Abstract
Lactobacillus-deficient cervicovaginal microbiota, including Gardnerella vaginalis, are implicated in cervical remodeling and preterm birth. Mechanisms by which microbes drives outcomes are not fully elucidated. We hypothesize that Gardnerella vaginalis induces matrix metalloproteinases through TLR-2, leading to epithelial barrier dysfunction and premature cervical remodeling. Cervicovaginal cells were treated with live Gardnerella vaginalis or Lactobacillus crispatus or their bacteria-free supernatants for 24 hours. For TLR-2 experiments, cells were pretreated with TLR-2 blocking antibody. A Luminex panel was run on cell media. For human data, we conducted a case-control study from a prospective pregnancy cohort of Black individuals with spontaneous preterm (sPTB) (n=40) or term (n=40) births whose vaginal microbiota had already been characterized. Cervicovaginal fluid was obtained between 20-24 weeks’ gestation. Short cervix was defined as <25mm by second trimester transvaginal ultrasound. MMP-9 was quantified by ELISA. Standard analytical approaches were used to determine differences across in vitro conditions, as well as MMP-9 and associations with clinical outcomes. Gardnerella vaginalis induced MMP-1 in cervical cells (p=0.01) and MMP-9 in cervical and vaginal (VK2) cells (p≤0.001 for all). TLR-2 blockade mitigated MMP-9 induction by Gardnerella vaginalis. MMP-9 in cervicovaginal fluid is higher among pregnant individuals with preterm birth, short cervix, and Lactobacillus-deficient microbiota (p<0.05 for all). MMP-9 is increased in the cervicovaginal fluid of pregnant individuals with subsequent sPTB. Our in vitro work ascribes a potential mechanism by which a cervicovaginal microbe, commonly associated with adverse pregnancy outcomes, may disrupt the cervicovaginal epithelial barrier and promote premature cervical remodeling in spontaneous preterm birth.
Keywords: Lactobacillus-deficient microbiota, matrix metalloproteinase, MMP-9, TLR-2, short cervix, spontaneous preterm birth
1. INTRODUCTION
Lactobacillus-deficient anaerobe-rich cervicovaginal microbial communities have been implicated in adverse reproductive outcomes, including bacterial vaginosis, acquisition of sexually transmitted infections, and spontaneous preterm birth (sPTB)(Holst et al., 1994, Ravel et al., 2011, Pflughoeft and Versalovic, 2012, DiGiulio et al., 2015, MacIntyre et al., 2015, Bretelle et al., 2015, Kindinger et al., 2016, Smith and Ravel, 2017, Stout et al., 2017, Gosmann et al., 2017, Kroon et al., 2018, Brown et al., 2018, Elovitz et al., 2019, Brown et al., 2019a, Chan et al., 2022). Gardnerella vaginalis (G. vaginalis)1 is a gram variable microbe prevalent in these communities. Mechanisms by which microbes drive outcomes are not fully elucidated, although it has been established that the presence of Lactobacillus species in the cervicovaginal microbiota confers protection in part through production of lactic acid and adherence to the cervicovaginal epithelial barrier (Kroon et al., 2018).
Existing data support the concept that G. vaginalis promotes cervical remodeling through disruption of the cervicovaginal epithelial barrier and activation of select host immune responses, and that this process lies on the pathway to sPTB (Elovitz et al., 2019, Sierra et al., 2018, Nallasamy and Mahendroo, 2017, Nold et al., 2012, Gonzalez et al., 2011, Anton et al., 2018, Anton et al., 2017). Components of G. vaginalis cell wall, including peptidoglycan, are known ligands for toll-like receptors (TLRs), which function as pathogen recognition proteins mediating epithelial innate immune responses (Benjelloun et al., 2020, Koga and Mor, 2010, Sadhu et al., 1989, Mares et al., 2008). Vaginal lavage containing G. vaginalis from individuals with bacterial vaginosis has been shown to initiate TLR-2 signal transduction, resulting in secretion of inflammatory mediators (Mares et al., 2008, Zariffard et al., 2005). In other biologic systems, TLRs induce matrix metalloproteinases (MMPs), which comprise a family of calcium-dependent zinc-containing endopeptidases involved in degradation of extracellular matrix proteins (Wong et al., 2011, Abdulkareem et al., 2018, Page-McCaw et al., 2007). In addition to proteolysis, MMPs differentially influence host immune response, and upregulation of specific MMPs occurs in response to microbial infection (Wong et al., 2011, Fingleton, 2017). Understanding this immunologic context, it is conceivable that MMPs are involved in regulation of host immune responses and cervical remodeling preceding sPTB.
We hypothesize that G. vaginalis drives cervical remodeling through TLR-2-mediated induction of MMPs. To interrogate this hypothesis and further elucidate molecular mechanisms underlying sPTB, we examined the effect of G. vaginalis on MMP protein expression in the cervicovaginal epithelium, and whether altered protein expression is mediated by TLR-2 activation. We further investigated the extent to which in vitro findings translate to human pregnancy utilizing cervicovaginal fluid specimens from a well-phenotyped prospective pregnancy cohort.
2. MATERIALS AND METHODS
2.1. Cell Culture
Ectocervical (Ect/E6E7, AATC# CRL-2614) (Ecto), endocervical (End1/E6E7, AATC# CRL-2615) (Endo) and vaginal (VK2/E6E7, ATCC# CRL-2616) (VK2) human epithelial cell lines (American Type Culture Collection, Manassas, VA) were cultured in keratinocyte serum-free media (K-SFM) supplemented with 0.1 ng/mL epidermal growth factor and 50μg/mL bovine pituitary extract (ScienCell Research Laboratories, Carlsbad, CA) in a 5% CO2 humidified incubator as previously described (Anton et al., 2018).
