Abstract
Background
Differences in the distribution of individual-level clinical risk factors across regions do not fully explain the observed global disparities in COVID-19 outcomes. We aimed to investigate the associations between environmental and societal factors and country-level variations in mortality attributed to COVID-19 among people with rheumatic disease globally.
Methods
In this observational study, we derived individual-level data on adults (aged 18–99 years) with rheumatic disease and a confirmed status of their highest COVID-19 severity level from the COVID-19 Global Rheumatology Alliance (GRA) registry, collected between March 12, 2020, and Aug 27, 2021. Environmental and societal factors were obtained from publicly available sources. The primary endpoint was mortality attributed to COVID-19. We used a multivariable logistic regression to evaluate independent associations between environmental and societal factors and death, after controlling for individual-level risk factors. We used a series of nested mixed-effects models to establish whether environmental and societal factors sufficiently explained country-level variations in death.
Findings
14 044 patients from 23 countries were included in the analyses. 10 178 (72·5%) individuals were female and 3866 (27·5%) were male, with a mean age of 54·4 years (SD 15·6). Air pollution (odds ratio 1·10 per 10 μg/m3 [95% CI 1·01–1·17]; p=0·0105), proportion of the population aged 65 years or older (1·19 per 1% increase [1·10–1·30]; p<0·0001), and population mobility (1·03 per 1% increase in number of visits to grocery and pharmacy stores [1·02–1·05]; p<0·0001 and 1·02 per 1% increase in number of visits to workplaces [1·00–1·03]; p=0·032) were independently associated with higher odds of mortality. Number of hospital beds (0·94 per 1-unit increase per 1000 people [0·88–1·00]; p=0·046), human development index (0·65 per 0·1-unit increase [0·44–0·96]; p=0·032), government response stringency (0·83 per 10-unit increase in containment index [0·74–0·93]; p=0·0018), as well as follow-up time (0·78 per month [0·69–0·88]; p<0·0001) were independently associated with lower odds of mortality. These factors sufficiently explained country-level variations in death attributable to COVID-19 (intraclass correlation coefficient 1·2% [0·1–9·5]; p=0·14).
Interpretation
Our findings highlight the importance of environmental and societal factors as potential explanations of the observed regional disparities in COVID-19 outcomes among people with rheumatic disease and lay foundation for a new research agenda to address these disparities.
Funding
American College of Rheumatology and European Alliance of Associations for Rheumatology.
Introduction
Research has identified demographic and clinical risk factors associated with poor COVID-19 outcomes in people with rheumatic disease.1, 2, 3 Although this evidence has facilitated individual risk stratification and guided management decisions in this patient group, neither the temporal dynamics of the COVID-19 pandemic nor the potential capacities of health-care systems have been assessed as additional factors of importance. In the general population, country-level estimates of the case fatality rate from COVID-19 have ranged from 0·5% to 20·0%.4, 5 Similarly, rates of excess death (from any cause) have varied considerably across countries during the pandemic.6 However, the underlying causes of global disparities in COVID-19 outcomes are not fully understood.
Research in context.
Evidence before this study
We searched PubMed for articles published from date of database inception to Nov 1, 2021, exploring the association between country-level policies and socioeconomic resources and COVID-19 outcomes in people with rheumatic disease. We first included the Medical Subject Heading (MeSH) “COVID-19” and the MeSH Major Topic “Global Health/statistics and numerical data”. We did not restrict this search by language or type of publication. We found multiple ecological studies investigating the association between country-level factors (eg, population-level burden of comorbidities, socioeconomic factors, environmental factors, and containment policies) and mortality rates from COVID-19. However, results were inconclusive across studies and reliable individual-level inferences might not be deducible from ecological study designs. We then performed a further unrestricted MeSH search with “COVID-19” AND “rheumatology” OR “rheumatic disease” AND title or abstract search with “disparity” OR “disparities” to identify relevant studies among people with rheumatic disease. One of the few identified studies was from Africa, and highlighted regional disparities in the availability of national rheumatology society COVID-19 recommendations (eg, locally agreed protocols on use of disease-modifying antirheumatic drugs). Another study showed disparate COVID-19 outcomes among minority ethnic populations with rheumatic disease in the USA. However, the effects of country-level policies and socioeconomic resources on global disparities in COVID-19 outcomes had not been characterised in people with rheumatic disease.
Added value of this study
In people with rheumatic disease, country-level characteristics—including exposure to air pollutants, low country socioeconomic status, high demands on or reduced capacity of health resources, few government-imposed containment policies, and increased population mobility—were associated with increased odds of death attributed to COVID-19, independent of individual-level risk factors (including age, rheumatic disease activity, immunosuppression, and comorbidities). Importantly, the inclusion of individuals as units of analysis in our study allowed for more reliable inferences about an individual's level of risk than those obtained from ecological study designs.
