Abstract
Diabetic ketoacidosis (DKA) is considered a medical emergency, most commonly associated with type 1 diabetes mellitus, and is relatively rare in type 2 diabetes mellitus (T2DM). We discuss a case of a 45-year-old woman with T2DM who presented to the emergency room with worsening lethargy and weakness. Before her presentation, her physician had recently added empagliflozin, a sodium-glucose cotransporter-2 (SGLT-2) inhibitor, to her anti-diabetic drug regimen along with glimepiride and a combination drug of vildagliptin and metformin. Based on the clinical examination and lab findings, DKA was suspected, but her glucose level was below the cutoff value for DKA diagnosis. However, her lab results showed significant metabolic acidosis and ketonemia with no clinical or laboratory features of sepsis. Therefore, the diagnosis of euglycemic diabetic ketoacidosis (eu-DKA) was made. She was successfully treated according to the DKA protocol and discharged in good condition. In this report, our aim is to discuss the relationship between SGLT-2 inhibitors with eu-DKA. Given the absence of significant hyperglycemia, recognition of this entity by clinicians may be delayed. Serum ketones should be obtained in diabetic patients with symptoms of nausea, vomiting, or malaise while taking SGLT-2 inhibitors, and SGLT-2 inhibitors should be discontinued if ketoacidosis is confirmed.
Keywords: : diabetes mellitus, diabetes mellitus type 2, empagliflozin, sodium-glucose cotransporter-2 (sglt-2) inhibitors, euglycemic diabetic ketoacidosis
Introduction
Diabetic ketoacidosis (DKA) is diagnosed by the presence of a triad of hyperglycemia or random blood sugar (RBS) >250 mg/dL, metabolic acidosis (pH <7.3, serum bicarbonate <18 mEq/L), and ketosis [1]. Rarely does the patient present with characteristics of DKA in the presence of blood sugar levels less than 200 mg/dL, and this is defined as euglycemic diabetic ketoacidosis (eu-DKA) [2]. eu-DKA was first described by Munro et al. in 1973 [3]. This case documents one of the rare side effects associated with empagliflozin. Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a newer generation of anti-diabetic drugs that ultimately inhibit sodium-dependent glucose uptake by the kidneys and increase glucose loss in urine [4]. However, the diagnosis of DKA can sometimes be missed due to the presence of an associated euglycemic effect. Therefore, physicians should be aware when prescribing SGLT-2 inhibitors about this rare side effect and the novel presentation of eu-DKA
Case presentation
We present here the case of a 45-year-old woman, a known case of type 2 diabetes mellitus (T2DM) for the last five years, poorly controlled with oral hypoglycemic medications including glimepiride, vildagliptin, and metformin. She saw her primary care physician, who added empagliflozin to the prescription five days prior to her presentation at the emergency department. She presented with complaints of increasing lethargy and weakness for the last two days. There were no preceding respiratory, gastrointestinal, or cardiovascular symptoms. She had no fever, cough, sore throat, chest pain, syncope, headache, vomiting, diarrhea, or urinary problems. She denied any history of chronic abdominal pain, weight loss, hypertension, ischemic heart disease, thyroid disease, stroke, substance abuse, or recent travel. She was married, a non-smoker, and a non-alcoholic. Her dietary habits were satisfactory. She had recently recovered from a COVID-19 infection two weeks ago.
On examination, she had no hyperthyroid or hypothyroid features. She had pallor and was slightly drowsy, but easily arousable; dehydrated but without jaundice and lymphadenopathy. Her pulse rate was 106 beats per minute (BPM) and regular, blood pressure (BP) was 110/70 mmHg with no postural drop, respiratory rate was 22 breaths per minute (BPM), peripheral capillary oxygen saturation (SpO2) was 88% at room air, and she was afebrile. Neurological examination showed normal tone, power, and reflexes, but she had bilaterally impaired vibration sense in her lower limbs. The rest of the systemic examination was unremarkable.
