -- | 0.5 | D |
| 50 | Salvati et al., 199335 | 45/M | SB | RI | Pituitary adenoma | 44 | 12 | -- | -- | -- | -- | Yes | STR | -- | Yes (50 Gy) | prog | 13 | 16 | D |
| 51 | Gnanalingham et al., 200236 | 67/F | Sella | RI | Pituitary adenoma | 52 | 14 | bitemporal hemianopsia and headache | High | -- | -- | yes | -- | No | No | prog | -- | -- | -- |
| 52 | Bembo et al., 200437 | 45/M | Sella | RI | Pituitary adenoma | 46 | 5 | headache, visual loss | -- | -- | -- | yes | -- | No | No | Prog | 1.5 | 1.75 | D |
| 53 | Patel et al., 201438 | 52/M | Sella + clivus | RI | Craniopharyngioma |
50 | 22 | Headache, sinusitis | -- | osteoblastic | -- | yes | -- | No | No | Prog | -- | 1 | D |
| 54 | Sundaresan et al., 198539 | 13/ F | Skull base (Not Specified) | Primary | -- | -- | -- | -- | -- | -- | yes | STR | MTX | No | No | 66 | 66 | A | |
| 55 | Mark et al., 199140 | 14/ M | ASB | Primary | -- | -- | -- | -- | -- | -- | -- | -- | yes | yes | prog | -- | 12 | D | |
| 56 | Potepan et al., 199945 | 13/ F | SB | Metastatic | -- | -- | -- | -- | -- | -- | -- | -- | MTX, VCN, CPN, IFD | No | prog | 7 | 12 | D | |
| 57 | Gadwal et al., 20012 | 9/ M | sphenoid | Primary | -- | -- | -- | -- | -- | -- | -- | -- | No | yes | prog | -- | 9 | D | |
| 58 | Kornreic et al., 198846 | 11/ F | MSB | Metastatic | -- | -- | Exomphalos | -- | osteoblastic | -- | yes | STR | YES | Yes | No | 24 | 24 | A | |
| 59 | Ohno et al., 201147 | 14/ F | ASB | Primary | -- | -- | -- | -- | -- | -- | yes | STR | yes | No | prog | -- | 26 | D | |
| 60 | Sen et al., 200548 | 28 y/F | SB, MSB, IF | Primary | -- | -- | exophthalmos, temporal bossing | -- | -- | yes | STR | No | No | No | 1 | 1 | A | ||
| 61 | Hayashi et al., 200049 | 28 y/M | Orbit, SB | Primary | -- | -- | Headacehe, exophthalmos | -- | -- | -- | yes | GTR | MTX/CPN/tetrahydropyranyl-Adriamycin | Yes (50 Gy) | prog | 2 | 10 | D | |
| 62 | Marks et al., 198750 | 59/F | SB | Primary | -- | -- | tender enlarged mass | grade II | -- | 7 | yes | GTR | -- | No | No | 16 | 16 | A | |
| 63 | Kleinsasser et al., 195741 | 48/F | Sella | primary | -- | -- | -- | -- | -- | -- | Yes | STR | No | No | Prog | -- | 0.2 | D | |
| 64 | Reichenthal et al., 198151 | 22/M | Floor and anterior clinoid process of sella turcica | primary | -- | -- | headache, diplopia, bilateral XI CN palsy | -- | -- | -- | Yes | Biopsy | Yes | Yes | No | 19 | 19 | A | |
| 65 | Park et al., 199542 | 56/F | Clivus | primary | -- | -- | Headache, and visual disturbance/L lateral gaze limitation | Low | Fibroblastic | -- | Yes | -- | No | No | -- | -- | -- | -- | |
| 66 | Geetha et al., 199843 | 38 /M | Sellar-suprasellar mass involving sphenoid sinus | primary | -- | -- | Diplopia and obesity/ decreased visual acuity | -- | -- | -- | Yes | STR | Yes | No | No | 12 | 12 | A | |
| 67 | Uysal et al., 200144 | 17/M | Nasal cavity, paranasal sinuses, and extending to clivus | primary | -- | -- | Epistaxis/L CN VII palsy | -- | Chondroblastic | -- | Yes | -- | No | Yes | No | 46 | 46 | A | |
PTF = Pituitary Fossa; TF = Temporal Fossa; SS = Sphenoid Sinus; SB = Sphenoid Bone; TB = Temporal Bone; TL = Temporal Lobe; MX = Maxilla; CS = Cavernous sinus; PPF = Pterygopalatine Fossa; ST = Sella Turcica; MS = Maxillary Sinus; MSB = Meddle Skull Base; IF = Infratemporal Fossa; ES = Ethmoidal Sinus; Ethmoidal Sinus; PSB = Posterior Skull Base; PS = Parapharyngeal Space; MN = Mandible; NC = Nasal Cavity; FO = Fronto-Orbital; ZB = Zygomatic Bone; SO = Supra-Orbital; OA = Orbital Apex; SOF = Superior Orbital Fissure; FB = Frontal Bone; CPS = Chief Presenting Symptoms; DOS = Duration of Symptoms; GTR = Gross-Total Resection; NTR = Near-Total Resection; STR = Subtotal Resection; VCR = Vincristine; BLMN = Bleomycin; MTX = Methotrexate; LCVN = Leucovorin; CPN = Cisplatin; DXCN = Doxorubicin; IFD = Ifosfamide; ETD = Etoposide; ADMN = Adriamycin; CFD = Cyclophosphamide; PDN = Prednisone; PFS = Progression- Free Survival; OS = Overall Survival; RI, Radiation Induced
Table 2.