Implications of all the available evidence
The findings from this study highlight the impact of societal policies and resources on COVID-19 outcomes in people with rheumatic disease globally. These findings lay the foundation for a new research agenda to address global disparities in COVID-19 outcomes in this patient group.
In people with rheumatic disease, in addition to regional differences in the distribution of individual-level risk factors associated with a poor COVID-19 prognosis (eg, age, comorbidities, rheumatic disease activity, and treatments), several other factors could explain the global disparities in the risk of poor outcomes. First, waves of SARS-CoV-2 infection occurred earlier in some countries; therefore, observed differences in outcomes might reflect differences in quickly evolving management strategies during the first several months of the pandemic. Second, differences in outcomes might reflect country-specific health-care capacity to handle surges in the number of patients requiring intensive care and other resources. Third, variations in risk might reflect country-level differences in wealth or governmental response with mitigation strategies. Given the heightened interest in global health equity, examining diverse country-level factors (including environmental and socioeconomic factors, health-care resources, population health, and demographics), COVID-19 containment policies, and individual behaviours is important to understand the potential mechanisms of disparate COVID-19 outcomes in people with rheumatic disease across nations.
Various ecological studies have investigated regional variations in poor COVID-19 outcomes in the general population. However, consistent with the ecological fallacy,7 inferences on individual-level risk might not be deducible from population-level measures of association due to loss of information. Individual-level databases, such as insurance claims or electronic health records, often do not provide readily accessible information on important clinical parameters (eg, rheumatic disease activity or glucocorticoid dose). Additionally, such databases usually operate nationally or subnationally, and thus cannot be used to evaluate the influence of country-level characteristics on health outcomes. By contrast, the COVID-19 Global Rheumatology Alliance (GRA) registry is unique in its inclusion of COVID-19 cases in people with prevalent rheumatic disease worldwide and of comprehensive data regarding each individual's characteristics of rheumatic disease and COVID-19 diagnosis and outcomes. Using data from the GRA registry linked to a robust array of country-level factors, we aimed to investigate the associations between environmental and societal factors and country-level variations in mortality attributed to COVID-19 among people with rheumatic disease globally.
Methods
Study design and participants
In this observational study, we derived individual-level data from the COVID-19 Global Rheumatology Alliance (GRA) registry collected between March 12, 2020, and Aug 27, 2021. Details of the GRA registry have been described previously.8 Briefly, data from adults with rheumatic disease diagnosed with confirmed or suspected COVID-19 were entered into the registry by rheumatology clinicians via two parallel international data entry portals. Entered data included patient demographics, characteristics of rheumatic disease, immunomodulatory medications used for the treatment of rheumatic disease, comorbidities, COVID-19 outcomes, and complications. A diagnosis of COVID-19 was indicated through one or more of the following methods: PCR, antigen testing, antibody testing, metagenomic testing, CT scan, laboratory assay, or a presumptive diagnosis based on symptoms or close contact alone. Quality was assessed by data validation teams, who removed all known or potential duplicates and addressed erroneous reports. The registry contains minimal data only; no personal identifiers, with the exception of dates of COVID-19 diagnosis, are included. Therefore, the UK Health Research Authority, the University of Manchester (Manchester, UK), and the University of California (San Francisco, CA, USA) deemed the registry to be “not human subjects research”, thus not requiring ethics committee approval or written informed consent. We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for reporting cohort studies.
We included adults (aged 18–99 years) with rheumatic disease from countries that reported 100 or more adults in the GRA registry. This cluster size was chosen to increase statistical power to detect regional variations in mortality attributed to COVID-19.9 For inclusion, patients were required to have a confirmed status of their highest COVID-19 severity level, which included one of the following: death, symptoms resolved at the time of data entry, not hospitalised for more than 30 days after initial diagnosis date, hospitalised and discharged, or not at risk of further interventions or death.
