Abstract
Simple Summary
Neuroendocrine tumors (NETs) are rare disorders of neuroendocrine cells with an increasing incidence. Surgical resection is the only cure; however, they are often found at an advanced stage, and many cases are unresectable because of being locally advanced or presenting as metastatic disease. Peptide receptor activation therapy (PRRT) has been found to be effective for metastatic NETs. Prediction of tumor shrinkage after PRRT for metastatic NETs is challenging and remains unclear. This study aimed to identify predictive factors associated with the rate of PRRT tumor shrinkage. This study performed both patient-based and lesion-based shrinkage analysis for metastatic NETs. We analyzed the relationship between pretreatment clinicopathological factors and the shrinkage rate per lesion (L-SR) in 20 patients. Previous treatment with cytotoxic agents and primary tumor of the pancreas were found to be significantly favorable factors; however, a primary tumor of the rectum was significantly more resistant to shrinkage.
Abstract
Peptide receptor activation therapy (PRRT) is a promising treatment option for metastatic neuroendocrine tumors (NETs). However, predicting tumor shrinkage before treatment is challenging. We analyzed the shrinkage rate of each metastatic tumor lesion to identify predictive factors related to shrinkage. Patients with metastatic NET who underwent PRRT were included in this retrospective study. For each patient, between one to five metastatic lesions were selected in descending order of size, and the change in the maximum tumor diameter after treatment was defined as the shrinkage rate per lesion (L-SR). We analyzed the relationship between pretreatment clinicopathological factors and L-SR. The median L-SR of all 75 lesions in 20 patients was 20% (95% CI: 4.8–26.1%). While previous treatment with cytotoxic agents (34.4%, p < 0.05) and primary tumor of the pancreas (27.8%, p < 0.05) were significantly favorable factors, a primary tumor of the rectum was significantly more resistant to shrinkage (−20.5%, p < 0.001). Therefore, lesion-based analysis of PRRT for NETs showed that pancreatic NET and previous treatment with cytotoxic agents were favorable factors for tumor shrinkage; however, rectal NET was a factor associated with resistance to shrinkage.
Keywords: lesion-based analysis, neuroendocrine tumors, peptide receptor radionuclide therapy
1. Introduction
Neuroendocrine tumors (NETs) are rare disorders of neuroendocrine cells that progress relatively slow, but their incidence is increasing [1,2]. Although surgical resection is the only curative treatment for NETs [3,4], nonfunctional NETs are often found at an advanced stage, and many cases are unresectable because of being locally advanced or presenting as metastatic disease. Many systemic treatments have been recently introduced for NETs, including somatostatin analogs, cytotoxic agents, and targeted molecular therapies. Although somatostatin analogs and targeted molecular therapies show promise in terms of progression-free survival (PFS), the response rate (RR) of sunitinib was 9.3% and that of everolimus was 4%, neither of which is high [5,6,7]. The RR of everolimus for pancreatic NETs (pan NETs) was 5%, and that for lung and gastrointestinal NETs was 2%. Targeted molecular therapy may also occasionally lead to relatively severe adverse events, and long-term continuation of therapy may worsen quality of life. In treatments with cytotoxic agents, the RR of streptozotocin-based chemotherapy is approximately 33–40% and that of temozolomide-based chemotherapy is 27.8–33.3% [8,9,10]; however, long-term continuation of therapy with these agents is difficult because of the moderate hematological and non-hematological damage that often results from such therapy.
In the NETTER-1 study, peptide receptor radionuclide therapy (PRRT) was shown to significantly prolong PFS (28.4 months vs. 8.4 months) and had a relatively high RR in comparison with high-dose octreotide therapy (18% vs. 3%) [11]. The RR of PRRT has been reported to be approximately 29–57% for pan NETs, and 17–73% for gastroenteropancreatic NET. Thus, PRRT has been shown to be a more reliable treatment option for tumor shrinkage than other systemic treatments.
Somatostatin receptor scintigraphy (SRS) is one of the most important and necessary modalities for determining PRRT adaptation. A retrospective study in 2008 revealed that the uptake score on pretreatment SRS was a prognostic factor for predicting tumor remission [12]. However, the RR in that study was only 60%, even with an intense uptake of 111In-pentetreotide; in contrast, approximately 40% of cases could not achieve tumor shrinkage rates over 30%. Another retrospective study revealed that the 68Ga-DOTATOC maximum standardized uptake value (SUVmax) cutoff of 16.5 showed a 95% sensitivity and 60% specificity for response prediction [13]. This low specificity indicated that predictive tumor shrinkage was not sufficient, even by pretreatment 68Ga-DOTATOC analysis.
Many other clinicopathological factors for predicting tumor shrinkage have been investigated, including tumor diameter, primary lesion, and pathological grade. More recent findings from the NETTER-1 study showed that the target lesions were divided into two groups based on tumor diameter, ≤30 mm and >30 mm [14]. The least squares mean shrinkage was 29% and 14%. There was a significant interaction of baseline tumor size and liver tumor size shrinkage. According to another report focused on tumor origin, tumors in the small intestine have a lower RR compared with particular pan NETs [15].
Other studies evaluated the RR of PRRT by pathological grade [11,16]. The NETTER-1 study showed no value of grade 1 versus grade 2 tumors for predicting response to PRRT. Sorbye et al. described PRRT in grade 3 as achieving high RR (31–41%); however, PFS was lower than in other studies for grade 1 and grade 2 NETs [16].
