Abstract
With the continuous advances in molecular biotechnology, many new cell death methods have been discovered. Pyroptosis is a programmed cell death process that differs from apoptosis and autophagy in cell morphology and function. Compared with apoptosis and autophagy, pyroptosis is primarily mediated by intracellular inflammasome and gasdermin D of the gasdermin protein family and involves the release of numerous inflammatory factors. Pyroptosis has been found to be involved in the occurrence and development of infectious diseases and other diseases involving the nervous system and the cardiovascular system. Recent studies have also reported the occurrence of pyroptosis in tumor cells. Accordingly, exploring its effect on tumors has become one of the research hotspots. Herein, recent research progress on pyroptosis is reviewed, especially its role in the development of gynecological tumors. As the pathogenesis of gynecological tumor is better understood, new targets have been introduced for the prevention and clinical treatment of gynecological tumors.
Keywords: pyroptosis, cervical cancer, ovarian cancer, endometrial carcinoma
1. Introduction
With the continuous advances in molecular biotechnology, many new cell death methods have been discovered, including necroptosis, ferroptosis, and pyroptosis [1]. Pan-apoptosis is also a new concept proposed by Maridi in 2019 which emphasizes the interaction and coordination among the pathways involved in pyroptosis, apoptosis, and necrosis. Research in recent years has highlighted the interconnectedness among the pathways of these three processes [2]. However, gynecological oncology focuses on the unique procedures and biochemical mechanisms related to each process. Pyroptosis is a type of programmed cell death that is characterized by changes in osmotic pressure inside and outside the cell, swelling and rupture of the cell, release of inflammatory factors, lysosomes, and other cell contents outside the cell, and induction of inflammatory cascade reaction [3]. Pyroptosis occurs when the nucleus of cells remains intact, despite chromatin shrinkage and DNA damage. Inflammasomes associated with pyroptosis have been found in cancer cells [4,5,6]. These inflammasomes induce caspase-1, which leads to pyroptosis [7].
Currently, cancer is considered to be one of the main public health problems. A gynecological tumor occurs in female reproductive organs and is associated with a wide variety of clinical outcomes and different prevention and treatment methods. Unlike solid tumors of other organs, the outcomes of gynecological tumors are not only related to the patients, but also their offspring. Therefore, more severe challenges are associated with gynecological tumors. In the 1950s, some doctors successfully cured a patient with choriocarcinoma using methotrexate, ultimately beginning the era of solid tumor chemotherapy [8]. To date, gestational trophoblastic tumors are cured with chemotherapy alone, while other gynecological tumors are treated with chemotherapy as the main adjuvant therapy [9,10,11]. The advantage of chemotherapy is that it can guarantee the structural integrity of the organ. Furthermore, the damage to the ovary due to chemotherapy is relatively smaller than that by other therapies. As a result, chemotherapy is unique in protecting the endocrine and reproductive functions of the ovary [12]. Targeted drugs shift drug targets from cells to molecules, and targeted therapy has been one of the hallmarks of precision medicine [13]. Several targeted drugs have led to remarkable outcomes in the treatment of various solid tumors. However, in the case of gynecologic tumors, especially ovarian cancer, only a few targeted drugs with statistically significant survival benefits exist; thus, the reasons behind the lack of targeted drugs for gynecologic tumors are worth exploring.
Physiological cell death can control cell proliferation and inhibit tumor progression. Tumor cells can escape the recognition and attack of the body’s immune system in a variety of ways to survive and proliferate [14]. Cell death is the irreversible cessation of life and the end of life that often occurs in normal tissues and is necessary to maintain the function and morphology of tissues, including programmed death, apoptosis, and cell necrosis [15]. Apoptosis is a process strictly controlled by multiple genes. With the development of molecular biology techniques, the mechanisms of many types of cell apoptosis have been elucidated. An imbalance in apoptosis may be directly or indirectly related to the occurrence of many diseases [16]. In recent years, many studies have sought to explore the molecular mechanism of pyroptosis in the formation and development of multiple tumors [17,18,19]. Pyroptosis is related to the progression of various diseases, especially the progression of human malignant tumors [20]. In 1996, pyroptosis was first reported in bacteria and is believed to be a form of gasdermin (GSDM)-mediated cell death [21]. Notably, pyroptosis may be an important natural immune response of the body, which plays an important role in the fight against infections [22]. Previously, a report published by Bergsbaken et al. revealed that pyroptosis can affect the clearance of pathogens, thereby reducing the adaptive immune response and causing extensive tissue damage [23]. In this review, the pathogenesis, tumor progression, and therapeutic strategies of pyroptosis in gynecological malignant tumors were systematically described, providing new ideas for the treatment of gynecological tumors.
2. Mechanism of Pyroptosis
In 1996, Cheng et al. [21] found that mouse macrophages could undergo apoptosis accompanied by the release of interleukin-1β (IL-1β) after being infected with Shigella spp., and subsequent studies revealed that this was a new type of programmed cell death which is independent of caspase-3 activity and is related to caspase-1 activity [24]. Although Shigella-induced death can still occur in caspase-3-deficient macrophages, it is inhibited by caspase-1 specific inhibitor or in caspase-1 gene knockout [25]. In 2000, Brennan and Cookson first described this particular type of programmed cell death as pyroptosis [26]. In 2006, Miao et al. [27] verified the above findings, confirming that caspase-1-dependent programmed cell death is accompanied by the release of numerous pro-inflammatory factors. Pyroptosis is a type of programmed cell death that activates inflammasomes [28]; this inflammatory cell death is harmful to the body, and the resulting disease is associated with chronic and continuous progress. Table 1 summarizes the difference between pyroptosis and other types of cell death.
Table 1.
Summary of studies on the difference between pyroptosis and other types of cell death.
| Pyroptosis | Apoptosis | Necrocytosis | Autophagy | Ferroptosis | |
|---|---|---|---|---|---|
| Death form | Programmed | Programmed | Programmed | Programmed | Programmed |
| induction | Pathological stimulus |
Gene regulation | Physical and chemical stimulation or pathogen infection |
Pathological irritation or nutritional deficiency |
Excessive lipid peroxidation |
| cytomembrane | Cell pore formation |
invagination | cleavage | integrity | integrity |
| cell nucleus | integrity | pyknosis and fragmentation |
pyknosis and fragmentation |
Fusion with lysosomes |
integrity |
| organelle | deformation | integrity | deformation | Autophagosome phagocytosis |
deformation |
| DNA | Random degradation |
Ladder banded degradation |
Random degradation | Random degradation | Random degradation |
| cell morphology |
Ballooning degeneration |
Cell shrinkage | cellular swelling | Crescent or cup shaped |
cellular swelling |
| Key molecules |
caspase-1/3/4/5/8/11, IL-18, IL-1β, GSDMD | Caspase-2/3/6/7/8/9/10,P53, Bcl-2, AOP-1, TNF, CHOP, JNK |
RIP1, RIP3, MLKL, TNF-α, TNFR1, FLIP, |
mTOR, RAS, MAPK, ERK, Bcl-2 |
RIP1, RIP3,MLKL, PKC, MAPK, AP-1, ROS |
GSDMs are expressed in a variety of cell types and tissues and belong to a family of proteins with multiple functions [29]. At present, six GSDM proteins have been identified in humans. Based on the crystal structure of mouse GSDM A (GSDMA), mouse GSDM D (GSDMD), and human GSDMD, it has been discovered that all GSDMs share the same self-inhibition mechanism. In fact, GSDM C-terminal domain (GSDM-CTD) can inhibit the oligomerization of GSDM N-terminal domain (GSDM-NTD) and thus inhibit its membrane perforation function [30,31,32].