Her laboratory results revealed increased white blood cell (WBC) count and raised glycosylated hemoglobin (HbA1c) levels (Table 1). Analysis of arterial blood gas (ABG) and serum electrolytes revealed a decrease in pH, with hypobicarbonatemia, hypokalemia, and hypocarbia. A euglycemic state was indicated in basal metabolic tests with an elevated anion gap and ketosis. The patient also had no history of methanol, ethanol, and paraldehyde ingestion. Chest X-ray was unremarkable. Therefore, based on the clinical and laboratory findings, the diagnosis of eu-DKA due to the use of the SGLT-2 inhibitor was made.
Table 1. Baseline and post-recovery laboratory investigations.
WBC: white blood cell; MCV: mean corpuscular volume, AST: aspartate aminotransferases; ALT: alanine aminotransferase; RBS: random blood sugar.
| Investigations | Day 1 - Before diagnosis | Day 4 - After recovery | Reference ranges |
| Complete blood count | |||
| Hemoglobin | 10.2 g/dL | 10.1 g/dL | 12-16 g/dL |
| WBC | 15.7 × 103 µL | 11.4 × 103 µL | 4-11 × 103 µL |
| Platelets | 215 × 103 µL | 215 × 103 µL | 150-400 × 103 µL |
| MCV | 91 fL | 91 fL | 80-100 fL |
| HbA1c | 10.4% | 10.4% | 4.8-5.7% |
| Electrolytes | |||
| Sodium | 140 mEq/L | 140 mEq/L | 135-145 mEq/L |
| Potassium | 2 mEq/L | 3.2 mEq/L | 3.5-5 mEq/L |
| Chloride | 98 mEq/L | 101 mEq/L | 95-105 mEq/L |
| Arterial blood gas analysis | |||
| pH | 7.03 | 7.4 | 7.35-7.45 |
| Bicarbonate | 5.9 mmol/L | 23.2 mmol/L | 24-28 mmol/L |
| PCO2 | 19 mmHg | 41 mmHg | 35-45 mmHg |
| Basic metabolic panel | |||
| RBS | 142 mg/dL | 133 mg/dL | <140 mg/dL |
| Ketones | >10 mmol/L | Negative | ≤1.5 mmol/L |
| Lactate | 13.8 mg/dL | 13.2 mg/dL | 4.5-19.8 mg/dL |
| Anion gap | 36 | 15.4 | 8-16 |
| Liver function tests | |||
| AST | 26 IU | 29 IU | 5-40 IU |
| ALT | 29 IU | 31 IU | 5-40 IU |
| Renal function tests | |||
| Creatinine | 0.5 mg/dL | 0.54 mg/dL | 0.5-1.30 mg/dL |
| Urea | 14 mg/dL | 14 mg/dL | 10-50 mg/dL |
| Urinalysis | |||
| Urinary ketones | +++ | Negative | <0.6 |
According to the local DKA protocol, the patient was treated with IV fluids, insulin, and potassium replacement. Four days later, the patient improved clinically. Her ABG, electrolyte levels, and basal metabolism returned to their normal values. After treatment, the patient was discharged in a stable condition.
Discussion
The SGLT-2 inhibitors are a relatively new class of oral hypoglycemic agents that can be used as an adjunctive agent or alternative monotherapy for patients who fail initial therapy with lifestyle interventions, metformin, and/or sulfonylureas. Due to the obvious cardiovascular and renal benefits, they are preferred in patients with atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease. These drugs dramatically reduce blood glucose and HbA1c level [5,6]. Additional benefits include promoting weight loss, decreasing blood pressure, reducing hypoglycemic episodes, and lowering exogenous insulin requirements [4]. However, several studies have estimated a three-fold increased risk of DKA compared to dipeptidyl peptidase-4 enzyme inhibitors [6]. We discuss the link between SGLT-2 inhibitors and eu-DKA.