In the past, many studies evaluated tumor shrinkage by RR according to the Response Evaluation Criteria in Solid Tumors guideline version 1.1 (RECIST) criteria; in other words, very few reports have described both patient-based and lesion-based analysis.
Prediction of tumor shrinkage after PRRT for metastatic NETs is challenging and needs to be better understood; accurate prediction could prove valuable to clinicians seeking to optimize treatment and determine prognosis. Therefore, this study aimed to identify predictive factors associated with the rate of PRRT tumor shrinkage. In this study, we performed both patient-based and lesion-based shrinkage analysis for metastatic NETs.
2. Materials and Methods
2.1. Patients
We retrospectively evaluated patients aged ≥20 years who were referred from the Yokohama City University Hospital (Yokohama, Japan) to the University Hospital Basel (Basel, Switzerland) for PRRT between 2011 and 2020 [17]. The diagnosis of NET was made by pathological tissue retrieval following the World Health Organization (WHO) 2019 classification [18]. The patients had a varied history of treatment before PRRT, including surgical treatment, somatostatin analog treatment, targeted molecular therapies, cytotoxic agent therapy, and topical therapy (transarterial chemoembolization and radionics wave ablation). Somatostatin analogs were discontinued six weeks prior to PRRT in patients who were still receiving them. This study was approved by the Ethics Committee of Yokohama City University (F210800004). Informed consent was acquired from all participants using an opt-out method.
2.2. PRRT Protocol
DOTATOC was synthesized according to a five-step procedure in accordance with good laboratory practices at the University of Basel [19]. 90Y-DOTATOC and 177Lu-DOTATOC were alternately used until December 2017, and 177Lu -DOTATOC single-agent treatment was used thereafter. Radiolabeling was performed using 3.7 GBq/m2 body surface of 90Y, a beta emitter, for therapeutic purposes, and 0.111 GBq of 111Indium, a gamma emitter, for internal imaging for therapeutic purposes. To inhibit tubular reabsorption of radiopeptides, an intravenous infusion of 1000 mL of saline containing 20.7 mg/mL of arginine and 20.0 mg/mL of lysine was started 30 min before the 90Y-DOTATOC injection and continued until 3 h later. The patients were hospitalized for 3 d per cycle in accordance with the Swiss requirements for legal radiation protection. Considering the patient burden associated with travel from Japan to Basel, three cycles of PRRT were performed in one treatment.
2.3. Somatostatin Receptor Imaging
The distribution of radiopeptides during the treatment was acquired using a dual-head hybrid single-photon emission computed tomography (SPECT)/computed tomography (CT) gamma camera combined with a SPECT unit and 16-slice CT unit, the Symbia T16 (Siemens Healthcare, Hoffman Estates, IL, USA) system. Planar images of the whole body were acquired 4 and 24 h after intravenous injection of 148–222 MBq of 111In-pentetreotide. Images were visually analyzed and abnormal SRS findings were defined as increased non-physiological uptake. The maximum grade was scored according to the Krenning scale on a five-point scale from 0 to 4 [20].
2.4. Image Analysis Method
All CT scans were performed using a multidetector helical CT system (Aquilion; Toshiba Medical Systems, Tokyo, Japan) with 16 or 64 detector rows, a spin time of 0.5 s, a radiation exposure dose coefficient of 120 kV, and 250–300 mAs. For post-treatment lesion evaluation, CT was performed approximately 10–12 weeks after completion of the three PRRT cycles. Lesions were selected from those with a score of 2 or higher on SRS, and 1–5 lesions were evaluated per patient. The longest diameter of the target lesion was measured by CT and compared before and after PRRT treatment to calculate the shrinkage rate. The growth rate was measured from the tumor size before and after PRRT, and the tumor doubling time was measured from the two-point CT imaging before PRRT.
2.5. Outcome Measurements
The primary outcome of this study was the median best shrinkage rate per lesion (L-SR) after PRRT. The secondary outcomes were the results obtained by analysis of factors associated with the best shrinkage rate. The primary lesion site; Krenning scale; Ki-67 labeling index (LI); previous treatment (cytotoxic anticancer agents, molecular targeted agents, somatostatin analogs); doubling time; and tumor size were evaluated as relevant factors. Tumor diameters were measured using up to five lesions (up to two lesions in each organ) as target lesions, based on RECIST, and the rate of change in the sum of tumor diameters before and after PRRT was determined as the shrinkage rate per patient (P-SR). The factors associated with P-SR were analyzed in the same manner for each lesion. In addition, the long-term course of P-SR, including the appearance of new lesions, was evaluated using RECIST.
2.6. Statistical Analysis
Continuous variables were compared using the Mann–Whitney U test or t-test, while categorical variables were compared using the chi-square test or Fisher’s exact test. A p value < 0.05 was considered significant. A logistic regression model was used for the multivariate analysis. Variables with p < 0.05 in the univariate analysis were entered into the multivariate analysis. Statistical analyses were performed using the JMP15.0 statistical software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient Characteristics
Twenty patients with NET (seven men; thirteen women) underwent SRS followed by PRRT, after excluding those patients who did not undergo SRS and those who could not undergo three sessions of PRRT. The median age at the time of the first PRRT session was 59 years (range: 39–68 years). The primary tumor sites were the pancreas (10 patients), rectum (5 patients), stomach (1 patient), small intestine (1 patient), thymus (1 patient), bile duct (1 patient), or unknown (1 patient). Grade 1 and 2 tumors according to WHO classification 2019 were reported in 3 and 17 patients, respectively; no grade 3 tumors were identified. The maximum accumulation score of SRS per case based on the Krenning scale was 0, 1 in no patients, 2 in 1 patient, 3 in 1 patient, and 4 in 18 patients. In the lesion-based analysis, the Krenning scale was 0, 1 for no lesions, 2 for 3 lesions, 3 for 10 lesions, and 4 for 62 lesions. Previous treatment comprised surgical resection in 16 patients, targeted molecular therapies in 11 patients, cytotoxic agent therapy in 5 patients, and somatostatin analog treatment in 16 patients (Table 1).