In humans, GSDMA is mainly expressed in the digestive tract, breast, and skin tissues. T cells also express GSDMA [33]. GSDMA is similar to other GSDMs, its N-terminal domain is responsible for the membrane perforation, and its C-terminal and N-terminal domains can form self-inhibited structures under physiological conditions [34]. The N-terminal domain of GSDMA can also interact with the tumor necrosis factor receptor-associated protein 1 (TRAP1) present on the mitochondria; TRAP1 regulates the permeability of the mitochondrial membrane. Since GSDMA can inhibit the function of TRAP1, GSDMA can damage cellular and mitochondrial membranes [35], thereby triggering the accumulation of intracellular reactive oxygen species (ROS), which initiates apoptosis through the mitochondrial pathway. To date, proteins with GSDMA cleavage ability and cytokines specifically released in GSDMA-induced pyroptosis have not been reported.
GSDM B (GSDMB) is mainly expressed in the human airway epithelium, esophagus, stomach, liver, small intestine, colon, and other tissues. Many alternative splicing variants of GSDMB have been detected in humans, and one transcript (encoded by exon 6) contains a caspase-1 cleavage site [36]. Studies have shown that caspase-1 cleaves this subtype and induces pyroptosis [37,38]. GSDMB releases its N-terminal domain through the hydrolysis of Lys229/Lys223, resulting in the pyroptosis of target cells [36].
GSDM C (GSDMC) is mainly expressed in the spleen, trachea, stomach, and digestive tract [39]. GSDMC is similar to other GSDMs, as its N-terminal domain can form holes in the cell membrane [40]. According to recent literature, the phosphorylated signal transducer and activator of transcription 3 (STAT3) and programmed cell death receptor ligand 1 (PDL1) complex can promote the expression of GSDMC in tumor cells [41]. In the presence of tumor necrosis factor-alpha (TNF-α), caspase-8 is activated in tumor cells. Activated caspase-8 cleaves GSDMC at D365, leading to the release of its N-terminal domain, which transforms cell apoptosis into tumor focal death [42]. A leucine zipper is located in the C-terminal domain of GSDMC that may recognize specific DNA sequences [43].
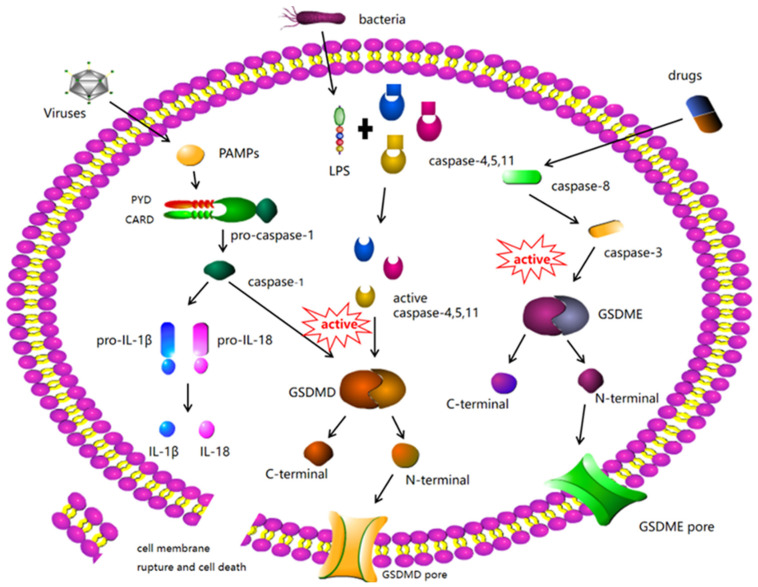
GSDMD is widely expressed in a variety of tissues and leukocytes [44]. GSDMD was the first executor to be identified in the process of pyroptosis [45]. According to current studies, GSDMD contains the cleavage sites of caspase-1 (human), caspase-11 (mouse), caspase-4, and caspase-5 [46]. Caspase-4, -5, and -11 are involved in non-classical inflammatory body signaling pathways that can be activated by viruses or lipopolysaccharide (LPS) [47]. After activation, caspases-4, 5, and 11 cleave GSDMD and release its N-terminal domain to induce pyroptosis. To date, no cytokines specifically released in caspase-11-GSDMD-mediated pyroptosis have been identified. Caspase-1-GSDMD-mediated pyroptosis is the most classical model of pyroptosis [48]. Caspase-1 belongs to the classic inflammasome signaling pathway. Pathogen-associated molecular patterns (PAMPs), abnormal cell homeostasis, and a variety of extracellular danger signals can activate caspase-1 [49,50]. Activated caspase-1 cleaves GSDMD to release its N-terminal domain to induce pyroptosis and cleaves IL-1β and interleukin-18 (IL-18) precursors for maturation [51]. Caspase-1-GSDMD-mediated pyroptosis is thus often accompanied by the release of activated IL-Iβ and IL-18 (Figure 1).
Figure 1.
Molecular mechanisms of pyroptosis-regulated cell death. The stimulated inflammasome components trigger the cleavage of Caspase-1. Then, Caspase-1 can significantly cleave GSDMD to form GSDMD N-fragment and plasma membrane pores formation, leading to pyroptosis-regulated cell death. Stimulated Caspase-1 leads to the maturation and secretion of IL-1β and IL-18 inflammatory factors. Besides, LPS can bind to the Caspase-4/5/11 precursor, leading to pyroptosis-regulated cell death. Caspase-3/GSDME can also lead to pyroptosis-regulated cell death. Notably, caspase-8 triggers Caspase-3. Then, the stimulated Caspase-3 cleaves GSDME to form GSDME N-fragments, leading to plasma membrane pores formation, cell swelling, and pyroptosis.
GSDM E (GSDME) was initially identified to be encoded by a gene responsible for autosomal dominant non-syndromic hearing loss diseases, but was later included in the GSDM protein family due to its sequence and structural similarity with that of the GSDM family proteins [52]. GSDME is expressed in a variety of human tissues, including the brain, endometrium, placenta, and intestine. Caspase-3 can activate GSDME through direct or indirect cleavage and initiate pyroptosis [53]. Caspase-3 cleaves interleukin-16 (IL-16) as well as GSDME [54]. IL-16 is similar to IL-Iβ and IL-18 because of the following reasons: it is a proinflammatory cytokine, it needs to be cleaved and activated to perform its biological function, and it also has no signaling peptides and cannot be released from the cell by the classic cytokine release process. Scientists have hypothesized that activated IL-16 might be a cytokine specifically released in GSDME-induced pyroptosis based on a comparison of the molecular mechanism of GSDME-IL-16 with that of GSDMD-IL-1β and -IL-18 [55].