Data summary of all pooled articles
| Characteristic | Value |
|---|---|
|
| |
| Cohort size (n) | 67 |
| Median age, range (yrs) | 31, 9–78 |
| gender | |
| Male | 40 (59.7%) |
| Female | 27 (40.3%) |
| Most common locations (n = 55) | N (%) |
|
| |
| Anterior skull base | 19/55 (34.5%) |
| Middle skull base | 29/55 (52.7%) |
| Posterior skull base | 7/55 (12.7 %) |
| Most common involved structures (n = 55) | N (%) |
|
| |
| Sphenoid bone | 15 (22.4%) |
| Sphenoid sinus | 8 (12%) |
| Temporal bone | 6 (9%) |
| others | 29 (43.4%) |
| Most common presenting symptoms (n = 51) | N (%) |
|
| |
| Headache | 18(27%) |
| Exophthalmos | 12(18%) |
| Diplopia | 5(10.4%) |
| Others | 16(46.6%) |
| Type of etiology (n = 67) | N (%) |
|
| |
| Primary osteosarcoma | 46 (68.4%) |
| Radiation induced osteosarcoma | 17 (25.4%) |
| Metastatic osteosarcoma | 4 (6%) |
| Metastases (n = 5) | N (%) |
|
| |
| Primary osteosarcoma (n = 2) | Parietal bone and liver; shoulder bones |
| Radiation induced osteosarcoma (n = 1) | Pulmonary |
| Metastatic osteosarcoma (n = 2) | Multiple facial bones; lung and maxilla |
| Histopathological type (n = 21) | N (%) |
|
| |
| Osteoblastic | 9 (42.9%) |
| Chondroblastic | 7 (33.3%) |
| Telangiectatic | 4 (19%) |
| Fibroblastic | 1 (4.8%) |
| Management | N (%) |
|
| |
| Surgery | 60 (89.6%) |
| Of reported surgical details (n = 53) | |
| Complete resection | 29 (54.7%) |
| Partial resection | 24 (45. 3%) |
| Chemotherapy | 44 (65.7 %) |
| Radiotherapy, | 34 (50.7%) |
| Median dose, IQR (cGy, n = 7) | 5000, 4500–5500 |
| Outcome | N (%) |
|
| |
| Median PFS, range (mos) (n = 31) | 12, 0.2–143 |
| Median OS, Range (mos) (n = 63) | 12, 1.5–66 |
| Recurrence (n/%) | 17 (25.4%) |
| Disease progression (n/%) | 13 (19.4%) |
| Status (n= 63) | N (%) |
|
| |
| Alive (n/%) | 30 (48%) |
| Dead (n/%) | 33, (52%) |
Most of the reported tumors were primary SBO (n=46; 68.4%), followed by radiation-induced SBO (n=17; 25.4%) and metastatic SBO (n=4; 6%). Among the radiation-induced SBO cohort, various initial pathologies were present, including: craniopharyngioma, adenoid cystic carcinoma, squamous cell carcinoma, and embryonal rhabdomyosarcoma, with pituitary adenoma being the most common pathology. Additionally, the median dose of radiation and latency period were 5100 cGy and 13 months, respectively. Of all included patients in this study, only 21 were found to have data on histopathologic tumor subtypes, of which 9 (43%) were osteoblastic, 7 (33.3%) chondroblastic, 4 (19%) telangiectatic, and 1 (4.8%) fibroblastic. Also, metastases were reported in 5 cases. Two primary skull-base osteosarcomas metastasized to the liver and shoulder bones, while other two metastatic cases of SBO showed further metastases to the lung and multiple facial bones. However, only one patient with radiation-induced SBO showed distal metastasis, involving the lungs. Initial management involved surgical resection in sixty patients (89.6%), out of which 53 patients reported surgical details. Out of those 53 patients with details on method of resection, 29 (54.7%) were complete resections, and 24 (45.3%) were partial resections. Out of the total study cohort, forty-four patients (65.7%) received chemotherapy, and 34 (51%) received radiotherapy with a median dose of 5000 cGy (interquartile range: 4500–5500).
Survival outcomes and analysis
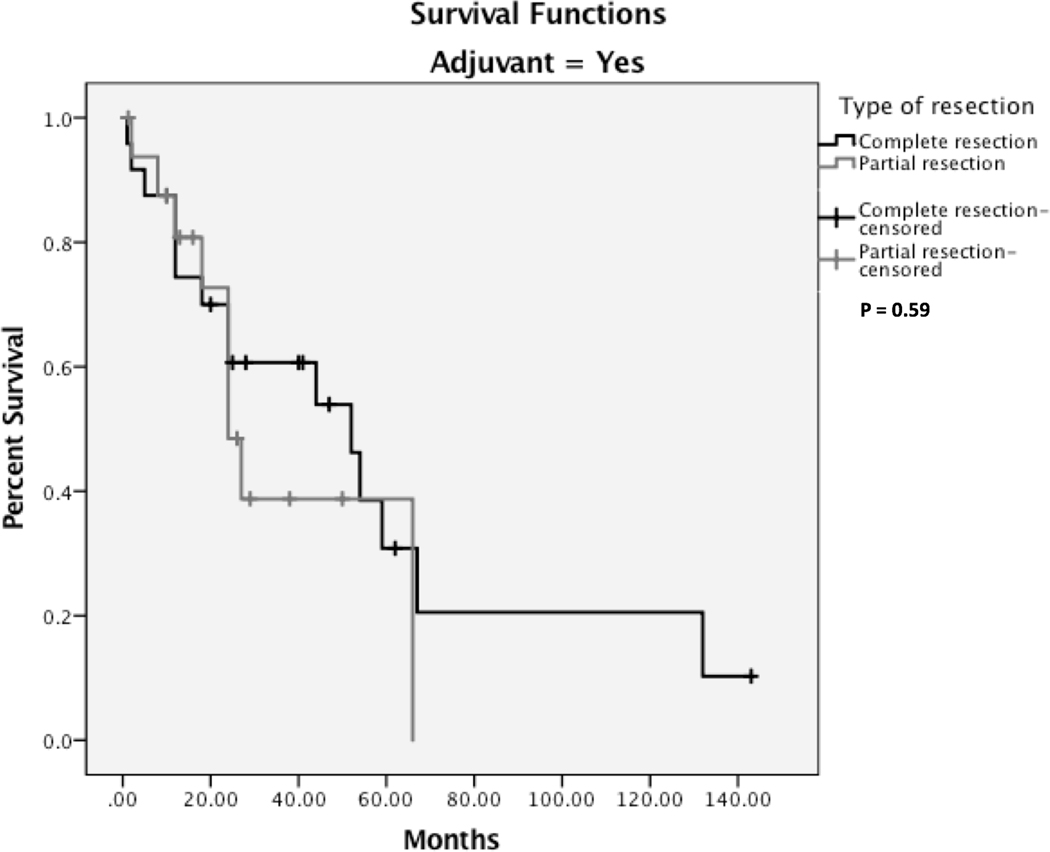
While 37 (55.2%) patients had a complete remission, disease progression was reported in 13 (19.4%) cases, and recurrence was reported in 17 (25.4%) cases with a median progression-free survival (PFS) of 12 months (range: 0.2 – 143 months). A five-year PFS is provided in Fig. 2A. There were 33 (52%) deaths reported with a median overall survival of 12 months (range: 1.5–66 months). Five-year overall survival is shown in Fig.2B. Additionally, a five-year survival analysis indicates that radiation-induced OS had a significantly better overall survival than the primary OS (log-rank test, P < 0.05, Fig. 3). Patients who received adjuvant therapy and underwent complete surgical resection had a better outcome than patients who received adjuvant therapy with partial resection; however, it was statistically insignificant (P = 0.59) (Fig. 4).