Table 1.
Patient characteristics.
| n | |
|---|---|
| Patients | 20 |
| Sex | |
| Male | 7 |
| Female | 13 |
| Age, median, (range) | 59 (39–68) |
| Primary site | |
| Pancreas | 10 |
| Rectum | 5 |
| Stomach | 1 |
| Small intestine | 1 |
| Thymus | 1 |
| Biliary tract | 1 |
| Unknown | 1 |
| WHO classification | |
| G1 | 3 |
| G2 | 17 |
| G3 | 0 |
| SRS score | |
| Score 0, 1 | 0 |
| Score 2 | 1 |
| Score 3 | 1 |
| Score 4 | 18 |
| Previous treatment | |
| Surgical operation | 16 |
| Cytotoxic agents | 5 |
| Targeted molecular therapies | 11 |
| Somatostatin analog | 16 |
| PS | |
| 0–1 | 20 |
| 2–4 | 0 |
| Period from diagnosis to PRRT, month, (range) | 61.5 (5–186) |
| Hereditary status | |
| Non-hereditary | 19 |
| MEN type 1 | 1 |
WHO, world health organization; SRS, somatostatin receptor scintigraphy; PS, performance status; PRRT, peptide receptor radionuclide therapy; MEN, multiple endocrine neoplasia.
3.2. Factors Associated with Best tumor Shrinkage Rate in Lesion-Based Analysis
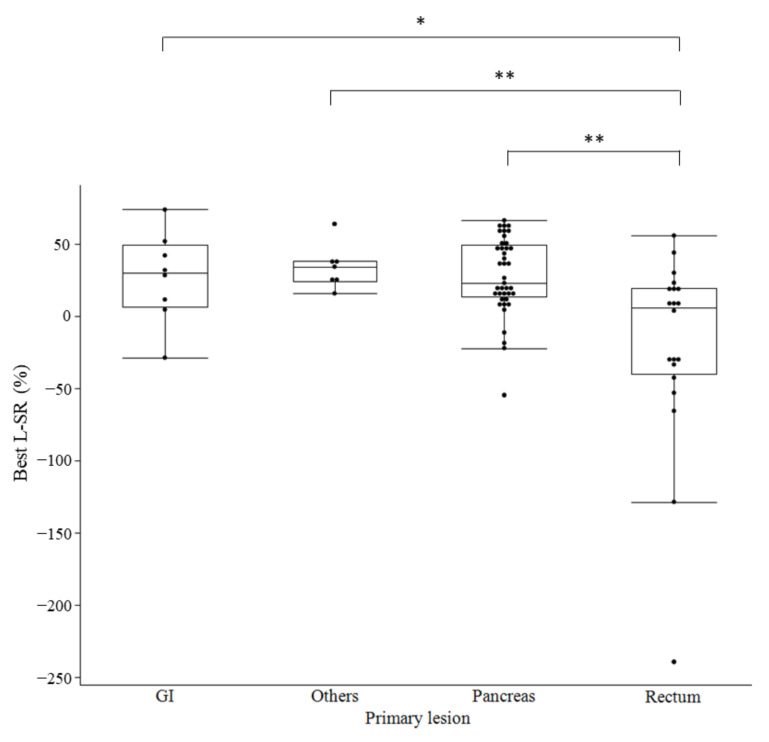
In total, 75 lesions with SRS scores of 2 or higher in 20 patients were evaluated for shrinkage and the median best L-SR was 20% (95% CI: 4.8–26.1%). The median time of best L-SR was 37.5 weeks (range: 19–231 weeks) after PRRT. The number of lesions and best L-SR are shown in Table 2, and no significant difference was noted in relation to the Krenning scale (score 2: 15.8%, 95% CI (−77.4% to 88.5%); score 3: 3.8% (−35.4% to 43.2%); score 4: 17.8% (6.3–29.4%)). In comparisons based on the primary lesion site, the best L-SR was significantly higher for pancreatic lesions (27.8% (19.2–36.3%) p = 0.0295) and significantly lower for rectal lesions (−20.5% (−51.9% to 11.0%) p = 0.0002). The box-and-whisker plot of L-SR by organ showed that PRRT was less effective in the rectum than in the pancreas and that there was greater variability among lesions (Figure 1). When the Ki-67 LI was compared with 10% as the cutoff, no significant difference was observed in the L-SR (Ki-67 LI < 10%: 22.9% (14.8–31.0%); Ki-67 LI > 10%: −2.48% (−33.5% to 29.5%); p = 0.1984). When doubling time was compared with 100 days as the cutoff time, no significant difference was observed in the L-SR (<100 days: 6.1% (−11.5% to 23.7%); >100 days: 28.1% (21.1–35.0%); p = 0.1141).
Table 2.