Procedures
Individual-level demographics, characteristics of rheumatic disease, and comorbidities were obtained from the GRA registry and reflected data at the time of COVID-19 diagnosis. A country-specific variable for follow-up time was generated, defined as time (in months) between the date of an individual's COVID-19 diagnosis (as entered in the GRA registry) and the index date of their respective country. For each country, index date was defined as the first date that a COVID-19 diagnosis was reported to the COVID-19 Data Repository by the Center for Systems Science and Engineering at Johns Hopkins University (Baltimore, MD, USA). The end of follow-up was Aug 27, 2021, or the most recent date of COVID-19 diagnosis reported to the GRA registry by the respective country, whichever occurred first. Follow-up time reflected how a health system had gained experience in treating COVID-19 over time. In addition to follow-up time, as covariates, we included time-dependent, country-level rates of cumulative and incident mortality attributed to COVID-19. These rates captured the multiple country-specific waves of the pandemic over time. We also included time-dependent, country-level estimates of SARS-CoV-2 reproduction rate, reflecting the country-specific spread of disease over time. All country-level covariates considered to be associated with an individual's odds of death were obtained from publicly available sources (table 1 ). A variance inflation factor analysis was used to remove country-level covariates that were highly intercorrelated in their associations with death. The GRA registry included data on reporting clinicians' country (and state if the reporting clinician was based in the USA). Therefore, we retrieved state-level data for a subset of regional covariates where these data were available (table 1). Individual-level and regional-level data were merged so that dates of all time-varying regional covariates corresponded with the diagnosis date or month of COVID-19 for each patient, as appropriate. Conceptualised relationships between outcome, individual-level, and regional-level covariates are depicted with a simplified directed acyclic graph (appendix p 2).
Table 1.
Regional covariate definitions and source datasets
| Definition | Type | Regional level | Source | Period | |
|---|---|---|---|---|---|
| Geographical | |||||
| Population density | Population divided by land area, km2 | Numeric, baseline | Country and US state | Countries: World Bank World Development Indicators, sourced from Food and Agriculture Organization and World Bank estimates; US states: United States Census Bureau | Countries: most recent year available; US states: 2020 |
| Climatic | |||||
| Precipitation | Average monthly precipitation, mm | Numeric, time-dependent | Country and US state | Countries: World Bank Climate Change Knowledge Portal; US states: World Bank Climate Change Knowledge Portal | 1991–2020 |
| Temperature | Average monthly temperature, °C | Numeric, time-dependent | Country and US state | Countries: World Bank Climate Change Knowledge Portal; US states: World Bank Climate Change Knowledge Portal | 1991–2020 |
| PM2·5 | Average monthly PM2·5, μg/m3 | Numeric, time-dependent | Country and US state | Countries: Air Quality Open Data Platform by the World Air Quality Project; US states: United States Environmental Protection Agency | Current, monthly |
| Social and economic measures of development | |||||
| Median age | Median age of the population, years | Numeric, baseline | Country and US state | Countries: UN Population Division, World Population Prospects (2017 Revision); US states: United States Census Bureau | Countries: UN projection for 2020; US states: 2019 |
| Life expectancy* | Life expectancy at birth, defined as the average number of years that a neonate could expect to live if they were to pass through life subject to the age-specific mortality rates of a given period | Numeric, baseline | Country and US state | Countries: Our World in Data and UN Population Division; US states: County Health Rankings and Roadmaps by the University of Wisconsin Population Health Institute (Madison, WI, USA) | Countries: 2019; US states: 2018 |
| Human development index | A composite index defined as the geometric mean of normalised indices in three dimensions (including life expectancy at birth, mean number of years of schooling for adults aged ≥25 years, expected years of schooling for children of school-entering age, and gross national income per capita); ranked on a scale from 0·0 to 1·0 | Numeric, baseline | Country and US state | Countries: UN Development Programme; US states: Global Data Lab by the Institute for Management Research at Radbound University (Nijmegen, Netherlands) | 2019 |
| Hospital beds | Number of hospital beds per 1000 people | Numeric, baseline | Country and US state | Countries: Our World In Data, sourced from the Organisation for Economic Co-operation and Development, Eurostat, World Bank, national government records and other sources; US states: Global Health Data Exchange by the IHME | Countries: most recent year available since 2010; US states: 2019 |
| Population demographic | |||||
| Proportion aged ≥65 years | Proportion of the population aged ≥65 years | Numeric, baseline | Country and US state | Countries: World Bank World Development Indicators based on age or sex distributions of UN World Population Prospects (2017 Revision); US states: Population Reference Bureau | Countries: most recent year available; US states: 2018 |
| Population burden of comorbidities | |||||
| Death rate from cardiovascular disease | Annual number of deaths per 100 000 people attributed to cardiovascular disease | Numeric, baseline | Country and US state | Countries: Global Burden of Disease Collaborative Network by the IHME; US states: Centers for Disease Control and Prevention, National Center for Health Statistics | Countries: 2017; US states: 2019 |
| Diabetes prevalence | Proportion of adults with diabetes in the population | Numeric, baseline | Country and US state | Countries: World Bank World Development Indicators, sourced from International Diabetes Federation Diabetes Atlas; US states: CDC, Diagnosed Diabetes | Countries: 2017; US states: 2018 |
| Death rate from air pollution | Annual number of deaths per 100 000 people attributed to outdoor and indoor air pollution | Numeric, baseline | Country | Global Burden of Disease Collaborative Network by the IHME | 2017 |
| COVID-19 measures | |||||