Figure 2.
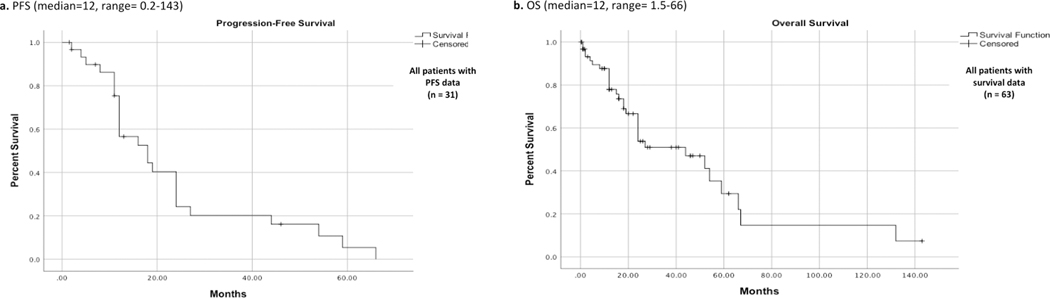
Kaplan-Meier survival curves for a. PFS (n = 31) and b. OS (n = 63) for the overall pooled cohort. PFS, progression free survival; OS, overall survival.
Figure 3.
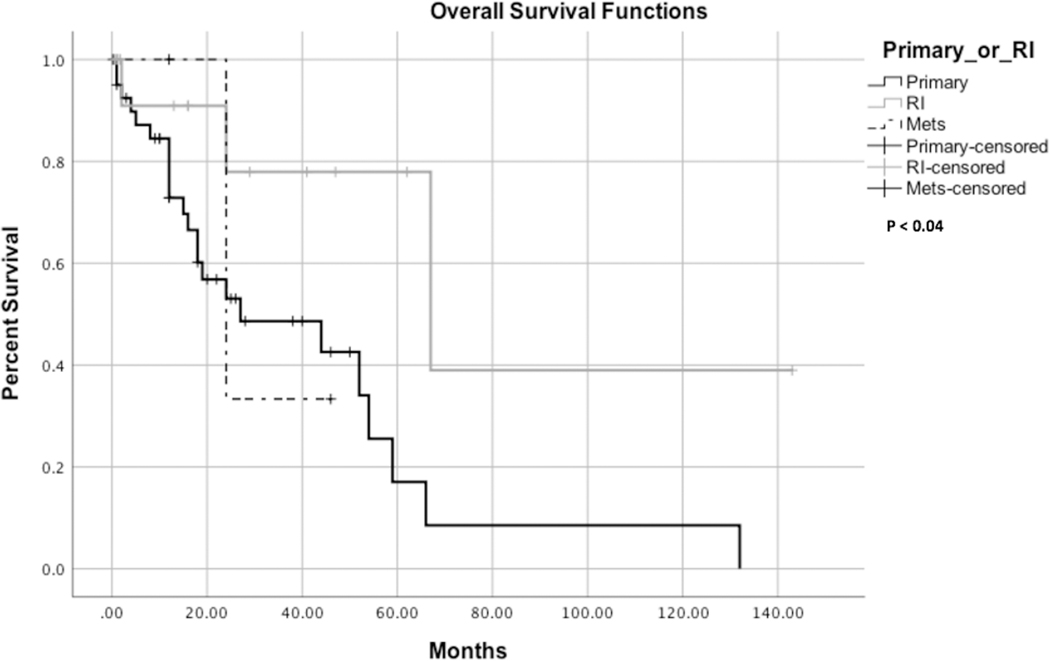
Kaplan-Meier survival curves comparing the overall survival of primary, metastatic and radiation induced osteosarcoma. RI, radiation induced; Mets, Metastatic.
Figure 4.
Kaplan-Meier survival curves comparing complete resection and partial resection in patients received adjuvant therapy.
Discussion
Osteosarcoma of the skull base is a rare and challenging condition. The pathogenesis of osteosarcoma remains obscure; however, various risk factors such as trauma, benign bone lesions, environmental factors, and genetic predisposition have been implicated in the literature.[6, 52, 53] This systematic review aimed to provide a comprehensive summary of the current literature regarding SBO. With a collective total of 67 patients, this study represents the largest analysis of SBO to date, examining the patient background, surgical management, clinical outcomes, recurrence, and survival associated with this tumor.
Clinical features and outcomes
The median age for the included cases of SBO was 31 years. This is unlike the incidence of long bone osteosarcoma (LBO), which is bimodally distributed by age, and peaks in adolescence (second and third decades) and in the elderly (seventh decade).[54] In addition, there was a male predominance (59.7%) seen across the included cases similar to long bone osteosarcoma.[54] The clinical symptoms and tumor locations in our study were intuitively associated: Exophthalmus, diplopia, and decreased visual acuity were seen with tumors involving the orbital cavity while nasal congestion and epistaxis were seen with tumors invading the nasal cavity. Overall, most patients presented with headaches, exophthalmos, and diplopia (27%, 18%, and 10%, respectively). In contrast, a case series by Guo et al. reported facial lump as the primary symptom.[4] This is likely attributed to the fact that most included cases were secondary osteosarcomas due to radiation’s direct impact on skull bones.