3. Pathways Associated with Pyroptosis
There are classical caspase-1 dependent pathways and non-classical caspase-4/-5/-11 dependent pathways. In the classical pyroptosis pathway, pro-caspase-1 contain caspase recruitment domain (CARD), apoptosis-associated speck-like protein (ASC), and nod-like receptor 1(NLRP1). Nod-like receptor protein 3(NLRP3), NLR family CARD domain-containing protein 4 (NLRC4), and absent in melanoma 2(AIM2) were indirectly linked to pyrin and other proteins [56]. Formation of the inflammasome, activation cleavage of pro-caspase-1, and formation of active cleaved caspase-1 promote membrane perforation and death. Further, cleaved caspase-1 cleaves IL-1β and IL-18 precursors, promoting IL-1β and IL-18 production and their release into the extracellular environment, thus amplifying the inflammatory response [57,58,59]. In the non-classical pyroptosis pathway, bacterial LPS and caspase-4/-5/-11 activate GSDMD and mediate cell membrane lysis and pyroptosis, eventually leading to the production of IL-1β [47].
The inflammasome is an intracellular multiprotein complex defined by its core protein pattern-recognition receptor (PRR). The main PRRs of inflammatory bodies are the nod-like receptor (NLR) family, ASC containing CARD, and pro-caspase-1. NLR is mainly composed of three domains: the end-effect binding domain of N can be divided into caspase recruitment domain, pyrin domain, or baculovirus apoptosis inhibition repeat domain; the center has a nucleotide-binding oligomerization domain; and the C-terminal consists of leucine-rich repeats. After recognizing endogenous or exogenous danger signals, NLR can recruit pro-caspase-1 by promoting CARD interaction or recruit CARD-containing adaptor protein ASC through pyrin domain. Further, ASC acts as a bridge to pro-caspase-1, thereby activating caspase-1 [60,61]. Mature caspase-1 cleaves pro-IL-1β and pro-IL-18 to further induce the maturation of IL-1β and IL-18. In general, cytokines, such as IL-1β and IL-18 have multiple biological functions and play a central role in immune response [62]. The assembly of NLRP3, AIM2, NLPR1, and NLRC4 inflammasome is strictly dependent on bridge ASC, NLRP1, and NLRC4, which have CARD domains that directly recruit caspase-1. Therefore, NLRP1 and NLRC4 can be assembled as a part of ASC, which is activated by canonical inflammatory bodies. In addition, caspase-11 and caspase-8 promote the activation of the non-classical inflammasome, and caspase-11 plays an important role in the activation of caspase-1 and caspase-3 [63].
In fact, Caspase 3 and Caspase 8 are major players in apoptosis. However, with progress in research, caspase-3 and/or caspase-8 have been found to induce pyroptosis. Caspase-3 is an important terminal shearing enzyme that can rapidly recognize and cleave GSDMD and form GSDMD-N, which promotes the formation of cell membrane pores and leads to pyroptosis [64]. Caspase-8 is involved in signaling pathways that regulate key molecules and can lyse GSDMC and GSDME to promote pyroptosis [65]. TGF-β-activated kinase-1 (TAK1) is a basic factor regulating NF-κB signal transduction. Evidence suggests that the inhibition of TAK1 by Yersinia exo-protein J (YOP-J) or other less critical inhibitory molecules promotes caspase-8-mediated cleavage of GSDMD and forms cell membrane pores, leading to pyroptosis [2].
4. Pyroptosis and Tumor
The pathogenesis of tumors is relatively complex. However, inflammasomes have also been found in tumor cells; these inflammasomes can promote and inhibit tumor growth [66]. As the inflammasome is a key molecule that induces caspase-1 to undergo pyroptosis, it may be an important node in the association between tumor cells and pyroptosis. Different tumors involve different inflammasomes. For example, NLRP3 is widely present in tumor cells and is associated with nasopharyngeal cancer, colorectal cancer, and lung adenocarcinoma [5]. In addition, liver cancer is associated with the AIM2 inflammasome [67]. Although pyroptosis can be presumed to be related to tumors, the relationship between pyroptosis and tumor is complicated. Pyroptosis can not only inhibit the occurrence and development of tumors, but also promote inflammatory cell death. Pyroptosis can also promote tumor growth by forming a suitable microenvironment for tumor cell growth [68]. In the tumor immune microenvironment, tumor cells are protected by mesenchymal cells, extranuclear cells, cytokines, chemokines, and metabolites so that tumor cells can survive. Pyroptosis can inhibit and promote tumor cells in the tumor immune microenvironment. It can reprogram tumor immune microenvironment into immune stimulation state through damage-associated molecular patterns (DAMPs) released after osmotic lysis, thus inhibiting tumor cell growth and metastasis. It can also promote the growth of tumor cells under the action of inflammatory factors. Pyrotopia may have specific effects on tumor immune microenvironment and promote immune surveillance. Thus, pyroptosis is a potential new target for cancer treatment.
Many molecules can induce pyroptosis of tumor cells, and their effects are not consistent in different tumors. Chemotherapeutic drugs have always played an important role in the treatment of tumors, especially extensive malignant tumors. Different types of chemotherapeutic drugs have different targets. Owing to a deeper understanding of pyroptosis, some chemotherapeutic drugs have been proven to induce cell pyroptosis. Some anticancer drugs that affect cancer by inducing different pyroptosis pathways are described in Table 2.
Table 2.
Drugs inducing pyroptosis signaling pathways for regulating and treating cancers.
| Cancer Types | Therapeutic Drugs | Mechanisms of Pyroptosis Induction | References |
|---|---|---|---|
| Lung cancer | Polyphyllin VI | ROS/NF-κB/NLRP3/GSDMD | [69] |
| Cisplatin and paclitaxel | Caspase-3/GSDME | [68] | |
| Colorectal cancer | Arsenic trioxide + Ascorbic acid | ROS/Caspase-1/IL-1β,IL-18 | [70] |
| Lobaplatin | Caspase- 3/GSDME | [54] | |
| Gastric cancer | 5-fluorouracil, cisplatin | Caspase-3/GSDME | [71] |
| Neuroblastoma | Dasatinib | Caspase-3/GSDME | [72] |
| Melanoma | Iron + CCCP | Caspase-3/GSDME | [73] |
| Breast cancer | Cisplatin | MEG3/NLRP3/caspase-1/GSDMD | [74] |
| Nasopharyngeal carcinoma | Taxol | Caspase-1/GSDMD | [75] |
| Skin cancer | Doxorubicin | Caspase-3/GSDME/eEF-2K | [76] |
| Esophageal cancer | Metformin | miR-497/PELP1/Caspase1/GSDMD | [77] |
In 2017, a study published in Nature revealed that topotecan, irinotecan, etoposide, and cisplatin can induce pyroptosis in Jurkat cells and Me Wo cells; adriamycin and fluorouracil can induce pyroptosis in HeLa cells; and adriamycin, actinomycin D, or topotecan can induce pyroptosis in NCI-H522 cells [78]. Notably, these pathways are induced by caspase-3 and GSDMD, and the widespread presence of GSDMD can lead to pyroptosis of normal cells during chemotherapy. Other studies have reported that paclitaxel and cisplatin can cause pyroptosis of lung cancer A549 cells, and cisplatin has a stronger pyroptosis ability than paclitaxel. Paclitaxel can also induce pyroptosis in nasopharyngeal carcinoma cells [71]. Loplatin has been confirmed to induce pyroptosis of HT-29 and HCT116 cells in the treatment of colon cancer [54]. Previously, chemotherapy drugs were believed to induce apoptosis of tumor cells; however, owing to a deeper understanding of pyroptosis, a process that was initially thought to be a part of apoptosis was later proven to be a part of pyroptosis.