Although SGLT-2 inhibitors are relatively safer drugs, they have been associated with side effects, such as urinary tract infection, genital mycotic infections, and volume depletion. eu-DKA has also been observed in rare cases [7]. Given the absence of significant hyperglycemia, the recognition of the problem may be delayed by both patients and clinicians. As noted in our case report, hyperglycemia was not present in our patient despite high anion gap metabolic acidosis and increased plasma and urinary ketones. Farjo et al. also studied a case of eu-DKA with empagliflozin in a 57-year-old man with T2DM with an RBS of 120 mg/dL after empagliflozin [8]. Hypoglycemia is associated with the use of SGLT-2 inhibitors, which in turn stimulate glucagon release and decrease glucose-dependent insulin release, thus altering the insulin-to-glucagon ratio and ketosis. Ketosis may be improved by reduced carbohydrate intake, starvation, acute illness, and decreasing exogenous insulin, as suggested by Burke et al. in their review [9]. Although SGLT-2 inhibitors increase glucose excretion in urine, this effect may be counterbalanced by its effect of increasing endogenous glucose production and glucagon release, which can eventually lead to ketosis [10,11]. In Pakistan, a case of eu-DKA after empagliflozin treatment was also reported [1]. Several similar cases have been reported in the literature. Some of them have been demonstrated in Table 2.
Table 2. Characteristics of similar reviewed cases.
DM: diabetes mellitus; T2DM: type 2 diabetes mellitus; HbA1c: glycosylated hemoglobin; WBC: white blood cells.
| Study name | Age, years | Type of DM | Sex | SGLT-2 inhibitor | Blood analysis | Arterial blood gas | Basal metabolic panel | Renal function tests |
| Mistry and Eschler (2021) [12] | 47 | T2DM | Female | Empagliflozin | HbA1C: 13.6% | pH: 7.24. Serum bicarbonate: 11 mmol/L, β-hydroxybutyrate: 6.78 mmol/L | Plasma glucose: 187 mg/dL, anion gap: 22 mmol/L | Not reported |
| 34 | T2DM | Male | Canagliflozin | HbA1C: 8.2% | pH: 7.27, serum bicarbonate: 12 mmol/L, β-hydroxybutyrate: 5 mmol/L | Serum glucose: 251 mg/dL, anion gap: 24 mmol/L | Not reported | |
| Brown and McColl (2018) [13] | 53 | T2DM | Male | Dapagliflozin | WBC count: 12 × 103 µL | pH: 7.24, β-hydroxybutyrate: 6.2 mmol/L | Blood glucose: 162 mg/dL, lactate: 4.5 mmol/L, anion gap: 30 | Not reported |
| Chou et al. (2018) [14] | 61 | T2DM | Female | Dapagliflozin | Not reported | pH: 6.986, CO2: 20.9 mmHg, serum bicarbonate: 7.0 mEq/L | Blood glucose: 180 mg/dL, blood ketones: 8.0 mmol/L, urine ketones: positive, serum lactate: 9.0 mg/dL, anion gap: 20 mEq/L | Blood urea nitrogen: 25 mg/dL, serum creatinine: 0.8 mg/dL |