2.2. Microbial Treatment
Bacteria were obtained from the American Type Culture Collection, Manassas, VA: L. crispatus (ATCC 33197) and G. vaginalis (ATCC 14019). Ectocervical, endocervical, and vaginal cells were plated in K-SFM without antibiotics then treated the following day with either live L. crispatus or G. vaginalis (1x106-1x104CFU/well) or 10% (v/v) bacteria-free supernatants (1x107-1x105 CFU/ml culture density) in K-SFM for 24 hours. Bacteria-free supernatants were utilized based on prior work demonstrating that microbial output is sufficient to induce epithelial barrier dysfunction (Anton et al., 2018). For supernatant preparation, bacteria were grown in NYCIII media with 10% horse serum at 37°C in an anaerobic jar in a 5% anaerobic CO2 incubator for 7 days. Cultures were centrifuged two times for 10 min each at 2,500 rpm at 4°C to remove bacteria. The resulting supernatants were sterile-filtered through a 0.22 μM membrane filter (EMD Millipore, Darmstadt, Germany) to remove any remaining bacterial components or debris. The bacteria-free supernatants and NYCIII media (negative control to determine the baseline measurements of background growth media) were then used for in vitro cell culture experiments. For experiments using L. crispatus supernatant, K-SFM was buffered as previously described (Anton et al., 2018). Briefly, K-SFM medium was supplemented with 50 mM HEPES and sodium bicarbonate (3000 mg/L) to produce a physiologic pH of 7.2 to permit cell survival, as these epithelial cells do not survive in acidic conditions. An anti-TLR-2 antibody (anti-hTLR-2-IgA, InvivoGen, San Diego, CA) was reconstituted in 100μL of sterile water to make a 100μg/mL solution. For TLR-2 experiments, cells were pre-treated with the neutralizing IgA monoclonal antibody to human TLR-2 (10μg/ml) for one hour prior to bacterial exposure. TLR-2 agonist FSL-1 was used as a positive control, an IgA2 antibody provided by the manufacturer was used as an isotype control, and anti-TLR-2 antibody treatment concentration was determined based on IL-8 activation as reported previously (Anton et. al. 2022, under revision at Microbiome). After 24 hours, culture media was collected for Luminex. Cell viability and characteristics following experimentation have been reported previously (Anton et al. 2018; Anton et al. 2022, under revision at Microbiome).
2.3. Luminex
A 5-plex MMP human magnetic bead Luminex panel (EMD Millipore, Billerica, MA) for MMP-1, MMP-2, MMP-7, MMP-9, and MMP-10 was run on cell culture media. Three technical replicates for each condition were run in duplicate per manufacturer protocol on the FLEXMAP3D Luminex platform (Luminex, Austin, TX). Samples were run on four plates. To account for inter-plate variation, internal controls were used across plates to ensure consistency of results. Fold change was calculated between treatment groups and non-treated control or NYC media control. Heatmaps were created using R version 4.1.10 (2021-08-10) (Team, 2021).
2.4. Human Study Setting
We performed a nested case-control study of 80 Black participants (n=40 sPTB cases and n=40 term controls) of the completed Motherhood and Microbiome (M&M) prospective cohort study in which 2,000 pregnant individuals enrolled from December 2013 through February 2017. Cases and controls were frequency matched 1:1 by Lactobacillus-deficient and Lactobacillus-dominant cervicovaginal microbiota, such that half of all sPTB cases had a Lactobacillus-deficient microbiota and half of all term birth controls had a Lactobacillus-deficient microbiota. Details of M&M have been published (Elovitz et al., 2019) and the study was approved by the Institutional Review Board at the University of Pennsylvania (IRB #818914) on October 23, 2013. Briefly, microbiota were analyzed by 16S rRNA gene sequencing via amplification of the V3-V4 regions of the 16S rRNA gene. Microbial communities were classified into community state types (CSTs) using hierarchical clustering with Jensen-Shannon divergence and Ward linkage. Lactobacillus-dominant microbiota included communities that were dominated by L. crispatus (CST I), L. gasseri (CST II), L. iners (CST III), or L. jensenii (CST V), while Lactobacillus-deficient communities included those with a paucity of Lactobacillus species and a diverse set of strict and facultative anaerobes (CST IV) (Elovitz et al., 2019). We focused on Black participants because of the disproportionately high rate of PTB and prevalence Lactobacillus-deficient cervicovaginal microbiota in this subset (Elovitz et al., 2019).
2.5. Human Biospecimen Collection and MMP-9 Quantification
This analysis included samples collected at 20-24 weeks’ gestation and processed as previously described (Elovitz et al., 2019). Briefly, cervicovaginal samples were self-collected or collected by a research coordinator if a clinical exam was indicated using Dacron swabs (Starplex, Thermofisher). Swabs were stored without buffer and were immediately frozen at −80°C until processing. Material on the swabs was subsequently eluted in sterile PBS with a protease inhibitor cocktail (Complete Mini) to release soluble proteins. MMP-9 was measured in eluted samples by ligand-specific commercially available ELISA kit that uses a quantitative sandwich enzyme immunoassay technique targeting 92 kDa pro-MMP-9 and 82 kDa active MMP-9 using regents from R&D Systems (Minneapolis, MN, United States). Absolute quantification in ng/mL was determined using a standard curve generated by a four-parameter logistic (4-PL) curve fit, with a mean MMP-9 protein concentration of 15.69 ng/mL. Total protein was quantified by BCA protein assay (Pierce, Rockford, IL) per manufacturer protocol with a mean total protein concentration of 306 μg/mL and standard error of the mean of 21 μg/mL across samples. MMP-9 concentrations were normalized to total protein content for individual samples.
2.6. Statistical Analyses
Statistical analyses were performed for all in vitro experiments with the GraphPad Prism Software (Version 9.0, San Diego, CA). Analysis of Luminex data, including TLR-2 experiments, was performed using a one-way analysis of variance (ANOVA) followed by a Dunnett’s multiple comparison test.
For human data, because of the skewed MMP-9 distribution, median (IQR) concentrations of MMP-9 by birth outcome (sPTB versus term), cervical length (short [<25 mm] versus normal), and microbiota (Lactobacillus-deficient versus Lactobacillus-dominant) were displayed, and statistical significance testing was performed on log2-transformed values using T-tests and ANOVA as appropriate. Multivariable logistic regression was used to model associations of one-fold change increase in MMP-9 with each outcome as well as a combined outcome of both short cervix and Lactobacillus-deficient vaginal microbiota. Analyses were performed using SAS 9.4, Cary, NC.
3. RESULTS
3.1. G. vaginalis induces MMP protein expression in cervicovaginal epithelial cells
To determine whether microbes common to a Lactobacillus-deficient cervicovaginal microbiota modify MMP protein expression, cervical and vaginal epithelial cell lines were treated with live G. vaginalis. L. crispatus, a commensal microbe dominant in a healthy cervicovaginal microbiota, served as a microbial control. Concentrations of MMP-1, MMP-2, MMP-7, MMP-9, and MMP-10 protein were measured in cell culture media. G. vaginalis increased MMP-1 in endocervical cells and MMP-9 in all cell lines (Fig. 1, Supplemental Table 1). Treatment of all cell lines with L. crispatus did not significantly alter protein expression of any of the MMPs.
Figure 1. Gardnerella vaginalis induces matrix metalloproteinases in cervicovaginal epithelial cell lines.