Some studies have shown that the cell membrane of gastric cancer cells is swollen and broken after 5-FU treatment. Further, GSDME was revealed to transform caspase-3-dependent apoptosis induced by anti-cancer drugs into pyroptosis of gastric cancer cells, which is helpful for re-understanding the effects of chemotherapy drugs [79]. However, whether combination therapy can improve patient outcomes is controversial. Pyroptosis has also been discussed in molecular studies, which suggests that it can be used to enhance the efficacy of chemotherapy drugs. For example, the polo-like kinase 1 (PLK1) inhibitor, BI2536, enhances the chemical sensitivity of cisplatin by inducing pyroptosis in esophageal squamous cell carcinoma, and cisplatin combined with BI2536 can induce pyroptosis in esophageal squamous cell carcinoma at low doses [80]. Eukaryotic plant factor 2 kinase (eEF-2K) plays a synergistic role with doxorubicin in the treatment of melanoma cells. Further, the inhibition of eEF-2K has been observed to inhibit pyroptosis of tumor cells [81]. eEF-2K indirectly enhances the antitumor effect of doxorubicin.
5. Gynecological Oncology
Gynecological tumors occur in female reproductive organs, with a wide variety of clinical outcomes and different prevention and treatment methods. Unlike solid tumors of other organs, the outcomes of gynecological tumors are not only related to the patients, but also their offspring. Therefore, more severe challenges are associated with gynecological tumors. Molecular targeted therapy has changed the treatment strategy and concept of tumors. Further, individualized and precise treatment of gynecological tumors has improved the prognosis and quality of life of patients. Research and screening of more efficient, accurate, and safe targeted drugs are the current focal points of gynecological tumor research. In recent years, the significance of pyroptosis in the treatment of gynecological tumors has been widely investigated, and many new achievements have been made. Pyroptosis can be a new target for the treatment of gynecological tumors [76]. A summary of studies on the role of pyroptosis and related compounds in gynecological oncology is provided in Table 3.
Table 3.
Summary of studies on the role of pyroptosis and related compounds in gynecological oncology.
| Gynecological Oncology | Interventions | Mechanism of Action | Application | In Vitro/In Vivo | Animal Model | References |
|---|---|---|---|---|---|---|
| endometrial cancer |
Hydrogen | ROS/NLRP3/caspase-1/ GSDMD |
Suppression | Both | female SPF grade BALB/c-nude mice |
[82] |
| cervical cancer |
MiRNA-214 | NLRP3 | Suppression | Both | wistar female rats | [83] |
| HPV E7 | inhibited the cleavage of GSDMD |
Promotion | In vitro | [84] | ||
| tanshinone II | regulating miR-145/ GSDMD |
Suppression | In vitro | [85] | ||
| SIRT1 | eliminate AIM2 | Promotion | Both | wistar female rats | [86] | |
| Lobaplatin | caspase-3/GSDME | Suppression | In vitro | [87] | ||
| ovarian cancer |
alpha-NETA | GSDMD/caspase-4 | Suppression | Both | female SPF grade BALB/c-nude mice |
[88] |
| Nobiletin | increased cleavage levels of GSDMD and GSDME | Suppression | In vitro | [89] | ||
| lncRNA GAS5 |
inhibits inflammasome formation and pyroptosis |
Suppression | Both | female SPF grade BALB/c-nude mice |
[90] | |
| LncRNA HOTTIP |
nhibits cell pyroptosis by targetingmiR-148a-3p/AKT2 axis |
Suppression | In vitro | [91] | ||
| Osthole | GSDME | Suppression | In vitro | [92] |
“Suppression” indicates that the intervention suppresses cancers. “Promotion” indicates that the intervention promotes cancers. “In vitro/in vivo” indicates whether the study was performed in vivo, in vitro, or both.
6. Pyroptosis and Endometrial Carcinoma
Endometrial carcinoma is a group of epithelial malignant tumors occurring in the endometrium, most commonly in perimenopausal and postmenopausal women. Recently, the incidence of this tumor has been increasing, with approximately 58,500 new cases and 7000 deaths worldwide each year [93]. A close relationship exists between inflammation and tumor. Several studies have found that inflammation is rich in inflammatory cells and cytokines. The inflammatory microenvironment of chemokines and DNA damage-promoting substances can lead to the occurrence, development, and metastasis of tumors. Endogenous and exogenous estrogen, genetic factors, and oncogenic mutations play a role in the development of endometrial carcinoma. Periodic changes in the endometrium are also related to chronic inflammation. Liu et al. [94] revealed that the expression of the inflammasome NLRP3, which is associated with pyroptosis, was significantly increased in endometrial cancer, and the increase was positively correlated with clinical and pathological stages of the cancer. Yang et al. [82] confirmed that hydrogen can induce the GSDMD pathway in endometrial cancer to mediate pyroptosis, thereby affecting the biological behavior of endometrial cancer cells. Chang et al. [95] found that in patients with endometrial cancer, the expression levels of pyroptosis-related inflammasomes AIM2 and NLRP3 were significantly higher than those in the normal group. The expression levels of NLRP3, GSDMD, caspase-1, and IL-1β were also significantly higher in atypical hyperplasia and carcinoma tissues than in benign endometrial tissues. Overactivated inflammasome and pyroptosis-related proteins in patients with endometrial cancer induce pyroptosis, inhibit the progression of endometrial cancer, and provide specific targets for clinical treatment.
7. Pyroptosis and Cervical Cancer
The incidence and mortality of cervical cancer is fourth among female malignant tumors worldwide and second in developing countries [96]. Persistent infection of human papillomavirus 16 (HPV16) and human papillomavirus 18 (HPV18) is the main cause of cervical cancer. In HPV-infected cells, the inflammasome AIM2 acts as a tumor suppressor by activating caspase-1 to promote pyroptosis of tumor cells [86]. The caspase-3/GSDME axis is involved in loplatin-mediated pyroptosis of cervical cancer cells. These findings suggest that GSDME-mediated pyroptosis is a novel mechanism to kill tumor cells, and the caspase-3/GSDME pathway provides a new approach for tumor chemotherapy [87]. HPV E7 recruits the estriol (E3) ligase recombinant protein of human tripartite motif-containing 21 (TRIM21) to ubiquitinate and degrade IL-16 inflammasome, leading to the inhibition of pyroptosis and self-evasion of immune surveillance. The upregulation of Mir-214 in cervical cancer patients and cervical cancer cell lines can promote pyroptosis of cervical cancer cells by enhancing NLRP3 expression [84]. Tong et al. [85] found that tanshinone II A could promote pyroptosis and inhibit tumor cell proliferation by regulating the Mir-145/GSDMD signaling pathway. After treatment with tanshinone II A, the expression levels of Mir-145, GSDMD, IL-18, and IL-1β, proteins related to pyroptosis, were found to be increased in Hela cells. Pyroptosis depends on the pore-forming activity of the GSDM protein family and inhibits the development of cervical cancer through the activity of caspase-1 and inflammatory factors.
