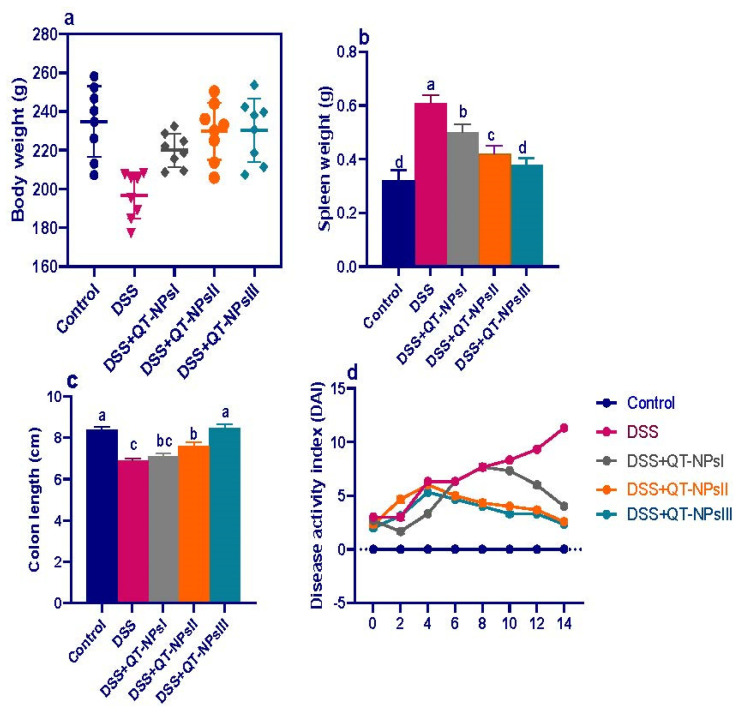
Figure 2.
Impacts of quercetin-loaded nanoparticles (QT-NPs) therapy on colitic signs. (a) Body weight gain. (b) Spleen weight, (c) colon length, (d) disease activity index score. Control: healthy non-colitic group (rats orally gavaged with PBS); colitic groups: DSS (rats orally gavaged with dextran sodium sulphate), DSS + QT-NPsI (rats orally gavaged with DSS and quercetin-loaded nanoparticles (QT-NPs at the level 10 mg/kg body weight for 14 days)), DSS + QT-NPsII (rats orally gavaged with DSS and quercetin-loaded nanoparticles (QT-NPs at the level 15 mg/kg body weight for 14 days)), DSS + QT-NPsIII (rats orally gavaged with DSS and quercetin-loaded nanoparticles (QT-NPs at the level 20 mg/kg body weight for 14 days)). All groups were orally gavaged by 3% DSS. a–d Means of the rows with different letters were significantly different among groups (p < 0.05). Control: healthy non-colitic group (rats orally gavaged with PBS); colitic groups: DSS (rats orally gavaged with dextran sodium sulphate), DSS + QT-NPsI (rats orally gavaged with DSS and quercetin-loaded nanoparticles (QT-NPs at the level 10 mg/kg body weight for 14 days)), DSS + QT-NPsII (rats orally gavaged with DSS and quercetin-loaded nanoparticles (QT-NPs at the level 15 mg/kg body weight for 14 days)), and DSS + QT-NPsIII (rats orally gavaged with DSS and quercetin-loaded nanoparticles (QT-NPs at the level 20 mg/kg body weight for 14 days)). All groups orally gavaged by 3% DSS.