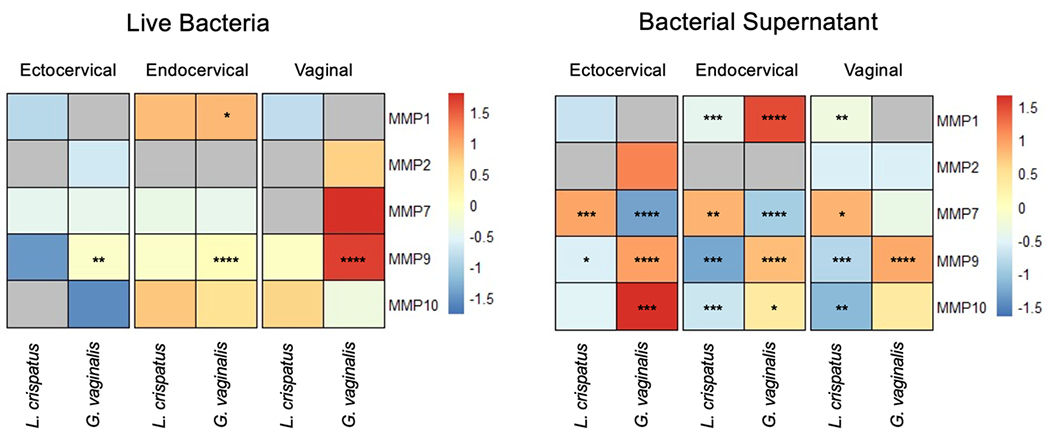
Matrix metalloproteinase (MMP) protein released from ectocervical, endocervical, and vaginal cells after exposure to live Lactobacillus crispatus (L. crispatus) and Gardnerella vaginalis (G. vaginalis) or bacteria-free supernatants. Heat map presents fold change of MMP-1, MMP-2, MMP-7, MMP-9, and MMP-10 protein in response to microbial treatment compared to non-treated control (NT) for live bacteria or NYC media control (NYC) for supernatants. Fold change for each condition is depicted relative to the mean fold change across individual rows with red denoting increase and blue denoting decrease. Grey indicates either non-treated and/or treated samples fell below the standard curve (undetectable) preventing accurate estimation of fold change. Three technical replicates for each condition were run in duplicate. A one-way ANOVA followed a Dunnett’s multiple comparison test (*p<0.05, **p<0.01, ***p<0.001, **** p<0.0001).
3.2. G. vaginalis supernatant induces MMP protein expression in cervicovaginal epithelial cells
We examined the effect of microbial supernatants on cervicovaginal epithelial cell lines to determine whether exposure to microbial output was sufficient to alter MMP protein expression. G. vaginalis supernatant induced MMP-1 in endocervical cells, MMP-9 in all cell lines, and MMP-10 in ectocervical and endocervical cells (Fig. 1, Supplemental Table 2). Although formal statistical analyses could not be performed for MMP-2 in ectocervical cells due to undetectable low levels in controls, MMP-2 was increased after G. vaginalis supernatant exposure (mean 293 pg/ml). G. vaginalis supernatant downregulated MMP-7 in ectocervical and endocervical cells. L. crispatus supernatant decreased MMP-1 in endocervical and VK2 cells, MMP-9 in all cell lines, and MMP-10 in endocervical and VK2 cells (Fig. 1, Supplemental Table 2). L. crispatus supernatant increased MMP-7 in all cell lines.
3.3. Induction of MMP-9 by G. vaginalis occurs in a TLR-2 dependent fashion
As TLR-2 is involved in pathogen recognition in the reproductive tract and can induce MMPs in other biologic settings, we sought to determine whether upregulation of MMP-9, which was observed in all cell lines in response to live G. vaginalis and bacteria-free supernatant, is dependent upon TLR-2. Treatment with the TLR-2 antibody alone did not induce cytotoxicity compared to nontreated cells (Supplemental Fig. 1). TLR-2 blockade mitigated induction of MMP-9 by G. vaginalis in all cell lines (Fig. 2). TLR-2 blockade did not alter MMP-9 protein expression after exposure to L. crispatus.
Figure 2. Blockade of TLR-2 receptor prevents induction of MMP-9 protein by Gardnerella vaginalis.
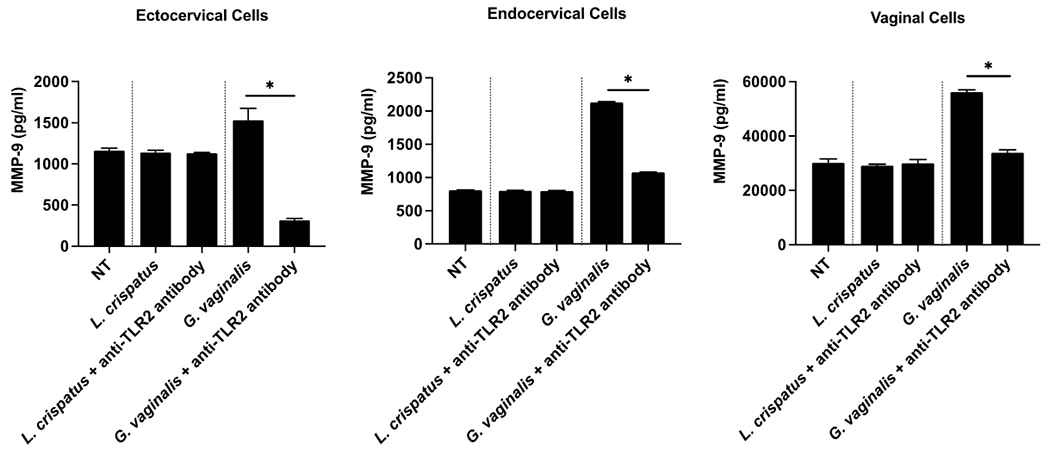
MMP-9 protein released from cervicovaginal epithelial cell lines (ectocervical, endocervical, VK2) are presented after treatment with live Lactobacillus crispatus (L. crispatus) or Gardnerella vaginalis (G. vaginalis), as well as in response to TLR-2 blockade prior to microbial treatment and a non-treated control (NT). Three technical replicates for each condition were run in duplicate. A one-way ANOVA was performed followed by a Dunnet’s test for multiple comparisons (*p<0.05).
3.4. Cervicovaginal MMP-9 is associated with a Lactobacillus-deficient microbiota, short cervix, and sPTB
To determine whether in vitro MMP-9 findings translated clinically, we examined MMP-9 in cervicovaginal fluid from a nested case-control study of Black participants (40 sPTB cases and 40 term controls) from a completed prospective pregnancy study for whom birth outcome and various clinical variables were well-phenotyped. Obstetric and clinical data are presented in Table 1. As per study design, 21 (52.5%) of individuals with sPTB and 21 (55.0%) of individuals with term births were colonized by Lactobacillus-deficient microbiota. There were 14 (36.8%) participants with short cervix among those with sPTB compared to 11 (27.5%) in the term birth group. Among the participants with sPTB, seven (17.5%) received progesterone compared to two (5%) among term births. Similarly, among those with sPTB, six (15.0%) underwent cerclage placement compared to three (7.5%) among those with term births.
Table 1.