| Cumulative death rate | Total number of cumulative deaths per 1 million people attributed to COVID-19 per day | Numeric, time-dependent | Country | Our World In Data, sourced from COVID-19 Data Repository by the Center for Systems Science and Engineering at Johns Hopkins University (Baltimore, MD, USA) | Current, daily |
| Incident death rate | Number of new deaths per 1 million people attributed to COVID-19 per day | Numeric, time-dependent | Country | Our World In Data, sourced from COVID-19 Data Repository by the Center for Systems Science and Engineering at Johns Hopkins University (Baltimore, MD, USA) | Current, daily |
| SARS-CoV-2 reproduction rate | Daily estimates of the effective SARS-CoV-2 reproduction rate | Numeric, time-dependent | Country | Our World In Data, based on Marioli et al (2020)10 | Current, daily |
| Government response | |||||
| Containment index | A composite index recorded daily based on 13 government response indicators: school closures, workplace closures, cancellation of public events, restrictions on public gatherings, closures of public transport, stay-at-home requirements, public information campaigns, restrictions on internal movements, international travel controls, testing policy, the extent of contact tracing, requirements to wear face coverings, and policies around vaccine rollout; rescaled to a value from 0 to 100, with 100 representing strictest response | Numeric, time-dependent | Country | Oxford COVID-19 Government Response Tracker, Blavatnik School of Government (Oxford, UK) | Current, daily |
| Population mobility trends | |||||
| Places of retail and recreation | Percentage change in the number of visitors per day (calculated as a rolling 7-day average) to places of retail and recreation compared with the median value for the 5week period from Jan 3 to Feb 6, 2020 | Numeric, time-dependent | Country | Our World In Data, sourced from Google | Current, daily |
| Grocery and pharmacy stores | Percentage change in the number of visitors per day (calculated as a rolling 7-day average) to grocery and pharmacy stores compared with the median value for the 5week period from Jan 3 to Feb 6, 2020 | Numeric, time-dependent | Country | Our World In Data, sourced from Google | Current, daily |
| Transit stations | Percentage change in the number of visitors per day (calculated as a rolling 7-day average) to transit stations compared with the median value for the 5week period from Jan 3 to Feb 6, 2020 | Numeric, time-dependent | Country | Our World In Data, sourced from Google | Current, daily |
| Workplaces | Percentage change in the number of visitors per day (calculated as a rolling 7-day average) to workplaces compared with the median value for the 5 week period from Jan 3 to Feb 6, 2020 | Numeric, time-dependent | Country | Our World In Data, sourced from Google | Current, daily |
PM2·5=fine particulate matter air pollutants. IHME=Institute for Health Metrics and Evaluation. CDC=Centers for Disease Control and Prevention.
Life expectancy was not used as a covariate due to strong collinearity with human development index.
Outcomes
The primary endpoint was mortality attributed to COVID-19, documented by the reporting clinician. The survey question relating to death as an outcome of COVID-19 was worded “Is the patient deceased?”.
Statistical analysis
We used descriptive statistics to summarise the characteristics of the study population and baseline regional characteristics, stratified by six global regions adapted from WHO regions: Eastern Mediterranean, Europe, Latin America, North America, South-East Asia, and the Western Pacific. We summarised changes in time-varying regional characteristics over the follow-up period using time series scatter plots.
We estimated independent associations between regional characteristics and an individual's odds of death, reported as odds ratios (OR) with 95% CIs, using multivariable logistic regression after accounting for individual-level demographics, characteristics of rheumatic disease, comorbidities, and follow-up time. Regional covariates included the following variables: population density; precipitation; temperature; air pollutants, as measured by fine particulate matter (PM2·5); median age, as a proxy for the country's socioeconomic status;11 human development index, a composite measure of life expectancy, mean number of years of education, and gross national income per capita; number of hospital beds; proportion of the population aged 65 years and older, as a proxy for population burden of comorbidities and burden on health resources; mortality attributed to cardiovascular disease; prevalence of diabetes; mortality attributed to air pollution; cumulative and incident mortality attributed to COVID-19, reflecting multiple waves of COVID-19 dynamics over time; SARS-CoV-2 reproduction rate; government response measured by containment index, a composite index based on 13 response indicators (eg, closures, travel controls, stay-at-home requirements, public information campaigns, face covering requirements, contact tracing, and testing and vaccination policies); and population mobility, measured by the application programming interface of the Google Maps app in tracking country-level movement of individuals (table 1). Individual-level demographics were age and sex. Individual-level characteristics of rheumatic disease included diagnosis (rheumatoid arthritis [reference], psoriatic arthritis, spondyloarthritis, other inflammatory arthritis, systemic lupus erythematosus [SLE], vasculitis, and other diagnoses); rheumatic disease activity (remission [reference], low, moderate, or high); level of immunosuppression inferred by use of disease-modifying antirheumatic drugs (conventional synthetic therapy [reference] or biological or targeted synthetic therapy, either alone or in combination, or none); and daily dose of prednisone-equivalent glucocorticoid (daily doses >60 mg were winsorised to 60 mg because they were considered to be clinically high doses). Individual-level comorbidities included morbid obesity (defined as a body-mass index ≥40 kg/m2), cardiovascular disease (eg, coronary artery disease and congestive heart failure) or hypertension, lung disease (eg, pulmonary hypertension, or interstitial, obstructive, or other lung disease), diabetes, and kidney disease (eg, chronic kidney insufficiency or end-stage kidney disease), each included as a dichotomous variable. Follow-up time was included both as a continuous variable and as a quadratic term to improve model fit.