Factors associated with tumor shrinkage in NET in lesion-based analysis.
| Variable | Number of Lesions, n (%) | Shrinkage Rate, % (Median [95%CI]) | p Value | |
|---|---|---|---|---|
| All lesion | 75 (100) | 20 [4.8~26.1] | ||
| SRS score | score 2 | 3 (4) | 15.8 [−77.4~88.5] | 0.3944 |
| score 3 | 10 (13.3) | 3.8 [−35.4~43.2] | 0.4782 | |
| score 4 | 62 (82.7) | 17.8 [6.3~29.4] | 0.2749 | |
| Primary lesion | Pancreas | 40 (53.3) | 27.8 [19.2~36.3] | 0.0295 |
| Rectum | 20 (26.7) | −20.5 [−51.9~11.0] | 0.0002 | |
| GI | 8 (10.7) | 27.1 [0.77~53.3] | 0.5480 | |
| Others | 7 (9.3) | 34.4 [20.3~48.6] | 0.1749 | |
| Ki67 labeling index | <10% | 53 (70.7) | 22.9 [14.8~31.0] | 0.1984 |
| >10% | 22 (29.3) | −2.48 [−33.5~29.5] | ||
| Doubling time | <100 days | 43 (57.3) | 6.1 [−11.5~23.7] | 0.1141 |
| >100 days | 32 (42.7) | 28.1 [21.1~35.0] | ||
| Therapeutic drug | 90Y and 177Lu-DOTATOC | 34 (45.3) | 10.6 [−9.5~30.6] | 0.7095 |
| 177Lu-DOTATOC | 41 (54.7) | 19.5 [8.7~30.3] | ||
| Previous treatment | ||||
| Cytotoxic agent | yes | 18 (24) | 34.4 [−23.6~45.2] | 0.0394 |
| no | 57 (76) | 9.49 [−3.8~22.8] | ||
| Target molecular therapy | yes | 48 (64) | 22.6 [14.3~31.1] | 0.2943 |
| no | 27 (36) | 2.66 [−23.2~28.6] | ||
| Somatostatin analog | yes | 60 (80) | 11.4 [−1.1~23.9] | 0.0523 |
| no | 15 (20) | 31.7 [13.3~50.2] | ||
| SSA maintenance treatment | yes | 22 (29.3) | 2.1 [−28.3~32.4] | 0.4887 |
| no | 53 (70.7) | 21.0 [12.2~29.9] | ||
| Tumor size before PRRT | <3.0 cm | 57 (76) | 13.8 [0.29~27.3] | 0.9062 |
| >3.0 cm | 18 (24) | 20.7 [7.57~33.9] |
NET, neuroendocrine tumor; SRS, somatostatin receptor scintigraphy; CI, confidence interval; GI, gastrointestinal tract; SSA, somatostatin analog; PRRT, peptide receptor radionuclide therapy.
Figure 1.
Box-and-whisker plot of lesion-based shrinkage rate for each organ. L-SR, Lesion-based shrinkage rate; GI, gastrointestinal tract; * p < 0.05, ** p < 0.01.
In comparisons based on the radionuclides used in the treatment, no significant difference was observed in the L-SR (90Y and 177Lu combination: 10.6% (−9.5% to 30.6%); 177Lu monotherapy: 19.5% (8.7–30.3%); p = 0.7095). No significant difference was observed in the L-SR between patients with and without a history of use of targeted molecular therapy (with use: 22.6% (14.3–31.1%); without use: 2.66% (−23.2% to 28.6%); p = 0.2943) or somatostatin analog therapy (with use: 11.4% (−1.1% to 23.9%); without use: 31.7% (13.3–50.2%); p = 0.0523), but the L-SR was significantly higher in patients with a history of cytotoxic agent use (with use: 25.2%; without use: 19.5%; p = 0.0394).
The L-SR showed no difference comparing patients with and without somatostatin analog (SSA) maintenance treatment after PRRT (with SSA: 2.1% (−28.3% to 32.4%); without SSA: 21.0% (12.2–29.9%); p = 0.4887). There was no difference in the L-SR on comparing pretreatment tumor diameters with a cutoff of 3 cm (13.8% (0.29–27.3%), >3 cm: 20.7% (7.57–33.9%), p = 0.9062).
Seventy-five tumor lesions were classified into two groups by the median value for the L-SR (20%), and clinicopathological factors associated with L-SR were analyzed using univariate and multivariate analyses. (Table 3). In univariate analysis, the factor with a significantly negative impact on tumor L-SR was rectal primary tumor (p = 0.0041). In multivariate analysis with rectal primary tumor, other primary tumor, history of somatostatin analog use, and tumor size, only rectal primary tumor had a significantly negative impact on the L-SR of PRRT (odds ratio, 0.21; 95% CI (0.05–0.76); p = 0.0184).
Table 3.
Univariate and multivariate analysis of factors affecting tumor shrinking effect of PRRT in lesion-based analysis.