In our analysis, the most common location for SBO was the middle skull-base (52.7%), and the most involved structure was the sphenoid bone (22.4%). Likewise, the sphenoid was reported as the most commonly involved bone by chondrosarcoma as well.[55] Due to the condensed anatomy and proximity to adjacent structures, most tumors in our study invaded multiple compartments with some tumors exhibiting facial, nasal, oral, or ophthalmic extensions. As such, a management strategy involving a multidisciplinary approach with otolaryngology, oral maxillofacial surgery or ophthalmology is warranted in these patients.
Imaging and Histopathology
Of the included cases, CT scans typically highlighted a mixture of osteoblastic-osteoclastic lesions with a predominance of one type, hypertonicity indicating calcification, and irregular margins. Similar findings were also reported in HNO as well as in LBO.[56–58] MR imaging predominantly revealed an iso-intense heterogeneous tumor on T1-weighted images and hypo-intense heterogeneous tumor on T2-weighted images with contrast enhancement. Likewise, a recent case series by Luo et al., reported parallel MRI features suggesting consistency among cases.[57] However, due to the ambiguity involved in imaging cues, biopsy is required for a definitive diagnosis.
Grossly, osteosarcoma shows osteoid production by neoblastic mesenchymal cells and heterogeneous areas of necrosis and hemorrhage.[59] Neoplastic cells, mainly osteoblasts, can present with considerable polymorphism, such as spindle cells, ovoid, small round cells, fusiform, epithelial, plasmacytoid, and round cells.[17, 60] Moreover, the pathological tumor grading system is based on the extent of local destruction and tumor cell differentiation level ranging from grade 1 and 2 (low-grades) to grade 3 and 4 (high-grades) osteosarcoma.[61] Osteosarcoma immunohistochemically is mainly positive for vimentin, S100, and neuron-specific enolase; however, it is negative for actin, myoglobin, and cytokeratin.[48] Histopathologically, osteosarcoma can be divided into four categories based on the predominant cell type: Osteoblastic, Chondroblastic, Telangiectatic, and Fibroblastic.[60] Of 21 cases that reported the histopathological diagnosis, the osteoblastic type was the most common (42.9%), followed by chondroblastic (33.3%), telangiectatic (19%), and fibroblastic (5%). This mirrors literature on LBO, which has the greatest prevalence of osteoblastic, followed by chondroblastic and fibroblastic, respectively.[62] However, the results of HNO were less concordant. While histological subtype distributions similar to this study were documented in HNO, other authors reported different distributions in HNO with chondroblastic as the most common type, followed by fibroblastic and osteoblastic.[63, 64]
Treatment and Survival
While most of our cohort underwent surgery, nearly 45% failed to achieve complete resection, which emphasizes the complex anatomy and surgical challenges of the skull-base. Generally, intra-operative estimation by the neurosurgeon has been used to determine partial, subtotal, or total tumor resection. However, neurosurgeons have begun to adopt objective measures, like post-op scanning, to determine the extent of resection. While most authors in our cohort relied on neurosurgeons’ estimation intraoperatively along with resection margin testing, almost none of the included articles reported employing objective measures like volumetric analysis on the post-operative scans.
Generally, the five-year survival rate in long bone osteosarcoma is between 70%−80%, and roughly 60% in HNO.[65, 66] In his literature review, Guo et al. reported that the 5-year survival rate in 47 patients with SBO was 37.8%, with a median of 42 months.[4] In contrast, our five-year overall survival rate was 22%, with a median of 12 months and a range of 0.2–143. The poor survival observed in our study is most likely attributed to the anatomic complexity and close proximity of SBO to vital intracranial structures, thus limiting the total resection in a considerable portion of cases. The choice and the effect of adjuvant therapy in SBO have not yet been investigated thoroughly in the current literature. However, data on HNO have shown controversial results. While some authors support adopting adjuvant therapy, including chemotherapy and radiotherapy, in conjunction with surgical resection in HNO, others presented results showing a significant survival benefit with surgical resection alone.[67, 68] These data contradict the results of LBO, which demonstrated a clear survival benefit of adjuvant therapy that allows limb-sparing procedures rather than traditional amputation in some cases.[69–71] On the other hand, some evidence exists that chemotherapy enhances survival outcomes in craniofacial osteosarcoma; therefore, several authors advocate employing the chemotherapy protocols used for OS of the long bones for craniofacial osteosarcoma.[72, 73] Similarly, an SBO case series found that patients who underwent comprehensive treatment––which includes surgical resection in conjunction with chemotherapy and/or radiotherapy––demonstrated a better overall survival rate than the patients who underwent resection alone and showed a significantly longer median survival duration.[4] These findings support adopting a comprehensive treatment approach in osteosarcoma of the skull base.
In our cohort, the authors’ choice of chemotherapy agents and radiotherapy concepts were variable and case-based due to the lack of standard protocol in treating SBO. While most articles did not specify the chosen chemotherapeutic agents, few adopt standard chemotherapy regimens like CDOP (Cyclophosphamide, Doxorubicin, Oncovin, Prednisone), which is typically employed to treat non-Hodgkin lymphoma.[16] Also, other commonly reported chemotherapeutic agents include Cisplatin, Ifosfamide, and Adriamycin. Similarly, radiotherapy modalities were rarely reported, and in our cohort, merely two authors reported the employed radiation concept, while the reset contented with mentioning the radiation dose only. One article reported employing 3-dimensional conformal radiotherapy and used 6MV photon-linear accelerator for dose delivery, whereas the other reported adopting CyberKnife radiotherapy.[18, 25] However, this modality resulted in cerebrospinal fluid leakage that needed further management.