We used mixed-effects regression12 to investigate whether the regional covariates included in the model sufficiently explained the proportion of the total variance in an individual's odds of death that was accounted for by country-level clustering. A series of nested multivariable mixed-effects logistic regression models were fitted with country included as random effects and all covariates successively added as fixed effects. The directed acyclic graph determined the order with which the covariates were added to the nested models. A likelihood ratio test was performed to compare each model with an equivalent logistic regression model that did not include country-random effects. An intraclass correlation coefficient (ICC) that approached 0% and a statistically non-significant likelihood ratio test indicated sufficient explanation of the observed country-level variations in mortality attributed to COVID-19.
Missing values in individual-level covariates were imputed using predictive mean matching across five nearest neighbours and multiple imputation by chained equations. All statistical analyses were done with Stata (version 16.0). The threshold for statistical significance was a two-sided p<0·05.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
14 044 people from 23 countries were included in the analyses, most of whom were from Europe (6369 [45·4%]) and North America (3506 [25·0%]; table 2 ; appendix p 8). 10 178 (72·5%) individuals were female and 3866 (27·5%) were male, with a mean age of 54·4 years (SD 15·6; appendix p 9). Rheumatoid arthritis was the most common diagnosis, followed by SLE, and psoriatic arthritis (table 2). The majority of people were in remission or had low disease activity, and conventional synthetic disease-modifying antirheumatic drugs were the most common treatment modality (table 2). Common comorbidities included cardiovascular disease or hypertension, lung disease, diabetes, and kidney disease. In total, 865 (6·2%) deaths were reported. The number of deaths and case fatality rate by country among patients included in the analyses are provided in the appendix (p 3). The mean time from date of COVID-19 diagnosis to death was 19 days (SD 19).
Table 2.
Patient characteristics grouped into six global regions
| Eastern Mediterranean (n=268) | Europe (n=6369) | South-East Asia (n=154) | Western Pacific (n=376) | North America (n=3506) | Latin America (n=3371) | Total (n=14 044) | ||
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age, years | 46·5 (13·1) | 56·2 (15·5) | 47·0 (13·5) | 55·1 (17·6) | 56·0 (15·8) | 50·2 (14·4) | 54·4 (15·6) | |
| Sex | ||||||||
| Male | 89 (33·2%) | 2080 (32·7%) | 38 (24·7%) | 144 (38·3%) | 905 (25·8%) | 610 (18·1%) | 3866 (27·5%) | |
| Female | 179 (66·8%) | 4289 (67·3%) | 116 (75·3%) | 232 (61·7%) | 2601 (74·2%) | 2761 (81·9%) | 10 178 (72·5%) | |
| Race or ethnicity | ||||||||
| White | 0 | 5491 (86·2%) | 0 | 1 (0·3%) | 2067 (59·0%) | 895 (26·5%) | 8454 (60·2%) | |
| Black | 2 (0·7%) | 77 (1·2%) | 0 | 0 | 402 (11·5%) | 3 (0·1%) | 484 (3·4%) | |
| Hispanic or Latinx | 0 | 22 (0·3%) | 0 | 1 (0·3%) | 673 (19·2%) | 2335 (69·3%) | 3031 (21·6%) | |