| Factor | n | Effectiveness (%) | Univariate p | Multivariate p | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Primary | Pancreas | 40 | 52.5 | 0.4893 | |||
| Rectum | 20 | 20 | 0.0041 | 0.0184 | 0.21 | 0.05~0.76 | |
| GI | 8 | 62.5 | 0.4692 | ||||
| Others | 7 | 85.7 | 0.0502 | 0.1466 | 0.19 | 0.02~1.78 | |
| SRS score | Score 2 | 3 | 33.3 | 1.000 | |||
| Score 3 | 10 | 50 | 1.000 | ||||
| Score 4 | 62 | 48.4 | 1.000 | ||||
| Ki67 LI | <10% | 53 | 50.9 | 0.4583 | |||
| >10% | 22 | 40.9 | |||||
| Doubling time | >100 days | 32 | 44.2 | 0.4897 | |||
| <100 days | 43 | 53.1 | |||||
| Therapeutic drug | 90Y and 177Lu DOTATOC | 34 | 44.1 | 0.6440 | |||
| 177Lu DOTATOC | 41 | 51.2 | |||||
| Previous treatment | |||||||
| Cytotoxic agent | Yes | 57 | 45.6 | 0.5901 | |||
| No | 18 | 55.7 | |||||
| Targeted molecular agent | Yes | 48 | 50.0 | 0.8101 | |||
| No | 27 | 44.4 | |||||
| Somatostatin analog | Yes | 60 | 43.3 | 0.1497 | 0.0736 | 0.30 | 0.89~1.12 |
| No | 15 | 66.7 | |||||
| SSA maintenance treatment | Yes | 22 | 50 | 1.000 | |||
| No | 53 | 47.1 | |||||
| Tumor size before PRRT | <3.0 cm | 57 | 52.6 | 0.1836 | 0.5044 | 0.65 | 0.19~2.24 |
| >3.0 cm | 18 | 33.3 |
SRS, somatostatin receptor scintigraphy; CI, confidence interval; GI, gastrointestinal tract; SSA, somatostatin analog; PRRT, peptide receptor radionuclide therapy.
3.3. Per-Patient Analysis of the Change in Tumor Size over Time Based on RECIST
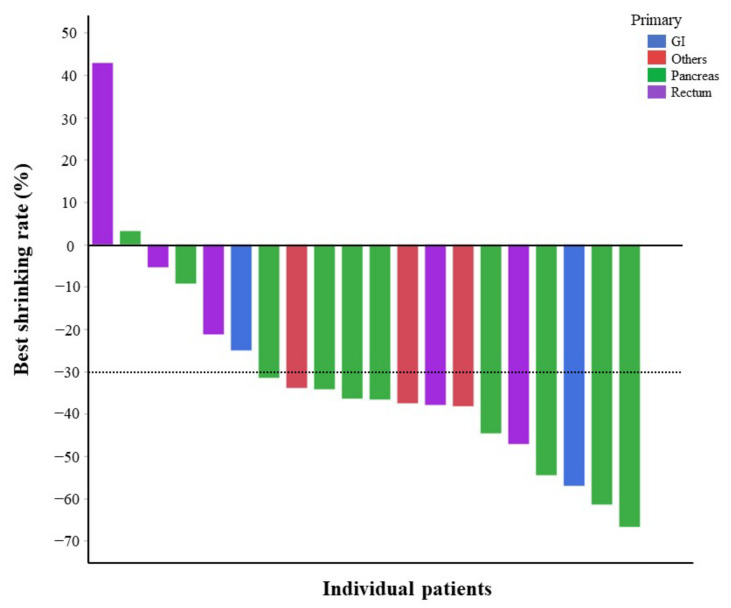
Based on RECIST, one to five lesions (up to two lesions in each organ) were selected as target lesions in each patient in the order of tumor size, and the rate of change in the sum of tumor diameters was defined as the P-SR for each patient. The best PRRT P-SR results are presented in Table S1. None of the factors were significantly related to the best P-SR in analyses with the Krenning scale, primary tumor site, Ki-67 LI, radiotherapeutic agents, prior therapy, SSA maintenance therapy, and tumor diameter. When the best P-SR was presented in a waterfall plot by primary site (Figure 2), primary pancreatic lesions tended to show more shrinkage than other primary lesions.
Figure 2.
Waterfall plot showing the best shrinkage rate by PRRT in patient-based analysis. Green line, primary site of pancreas; blue line, primary site of gastrointestinal tract; purple line, primary site of rectum; red line, primary site of others. PRRT, peptide receptor activation therapy.
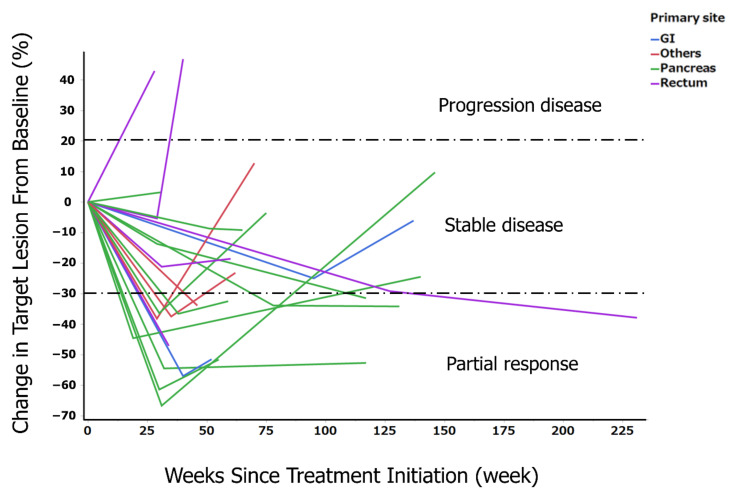
The long-term course of P-SR was examined in each patient. The rate of change from the baseline was clearly more sustained in the patients with primary pancreatic tumors than in those with a primary rectal tumor (Figure 3). The long-term response rate of PRRT was 40% (patients with CR0, PR8, SD11, PD1) at 25 weeks after PRRT; 35% (patients with CR0, PR7, SD7, PD4, two unevaluable patients) at 50 weeks after PRRT; and 20% (patients with CR0, PR4, SD3, PD9, four unevaluable patients) at 100 weeks after PRRT (Table 4).