In patients who received adjuvant therapy, our analysis indicated a minimal difference in 5-year survival rates between the complete surgical resection and partial surgical resection groups. However, two-year survival rates showed a noticeable survival difference between the complete surgical resection (63%) and partial surgical resection (42%) groups, although this failed to meet statistical significance (P = 0.59). Likewise, some authors reported similar survival outcomes in HNO.[66, 74] In an article by Smith et al., patients who underwent surgical resection with negative margins had a five-year survival of 64%, in contrast to only 32% for patients with a positive margin.[66] Our cohort demonstrates a metastasis rate of 7%, in concordance with the current data on head and neck osteosarcoma.[2, 12] In contrast, literature illustrated higher micrometastasis and overt metastasis rates in long bone osteosarcoma, reaching 80% rate of pulmonary micrometastasis.[75] Although our pool included only one case of radiation-induced SBO with pulmonary metastasis, data showed that the lungs are the primary organs for metastasis in radiation-induced SBO.[7]
Besides tumor progression, local recurrence was the leading cause of death and was reported in 25% of our patients. This high percent can potentially be attributed to limited tumor resection and the limited use of intraoperative frozen sections to confirm margin negativity.[4]
Primary vs. radiation-induced osteosarcoma of the skull base
Although widely recognized an effective modality for treatment of HNO, radiation therapy has been associated with short- and long-term morbidity and may predispose to the development of a secondary malignancy.[76, 77] Few cases of radiation-induced SBO have been reported in the literature, and a comprehensive clinical understanding of this condition remains indistinct. According to Salvati et al., the incidence of radiation-induced osteosarcoma is estimated to range from 0.01% to 0.03% of all irradiated patients.[28] In contrast, our study reported 17 cases (25%) presenting with radiation-induced SBO. Our pooled analysis indicates that pituitary adenoma is the most common pathology of initial radiation therapy, contrasting the most comprehensive case series, which reported retinoblastoma as the most common initial pathology.[7] Initially, the diagnosis criteria of radiation-induced SBO indicated a latency period of at least 5 years.[78] However, shorter latency periods have been reported by serval studies including our study which indicates a short latency period with a median of 13 months.[79–81]Moreover, the median overall survival in radiation-induced SBO cases was reported in the literature as 41 months.[47] However, our pooled analysis indicated a median overall survival of 29 months among radiation-induced SBO cases. In comparison, radiation induced osteosarcoma of the long bones has a 5-year survival rate of 17%, which is worse than the survival rates seen in primary LBO (70%).[82],[83] However, our study showed that radiation-induced SBO has a better 5-year survival rate than primary SBO with statistical significance (p<0.04) (Figure 3). Although our overall 5-year survival rate was 22%, stratifying our data indicated an excellent 5-year survival rate of 39% in the radiation induced SBO group comparing to the primary SBO group (16%). The most likely explanation is that the radiation-induced tumors tend to be lateral and superficial, making complete excision more feasible and accessible.[4]
Limitations
The limitations of this study – many of which stem from the paucity of SBO in the literature – warrant further discussion. In addition to the small sample size, there was heterogeneity in the outcome data which challenged the statistical power. Unstratified factors limited survival rates; a specific example was the difference between the survival rates of radiation-induced SBO and primary SBO. Although our analysis showed a better 5-year survival rate in the radiation-induced SBO group, the factor of radiation could be confounded with better management courses and more favorable tumors for resection.
Conclusion
SBO is a rare, debilitating neoplasm that may arise as a result of a de novo mutation, metastasis from another location, or radiation exposure. In addition to adjuvant radiation therapy and chemotherapy, complete surgical resection should be pursued as a means of treating this tumor. SBO demonstrated a poor five-year survival rate at 21%. However, radiation-induced SBO was shown to have a better overall survival in contrast to primary SBO. In order to have a clear understanding and an agreement on treatment protocols, further prospective studies with sufficient sample size are necessary.
Funding:
No funds, grants, or other support was received.
Footnotes
Declaration:
Conflicts of interest/Competing interests: The authors have no relevant financial or non-financial interests to disclose.
Availability of data and material: All authors confirm the appropriateness of all dataset and software used for supporting the conclusion.
Code availability: Not applicable
Ethics approval: Not applicable
Consent to participate: Not applicable
Consent for publication: Not applicable
References
- 1.Mirabello L, Troisi RJ, Savage SA (2009) Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 115:1531–1543. 10.1002/cncr.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadwal SR, Gannon FH, Fanburg-Smith JC, et al. (2001) Primary osteosarcoma of the head and neck in pediatric patients: A clinicopathologic study of 22 cases with a review of the literature. Cancer 91:598–605. [DOI] [PubMed] [Google Scholar]