Descriptive characteristics among n=80 Black participants of the Motherhood & Microbiome Cohort stratified by spontaneous preterm birth and term birth.
| Term birth n=40 | Spontaneous preterm birth n=40 | p-value | |
|---|---|---|---|
| mean (SD) | |||
| Maternal age in years | 27.7 (5.4) | 27.6 (6.4) | 0.96 |
| Body mass index (kg/m2) | 30.3 (7.6) | 31.0 (8.8) | 0.72 |
| Hispanic ethnicity | 0 (0) | 1 (2.5) | 0.31 |
| Gestational age at delivery in weeks | 39.0 (0.8) | 31.4 (4.8) | <0.0001 |
| Infant weight (g) | 3218 (732) | 1882 (951) | <0.0001 |
| n (column %) | |||
| Obstetrical history | 0.008 | ||
| Nulliparous | 19 (47.5) | 15 (37.5) | |
| Prior spontaneous preterm birth | 5 (12.5) | 17 (42.5) | |
| Multiparous term delivery | 16 (40.0) | 8 (20.0) | |
| Married | 7 (17.5) | 10 (25.0) | 0.41 |
| Publicly insured | 24 (60.0) | 28 (70.0) | 0.35 |
| Smoked during pregnancy | 4 (10.0) | 7 (17.5) | 0.33 |
| Short cervix (<25 mm) | 11 (27.5) | 14 (36.8) | 0.38 |
| Cerclage placement | 6 (15.0) | 3 (7.5) | 0.28 |
| Vaginal progesterone use | 2 (5.0) | 7 (17.5) | 0.08 |
| Lactobacillus-deficient vaginal microbiota* | 21 (52.5) | 21 (55.3) | 0.81 |
Continuous variables compared using two-sided t-tests; categorical variables compared using chi-square tests.
Frequency matching factor (2 participants were missing microbiota data).
MMP-9 concentrations were higher among participants with subsequent sPTB than term births (1.86-fold, p=0.0013) (Table 2). MMP-9 concentrations were also higher among participants with short cervix compared to normal cervical length (1.41-fold, p=0.029) and a Lactobacillus-deficient microbiota compared to Lactobacillus-dominant microbiota (1.87-fold, p=0.0017). Participants with both a short cervix and Lactobacillus-deficient microbiota had higher MMP-9 concentrations than other participants (3.21-fold, p=0.0018, ANOVA comparing the four combinations of cervical length and microbiota). These associations were similar in adjusted analyses (Table 3). Per fold change increase in MMP-9, participants had higher adjusted odds of sPTB (aOR 1.35, 95% CI: 1.11-1.65) and Lactobacillus-deficient microbiota (aOR 1.34, 95% CI: 1.10-1.63). MMP-9 and short cervix adjusted analyses were not statistically significant (aOR 1.27, 95% CI: 0.99-1.62). Finally, MMP-9 was associated with the combination of a short cervix and Lactobacillus-deficient microbiota (aOR 1.63, 95% CI: 1.14-2.32).
Table 2.
MMP-9 in n=80 participants of the Motherhood & Microbiome Cohort by spontaneous preterm birth, cervical length, and cervicovaginal microbiota classification.
| Participant outcome | Median (IQR) MMP-9/total protein (ng/μg) | Fold change | P value* |
|---|---|---|---|
| Birth outcome | 0.0013 | ||
| Term (n=40) | 0.022 (0.060) | reference | |
| sPTB (n=40) | 0.101 (0.188) | 1.86 | |
| Cervical length | 0.029 | ||
| Normal (n=53) | 0.029 (0.094) | reference | |
| Short <25 mm (n=25) | 0.138 (0.319) | 1.41 | |
| Vaginal microbiota | 0.0017 | ||
| Lactobacillus-dominant (n=36) | 0.017 (0.071) | reference | |
| Lactobacillus-deficient (n=42) | 0.083 (0.169) | 1.87 | |
| Microbiota and cervical length combined | 0.0018 | ||
| Lactobacillus-dominant & normal cervix (n=26) | 0.014 (0.042) | reference | |
| Lactobacillus-dominant & short cervix (n=9) | 0.021 (0.130) | 0.58 | |
| Lactobacillus-deficient & normal cervix (n=26) | 0.048 (0.093) | 0.91 | |
| Lactobacillus-deficient & short cervix (n=15) | 0.197 (0.441) | 3.21 |
MMP-9, matrix metalloproteinase-9; IQR, interquartile range; sPTB, spontaneous preterm birth.
P value from t-test of log2-transformed MMP-9 values or ANOVA as appropriate
Table 3.
Associations of one fold-change increase in vaginal fluid MMP-9 with spontaneous preterm birth (sPTB), short cervix, and Lactobacillus-deficient cervicovaginal microbiota
| Unadjusted | Adjusted* | |
|---|---|---|
| Model | OR (95% CI) | OR (95% CI) |
| sPTB (n=40) vs. term (n-40) | 1.35 (1.11-1.65) | 1.43 (1.14-1.81) |
| Short cervix (n=25) vs. normal length (n=53) | 1.24 (1.02-1.52) | 1.27 (0.99-1.62) |
| Lactobacillus-deficient (n=42) vs. Lactobacillus-dominant (n=36) | 1.34 (1.10-1.63) | 1.29 (1.03-1.61) |
| Short cervix & Lactobacillus-deficient (n=15) vs. others (n=65) | 1.57 (1.17-2.11) | 1.63 (1.14-2.32) |
Adjusted for age, nulliparity, body mass index at initial prenatal visit, public insurance, and smoking during pregnancy.
4. DISCUSSION
Our data demonstrate that G. vaginalis, which is implicated in sPTB and multiple other adverse reproductive outcomes, induces expression of MMP proteins in the cervicovaginal epithelium. Upregulation of MMP-9 specifically occurs in a TLR-2-dependent manner. MMP-9 protein concentrations in the cervicovaginal space are higher among individuals who deliver preterm and among those who have known risk factors for sPTB, including a Lactobacillus-deficient cervicovaginal microbiota and short cervix.
Our findings corroborate published literature examining MMPs in pregnancy. Elevated MMP-9 expression has been reported in both vaginal secretions and cervical mucus among individuals who have sPTB (Becher et al., 2010, Duran-Chavez et al., 2021). MMP-9 was also increased in amniotic fluid among individuals with cervical dilation, spontaneous rupture of membranes, labor, intra-amniotic infection, and sPTB (Locksmith et al., 2001, Athayde et al., 1999). Though only MMP-9 and MMP-1 were induced by G. vaginalis in our in vitro studies, elevations in other MMPs in cervicovaginal fluid have been detected in association with short cervix and labor, both term and preterm, including MMP-8 (Sisti et al., 2020). Contrary to our findings, several groups have failed to identify an association between cervicovaginal MMP-9 and short cervix or sPTB (Yoo et al., 2017, Duran-Chavez et al., 2021). These differences may be attributable to retrospective study design and clinical heterogeneity among participants with respect to cervical dilation, progesterone therapy, and cerclage placement. Racial diversity across studies is likely another significant contributing factor to discrepant findings. As we have established previously that expression of certain immune factors in the cervicovaginal space, such as beta defensin, differ between Black and non-Black individuals (Elovitz et al., 2019), it is possible that expression of MMP-9 may similarly differ by race.