| Asian | 178 (66·4%) | 108 (1·7%) | 154 (100·0%) | 365 (97·1%) | 109 (3·1%) | 1 (<0·1%) | 915 (6·5%) | |
| Other | 86 (32·1%) | 12 (0·2%) | 0 | 8 (2·1%) | 94 (2·7%) | 6 (0·2%) | 206 (1·5%) | |
| Missing data | 2 (0·7%) | 659 (10·3%) | 0 | 1 (0·3%) | 161 (4·6%) | 131 (3·9%) | 954 (6·8%) | |
| Characteristics of rheumatic disease | ||||||||
| Diagnosis | ||||||||
| Rheumatoid arthritis | 125 (46·6%) | 2607 (40·9%) | 68 (44·2%) | 124 (33·0%) | 1413 (40·3%) | 1359 (40·3%) | 5696 (40·6%) | |
| Psoriatic arthritis | 16 (6·0%) | 960 (15·1%) | 9 (5·8%) | 9 (2·4%) | 359 (10·2%) | 77 (2·3%) | 1430 (10·2%) | |
| Spondyloarthritis | 20 (7·5%) | 850 (13·3%) | 14 (9·1%) | 6 (1·6%) | 178 (5·1%) | 293 (8·7%) | 1361 (9·7%) | |
| Other inflammatory arthritis | 9 (3·4%) | 109 (1·7%) | 2 (1·3%) | 3 (0·8%) | 161 (4·6%) | 8 (0·2%) | 292 (2·1%) | |
| SLE | 45 (16·8%) | 380 (6·0%) | 15 (9·7%) | 82 (21·8%) | 434 (12·4%) | 694 (20·6%) | 1650 (11·7%) | |
| Vasculitis | 6 (2·2%) | 195 (3·1%) | 6 (3·9%) | 26 (6·9%) | 135 (3·9%) | 95 (2·8%) | 463 (3·3%) | |
| Other diagnoses | 47 (17·5%) | 1268 (19·9%) | 40 (26·0%) | 126 (33·5%) | 826 (23·6%) | 845 (25·1%) | 3152 (22·4%) | |
| Disease activity | ||||||||
| Remission | 140 (52·2%) | 2704 (42·5%) | 54 (35·1%) | 154 (41·0%) | 837 (23·9%) | 1443 (42·8%) | 5332 (38·0%) | |
| Low | 72 (26·9%) | 2644 (41·5%) | 78 (50·6%) | 150 (39·9%) | 1885 (53·8%) | 1211 (35·9%) | 6040 (43·0%) | |
| Moderate | 44 (16·4%) | 839 (13·2%) | 13 (8·4%) | 46 (12·2%) | 669 (19·1%) | 573 (17·0%) | 2184 (15·6%) | |
| High | 12 (4·5%) | 182 (2·9%) | 9 (5·8%) | 26 (6·9%) | 115 (3·3%) | 144 (4·3%) | 488 (3·5%) | |
| Disease-modifying antirheumatic drugs | ||||||||
| Conventional synthetic therapy only | 180 (67·2%) | 2608 (40·9%) | 132 (85·7%) | 185 (49·2%) | 1335 (38·1%) | 2051 (60·8%) | 6491 (46·2%) | |
| Biological or targeted synthetic therapy only | 23 (8·6%) | 1687 (26·5%) | 0 | 20 (5·3%) | 768 (21·9%) | 506 (15·0%) | 3004 (21·4%) | |
| Conventional synthetic plus biological or targeted synthetic therapy | 17 (6·3%) | 1094 (17·2%) | 16 (10·4%) | 20 (5·3%) | 689 (19·7%) | 447 (13·3%) | 2283 (16·3%) | |
| None | 48 (17·9%) | 980 (15·4%) | 6 (3·9%) | 151 (40·2%) | 714 (20·4%) | 367 (10·9%) | 2266 (16·1%) | |
| Use of glucocorticoids | 59 (22·0%) | 1952 (30·6%) | 82 (53·2%) | 174 (46·3%) | 879 (25·1%) | 1201 (35·6%) | 4347 (31·0%) | |
| Comorbidities* | ||||||||
| Morbid obesity† | 3 (1·1%) | 50 (0·8%) | 2 (1·3%) | 2 (0·5%) | 281 (8·0%) | 51 (1·5%) | 389 (2·8%) | |
| Cardiovascular disease or hypertension | 93 (34·7%) | 2417 (37·9%) | 41 (26·6%) | 137 (36·4%) | 1504 (42·9%) | 1062 (31·5%) | 5254 (37·4%) | |
| Lung disease | 35 (13·1%) | 916 (14·4%) | 10 (6·5%) | 69 (18·4%) | 691 (19·7%) | 297 (8·8%) | 2018 (14·4%) | |
| Diabetes | 71 (26·5%) | 675 (10·6%) | 25 (16·2%) | 70 (18·6%) | 596 (17·0%) | 326 (9·7%) | 1763 (12·6%) | |
| Kidney disease | 22 (8·2%) | 335 (5·3%) | 0 | 30 (8·0%) | 306 (8·7%) | 130 (3·9%) | 823 (5·9%) | |
| Cancer | 4 (1·5%) | 244 (3·8%) | 0 | 11 (2·9%) | 194 (5·5%) | 61 (1·8%) | 514 (3·7%) | |
| Death | 20 (7·5%) | 408 (6·4%) | 7 (4·5%) | 44 (11·7%) | 178 (5·1%) | 208 (6·2%) | 865 (6·2%) | |
Data are mean (SD) or n (%). SLE=systemic lupus erythematosus.