8. Pyroptosis and Ovarian Cancer
Ovarian cancer is one of the most common cancers in women, with a five-year survival rate of less than 45% [97]. Studies have shown that the development of ovarian cancer can be inhibited by inducing pyroptosis. Further, long non-coding RNA growth arresting-specific transcript 5 (LNC-RNA-GAS5) can activate apoptosis-related blotk-like protein, caspase-1, and IL-1β. Caspase-1 has been demonstrated to activate GSDMD to form the GSDMD-N terminal fragment. GSDMD-N specifically binds to the cell membrane and oligomerizes to form membrane pores, causing cell swelling and pyroptosis, which inhibits the proliferation of ovarian cancer cells [90]. According to Qiao et al. [88], α-Neta inhibited the proliferation of epithelial ovarian cancer cells by directly inducing pyroptosis via the activation of caspase-4 cleavage of GSDMD. With further elucidation of the mechanisms involved in pyroptosis, pyroptosis can be considered as an efficient target for the diagnosis and treatment of ovarian cancer. Nobilin reduces mitochondrial membrane potential and induces the generation of reactive oxygen species and autophagy of human ovarian cancer cells (HOCCs), leading to GSDMD/GSDME-mediated pyroptosis [89].
9. Prospects and Summary
In conclusion, pyroptosis is a pro-inflammatory programmed cell death process that relies on the pore-forming activity of the GSDM protein family and plays a role in the occurrence and development of gynecological tumors through the activation of the caspase proteins and inflammatory factors. However, the specific pathways and mechanisms associated with pyroptosis in gynecologic tumors have not been fully elucidated. Current studies have shown that the inflammasome is closely related to gynecological malignant tumors and causes thermal death of tumor cells, which can be used as a feasible therapeutic target for gynecological tumors. Therefore, the regulation of GSDMs, key effectors of pyroptosis, is a new strategy and effective approach for the treatment of gynecological tumors. For example, certain drugs or molecules (metformin, anthocyanin, DHA(Docosahexaenoic Acid), 2-(naphthoyl) ethyl trimethylammonium iodide) can promote pyroptosis of cancer cells by activating GSDMD. Most tumor cells inhibit GSDME via methylation modification with a DNA methyltransferase inhibitor. Decitabine can promote the expression of GSDME and induce pyroptosis of tumor cells. Further, the increase in intracellular reactive oxygen species can stimulate caspase-3 to cut off GSDME and induce pyroptosis of tumor cells. The occurrence of pyroptosis also increases the sensitivity of tumor cells to chemotherapeutic drugs; thus, the combination of chemotherapeutic drugs and GSDM protein activator may be beneficial for tumor treatment. The braftovi/mektovi (BRAF/MEK) inhibitor-induced pyroptosis can induce changes in the tumor immune microenvironment and improve the therapeutic effect of drug-resistant melanoma, which provides a new direction for scorch-mediated anti-tumor immunity and treatment of drug-resistant tumors. Although some members of the GSDM protein family are known to induce pyroptosis and participate in the treatment of tumors, how they precisely regulate its expression level and promote tumor cell death are unknown. Further, avoiding the generation of a tumor-friendly microenvironment remains key in future cancer therapy. The inflammatory response caused by pyroptosis can cause strong tissue damage. Thus, how to kill tumor cells and avoid an inflammatory cytokine storm are current challenges in the application of heat death.
Pyroptosis is a newly discovered way of cell death in recent years, and its regulatory mechanism needs to be further explored. LncRNAs (long noncoding RNA) are RNA transcripts over 200 nucleotides in length that are generally considered incapable of encoding amino acids and are involved in many physiological and pathological processes. Stimulated by exogenous and endogenous factors, abnormal expression of lncRNAs affects the expression of pyroptosis pathway-related proteins through two indirect ways: miRNAs and downstream proteins/pathways, and they directly regulate the inflammasome, which plays an important role in pyroptosis, thus regulating the pathological process of various diseases. In addition, lncRNAs often act as endogenous sponges for miRNAs, affecting the levels of their targeted proteins, including those associated with the pyroptosis pathway [98]. Accumulating evidence suggests that lncRNAs play important roles in processes such as inflammation, oxidative stress, and pyroptosis. LncRNA can increase the expression of NLRP3, and up-regulation of lncRNA expression can activate the NLRP3, trigger tumor pyroptosis, and lead to cell death. With more research, more lncRNAs related to pyroptosis will be discovered, and the regulatory mechanisms of their pyroptotic pathways will become clearer [99]. Therefore, altering lncRNAs in vivo through novel therapies may be a promising approach for the diagnosis and treatment of pyroptosis-related diseases.
More and more studies have focused on pyroptosis in tumors. Currently, the research mainly focuses on compounds or molecules that activate inflammasome such as NLRP3 and AIM2 and promote pyroptosis, which are promising as new drugs for the treatment of tumors. However, there are still many pending issues in the study of pyroptosis. For example, cell death may coexist in various ways in the state of disease, and activation of inflammasome and caspase and other proteins may not all cause pyroptosis. How to accurately distinguish and identify different types of cell death? This requires the development of new identification methods. In addition, as a key pyroptosis executor, there are few studies on the molecular regulatory mechanism of GSDMD itself. Besides upstream inflammasome and caspase protein, whether there are other effector targets remains to be discovered. Owing to differences in the expression and activity of pyroptosis in different gynecological diseases, its role is also different. Therefore, the application of pyroptosis in the pathogenesis, diagnosis, and treatment of gynecological tumors requires further comprehensive research.