In other biologic systems, MMPs have well established roles in tissue remodeling. These proteases can be characterized based on their specificity for extracellular matrix (ECM) proteins and include collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs (Cui et al., 2017). MMP-9 is a gelatinase MMP with substrates that include gelatin, collagens, fibronectin, elastin, laminin, and vitronectin (Bassiouni et al., 2021). In addition to degradation and remodeling of the ECM, this protease has been implicated in wound healing and inflammation. At other mucosal interfaces, MMP-9 increases barrier permeability through disruption of epithelial tight junctions (Al-Sadi et al., 2021). Other data suggest that MMP-9 triggers apoptosis in various cell types, and that this may contribute to leaky epithelial barriers in inflammatory bowel disease (Abdel-Hamid et al., 2017, Chen et al., 2019, Goretsky et al., 2012, Hering and Schulzke, 2009, Hu et al., 2015, Vu et al., 1998). Given its established role in other systems, it is plausible that MMP-9 may function in similar capacities in the cervicovaginal space. Further research exploring these potential mechanisms is warranted.
While live G. vaginalis upregulated MMPs in the cervicovaginal epithelium, it is notable that bacteria-free supernatants were also sufficient to induce MMP protein expression. These observations raise questions as to the mechanisms by which different microbial exposures result in common molecular outcomes. Our data support a role for TLR-2 in MMP-9 regulation by G. vaginalis, consistent with the knowledge that this pathogen recognition receptor is activated by components present in the bacterial wall of G. vaginalis (Koga and Mor, 2010, Sadhu et al., 1989). Though our findings suggest that TLR-2 blockade may be beneficial in mitigating MMP-9 induction by G. vaginalis, there is known redundancy in the innate immune system, and other factors in the cervicovaginal space are likely to play a role in modulating expression of this protease and other MMPs. It remains unclear what factors in G. vaginalis supernatant distinct from cell wall components are responsible for MMP-9 regulation. It is well established that virulence factors secreted by G. vaginalis, such as vaginolysin, contribute to damage of the cervicovaginal epithelium by producing lytic and non-lytic pores in cell membranes leading to cell lysis (Pleckaityte, 2019). It is possible that process serves as a trigger for MMP-9 induction, as synthesis of this protease by epithelial cells in other biologic settings is stimulated by cell injury and disruption of the epithelial barrier (Fini et al., 1998).
In addition to variation in MMP concentrations observed between live bacteria and bacteria-free supernatants, we also detected epithelial cell-specific effects, with ectocervical, endocervical, and vaginal epithelial cells responding uniquely to bacterial exposure. These findings are consistent with previously published work from our laboratory examining epithelial barrier integrity and cytokine expression (Anton et al., 2018). Indeed, the various types of epithelial cells lining the cervicovaginal space derive from different embryonic origins and are histologically, biochemically, and functionally unique. These differences enhance our understanding of mechanisms underlying acquisition of sexually transmitted infections, including HIV (Blaskewicz et al., 2011, Cherne et al., 2020, Murall et al., 2019). It follows that these cells may produce different responses to factors in their shared microenvironment.
Our findings underscore the need for future research defining bioactive components in the microbial output of G. vaginalis and L. crispatus, as well as other common cervicovaginal bacteria. Outside of the cervicovaginal space, characterization of the genetic and biochemical footprints of microbial ecosystems has illuminated host-microbial interactions (Levy et al., 2015, Shapiro et al., 2014, Blacher et al., 2017). Different functional phenotypes and clinical outcomes are observed across similar microbial communities. As this concept pertains to reproductive biology, only a proportion of individuals colonized by a Lactobacillus-deficient cervicovaginal microbiota deliver preterm despite risk (Elovitz et al., 2019). Different strains of G. vaginalis demonstrate variation in virulence, which has been linked to risk of bacterial vaginosis (Muzny et al., 2019). Genetic diversity among group B streptococcus strains has similarly been shown to play a role in the pathogenesis of neonatal sepsis, with invasive isolates being more strongly associated with disease (Fluegge et al., 2011). These differences in outcomes are likely attributable to variation in genetic strains of the bacteria, host interactions, and immunologic responses. Investigation of the cervicovaginal metabolome is a new and active area of research. Recent studies have unveiled metabolites and metabolic pathways in association with adverse reproductive outcomes, including sPTB (Ghartey et al., 2015, Ghartey et al., 2017, Oliver et al., 2020, Ansari et al., 2020, Flaviani et al., 2021, Marangoni et al., 2021, Pruski et al., 2021, Gerson et al., 2021, Gerson et al., 2022). Understanding the implications of diverse metabolomic profiles within the cervicovaginal space offers potential to leverage these biochemical footprints to gain insight into mechanisms underlying adverse reproductive outcomes.
Our work has several strengths, including the in vitro experimental approaches coupled with validation in a human cohort. Inclusion of bacteria-free supernatants in addition to live bacteria offers greater insight into the diverse pathways through which G. vaginalis influences host immunity. As the cervicovaginal epithelium constitutes multiple epithelial cell types, our use of ectocervical, endocervical, and vaginal cells for in vitro experiments expands our understanding of epithelial biology and specificity in response to microbial challenge within this space. The use of individual cell lines, however, also has limitations, as they may not accurately replicate primary cells or in vivo conditions. As epithelial cell lines used for this study were derived from individuals without reported race or ethnicity, we are limited in our ability to generalize in vitro findings given the potential for ancestry-associated genetic diversity to influence biologic responses. We were also unable to examine the impact of an entire microbial community or select G. vaginalis strains on MMP protein expression. Such experimental approaches are more likely to recapitulate the complexity of in vivo conditions, and co-culture of L. crispatus and G. vaginalis is a key next step in refining our understanding of the cervicovaginal ecosystem. Nonetheless, our in vitro findings were corroborated in the human cohort suggesting biologic and translational relevance.
With respect to the human cohort, we recognize limitations of the cohort sample size and availability of biospecimens. Given the discovery approach of this study, power calculations were not performed a priori. As presented in Table 3, the large width of certain confidence intervals suggests that comparisons cannot rule out an association, e.g., short cervix versus normal cervical length (aOR 1.27 [CI 0.99-1.62]). Such nonsignificant findings do not preclude the possibility that statistical significance may have been achieved with a larger sample size. As Lactobacillus-dominant cervicovaginal microbiota among study participants included differing predominant Lactobacillus species (i.e., L. crispatus, L. gasseri, L. iners, or L. jensenii), findings from the human analyses may not correlate precisely to the in vitro work, in which only L. crispatus bacteria and bacteria-free supernatants were used. However, our prior work has demonstrated that L. crispatus-dominant microbial communities are similar with respect to functional phenotypes when compared to communities dominated by other Lactobacillus species (Elovitz et al., 2019), and these data are corroborated by our in vitro studies (Anton et al., 2019). Furthermore, this study is cross sectional and cannot establish causation. We cannot rule out that possibility that clinical interventions influenced MMP measurements.