Figure 3.
Spider plot showing the change in sum of tumor diameters based on RECIST in the 20 patients. RECIST, Response Evaluation Criteria in Solid Tumors guideline version 1.1; green line, primary site of pancreas; blue line, primary site of gastrointestinal tract; purple line, primary site of rectum; red line, primary site of others.
Table 4.
Long-term outcomes after PRRT based on RECIST in the 20 patients.
| Response Criteria | 25 Weeks | 50 Weeks | 100 Weeks |
|---|---|---|---|
| Response, n (%) | 8 (40) | 7 (35) | 4 (20) |
| CR, n (%) | 0 (0) | 0 (0) | 0 (0) |
| PR, n (%) | 8 (40) | 7 (35) | 4 (20) |
| SD, n (%) | 11 (55) | 7 (35) | 3 (15) |
| PD, n (%) | 1 (5) | 4 (20) | 9 (45) |
| Not evaluated, n (%) | 0 (0) | 2 (10) | 4 (20) |
PRRT, peptide receptor radionuclide therapy; RECIST, Response Evaluation Criteria in Solid Tumors guideline version 1.1; CR, complete response; PR, partial response; SD, stable disease; PD, progression disease.
4. Discussion
In this lesion-based analysis of the efficacy of PRRT, the median rate of maximum L-SR was 20% and the time of maximum shrinkage was 37.5 weeks (range: 19–231 weeks) after PRRT. Multivariate analysis showed that the location of the primary tumor in the rectum was a poor response factor. In the patient-based analysis, no factors were significantly associated with P-SR. The novelty of this study is that the shrinkage rate of PRRT differs for each lesion. In addition, the shrinkage rate may differ depending on the primary site. It may be possible to predict treatment efficacy, including the primary site and the time of maximum shrinkage, when conversion surgery using PRRT is considered.
The RR to PRRT was relatively high (26–56%) in previous reports [21,22,23,24,25]. In the present study, the RR per patient was 40% (at 25 weeks), which is similar to findings of previous reports on PRRT. Previous research has also examined RR per primary organ, with Brabander et al. describing an RR of 55% for pan NET, 31% for midgut NET, and 33% for hindgut NET [25].
In the present study, when the PRRT reduction rate was evaluated according to the primary organ, a favorable trend in pan NET was observed, while rectal NET was found to be a significantly poor factor. One of the reasons for this may be that the L-SR of the patients with primary rectal tumors in this study varied greatly across lesions and was more heterogeneous than in other organs according to the box-and-whisker plot. In addition, the pancreas originates from the foregut and the rectum from the hindgut, and the different origins of the organs may have influenced the results of different tumor shrinkage. In fact, the response rate of PRRT for midgut NET (18%) is lower than that of other primary lesions (26–56%) [11,21,22,23,24,25]. Primary lesions may be one of the most reliable predictive factors of tumor shrinkage; however, there may be other factors related to the response of PRRT that have not yet been identified. There was no significant difference in the duration of disease among organs (detailed data not shown) in this study, although the overall duration of disease before PRRT was long and may have been greatly influenced by previous treatment.
The efficacy of PRRT was retrospectively analyzed in 27 patients with rectal NET in a previously reported series [26]. Partial response was observed in 70% of the patients; the median PFS was 29 months; and the median overall survival (OS) was 81 months. In this study, a high morphological response and long PFS and OS were achieved in patients with metastatic rectal NETs. However, 15 patients (51.9%) received PRRT with radio sensitizer (5-FU or capecitabine), and one sigmoid NET was included in this study. According to another study on the efficacy of PRRT in 12 rectal NETs, the partial RR was 33%, with a median PFS of 29 months [25]. Another study reported that the overall RR was only 15.7% in hindgut NETs, including rectal NETs [27]. In general, rectal NETs with distant metastasis have a poor prognosis with a median OS of 4–11 months [2,28]. The efficacy of PRRT for rectal NETs remains controversial because the number of reported cases is very small, including our data. Therefore, we believe that the accumulation of more cases of rectal NETs is necessary for future research.
Uptake of SRS before PRRT is one of the most important significant prognostic factors for predicting tumor remission [12]. However, in the present study, there was no significant correlation between SRS score and tumor shrinkage. The most important reason for this finding was the insufficient number of lesions to analyze the correlation between SRS score and tumor shrinkage. Nevertheless, the SRS score alone cannot provide adequate information for predicting treatment response. Ga-DOTATOC-PET is a more reliable imaging modality for quantity analysis and prediction of tumor response than SRS [13].
Regarding the presence or absence of large lesions (>3 cm), a significant difference in PFS was reported in the NETTER-1 study [15]. However, the present study showed no difference in tumor L-SR based on the presence or absence of large lesions (>3 cm). In this study, half of all patients received 90Y and 177Lu combination PRRT; therefore, 90Y may be more effective for large lesions. The combination of 90Y and 177Lu has been reported as being more effective than 177Lu monotherapy [15].
In the lesion-based analysis, previous treatment with cytotoxic agents was favorable for the therapeutic effect of PRRT. No difference in PRRT efficacy related to prior treatment has been reported in previous studies. The reason for this discrepancy was that the median time from diagnosis to PRRT in this study was 61.5 months, which was much longer than that of previously reported studies. It is possible that cytotoxic agent usage was extracted as a significant factor as a result of the implementation of various multidisciplinary therapies for NETs in comparison with other reports.