Abbreviations
| NLRP3 | nod-like receptor protein 3 |
| AIM2 | absent in melanoma 2 |
| IL-1β | interleukin-1β |
| GSDMs | Gasdermins |
| GSDMA | GasderminA;GSDMB:Gasderminb |
| GSDMC | GasderminC |
| GSDMD | GasderminD |
| GSDME | GasderminE |
| DFNB59 | autosomal recessive deafness-59 |
| GSDMs-CTD | Gasdermins C-terminal domain |
| GSDMS-NTD | Gasdermins D-terminal domain |
| TRAP1 | tumor necrosis factor receptor-associated protein 1 |
| ROS | reactive oxygen species |
| CTLs | cytotoxic T lymphocytes |
| STAT3 | signal transducer and activator of transcription 3 |
| PDL1 | programmed cell death receptor ligand 1 |
| TNF-α | tumor necrosis factor alpha |
| IL-18 | interleukin-18 |
| IL-16 | interleukin-16 |
| CARD | caspase recruitment domain |
| ASC | apoptosis-associated speck-like protein |
| NLRP1 | nod-like receptor 1 |
| NLRP3 | nod-like receptor protein 3 |
| NLRC4 | NLR Family CARD domain-containing protein 4 |
| LPS | lipopolysaccharide |
| PRR | protein pattern-recognition receptor |
| NLR | nod-like receptor |
| APAF-1 | apoptotic peptidase activating factor-1 |
| TAK1 | TGF-β-activated kinase-1 |
| YOP-J | Yersinia exo-protein J |
| PLK1 | polo-like kinase 1 |
| eEF-2K | Eukaryotic plants factor 2 kinase |
| HPV16 | human papillomavirus 16 |
| HPV18 | human papillomavirus 18 |
| TRIM21 | recombinant protein of human tripartite motif-containing 21 |
| LNC-RNA-GAS5 | long non-coding RNA growth arresting-specific transcript 5 |
| HOCCs | human ovarian cancer cells |
| BRAF/MEK | braftovi/mektovi |
| LncRNAs | long noncoding RNA. |
Author Contributions
Conceptualization, Y.H. and Y.Y.; writing—original draft preparation, Y.H.; writing—review and editing, R.L.; supervision, Y.Y.; project administration, Y.Y.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by The First Hospital of Lanzhou University Research Ethics Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was supported by the The First Hospital of Lanzhou University Foundation (No. ldyyyn2019-62); the Regional Scientists Fund of the National Natural Science Foundation of China (No. 81960275); Gansu Provincial Department of Science and Technology Foundation (21YF5FA119).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tang R., Xu J., Zhang B., Liu J., Liang C., Hua J., Meng Q., Yu X., Shi S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020;13:110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malireddi R., Kesavardhana S., Kanneganti T.D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis) Front. Cell. Infect. Microbiol. 2019;9:406. doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank D., Vince J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99–114. doi: 10.1038/s41418-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma B.R., Kanneganti T.-D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021;22:550–559. doi: 10.1038/s41590-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamarsheh S., Zeiser R. NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front. Immunol. 2020;11:1444. doi: 10.3389/fimmu.2020.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey Sarkar R., Sinha S., Biswas N. Manipulation of Inflammasome: A Promising Approach Towards Immunotherapy of Lung Cancer. Int. Rev. Immunol. 2021;40:171–182. doi: 10.1080/08830185.2021.1876044. [DOI] [PubMed] [Google Scholar]
- 7.Downs K.P., Nguyen H., Dorfleutner A., Stehlik C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020;76:100924. doi: 10.1016/j.mam.2020.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isonishi S., Terashima Y. Methotrexate in gynecologic oncology. Gan Kagaku Ryoho. Cancer Chemother. 1996;23:1896–1900. [PubMed] [Google Scholar]
- 9.Kasius J.C., Velden J., Denswil N.P., Tromp J.M., Mom C.H. Neo-adjuvant chemotherapy in fertility-sparing cervical cancer treatment. Best Pract. Res. Clin. Obstet. Gynaecol. 2021;75:82–100. doi: 10.1016/j.bpobgyn.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Patel A., Iyer P., Matsuzaki S., Matsuo K., Sood A.K., Fleming N.D. Emerging Trends in Neoadjuvant Chemotherapy for Ovarian Cancer. Cancers. 2021;13:626. doi: 10.3390/cancers13040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Raposo C., Merino S.M., Aguayo Z.C., Casado S.E. Adjuvant chemotherapy in endometrial cancer. Cancer Chemother. Pharmacol. 2020;85:477–486. doi: 10.1007/s00280-019-04027-6. [DOI] [PubMed] [Google Scholar]
- 12.Bedoschi G.M., Navarro P.A., Oktay K.H. Novel insights into the pathophysiology of chemotherapy-induced damage to the ovary. Panminerva Med. 2019;61:68–75. doi: 10.23736/S0031-0808.18.03494-8. [DOI] [PubMed] [Google Scholar]
- 13.Jackson S.E., Chester J.D. Personalised cancer medicine. Int. J. Cancer. 2015;137:262–266. doi: 10.1002/ijc.28940. [DOI] [PubMed] [Google Scholar]
- 14.Cree I.A. Cancer biology. Methods Mol. Biol. 2011;731:1–11. doi: 10.1007/978-1-61779-080-5_1. [DOI] [PubMed] [Google Scholar]
- 15.Bedoui S., Herold M.J., Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020;21:678–695. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 16.Castillo Ferrer C., Berthenet K., Ichim G. Apoptosis–Fueling the oncogenic fire. FEBS J. 2021;288:4445–4463. doi: 10.1111/febs.15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang Y., Tian S., Pan Y., Li W., Wang Q., Tang Y., Shu Y. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020;121:109595. doi: 10.1016/j.biopha.2019.109595. [DOI] [PubMed] [Google Scholar]
- 18.Xia X., Wang X., Cheng Z., Qin W., Lei L., Jiang J., Hu J. The role of pyroptosis in cancer: Pro-cancer or pro-“host”? Cell Death Dis. 2019;10:650. doi: 10.1038/s41419-019-1883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Jiang M., Qi L., Wu Y., Song D., Gan J., Bai Y. Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 2021;112:3979–3994. doi: 10.1111/cas.15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju X., Yang Z., Zhang H., Wang Q. Role of pyroptosis in cancer cells and clinical applications. Biochimie. 2021;185:78–86. doi: 10.1016/j.biochi.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Smith M.R., Thirumalai K., Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. doi: 10.1002/j.1460-2075.1996.tb00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou J., Hsu J.M., Hung M.C. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol. Cell. 2021;81:4579–4590. doi: 10.1016/j.molcel.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergsbaken T., Fink S.L., Hartigh A.B., Loomis W.P., Cookson B.T. Coordinated host responses during pyroptosis: Caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J. Immunol. 2011;187:2748–2754. doi: 10.4049/jimmunol.1100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball D.P., Taabazuing C.Y., Griswold A.R., Orth E.L., Rao S.D., Kotliar I.B., Bachovchin D.A. Caspase-1 interdomain linker cleavage is required for pyroptosis. Life Sci. Alliance. 2020;3:1–11. doi: 10.26508/lsa.202000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Sun J., Wan L., Yang X., Lin H., Zhang Y., Wei C. The Shigella Type III Secretion Effector IpaH4.5 Targets NLRP3 to Activate Inflammasome Signaling. Front. Cell. Infect. Microbiol. 2020;10:511798. doi: 10.3389/fcimb.2020.511798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan M.A., Cookson B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 27.Miao E.A., Alpuche-Aranda C.M., Dors M., Clark A.E., Bader M.W., Miller S.I., Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 28.Briard B., Malireddi R., Kanneganti T.D. Role of inflammasomes/pyroptosis and PANoptosis during fungal infection. PLoS Pathog. 2021;17:e1009358. doi: 10.1371/journal.ppat.1009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacs S.B., Miao E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J., Gao W., Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Broz P., Pelegrín P., Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020;20:143–157. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 32.Shao F. Gasdermins: Making pores for pyroptosis. Nat. Rev. Immunol. 2021;21:620–621. doi: 10.1038/s41577-021-00602-2. [DOI] [PubMed] [Google Scholar]