This study is also limited by the measurement of total MMP-9 concentrations in human cervicovaginal swabs. As such, we are unable to discern active MMP-9. Investigating enzymatic function of this protease in the future is warranted. Finally, biospecimens were immediately frozen following collection, and subsequent processing likely resulted in elution of proteins not only in the extracellular fluid but also released due to cell lysis. Thus, measurement of MMP-9 may not accurately capture absolute concentrations in cervicovaginal fluid. Understanding that redundancy exists within the host immune system, MMP-9 is likely a single player in a large network, yet to be fully elucidated, of microbial-host crosstalk influencing biochemical profiles and functional outcomes.
Conclusions
Our data establish that G. vaginalis live bacteria and microbial output produce biologically distinct and significant effects on MMP protein expression, and that epithelial cell specificity in response to microbial exposure underlies host immunity in the cervicovaginal space. We provide evidence that TLR-2 plays a key role in the regulation of MMP-9 specifically. This research carries significant translational implications, as in vitro observations were corroborated in a prospective human pregnancy cohort. Our findings provide insight into potential mechanisms by which G. vaginalis may promote cervical remodeling through production of inflammatory proteases.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge Amy Brown, PhD and Yusra Gimie for their technical contributions to in vitro experiments. We also acknowledge The PennCHOP Microbial Culture & Metabolomics Core, including Elliot Friedman, PhD and Dillon Murphy, for preparation of microbial cultures. We acknowledge the Human Immunology Core (P30-CA016520) at the University of Pennsylvania for help with Luminex assays performed in this study.
FUNDING:
SMFM/AAOGF Award (KG); Danielle Peress, MD Memorial Fund Award (KG); R01NR014784 (ME); R01HD098867 (ME); R01HD102318 (ME). Funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
DISCLOSURE STATEMENT:
MAE receives salary support from National Institutes of Health (NINR, NIAID, and NICHD). She is also a consultant for Mirvie. The other authors report no conflict of interest.
Footnotes
PRESENTATION: This work was presented as an Oral Abstract (#37) in the Concurrent Session 3 – Prematurity & Infection of the Society for Maternal-Fetal Medicine’s 42nd Annual Pregnancy Meeting, Orlando, FL, January 31-February 5, 2022.
ABBREVIATIONS: Gardnerella vaginalis (G. vaginalis), spontaneous preterm birth (sPTB), matrix metalloproteinases (MMPs), toll-like receptors (TLRs), keratinocyte serum free media (K-SFM), Motherhood & Microbiome (M&M), non-treated control (NTC), Lactobacillus crispatus (L. crispatus)
REFERENCES
- ABDEL-HAMID NI, EL-AZAB MF & MOUSTAFA YM 2017. Macrolide antibiotics differentially influence human HepG2 cytotoxicity and modulate intrinsic/extrinsic apoptotic pathways in rat hepatocellular carcinoma model. Naunyn Schmiedebergs Arch Pharmacol, 390, 379–395. [DOI] [PubMed] [Google Scholar]
- ABDULKAREEM AA, SHELTON RM, LANDINI G, COOPER PR & MILWARD MR 2018. Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial-mesenchymal transition. Journal of Periodontal Research. [DOI] [PubMed] [Google Scholar]
- AL-SADI R, ENGERS J, HAQUE M, KING S, AL-OMARI D & MA TY 2021. Matrix Metalloproteinase-9 (MMP-9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF-kappaB activation. PLoS One, 16, e0249544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSARI A, LEE H, YOU YA, JUNG Y, PARK S, KIM SM, HWANG GS & KIM YJ 2020. Identification of Potential Biomarkers in the Cervicovaginal Fluid by Metabolic Profiling for Preterm Birth. Metabolites, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTON L, DEVINE A, SIERRA LJ, BROWN AG & ELOVITZ MA 2017. MIR-143 and miR-145 disrupt the cervical epithelial barrier through dysregulation of cell adhesion, apoptosis and proliferation. Scientific Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTON L, SIERRA LJ, DE VINE A, BARILA G, HEISER L, BROWN AG & ELOVITZ MA 2018. Common cervicovaginal microbial supernatants alter cervical epithelial function: Mechanisms by which lactobacillus crispatus contributes to cervical health. Frontiers in Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHAYDE N, ROMERO R, GOMEZ R, MAYMON E, PACORA P, MAZOR M, YOON BH, FORTUNATO S, MENON R, GHEZZI F & EDWIN SS 1999. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med, 8, 213–9. [DOI] [PubMed] [Google Scholar]
- BASSIOUNI W, ALI MAM & SCHULZ R 2021. Multifunctional intracellular matrix metalloproteinases: implications in disease. FEBS J. [DOI] [PubMed] [Google Scholar]
- BECHER N, HEIN M, DANIELSEN CC & ULDBJERG N 2010. Matrix metalloproteinases in the cervical mucus plug in relation to gestational age, plug compartment, and preterm labor. Reprod Biol Endocrinol, 8, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENJELLOUN F, QUILLAY H, CANNOU C, MARLIN R, MADEC Y, FERNANDEZ H, CHRETIEN F, LE GRAND R, BARRE-SINOUSSI F, NUGEYRE MT & MENU E 2020. Activation of Toll-Like Receptors Differentially Modulates Inflammation in the Human Reproductive Tract: Preliminary Findings. Front Immunol, 11, 1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACHER E, LEVY M, TATIROVSKY E & ELINAV E 2017. Microbiome-Modulated Metabolites at the Interface of Host Immunity. The Journal of Immunology. [DOI] [PubMed] [Google Scholar]
- BLASKEWICZ CD, PUDNEY J & ANDERSON DJ 2011. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod, 85, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRETELLE F, ROZENBERG P, PASCAL A, FAVRE R, BOHEC C, LOUNDOU A, SENAT MV, AISSI G, LESAVRE N, BRUNET J, HECKENROTH H, LUTON D, RAOULT D, FENOLLAR F & GROUPE DE RECHERCHE EN OBSTETRIQUE, G. 2015. High Atopobium vaginae and Gardnerella vaginalis vaginal loads are associated with preterm birth. Clin Infect Dis, 60, 860–7. [DOI] [PubMed] [Google Scholar]