In recent years, there have been scattered reports of the usefulness of PRRT as neoadjuvant therapy for locally advanced unresectable NETs. Parghane et al. reported that after 177Lu-DOTATATE therapy, the unresectable primary tumor became resectable in 15 of 57 (26.3%) patients [29]. PRRT may be considered not only as a palliative but also as a neoadjuvant therapy; therefore, the prediction of tumor shrinkage before PRRT is a meaningful evaluation that may lead to curative treatment. Although further accumulation of cases is essential, the primary tumor site and previous treatment history may provide some guidance in this regard.
This study had several limitations. First, this was a single-center retrospective study with a small number of patients. Second, the treatment comprised three PRRT sessions, fewer than the 4–6 sessions reported previously. Third, pathological factors were not always evaluated in the target lesions, and Ki-67 LI values included the results for some patients without liver metastases. Furthermore, the evaluation time of the images varied from case to case, making uniform evaluation difficult.
5. Conclusions
Despite the limitations, this is a valuable report evaluating the lesion-based therapeutic efficacy of PRRT in patients with NETs. In this study, pan NET and previous cytotoxic anticancer drug use were good predictors of response to PRRT, while rectal NET was a poor predictor.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14143317/s1, Table S1: Factors associated with tumor shrinkage in NET in patient-based analysis.
Author Contributions
Conceptualization: S.H., N.K., D.W., F.K., N.O., A.S., Y.K., S.T., A.N. and Y.I.; data curation: S.H. and N.K.; resources: N.K., D.W. and F.K.; formal analysis: S.H., N.K., N.O., A.S., Y.K. and S.T.; Investigation: S.H. and N.K.; supervision: A.N. and Y.I.; writing—original draft: S.H. and N.K.; writing—review and editing: D.W., F.K., N.O., A.S., Y.K., S.T., A.N. and Y.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Yokohama City University (protocol code F210800004 and date of approval was 6 September 2021).
Informed Consent Statement
Patient consent was waived due to REASON (The study was a retrospective, observational study and followed an opt-out approach, whereby information about the conduct of the study was made available to the public, and in the event of refusal, the participants were asked to provide it.).
Data Availability Statement
The data presented in this study is available in this article (and Supplementary Materials).
Conflicts of Interest
Noriroshi Kobayashi received an honorarium from PDRadiopharma Inc. (successor to the radiopharmaceutical business of Fujifilm Toyama Chemical Co., Ltd.). All other authors have no conflict of interest to declare. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ito T., Lee L., Hijioka M., Kawabe K., Kato M., Nakamura K., Ueda K., Ohtsuka T., Igarashi H. The up-to-date review of epidemiological pancreatic neuroendocrine tumors in Japan. J. Hepato-Bil. Pancreat. Sci. 2015;22:574–577. doi: 10.1002/jhbp.225. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A., Shen C., Halperin D., Zhao B., Zhou S., Xu Y., Shih T., Yao J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz D.C., Jensen R.T. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Partelli S., Bartsch D.K., Capdevila J., Chen J., Knigge U., Niederle B., Nieveen van Dijkum E.J.M., Pape U.F., Pascher A., Ramage J., et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: Surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology. 2017;105:255–265. doi: 10.1159/000464292. [DOI] [PubMed] [Google Scholar]
- 5.Raymond E., Dahan L., Raoul J.L., Bang Y.J., Borbath I., Lombard-Bohas C., Valle J., Metrakos P., Smith D., Vinik A., et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 6.Yao J.C., Shah M.H., Ito T., Bohas C.L., Wolin E.M., Van Cutsem E., Hobday T.J., Okusaka T., Capdevila J., de Vries E.G., et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinik A.I., Wolin E.M., Liyanage N., Gomez-Panzani E., Fisher G.A., ELECT Study Group Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (ELECT): A randomized, double-blind, placebo-controlled trial. Endocr. Pract. 2016;22:1068–1080. doi: 10.4158/EP151172.OR. [DOI] [PubMed] [Google Scholar]
- 8.Fjallskog M.L., Janson E.T., Falkmer U.G., Vatn M.H., Oberg K.E., Eriksson B.K. Treatment with combined streptozotocin and liposomal doxorubicin in metastatic endocrine pancreatic tumors. Neuroendocrinology. 2008;88:53–58. doi: 10.1159/000117575. [DOI] [PubMed] [Google Scholar]
- 9.Dahan L., Bonnetain F., Rougier P., Raoul J.L., Gamelin E., Etienne P.L., Cadiot G., Mitry E., Smith D., Cvitkovic F., et al. Phase III trial of chemotherapy using 5-fluorouracil and streptozotocin compared with interferon alpha for advanced carcinoid tumors: FNCLCC-FFCD 9710. Endocr. Relat. Cancer. 2009;16:1351–1361. doi: 10.1677/ERC-09-0104. [DOI] [PubMed] [Google Scholar]
- 10.Turner N.C., Strauss S.J., Sarker D., Gillmore R., Kirkwood A., Hackshaw A., Papadopoulou A., Bell J., Kayani I., Toumpanakis C., et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br. J. Cancer. 2010;102:1106–1112. doi: 10.1038/sj.bjc.6605618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strosberg J., El-Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P.L., Kulke M.H., Jacene H., et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwekkeboom D.J., de Herder W.W., Kam B.L., van Eijck C.H., van Essen M., Kooij P.P., Feelders R.A., van Aken M.O., Krenning E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 13.Kratochwill C., Stefanova M., Mavriopulou E., Holland-Letz T., Dimitrakopoulou-Strauss A., Afshar-Oromieh A., Mier W., Haberkorn U., Giesel F.L. SUV of [68Ga] DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol. Imaging Biol. 2015;17:313–318. doi: 10.1007/s11307-014-0795-3. [DOI] [PubMed] [Google Scholar]