- 3.Takahama A, De Abreu Alves F, Lopes Pinto CA, et al. (2003) Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol 39:521–530. 10.1016/S1368-8375(03)00017-4 [DOI] [PubMed] [Google Scholar]
- 4.Guo Z, Hu K, Zhao B, et al. (2017) Osteosarcoma of the skull base: An analysis of 19 cases and literature review. J Clin Neurosci 44:133–142. 10.1016/j.jocn.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 5.Sturgis EM, Potter BO (2003) Sarcomas of the head and neck region. Curr. Opin. Oncol. 15:239–252 [DOI] [PubMed] [Google Scholar]
- 6.Nissanka EH, Amaratunge EAPD, Tilakaratne WM (2007) Clinicopathological analysis of osteosarcoma of jaw bones. Oral Dis 13:82–87. 10.1111/j.1601-0825.2006.01251.x [DOI] [PubMed] [Google Scholar]
- 7.Patel AJ, Rao VY, Fox BD, et al. (2011) Radiation-induced osteosarcomas of the calvarium and skull base. Cancer 117:2120–2126. 10.1002/cncr.25734 [DOI] [PubMed] [Google Scholar]
- 8.De S, Ghosh S, Mondal D, Sur PK (2010) Osteosarcoma of the mandible-second cancer in a case of Hodgkin’s lymphoma post-chemotherapy. J Cancer Res Ther 6:336–338. 10.4103/0973-1482.73349 [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary M, Chaudhary SD (2012) Osteosarcoma of jaws. J. Oral Maxillofac. Pathol. 16:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahrari A, Labib M, Gravel D, Macdonald K (2015) Primary Osteosarcoma of the Skull Base Treated with Endoscopic Endonasal Approach: A Case Report and Literature Review. J Neurol Surg Reports 76:e270–e274. 10.1055/s-0035-1564606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakley GM, Costa DJ, Mitchell RB, Sotelo C (2011) Osteosarcoma of the skull base in a 15-year-old boy. Ear, Nose Throat J 90:479–480. 10.1177/014556131109001006 [DOI] [PubMed] [Google Scholar]
- 12.Chennupati SK, Norris R, Dunham B, Kazahaya K (2008) Osteosarcoma of the skull base: Case report and review of literature. Int J Pediatr Otorhinolaryngol 72:115–119. 10.1016/j.ijporl.2007.08.015 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6 [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine, Eden J, Levit L, et al. (2011) Standards for Finding and Assessing Individual Studies [Google Scholar]
- 15.Lin PY, Chen WM, Hsieh YL, et al. (2005) Orbital metastatic osteosarcoma. J Chinese Med Assoc 68:286–289. 10.1016/S1726-4901(09)70153-4 [DOI] [PubMed] [Google Scholar]
- 16.Lee KBL, Ang ESW, Tan KC (2001) Reconstructive challenges in the management of a rare case of sphenoid osteosarcoma - A case report. Singapore Med J 42:586–589 [PubMed] [Google Scholar]
- 17.Mathkour M, Garces J, Beard B, et al. (2016) Primary High-Grade Osteosarcoma of the Clivus: A Case Report and Literature Review. World Neurosurg 89:730.e9–730.e13. 10.1016/j.wneu.2016.01.054 [DOI] [PubMed] [Google Scholar]
- 18.Meel R, Thulkar S, Sharma MC, et al. (2012) Childhood osteosarcoma of greater wing of sphenoid: Case report and review of literature. J Pediatr Hematol Oncol 34:59–62. 10.1097/MPH.0b013e3182331f5a [DOI] [PubMed] [Google Scholar]
- 19.Mohadjer Y, Wilson MW, Fuller CE, Haik BG (2004) Primary Pelvic Telagiectatic Osteosarcoma Metastic to Both Orbits. Ophthal Plast Reconstr Surg 20:77–79. 10.1097/01.IOP.0000103002.04762.6E [DOI] [PubMed] [Google Scholar]
- 20.Mohindra S, Savardekar A, Mahalingam SS, et al. (2014) Primary osteosarcoma of clivus: A short report. Br J Neurosurg 28:531–533. 10.3109/02688697.2013.841852 [DOI] [PubMed] [Google Scholar]
- 21.Kohyama K, Yamada K, Sugiura H, et al. (2015) Salvage surgery and microsurgical reconstruction for recurrence of skull base osteosarcoma after carbon ion radiotherapy. Nagoya J Med Sci 77:667–673. 10.18999/nagjms.77.4.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitehead RE, Melhem ER, Kasznica J, Eustace S (1998) Telangiectatic osteosarcoma of the skull base. Am J Neuroradiol 19:754–757 [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada SM, Ishii Y, Yamada S, et al. (2012) Advanced therapeutic strategy for radiation-induced osteosarcoma in the skull base: A case report and review. Radiat Oncol 7:1–5. 10.1186/1748-717X-7-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazarika P, Nayak DR, Sahota JS, et al. (1995) Osteogenic sarcoma of sphenoid bone: An extended lateral skull base approach. J Laryngol Otol 109:1101–1104. 10.1017/S002221510013213X [DOI] [PubMed] [Google Scholar]
- 25.Yamada SM, Ishii Y, Yamada S, et al. (2013) Skull base osteosarcoma presenting with cerebrospinal fluid leakage after CyberKnife® treatment: A case report. J Med Case Rep 7:3–7. 10.1186/1752-1947-7-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amine ARC, Sugar O (1976) Suprasellar osteogenic sarcoma following radiation for pituitary adenoma. Case report. J Neurosurg 44:88–91. 10.3171/jns.1976.44.1.0088 [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Nishio S, Morioka T, et al. (1989) Radiation-induced osteosarcoma of the sphenoid bone. Neurosurgery. 10.1227/00006123-198910000-00021 [DOI] [PubMed] [Google Scholar]
- 28.Salvati M, Ciappetta P, Raco A (1993) Osteosarcomas of the skull. Clinical remarks on 19 cases. Cancer 71:2210–2216. [DOI] [PubMed] [Google Scholar]
- 29.Gnanalingham KK, Chakraborty A, Galloway M, et al. (2002) Osteosarcoma and fibrosarcoma caused by postoperative radiotherapy for a pituitary adenoma: Case report. J Neurosurg. 10.3171/jns.2002.96.5.0960 [DOI] [PubMed] [Google Scholar]