- BROWN RG, AL-MEMAR M, MARCHESI JR, LEE YS, SMITH A, CHAN D, LEWIS H, KINDINGER L, TERZIDOU V, BOURNE T, BENNETT PR & MACINTYRE DA 2019a. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl Res, 207, 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN RG, MARCHESI JR, LEE YS, SMITH A, LEHNE B, KINDINGER LM, TERZIDOU V, HOLMES E, NICHOLSON JK, BENNETT PR & MACINTYRE DA 2018. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN D, BENNETT PR, LEE YS, KUNDU S, TEOH TG, ADAN M, AHMED S, BROWN RG, DAVID AL, LEWIS HV, GIMENO-MOLINA B, NORMAN JE, STOCK SJ, TERZIDOU V, KROPF P, BOTTO M, MACINTYRE DA & SYKES L 2022. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat Commun, 13, 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN YT, YANG CC, LIN KC, CHEN KH, SUNG PH, SHAO PL, LI YC, CHIANG JY & YIP HK 2019. Preactivated and disaggregated shape-changed platelets protect kidney against from ischemia-reperfusion injury in rat through attenuating inflammation reaction. J Tissue Eng Regen Med, 13, 2155–2168. [DOI] [PubMed] [Google Scholar]
- CHERNE MD, COLE AL, NEWBERRY L, SCHMIDT-OWENS M, DEICHEN M & COLE AM 2020. Matrix Metalloproteinases Expressed in Response to Bacterial Vaginosis Disrupt the Endocervical Epithelium, Increasing Transmigration of HIV. Infect Immun, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUI N, HU M & KHALIL RA 2017. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci, 147, 1–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIGIULIO DB, CALLAHAN BJ, MCMURDIE PJ, COSTELLO EK, LYELL DJ, ROBACZEWSKA A, SUN CL, GOLTSMAN DSA, WONG RJ, SHAW G, STEVENSON DK, HOLMES SP & RELMAN DA 2015. Temporal and spatial variation of the human microbiota during pregnancy. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURAN-CHAVEZ J, GRANDI C, DOS SRL, DE FREITAS SF, CARDOSO VC & CARVALHO CAVALLI R 2021. Relationship between metalloproteinase-2 and -9 levels in plasma and vaginal secretion with preterm birth. Eur J Obstet Gynecol Reprod Biol, 261, 217–221. [DOI] [PubMed] [Google Scholar]
- ELOVITZ MA, GAJER P, RIIS V, BROWN AG, HUMPHRYS M, HOLM J & RAVEL J 2019. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINGLETON B 2017. Matrix metalloproteinases as regulators of inflammatory processes. Biochim Biophys Acta Mol Cell Res, 1864, 2036–2042. [DOI] [PubMed] [Google Scholar]
- FINI ME, COOK JR & MOHAN R 1998. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res, 290 Suppl, S12–23. [DOI] [PubMed] [Google Scholar]
- FLAVIANI F, HEZELGRAVE NL, KANNO T, PROSDOCIMI EM, CHIN-SMITH E, RIDOUT AE, VON MAYDELL DK, MISTRY V, WADE WG, SHENNAN AH, DIMITRAKOPOULOU K, SEED PT, MASON AJ & TRIBE RM 2021. Cervicovaginal microbiota and metabolome predict preterm birth risk in an ethnically diverse cohort. JCI Insight, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLUEGGE K, WONS J, SPELLERBERG B, SWOBODA S, SIEDLER A, HUFNAGEL M & BERNER R 2011. Genetic differences between invasive and noninvasive neonatal group B streptococcal isolates. Pediatr Infect Dis J, 30, 1027–31. [DOI] [PubMed] [Google Scholar]
- GERSON KD, LIAO L, MCCARTHY C, BURRIS HH, KOREM T, LEVY M, .RAVEL J & ELOVITZ MA. 2021. A non-optimal cervicovaginal microbiota in pregnancy is associated with a distinct metabolomic signature among non-Hispanic Black individuals. Sci Rep, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSON KD, YANG N, ANTON L, LEVY M, RAVEL J, ELOVITZ MA & BURRIS HH 2022. Second trimester short cervix is associated with decreased abundance of cervicovaginal lipid metabolites. Am J Obstet Gynecol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHARTEY J, ANGLIM L, ROMERO J, BROWN A & ELOVITZ MA 2017. Women with Symptomatic Preterm Birth Have a Distinct Cervicovaginal Metabolome. American Journal of Perinatology. [DOI] [PubMed] [Google Scholar]
- GHARTEY J, BASTEK JA, BROWN AG, ANGLIM L & ELOVITZ MA 2015. Women with preterm birth have a distinct cervicovaginal metabolome. American Journal of Obstetrics and Gynecology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ JM, DONG Z, ROMERO R & GIRARDI G 2011. Cervical remodeling/ripening at term and preterm delivery: The same mechanism initiated by different mediators and different effector cells. PLoS ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORETSKY T, DIRISINA R, SINH P, MITTAL N, MANAGLIA E, WILLIAMS DB, POSCA D, RYU H, KATZMAN RB & BARRETT TA 2012. p53 mediates TNF-induced epithelial cell apoptosis in IBD. Am J Pathol, 181, 1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSMANN C, ANAHTAR MN, HANDLEY SA, FARCASANU M, ABU-ALI G, BOWMAN BA, PADAVATTAN N, DESAI C, DROIT L, MOODLEY A, DONG M, CHEN Y, ISMAIL N, NDUNG’U T, GHEBREMICHAEL MS, WESEMANN DR, MITCHELL C, DONG KL, HUTTENHOWER C, WALKER BD, VIRGIN HW & KWON DS 2017. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERING NA & SCHULZKE JD 2009. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis, 27, 450–4. [DOI] [PubMed] [Google Scholar]
- HOLST E, GOFFENG AR & ANDERSCH B 1994. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol, 32, 176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU CA, HOU Y, YI D, QIU Y, WU G, KONG X & YIN Y 2015. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim Nutr, 1, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINDINGER LM, MACINTYRE DA, LEE YS, MARCHESI JR, SMITH A, MCDONALD JAK, TERZIDOU V, COOK JR, LEES C, ISRAFIL-BAYLI F, FAIZA Y, TOOZS-HOBSON P, SLACK M, CACCIATORE S, HOLMES E, NICHOLSON JK, TEOH TG & BENNETT PR 2016. Relationship between vaginal microbial dysbiosis, inflammation, and pregnancy outcomes in cervical cerclage. Science Translational Medicine. [DOI] [PubMed] [Google Scholar]
- KOGA K & MOR G 2010. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol, 63, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROON SJ, RAVEL J & HUSTON WM 2018. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertility and Sterility. [DOI] [PubMed] [Google Scholar]