| Diaz-Ramos et al. (2019) [15] | 44 | T2DM | Female | Canagliflozin | Not reported | pH: 7.27, PCO2: 29 mm/Hg | Serum glucose: 163 mg/dL, serum bicarbonate: 14 mmol/L, anion gap: 18 mmol/L, urinary ketones: positive, serum acetone: positive | Not reported |
| Gajjar and Luthra (2019) [16] | 28 | T2DM | Female | Dapagliflozin | HbA1c: 10% | pH: 7.27, bicarbonate: 18 mmol/L, β-hydroxybutyrate: 5.29 mmol/L | Serum glucose: 111 mg/dL, anion gap: 20 | Creatinine: 0.4 mg/dL |
| Lee and Ahn (2020) [17] | 76 | T2DM | Female | Dapagliflozin | WBC count: 11,800/μL, Hb: 13 g/dL, platelet count: 173,000/μL, erythrocyte sedimentation rate: 12 mm/hour, HbA1C: 8.1% | pH: 6.904, pCO2: 12.0 mmHg, serum bicarbonate: 3.1 mmol/L | Serum glucose: 410 mg/dL, insulin, 3.3 μIU/mL, anion gap; 37, serum lactate: 1.1 mmol/L, serum ketone: 2.7 mg/dL | Blood urea nitrogen: 41.7 mg/dL, creatinine: 3.2 mg/dL |
| Turner et al. (2016) [18] | 62 | T2DM | Female | Canagliflozin | HbA1c: 11.1 % | pH: 7.08, CO2: <5 mEq/L | Serum glucose: 213 mg/dL, anion gap: >17, serum lactate: 0.8 mmol/L | Blood urea nitrogen: 22 mg/dL, creatinine: 1.3 mg/dL |
| Steinmetz-Wood et al. (2020) [19] | 47 | T2DM | Male | Empagliflozin | HbA1c: 9.1% | pH: 6.94, serum bicarbonate: 5 mmol/L, β-hydroxybutyrate: 8.9 mmol/L | Serum glucose: 269 mg/dL, urinary ketones: +++, anion gap: 28 | Creatinine: 1.21 mg/dL |
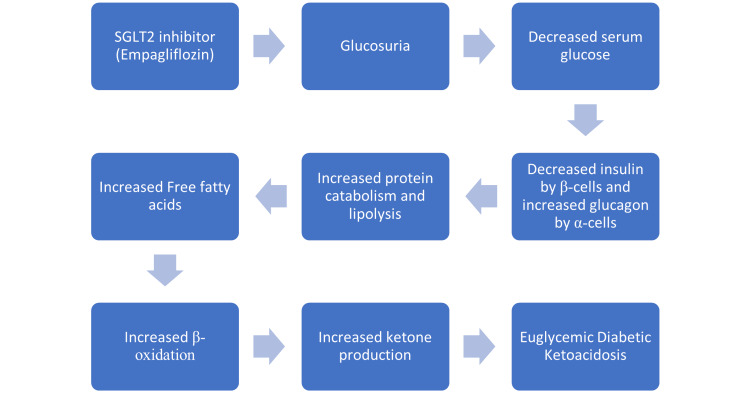
This rare and unique form of DKA may be due to an altered physiological process. Usually, a decrease in insulin or insulin resistance causes a surge of glucagon release, leading to increased glucose levels and lipolysis, i.e., fatty acid oxidation and, hence, ketosis (Figure 1). A recent meta-analysis was able to develop a dose-dependent association between eu-DKA and SGLT-2 inhibitors. Patients taking high doses of SGLT-2 inhibitors have an increased risk of developing eu-DKA [20].
Figure 1. Mechanism of eu-DKA with SGLT-2 inhibitors.
eu-DKA: euglycemic diabetic ketoacidosis; SGLT-2: sodium-glucose cotransporter-2.
Conclusions
The case report discusses a rare and life-threatening side effect of SGLT-2 inhibitors. Given the potential adverse consequences of eu-DKA, it is important that emergency physicians should consider the reasonable suspicion of eu-DKA in patients on SGLT-2 inhibitors, especially if the patient presents with nausea, vomiting, lethargy, dyspnea, and severe dehydration. Ketone studies and blood gas analyses would be performed on these patients, regardless of their blood glucose levels for early diagnosis and better prognostic outcomes. Patients taking SGLT-2 inhibitors should be educated to perform urine dipstick tests to check for ketonuria and seek immediate medical attention if positive.