- 33.Feng S., Fox D., Man S.M. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J. Mol. Biol. 2018;430:3068–3080. doi: 10.1016/j.jmb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka S., Mizushina Y., Kato Y., Tamura M., Shiroishi T. Functional conservation of Gsdma cluster genes specifically duplicated in the mouse genome. G3 Genes Genomes Genet. 2013;3:1843–1850. doi: 10.1534/g3.113.007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin P.H., Lin H.Y., Kuo C.C., Yang L.T. N-terminal functional domain of Gasdermin A3 regulates mitochondrial homeostasis via mitochondrial targeting. J. Biomed. Sci. 2015;22:44. doi: 10.1186/s12929-015-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L., Li Y., Bai Y. Role of GSDMB in Pyroptosis and Cancer. Cancer Manag. Res. 2020;12:3033–3043. doi: 10.2147/CMAR.S246948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das S., Miller M., Broide D.H. Chromosome 17q21 Genes ORMDL3 and GSDMB in Asthma and Immune Diseases. Adv. Immunol. 2017;135:1–52. doi: 10.1016/bs.ai.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Panganiban R.A., Sun M., Dahlin A., Park H.R., Kan M., Himes B.E., Lu Q. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J. Allergy Clin. Immunol. 2018;142:1469–1478.e2. doi: 10.1016/j.jaci.2017.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia S., Ruan J., Wu H. Monitoring gasdermin pore formation in vitro. Methods Enzymol. 2019;625:95–107. doi: 10.1016/bs.mie.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruan J. Structural Insight of Gasdermin Family Driving Pyroptotic Cell Death. Adv. Exp. Med. Biol. 2019;1172:189–205. doi: 10.1007/978-981-13-9367-9_9. [DOI] [PubMed] [Google Scholar]
- 41.Hou J., Zhao R., Xia W., Chang C.W., You Y., Hsu J.M., Hung M.C. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020;22:1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J.Y., Zhou B., Sun R.Y., Ai Y.L., Cheng K., Li F.N., Wu Q. The metabolite alpha-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 2021;31:980–997. doi: 10.1038/s41422-021-00506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Z., Deng W., Lou X., Bai Y., Wang J., Zeng H., Liu X. Gasdermins: Pore-forming activities and beyond. Acta Biochim. Biophys. Sin. 2020;52:467–474. doi: 10.1093/abbs/gmaa016. [DOI] [PubMed] [Google Scholar]
- 44.Pandeya A., Li L., Li Z., Wei Y. Gasdermin D (GSDMD) as a new target for the treatment of infection. MedChemComm. 2019;10:660–667. doi: 10.1039/C9MD00059C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burdette B.E., Esparza A.N., Zhu H., Wang S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B. 2021;11:2768–2782. doi: 10.1016/j.apsb.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X., Ding S., Liu P. The Roles of Gasdermin D in Coronavirus Infection and Evasion. Front. Microbiol. 2021;12:784009. doi: 10.3389/fmicb.2021.784009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matikainen S., Nyman T.A., Cypryk W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J. Immunol. 2020;204:3063–3069. doi: 10.4049/jimmunol.2000373. [DOI] [PubMed] [Google Scholar]
- 48.Miao E.A., Rajan J.V., Aderem A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M.Y., Ye X.J., He X.H., Ouyang D.Y. The Signaling Pathways Regulating NLRP3 Inflammasome Activation. Inflammation. 2021;44:1229–1245. doi: 10.1007/s10753-021-01439-6. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Tang W., Yi F. Role of Inflammasome in Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019;1165:407–421. doi: 10.1007/978-981-13-8871-2_19. [DOI] [PubMed] [Google Scholar]
- 51.Xia S., Zhang Z., Magupalli V.G., Pablo J.L., Dong Y., Vora S.M., Wu H. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593:607–611. doi: 10.1038/s41586-021-03478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., Peng J., Xie X., Zhang Z., Li M., Yang M. Gasdermin E-mediated programmed cell death: An unpaved path to tumor suppression. J. Cancer. 2021;12:5241–5248. doi: 10.7150/jca.48989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu W.F., Zhang Q., Ding C.J., Sun H.Y., Che Y., Huang H., Cao L.J. Gasdermin E-derived caspase-3 inhibitors effectively protect mice from acute hepatic failure. Acta Pharmacol. Sin. 2021;42:68–76. doi: 10.1038/s41401-020-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu J., Li S., Qi J., Chen Z., Wu Y., Guo J., Zheng J. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:193. doi: 10.1038/s41419-019-1441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J., Wu H., Yao X., Zhang D., Zhou Y., Fu B., Wei H. Pyroptotic macrophages stimulate the SARS-CoV-2-associated cytokine storm. Cell. Mol. Immunol. 2021;18:1305–1307. doi: 10.1038/s41423-021-00665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kist M., Vucic D. Cell death pathways: Intricate connections and disease implications. EMBO J. 2021;40:e106700. doi: 10.15252/embj.2020106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsuchiya K. Inflammasome-associated cell death: Pyroptosis, apoptosis, and physiological implications. Microbiol. Immunol. 2020;64:252–269. doi: 10.1111/1348-0421.12771. [DOI] [PubMed] [Google Scholar]
- 58.He Y., Hara H., Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue Y., Enosi T.D., Tan W.H., Kay C., Man S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019;40:1035–1052. doi: 10.1016/j.it.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Zheng Z., Wang T., Chen J., Qiu H., Zhang C., Liu W., Guo J. Inflammasome-Induced Osmotic Pressure and the Mechanical Mechanisms Underlying Astrocytic Swelling and Membrane Blebbing in Pyroptosis. Front. Immunol. 2021;12:688674. doi: 10.3389/fimmu.2021.688674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Cui J., Zhang G., Wu C., Abdel-Latif A., Smyth S.S., Li Z. Inflammasome activation promotes venous thrombosis through pyroptosis. Blood Adv. 2021;5:2619–2623. doi: 10.1182/bloodadvances.2020003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao W., Yang H., Lyu L., Zhang J., Xu Q., Jiang N., Che C. GSDMD, an executor of pyroptosis, is involved in IL-1beta secretion in Aspergillus fumigatus keratitis. Exp. Eye Res. 2021;202:108375. doi: 10.1016/j.exer.2020.108375. [DOI] [PubMed] [Google Scholar]
- 63.Cheng Q., Pan J., Zhou Z.L., Yin F., Xie H.Y., Chen P.P., Lu L.M. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol. Sin. 2021;42:954–963. doi: 10.1038/s41401-020-00525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang M., Qi L., Li L., Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112. doi: 10.1038/s41420-020-00349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fritsch M., Gunther S.D., Schwarzer R., Albert M.C., Schorn F., Werthenbach J.P., Kashkar H. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683–687. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]
- 66.Lee C., Do H., Her J., Kim Y., Seo D., Rhee I. Inflammasome as a promising therapeutic target for cancer. Life Sci. 2019;231:116593. doi: 10.1016/j.lfs.2019.116593. [DOI] [PubMed] [Google Scholar]