- 30.Bembo SA, Pasmantier R, Davis RP, et al. (2004) Osteogenic sarcoma of the sella after radiation treatment of a pituitary adenoma. Endocr Pract 10:335–338. 10.4158/EP.10.4.335 [DOI] [PubMed] [Google Scholar]
- 31.Patel RD, Gadgil NM, Khare M, Majethia N (2014) Radiation-induced intracranial osteosarcoma: A case report. J. Postgrad. Med. 60:218–219 [DOI] [PubMed] [Google Scholar]
- 32.Sundaresan N, Huvos AG, Rosen G, Galicich JH (1985) Combined-modality treatment of osteogenic sarcoma of the skull. J Neurosurg 63:562–567. 10.3171/jns.1985.63.4.0562 [DOI] [PubMed] [Google Scholar]
- 33.Mark RJ, Sercarz JA, Tran L, et al. (1991) Osteogenic Sarcoma of the Head and Neck: The UCLA Experience. Arch Otolaryngol Neck Surg 117:761–766. 10.1001/archotol.1991.01870190073015 [DOI] [PubMed] [Google Scholar]
- 34.Kleinsasser O, Albrecht H (1957) Zur Kenntnis der Osteosarkome des Stirn- und Keilbeines. Arch für Ohren- Nasen- und Kehlkopfheilkd. 10.1007/BF02115761 [DOI] [Google Scholar]
- 35.Alleyne CH, Theodore N, Spetzler RF, Coons SW (2000) Osteosarcoma of the temporal fossa with hemorrhagic presentation: Case report. Neurosurgery 47:447–451. 10.1097/00006123-200008000-00036 [DOI] [PubMed] [Google Scholar]
- 36.Park YK, Yang MH, Choi WS LY Well-differentiated, low-grade osteosarcoma of the clivus. Skelet Radiol 24:386–8. 10.1007/BF00197075. [DOI] [PubMed] [Google Scholar]
- 37.Geetha N, Kumar A, Ramachandran K, et al. (1999) Osteosarcoma of the sella. Australas Radiol. 10.1046/j.1440-1673.1999.00719.x [DOI] [PubMed] [Google Scholar]
- 38.Uysal KM, Koyuncuoǧlu M, Akman F, et al. (2001) A rare tumor of craniofacial bones in children: A pediatric chondroblastic osteosarcoma case with diagnostic and therapeutic problems. Pediatr Hematol Oncol. 10.1080/088800101300002991 [DOI] [PubMed] [Google Scholar]
- 39.Potepan P, Luksch R, Sozzi G, et al. (1999) Multifocal osteosarcoma as second tumor after childhood retinoblastoma. Skeletal Radiol 28:415–421. 10.1007/s002560050540 [DOI] [PubMed] [Google Scholar]
- 40.Kornreich L, Grunebaum M, Ziv N, Cohen Y (1988) Osteogenic sarcoma of the calvarium in children: CT manifestations. Neuroradiology. 10.1007/BF00404110 [DOI] [PubMed] [Google Scholar]
- 41.Ohno K, Tsunoda A, Shirakura S, et al. (2011) The approaches and outcomes of skull base surgery for pediatric sarcoma after initial therapy. Auris Nasus Larynx 38:208–214. 10.1016/j.anl.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 42.Sen O, Atalay B, Ozerdem OR, et al. (2005) Management of pronto-orbital sphenoidal and facial osteosarcoma: A case with uncommon localization. J Craniofac Surg 16:470–473. 10.1097/01.SCS.0000157247.86084.79 [DOI] [PubMed] [Google Scholar]
- 43.Hayashi T, Kuroshima Y, Yoshida K, et al. (2000) Primary osteosarcoma of the sphenoid bone with extensive periosteal extension. Neurol Med Chir (Tokyo). 10.2176/nmc.40.419 [DOI] [PubMed] [Google Scholar]
- 44.Marks MP, Marks SC, Segall HD, et al. (1987) Case report 420: Parosteal osteosarcoma. Skeletal Radiol 16:246–251. 10.1007/BF00356962 [DOI] [PubMed] [Google Scholar]
- 45.Reichenthal E, Cohen ML, Manor R, et al. (1981) Primary osteogenic sarcoma of the sellar region. Case report. J Neurosurg. 10.3171/jns.1981.55.2.0299 [DOI] [PubMed] [Google Scholar]
- 46.Ashkan K, Pollock J, D’Arrigo C, Kitchen ND (1998) Intracranial osteosarcomas: Report of four cases and review of the literature. J Neurooncol 40:87–96. 10.1023/A:1006007411312 [DOI] [PubMed] [Google Scholar]
- 47.Echchikhi Y, Loughlimi H, Touil A, et al. (2016) Radiation-induced osteosarcoma of the skull base after radiation therapy in a patient with nasopharyngeal carcinoma: a case report and review of the literature. J Med Case Rep 10:1–6. 10.1186/s13256-016-1112-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellison DA, Silverman JF, Strausbach PS, Joshi VV. (1996) Fine-needle aspiration of chondroblastic osteosarcoma of the skull: Report of a case in an 11-year-old girl. Diagn Cytopathol 14:51–55. [DOI] [PubMed] [Google Scholar]
- 49.Hadley C, Gressot LV, Patel AJ, et al. (2014) Osteosarcoma of the cranial vault and skull base in pediatric patients: Report of 3 cases. J Neurosurg Pediatr 13:380–387. 10.3171/2013.12.PEDS13359 [DOI] [PubMed] [Google Scholar]
- 50.Hettmer S, Fleischhack G, Hasan C, et al. (2002) Intracranial manifestation of osteosarcoma. Pediatr Hematol Oncol 19:347–354. 10.1080/08880010290057363 [DOI] [PubMed] [Google Scholar]
- 51.Kachhara R, Nair S, Sandhyamani S, Bhattacharya RN (1999) Primary Osteogenic Sarcoma Involving Sella-Sphenoid Sinus: Case Report. Neurol Med Chir (Tokyo) 39:534–538. 10.2176/nmc.39.534 [DOI] [PubMed] [Google Scholar]
- 52.Savage SA, Mirabello L (2011) Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gil Z, Orr-Urtreger A, Voskoboinik N, et al. (2008) Cytogenetic analysis of 101 skull base tumors. Head Neck 30:567–581. 10.1002/hed.20741 [DOI] [PubMed] [Google Scholar]
- 54.Whelan J, McTiernan A, Cooper N, et al. (2012) Incidence and survival of malignant bone sarcomas in England 1979–2007. Int J Cancer 131:E508–E517. 10.1002/ijc.26426 [DOI] [PubMed] [Google Scholar]