Categories are not mutually exclusive.
Body-mass index ≥40 kg/m2.
The distribution of baseline country characteristics varied considerably across global regions (table 3 ). The minimum country-specific follow-up time was 17 months; therefore, time-series plots are presented over a period of 17 months from the index date (appendix p 4). The fastest increases in cumulative COVID-19 death rates over time occurred in Latin America, followed by North America and Europe (appendix p 4). Temporal trends for containment index, estimates of SARS-CoV-2 reproduction rate, and population mobility were similar across regions (appendix pp 4–5). Temporal trends in climatic factors (including temperature, precipitation, and PM2·5) between March, 2020, and July, 2021, are provided in the appendix (p 6).
Table 3.
Baseline regional characteristics grouped into six global regions
| Eastern Mediterranean (n=2) | Europe (n=11) | South-East Asia (n=1) | Western Pacific (n=2) | North America (n=2) | Latin America (n=5) | Total (n=23) | |
|---|---|---|---|---|---|---|---|
| Annual temperature, °C | 24 (8) | 10 (7) | 25 (4) | 19 (10) | 13 (10) | 21 (5) | 19 (6) |
| Annual precipitation, mm | 15 (15) | 71 (27) | 88 (93) | 172 (77) | 85 (35) | 118 (82) | 91 (52) |
| Population density, people per km2 | 241 (20) | 125 (78) | 450 (NA) | 350 (3) | 193 (675) | 35 (20) | 179 (552) |
| Median age, years | 28 (6) | 44 (3) | 28 (NA) | 37 (16) | 39 (2) | 31 (2) | 38 (5) |
| Human development index* | 0·70 (0·21) | 0·90 (0·04) | 0·65 (NA) | 0·82 (0·14) | 0·92 (0·02) | 0·79 (0·03) | 0·89 (0·08) |
| Number of hospital beds, per 1000 people | 0·9 (0·4) | 4·3 (1·9) | 0·5 (NA) | 7·0 (8·5) | 2·5 (0·7) | 2·4 (1·5) | 2·9 (1·9) |
| Proportion of population aged ≥65 years, % | 3 (2) | 19 (3) | 6 (NA) | 16 (16) | 16 (2) | 8 (2) | 16 (5) |
| Annual death rate from cardiovascular disease, per 100 000 people | 300 (174) | 179 (87) | 282 (NA) | 225 (206) | 162 (30) | 146 (42) | 173 (65) |
| Prevalence of adults with diabetes, % | 12 (6) | 6 (2) | 10 (NA) | 6 (1) | 11 (2) | 8 (3) | 10 (3) |
| Annual death rate from air pollution, per 100 000 people | 93 (43) | 22 (10) | 132 (NA) | 59 (68) | 19 (1) | 32 (8) | 26 (23) |
Data are mean (SD). Regional characteristics include country-level and US state-level characteristics. NA=not applicable.
Human development index is a composite index defined as the geometric mean of normalised indices in three dimensions (including life expectancy at birth, mean number of years of schooling for adults aged ≥25 years, expected years of schooling for children of school-entering age, and gross national income per capita); ranked on a scale from 0·0 to 1·0.
All individual-level covariates had less than 10% missing data and all country-level covariates had less than 5% missing data. Record entry date was used to impute the date of COVID-19 diagnosis in 1300 (9·3%) of 14 044 patients with a missing diagnosis date.