Acknowledgments
RSM and MKK contributed to the concept and design of the manuscript. RSM and HAH contributed to the review of literature and extraction of data. RSM and AK contributed to the design and construction of tables and figures. RSM drafted the manuscript. All authors critically revised the work and gave final approval and agreed to be accountable for all aspects of the work, ensuring integrity and accuracy.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Euglycaemic diabetic ketoacidosis in a patient with type 2 diabetes started on empagliflozin. Rashid O, Farooq S, Kiran Z, Islam N. BMJ Case Rep. 2016;2016:0. doi: 10.1136/bcr-2016-215340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Euglycemic diabetic ketoacidosis. Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A. Eur J Intern Med. 2019;63:9–14. doi: 10.1016/j.ejim.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Euglycaemic diabetic ketoacidosis. Munro JF, Campbell IW, McCuish AC, Duncan LJ. Br Med J. 1973;2:578–580. doi: 10.1136/bmj.2.5866.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sodium-glucose cotransporter 2 inhibitors with insulin in type 2 diabetes: clinical perspectives. John M, Gopinath D, Jagesh R. Indian J Endocrinol Metab. 2016;20:22–31. doi: 10.4103/2230-8210.172268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodium-glucose cotransporter-2 inhibitors and the risk for diabetic ketoacidosis: a multicenter cohort study. Douros A, Lix LM, Fralick M, et al. Ann Intern Med. 2020;173:417–425. doi: 10.7326/M20-0289. [DOI] [PubMed] [Google Scholar]
- 6.Sodium glucose co-transporter 2 inhibitors and their mechanism for improving glycemia in patients with type 2 diabetes. Davidson JA, Kuritzky L. Postgrad Med. 2014;126:33–48. doi: 10.3810/pgm.2014.10.2819. [DOI] [PubMed] [Google Scholar]
- 7.Euglycemic diabetic ketoacidosis in a patient with type 2 diabetes after treatment with empagliflozin. Roach P, Skierczynski P. Diabetes Care. 2016;39:0. doi: 10.2337/dc15-1797. [DOI] [PubMed] [Google Scholar]
- 8.A case of euglycemic diabetic ketoacidosis following long-term empagliflozin therapy. Farjo PD, Kidd KM, Reece JL. Diabetes Care. 2016;39:0–6. doi: 10.2337/dc16-0728. [DOI] [PubMed] [Google Scholar]
- 9.SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Burke KR, Schumacher CA, Harpe SE. Pharmacotherapy. 2017;37:187–194. doi: 10.1002/phar.1881. [DOI] [PubMed] [Google Scholar]
- 10.Euglycemic diabetic ketoacidosis: a missed diagnosis. Nasa P, Chaudhary S, Shrivastava PK, Singh A. World J Diabetes. 2021;12:514–523. doi: 10.4239/wjd.v12.i5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. Ferrannini E, Muscelli E, Frascerra S, et al. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Euglycemic diabetic ketoacidosis caused by SGLT2 inhibitors and a ketogenic diet: a case series and review of literature. Mistry S, Eschler DC. AACE Clin Case Rep. 2021;7:17–19. doi: 10.1016/j.aace.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Euglycemic diabetic ketoacidosis secondary to dapagliflozin use: a case report. Brown F, McColl T. J Emerg Med. 2018;54:109–111. doi: 10.1016/j.jemermed.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Euglycemic diabetic ketoacidosis caused by dapagliflozin: a case report. Chou YM, Seak CJ, Goh ZN, Seak JC, Seak CK, Lin CC. Medicine (Baltimore) 2018;97:0. doi: 10.1097/MD.0000000000011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter-2 inhibitor use: a case report and review of the literature. Diaz-Ramos A, Eilbert W, Marquez D. Int J Emerg Med. 2019;12:27. doi: 10.1186/s12245-019-0240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Euglycemic diabetic ketoacidosis in the setting of SGLT2 inhibitor use and hypertriglyceridemia: a case report and review of literature. Gajjar K, Luthra P. Cureus. 2019;11:0. doi: 10.7759/cureus.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dapagliflozin-associated euglycemic diabetic ketoacidosis in a patient with type 2 diabetes mellitus: a case report. Lee IH, Ahn DJ. Medicine (Baltimore) 2020;99:0. doi: 10.1097/MD.0000000000020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canagliflozin-induced diabetic ketoacidosis: case report and review of the literature. Turner J, Begum T, Smalligan RD. J Investig Med High Impact Case Rep. 2016;4:2324709616663231. doi: 10.1177/2324709616663231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A case of diabetic ketoacidosis in a patient on an SGLT2 inhibitor and a ketogenic diet: a critical trio not to be missed. Steinmetz-Wood S, Gilbert M, Menson K. Case Rep Endocrinol. 2020;2020:8832833. doi: 10.1155/2020/8832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Euglycemic diabetic ketoacidosis associated with SGLT2 inhibitors: a systematic review and quantitative analysis. Dutta S, Kumar T, Singh S, Ambwani S, Charan J, Varthya SB. J Family Med Prim Care. 2022;11:927–940. doi: 10.4103/jfmpc.jfmpc_644_21. [DOI] [PMC free article] [PubMed] [Google Scholar]