- 14.Strosberg J., Kunz P.L., Hendifar A., Yao J., Bushnell D., Kulke M.H., Baum R.P., Caplin M., Ruszniewski P., Delpassand E., et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-Dotatate: An analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:2372–2382. doi: 10.1007/s00259-020-04709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum R.P., Kulkarni H.R., Singh A., Kaemmerer D., Mueller D., Prasad V., Hommann M., Robiller F.C., Niepsch K., Franz H., et al. Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9:16932–16950. doi: 10.18632/oncotarget.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorbye H., Kong G., Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3) Endocr. Relat. Cancer. 2020;27:R67–R77. doi: 10.1530/ERC-19-0400. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N., Wild D., Kaul F., Shimamura T., Takano S., Takeda Y., Okubo N., Suzuki A., Tokuhisa M., Ichikawa Y. Retrospective study of peptide receptor radionuclide therapy for Japanese patients with advanced neuroendocrine tumors. J. Hepato-Bil. Pancreat. Sci. 2021;28:727–739. doi: 10.1002/jhbp.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., Washington K.M., Carneiro F., Cree I.A., The WHO Classification of Tumours Editorial Board The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imhof A., Brunner P., Marincek N., Briel M., Schindler C., Rasch H., Mäcke H.R., Rochlitz C., Müller-Brand J., Walter M.A. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 20.Krenning E.P., Bakker W.H., Kooij P.P., Breeman W.A., Oei H.Y., de Jong M., Reubi J.C., Visser T.J., Bruns C., Kwekkeboom D.J., et al. Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: Metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J. Nucl. Med. 1992;33:652–658. [PubMed] [Google Scholar]
- 21.Waldherr C., Pless M., Maecke H.R., Schumacher T., Crazzolara A., Nitzsche E.U., Haldemann A., Mueller-Brand J. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90) Y-DOTATOC. J. Nucl. Med. 2002;43:610–616. [PubMed] [Google Scholar]
- 22.Pfeifer A.K., Gregersen T., Grønbæk H., Hansen C.P., Müller-Brand J., Herskind Bruun K., Krogh K., Kjær A., Knigge U. Peptide receptor radionuclide therapy with Y-DOTATOC and (177)Lu-DOTATOC in advanced neuroendocrine tumors: Results from a Danish cohort treated in Switzerland. Neuroendocrinology. 2011;93:189–196. doi: 10.1159/000324096. [DOI] [PubMed] [Google Scholar]
- 23.Campana D., Capurso G., Partelli S., Nori F., Panzuto F., Tamburrino D., Cacciari G., Delle Fave G., Falconi M., Tomassetti P. Radiolabelled somatostatin analogue treatment in gastroenteropancreatic neuroendocrine tumours: Factors associated with response and suggestions for therapeutic sequence. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:1197–1205. doi: 10.1007/s00259-013-2402-2. [DOI] [PubMed] [Google Scholar]
- 24.Ezziddin S., Khalaf F., Vanezi M., Haslerud T., Mayer K., Al Zreiqat A., Willinek W., Biersack H.J., Sabet A. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:925–933. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]
- 25.Brabander T., van der Zwan W.A., Teunissen J.J.M., Kam B.L.R., Feelders R.A., de Herder W.W., van Eijck C.H.J., Franssen G.J.H., Krenning E.P., Kwekkeboom D.J. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin. Cancer Res. 2017;23:4617–4624. doi: 10.1158/1078-0432.CCR-16-2743. [DOI] [PubMed] [Google Scholar]
- 26.Kong G., Grozinsky-Glasberg S., Hofman M.S., Akhurst T., Meirovitz A., Maimon O., Krausz Y., Godefroy J., Michael M., Gross D.J., et al. Highly favourable outcomes with peptide receptor radionuclide therapy (PRRT) for metastatic rectal neuroendocrine neoplasia (NEN) Eur. J. Nucl. Med. Mol. Imaging. 2019;46:718–727. doi: 10.1007/s00259-018-4196-8. [DOI] [PubMed] [Google Scholar]
- 27.Wetz C., Genseke P., Apostolova I., Furth C., Ghazzawi S., Rogasch J.M.M., Schatka I., Kreissl M.C., Hofheinz F., Grosser O.S., et al. The association of intra-therapeutic heterogeneity of somatostatin receptor expression with morphological treatment response in patients undergoing PRRT with [177Lu]-DOTATATE. PLoS ONE. 2019;14:e0216781. doi: 10.1371/journal.pone.0216781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z., Wang L., Dai S., Chen M., Li F., Sun J., Luo F. Epidemiologic trends of and factors associated with overall survival for patients with gastroenteropancreatic neuroendocrine tumors in the United States. JAMA Netw. Open. 2021;4:e2124750. doi: 10.1001/jamanetworkopen.2021.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parghane R.V., Bhandare M., Chaudhari V., Ostwal V., Ramaswamy A., Talole S., Shrikhande S.V., Basu S. Surgical feasibility, determinants, and overall efficacy of neoadjuvant 177Lu-DOTATATE PRRT for locally advanced unresectable gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2021;62:1558–1563. doi: 10.2967/jnumed.120.258772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study is available in this article (and Supplementary Materials).