- LEVY M, THAISS CA, ZEEVI D, DOHNALOVÁ L, ZILBERMAN-SCHAPIRA G, MAHDI JA, DAVID E, SAVIDOR A, KOREM T, HERZIG Y, PEVSNER-FISCHER M, SHAPIRO H, CHRIST A, HARMELIN A, HALPERN Z, LATZ E, FLAVELL RA, AMIT I, SEGAL E & ELINAV E 2015. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKSMITH GJ, CLARK P, DUFF P, SAADE GR & SCHULTZ GS 2001. Amniotic fluid concentrations of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 during pregnancy and labor. Am J Obstet Gynecol, 184, 159–64. [DOI] [PubMed] [Google Scholar]
- MACINTYRE DA, CHANDIRAMANI M, LEE YS, KINDINGER L, SMITH A, ANGELOPOULOS N, LEHNE B, ARULKUMARAN S, BROWN R, TEOH TG, HOLMES E, NICOHOLSON JK, MARCHESI JR & BENNETT PR 2015. The vaginal microbiome during pregnancy and the postpartum period in a European population. Scientific Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARANGONI A, LAGHI L, ZAGONARI S, PATUELLI G, ZHU C, FOSCHI C, MORSELLI S, PEDNA MF & SAMBRI V 2021. New Insights into Vaginal Environment During Pregnancy. Front Mol Biosci, 8, 656844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARES D, SIMOES JA, NOVAK RM & SPEAR GT 2008. TLR2-mediated cell stimulation in bacterial vaginosis. J Reprod Immunol, 77, 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURALL CL, JACKSON R, ZEHBE I, BOULLE N, SEGONDY M & ALIZON S 2019. Epithelial stratification shapes infection dynamics. PLoS Comput Biol, 15, e1006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZNY CA, TAYLOR CM, SWORDS WE, TAMHANE A, CHATTOPADHYAY D, CERCA N & SCHWEBKE JR 2019. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J Infect Dis, 220, 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NALLASAMY S & MAHENDROO M 2017. Distinct Roles of Cervical Epithelia and Stroma in Pregnancy and Parturition. Seminars in Reproductive Medicine. [DOI] [PubMed] [Google Scholar]
- NOLD C, ANTON L, BROWN A & ELOVITZ M 2012. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: A possible mechanism of premature cervical remodeling and preterm birth. American Journal of Obstetrics and Gynecology. [DOI] [PubMed] [Google Scholar]
- OLIVER A, LAMERE B, WEIHE C, WANDRO S, LINDSAY KL, WADHWA PD, MILLS DA, PRIDE DT, FIEHN O, NORTHEN T, DE RAAD M, LI H, MARTINY JBH, LYNCH S & WHITESON K 2020. Cervicovaginal Microbiome Composition Is Associated with Metabolic Profiles in Healthy Pregnancy. mBio, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE-MCCAW A, EWALD AJ & WERB Z 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol, 8, 221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFLUGHOEFT KJ & VERSALOVIC J 2012. Human microbiome in health and disease. Annu Rev Pathol, 7, 99–122. [DOI] [PubMed] [Google Scholar]
- PLECKAITYTE M 2019. Cholesterol-Dependent Cytolysins Produced by Vaginal Bacteria: Certainties and Controversies. Front Cell Infect Microbiol, 9, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRUSKI P, CORREIA GDS, LEWIS HV, CAPUCCINI K, INGLESE P, CHAN D, BROWN RG, KINDINGER L, LEE YS, SMITH A, MARCHESI J, MCDONALD JAK, CAMERON S, ALEXANDER-HARDIMAN K, DAVID AL, STOCK SJ, NORMAN JE, TERZIDOU V, TEOH TG, SYKES L, BENNETT PR, TAKATS Z & MACINTYRE DA 2021. Direct on-swab metabolic profiling of vaginal microbiome host interactions during pregnancy and preterm birth. Nat Commun, 12, 5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVEL J, GAJER P, ABDO Z, SCHNEIDER GM, KOENIG SSK, MCCULLE SL, KARLEBACH S, GORLE R, RUSSELL J, TACKET CO, BROTMAN RM, DAVIS CC, AULT K, PERALTA L & FORNEY LJ 2011. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADHU K, DOMINGUE PA, CHOW AW, NELLIGAN J, CHENG N & COSTERTON JW 1989. Gardnerella vaginalis has a gram-positive cell-wall ultrastructure and lacks classical cell-wall lipopolysaccharide. J Med Microbiol, 29, 229–35. [DOI] [PubMed] [Google Scholar]
- SHAPIRO H, THAISS CA, LEVY M & ELINAV E 2014. The cross talk between microbiota and the immune system: Metabolites take center stage. Current Opinion in Immunology. [DOI] [PubMed] [Google Scholar]
- SIERRA L, BROWN A, BARILÁ G, ANTON L, BARNUM C, SHETYE S, SOSLOWSKY L & ELOVITZ M 2018. Colonization of the cervicovaginal space with Gardnerella vaginalis leads to local inflammation and cervical remodeling in pregnant mice. PLoS ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTI G, PACCOSI S, PARENTI A, SERAVALLI V, LINARI C, DI TOMMASO M & WITKIN S 2020. Pro-inflammatory mediators in vaginal fluid and short cervical length in pregnancy. Bratisl Lek Listy, 121, 278–281. [DOI] [PubMed] [Google Scholar]
- SMITH SB & RAVEL J 2017. The vaginal microbiota, host defence and reproductive physiology. Journal of Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOUT MJ, ZHOU Y, WYLIE KM, TARR PI, MACONES GA & TUULI MG 2017. Early pregnancy vaginal microbiome trends and preterm birth. American Journal of Obstetrics and Gynecology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEAM, R. C. 2021. R: A language and environment fr statistical computing [Online]. Vienna, Austria. Available: https://www.R-project.org/ [Accessed]. [Google Scholar]
- VU TH, SHIPLEY JM, BERGERS G, BERGER JE, HELMS JA, HANAHAN D, SHAPIRO SD, SENIOR RM & WERB Z 1998. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell, 93, 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG Y, SETHU C, LOUAFI F & HOSSAIN P 2011. Lipopolysaccharide regulation of toll-like receptor-4 and matrix metalloprotease-9 in human primary corneal fibroblasts. Invest Ophthalmol Vis Sci, 52, 2796–803. [DOI] [PubMed] [Google Scholar]
- YOO HN, PARK KH, JUNG EY, KIM YM, KOOK SY & JEON SJ 2017. Non-invasive prediction of preterm birth in women with cervical insufficiency or an asymptomatic short cervix (</=25 mm) by measurement of biomarkers in the cervicovaginal fluid. PLoS One, 12, e0180878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZARIFFARD MR, NOVAK RM, LURAIN N, SHA BE, GRAHAM P & SPEAR GT 2005. Induction of tumor necrosis factor- alpha secretion and toll-like receptor 2 and 4 mRNA expression by genital mucosal fluids from women with bacterial vaginosis. J Infect Dis, 191, 1913–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