- 55.Muhammed A, Meshneb M, Saro H, et al. (2020) Management of cranial chondroblastoma in adults; a pooled analysis. Am. J. Otolaryngol. - Head Neck Med. Surg. 41 [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Shi H, Yu Q (2012) Osteosarcoma of the jaws: Demographic and CT imaging features. Dentomaxillofacial Radiol 41:37–42. 10.1259/dmfr/86834844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo Z, Chen W, Shen X, et al. (2020) Head and neck osteosarcoma: Ct and MR imaging features. Dentomaxillofacial Radiol 49:20190202. 10.1259/dmfr.20190202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gangadhar K, Santhosh D (2012) Primary skull osteosarcoma: MDCT evaluation and histopathological. Correlation in two cases. Neuroradiol J 25:188–192. 10.1177/197140091202500206 [DOI] [PubMed] [Google Scholar]
- 59.Gorlick R, Khanna C (2010) Osteosarcoma. J. Bone Miner. Res. 25:683–691 [DOI] [PubMed] [Google Scholar]
- 60.Klein MJ, Siegal GP (2006) Osteosarcoma: Anatomic and histologic variants. Am. J. Clin. Pathol. 125:555–581 [DOI] [PubMed] [Google Scholar]
- 61.Righi A, Paioli A, Dei Tos AP, et al. (2015) High-grade focal areas in low-grade central osteosarcoma: high-grade or still low-grade osteosarcoma? Clin Sarcoma Res 5:. 10.1186/s13569-015-0038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rozeman LB, Cleton-Jansen AM, Hogendoorn PCW (2006) Pathology of primary malignant bone and cartilage tumours. Int. Orthop. 30:437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paparella ML, Olvi LG, Brandizzi D, et al. (2013) Osteosarcoma of the jaw: An analysis of a series of 74 cases. Histopathology 63:551–557. 10.1111/his.12191 [DOI] [PubMed] [Google Scholar]
- 64.Ha PK, Eisele DW, Frassica FJ, et al. (1999) Osteosarcoma of the head and neck: A review of the Johns Hopkins experience. Laryngoscope 109:964–969. 10.1097/00005537-199906000-00023 [DOI] [PubMed] [Google Scholar]
- 65.Cai Y, Niu X, Zhang Q, et al. (2000) Long-term results of combined therapy for primary osteosarcoma in extremities. Zhonghua Wai Ke Za Zhi 38:329–331 [PubMed] [Google Scholar]
- 66.Smith RB, Apostolakis LW, Karnell LH, et al. (2003) National cancer data base report on osteosarcoma of the head and neck. Cancer 98:1670–1680. 10.1002/cncr.11716 [DOI] [PubMed] [Google Scholar]
- 67.Smith RB, Apostolakis LW, Karnell LH, et al. (2003) National cancer data base report on osteosarcoma of the head and neck. Cancer. 10.1002/cncr.11716 [DOI] [PubMed] [Google Scholar]
- 68.Kassir RR, Rassekh CH, Kinsella JB, et al. (1997) Osteosarcoma of the head and neck: Meta-analysis of nonrandomized studies. Laryngoscope. 10.1097/00005537-199701000-00013 [DOI] [PubMed] [Google Scholar]
- 69.Eilber F, Giuliano A, Eckardt J, et al. (1987) Adjuvant chemotherapy for osteosarcoma: A randomized prospective trial. J Clin Oncol. 10.1200/JCO.1987.5.1.21 [DOI] [PubMed] [Google Scholar]
- 70.Eilber FR, Morton DL, Eckardt J, et al. (1984) Limb salvage for skeletal and soft tissue sarcomas multidisciplinary preoperative therapy. Cancer. [DOI] [PubMed] [Google Scholar]
- 71.Seidensaal K, Mattke M, Haufe S, et al. (2021) The role of combined ion-beam radiotherapy (CIBRT) with protons and carbon ions in a multimodal treatment strategy of inoperable osteosarcoma. Radiother Oncol. 10.1016/j.radonc.2021.01.029 [DOI] [PubMed] [Google Scholar]
- 72.Smeele LE, Kostense PJ, van der Waal I, Snow GB (1997) Effect of chemotherapy on survival of craniofaciai osteosarcoma: A systematic review of 201 patients. J. Clin. Oncol. [DOI] [PubMed] [Google Scholar]
- 73.König M, Osnes T, Bruland Ø, et al. (2020) The Role of Adjuvant Treatment in Craniofacial Malignancy: A Critical Review. Front. Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boon E, van der Graaf WTA, Gelderblom H, et al. (2017) Impact of chemotherapy on the outcome of osteosarcoma of the head and neck in adults. Head Neck 39:140–146. 10.1002/hed.24556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaffe N (2009) Osteosarcoma: Review of the past, impact on the future. The American experience. In: Cancer Treatment and Research [DOI] [PubMed] [Google Scholar]
- 76.Thiagarajan A, Iyer NG (2014) Radiation-induced sarcomas of the head and neck. World J Clin Oncol 5:973–981. 10.5306/wjco.v5.i5.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kansara M, Leong HS, Lin DM, et al. (2013) Immune response to rb1-Regulated senescence limits radiation-Induced osteosarcoma formation. J Clin Invest 123:5351–5360. 10.1172/JCI70559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.CAHAN WG, WOODARD HQ (1948) Sarcoma arising in irradiated bone; report of 11 cases. Cancer [DOI] [PubMed] [Google Scholar]
- 79.Matsuyama A, Yonemitsu N, Hayashida S, et al. (2003) Case of postradiation osteosarcoma with a short latency period of 3 years. Pathol Int 53:46–50. 10.1046/j.1440-1827.2003.01427.x [DOI] [PubMed] [Google Scholar]
- 80.Sale KA, Wallace DI, Girod DA, Tsue TT (2004) Radiation-induced malignancy of the head and neck. Otolaryngol - Head Neck Surg 131:643–645. 10.1016/j.otohns.2004.05.012 [DOI] [PubMed] [Google Scholar]
- 81.Murray EM, Werner D, Greeff EA, Taylor DA (1999) Postradiation sarcomas: 20 Cases and a literature review. Int J Radiat Oncol Biol Phys 45:951–961. 10.1016/S0360-3016(99)00279-5 [DOI] [PubMed] [Google Scholar]
- 82.Gharbi O, Chabchoub I, Remadi S, et al. (2009) Postirradiation osteosarcoma of the maxilla: A case report and current review of literature. J Oncol. 10.1155/2009/876138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel SG, Meyers P, Huvos AG, et al. (2002) Improved outcomes in patients with osteogenic sarcoma of the head and neck. Cancer 95:1495–1503. 10.1002/cncr.10849 [DOI] [PubMed] [Google Scholar]