- 67.Sharma B.R., Karki R., Kanneganti T.D. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur. J. Immunol. 2019;49:1998–2011. doi: 10.1002/eji.201848070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Du T., Gao J., Li P., Wang Y., Qi Q., Liu X., Du L. Pyroptosis, metabolism, and tumor immune microenvironment. Clin. Transl. Med. 2021;11:e492. doi: 10.1002/ctm2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teng J.F., Mei Q.B., Zhou X.G., Tang Y., Xiong R., Qiu W.Q., Wu A.G. Polyphyllin VI Induces Caspase-1-Mediated Pyroptosis via the Induction of ROS/NF-kappaB/NLRP3/GSDMD Signal Axis in Non-Small Cell Lung Cancer. Cancers. 2020;12:193. doi: 10.3390/cancers12010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian W., Wang Z., Tang N.N., Li J.T., Liu Y., Chu W.F., Yang B.F. Ascorbic Acid Sensitizes Colorectal Carcinoma to the Cytotoxicity of Arsenic Trioxide via Promoting Reactive Oxygen Species-Dependent Apoptosis and Pyroptosis. Front. Pharmacol. 2020;11:123. doi: 10.3389/fphar.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang C.C., Li C.G., Wang Y.F., Xu L.H., He X.H., Zeng Q.Z., Ouyang D.Y. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24:312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J., Chen Y., He Q. Distinct characteristics of dasatinib-induced pyroptosis in gasdermin E-expressing human lung cancer A549 cells and neuroblastoma SH-SY5Y cells. Oncol. Lett. 2020;20:145–154. doi: 10.3892/ol.2020.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou B., Zhang J.Y., Liu X.S., Chen H.Z., Ai Y.L., Cheng K., Wu Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28:1171–1185. doi: 10.1038/s41422-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan H., Luo B., Wu X., Guan F., Yu X., Zhao L., Yuan J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Biol. Sci. 2021;17:2606–2621. doi: 10.7150/ijbs.60292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X., Li H., Li W., Xie J., Wang F., Peng X., Tan G. The role of Caspase-1/GSDMD-mediated pyroptosis in Taxol-induced cell death and a Taxol-resistant phenotype in nasopharyngeal carcinoma regulated by autophagy. Cell Biol. Toxicol. 2020;36:437–457. doi: 10.1007/s10565-020-09514-8. [DOI] [PubMed] [Google Scholar]
- 76.Yu S.Y., Li X.L. Pyroptosis and inflammasomes in obstetrical and gynecological diseases. Gynecol. Endocrinol. 2021;37:385–391. doi: 10.1080/09513590.2021.1871893. [DOI] [PubMed] [Google Scholar]
- 77.Wang L., Li K., Lin X., Yao Z., Wang S., Xiong X., Zhang H. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Lett. 2019;450:22–31. doi: 10.1016/j.canlet.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y., Gao W., Shi X., Ding J., Liu W., He H., Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y., Yin B., Li D., Wang G., Han X., Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem. Biophys. Res. Commun. 2018;495:1418–1425. doi: 10.1016/j.bbrc.2017.11.156. [DOI] [PubMed] [Google Scholar]
- 80.Wu M., Wu Y., Yang D., Gong Y., Rao F., Liu R., Zhan Q. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. EBioMedicine. 2019;41:244–255. doi: 10.1016/j.ebiom.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu P., Wang H.Y., Tian M., Li A.X., Chen X.S., Wang X.L., Cheng Y. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharmacol. Sin. 2019;40:1237–1244. doi: 10.1038/s41401-019-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y., Liu P.Y., Bao W., Chen S.J., Wu F.S., Zhu P.Y. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer. 2020;20:28. doi: 10.1186/s12885-019-6491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu S., Zhao N., He M., Zhang K., Bi X. MiRNA-214 promotes the pyroptosis and inhibits the proliferation of cervical cancer cells via regulating the expression of NLRP3. Cell. Mol. Biol. 2020;66:59–64. doi: 10.14715/cmb/2020.66.6.11. [DOI] [PubMed] [Google Scholar]
- 84.Song Y., Wu X., Xu Y., Zhu J., Li J., Zou Z., Cheng H. HPV E7 inhibits cell pyroptosis by promoting TRIM21-mediated degradation and ubiquitination of the IFI16 inflammasome. Int. J. Biol. Sci. 2020;16:2924–2937. doi: 10.7150/ijbs.50074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tong W., Guo J., Yang C. Tanshinone II A enhances pyroptosis and represses cell proliferation of HeLa cells by regulating miR-145/GSDMD signaling pathway. Biosci. Rep. 2020;40:BSR20200259. doi: 10.1042/BSR20200259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.So D., Shin H.W., Kim J., Lee M., Myeong J., Chun Y.S., Park J.W. Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense. Oncogene. 2018;37:5191–5204. doi: 10.1038/s41388-018-0339-4. [DOI] [PubMed] [Google Scholar]
- 87.Chen J., Ge L., Shi X., Liu J., Ruan H., Heng D., Ye C. Lobaplatin induces pyroptosis in cervical cancer cells via caspase-3/GSDME pathway. Anti-Cancer Agents Med. Chem. 2021;22:2091–2097. doi: 10.2174/1871520621666211018100532. [DOI] [PubMed] [Google Scholar]
- 88.Qiao L., Wu X., Zhang J., Liu L., Sui X., Zhang R., Xi X. alpha-NETA induces pyroptosis of epithelial ovarian cancer cells through the GSDMD/caspase-4 pathway. FASEB J. 2019;33:12760–12767. doi: 10.1096/fj.201900483RR. [DOI] [PubMed] [Google Scholar]
- 89.Zhang R., Chen J., Mao L., Guo Y., Hao Y., Deng Y., Yuan M. Nobiletin Triggers Reactive Oxygen Species-Mediated Pyroptosis through Regulating Autophagy in Ovarian Cancer Cells. J. Agric. Food Chem. 2020;68:1326–1336. doi: 10.1021/acs.jafc.9b07908. [DOI] [PubMed] [Google Scholar]
- 90.Li J., Yang C., Li Y., Chen A., Li L., You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Biosci. Rep. 2018;38:BSR20171150. doi: 10.1042/BSR20171150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan C., Liu W., Zheng Z.H., Wan X.G. LncRNA HOTTIP inhibits cell pyroptosis by targeting miR-148a-3p/AKT2 axis in ovarian cancer. Cell Biol. Int. 2021;45:1487–1497. doi: 10.1002/cbin.11588. [DOI] [PubMed] [Google Scholar]
- 92.Liang J., Xu Y., Huang X., Wang X., Huang W., Li H. Osthole inhibits ovarian carcinoma cells through LC3-mediated autophagy and GSDME-dependent pyroptosis except for apoptosis. Eur. J. Pharmacol. 2020;874:172990. doi: 10.1016/j.ejphar.2020.172990. [DOI] [PubMed] [Google Scholar]
- 93.Lu K.H., Broaddus R.R. Endometrial Cancer. N. Engl. J. Med. 2020;383:2053–2064. doi: 10.1056/NEJMra1514010. [DOI] [PubMed] [Google Scholar]
- 94.Liu S.G., Wu X.X., Hua T., Xin X.Y., Feng D.L., Chi S.Q., Wang H.B. NLRP3 inflammasome activation by estrogen promotes the progression of human endometrial cancer. OncoTargets Ther. 2019;12:6927–6936. doi: 10.2147/OTT.S218240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang Z., Talukdar S., Mullany S.A., Winterhoff B. Molecular characterization of endometrial cancer and therapeutic implications. Curr. Opin. Obstet. Gynecol. 2019;31:24–30. doi: 10.1097/GCO.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 96.Cohen P.A., Jhingran A., Oaknin A., Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 97.Matulonis U.A. Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018;32:xiii–xiv. doi: 10.1016/j.hoc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 98.He D., Zheng J., Hu J., Chen J., Wei X. Long non-coding RNAs and pyroptosis. Clin. Chim. Acta. 2020;504:201–208. doi: 10.1016/j.cca.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 99.Ren N., Jiang T., Wang C., Xie S., Xing Y., Piao D., Zhu Y. LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis. Aging. 2020;12:11025–11041. doi: 10.18632/aging.103314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.