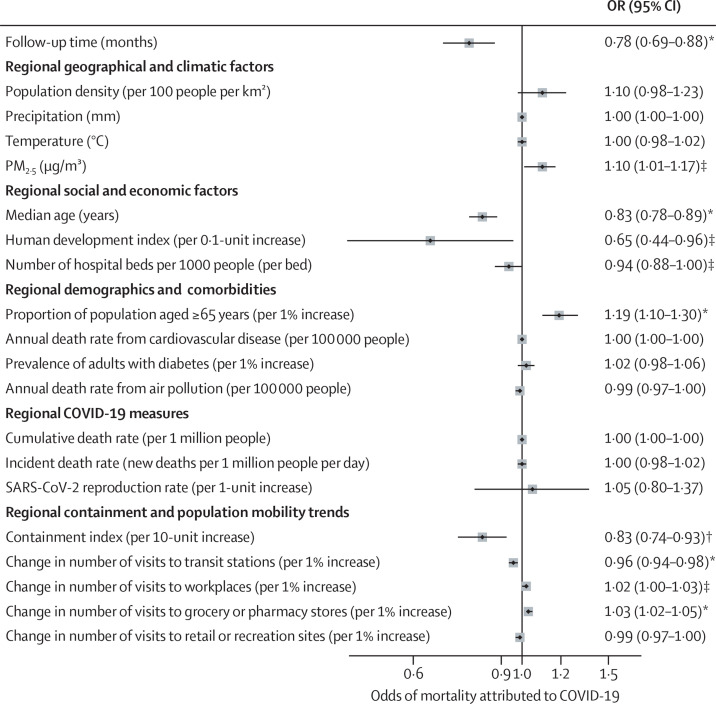
The multivariable logistic regression showed that PM2·5 (OR 1·10 per 10 μg/m3 [95% CI 1·01–1·17]; p=0·0105), proportion of the population aged 65 years or older (1·19 per 1% increase [1·10–1·30]; p<0·0001), number of visits to grocery and pharmacy stores (1·03 per 1% increase in number of visits [1·02–1·05]; p<0·0001), and number of visits to workplaces (1·02 per 1% increase in number of visits [1·00–1·03]; p=0·032) were independently associated with higher odds of mortality attributed to COVID-19 in people with rheumatic disease, after controlling for individual demographics and clinical characteristics. By contrast, median population age (0·83 per year [0·78–0·89]; p<0·0001), number of hospital beds (0·94 per 1-unit increase per 1000 people [0·88–1·00]; p=0·046), human development index (0·65 per 0·1-unit increase [0·44–0·96]; p=0·032), containment index (0·83 per 10-unit increase [0·74–0·93]; p=0·0018), number of visits to transit stations (0·96 per 1% increase in number of visits [0·94–0·98]; p<0·0001), and follow-up time (0·78 per month [0·69–0·88]; p<0·0001) were independently associated with lower odds of mortality attributed to COVID-19 (figure ).
Figure.
Associations between regional-level characteristics and odds of mortality attributed to COVID-19
Odds ratios derived from a multivariable logistic regression model, including all covariates shown, individual-level demographics (ie, age and sex), clinical characteristics, and follow-up time as a polynomial term. Clinical characteristics were diagnosis of rheumatic disease (eg, rheumatoid arthritis, psoriatic arthritis, spondyloarthritis, other inflammatory arthritis, systemic lupus erythematosus, and vasculitis), rheumatic disease activity (ie, remission, low, moderate, or high), clinically significant comorbidities (eg, cardiovascular disease or hypertension, lung disease, morbid obesity, diabetes, and kidney disease), use of disease-modifying antirheumatic drugs (ie, conventional systemic therapy or biological or targeted synthetic therapy, either alone or in combination, or none), and average daily dose of prednisone-equivalent glucocorticoid. Regional characteristics include country-level and US state-level characteristics. PM2·5=fine particulate matter air pollutants. *p<0·0001. †p<0·01. ‡p<0·05.
To establish whether the identified regional factors sufficiently explained the observed country-level variations in death, ICCs were derived from nested mixed-effects logistic regression models that sequentially incorporated regional covariates as fixed effects (table 4 ). A base model including only patient demographics as fixed effects had an ICC of 14·2% (95% CI 7·5–25·2). Addition of individual-level characteristics of rheumatic disease and comorbidities reduced the ICC to 10·1% (5·1–19·1). Addition of follow-up time and regional factors further reduced the ICC to 1·2% (0·1–9·5). The resulting model was no longer favourable over a logistic regression model without country random effects (p=0·14), indicating that the identified regional factors sufficiently explained the observed country-level variations in mortality attributed to COVID-19 that remained after accounting for individual-level characteristics. The ORs of mortality attributed to COVID-19 corresponding to individual-level characteristics are provided in the appendix (p 7).
Table 4.
Inclusion of temporal and regional covariates as fixed effects, and corresponding shrinkage in ICCs
| ICC (95% CI) | p value | |
|---|---|---|
| Base model with individual-level demographics as fixed effects | 14·2% (7·5–25·2) | <0·0001 |
| Addition of individual-level rheumatic disease characteristics and comorbidities | 10·1% (5·1–19·1) | <0·0001 |
| Addition of follow-up time (as main term and squared term) | 8·6% (4·2–16·7) | <0·0001 |
| Addition of regional geographical and climatic covariates | 6·9% (3·0–15·0) | <0·0001 |
| Addition of regional social and economic covariates | 4·2% (1·7–9·8) | <0·0001 |
| Addition of regional demographics and burden of comorbidities | 3·8% (1·4–10·2) | <0·0001 |
| Addition of regional cumulative COVID-19 deaths, containment efforts, and population mobility trends | 1·2% (0·1–9·5) | 0·14 |
Regional characteristics include country-level and US state-level characteristics. Covariates were added successively to nested mixed-effects models. All models include country-random effects. p values for a likelihood ratio test comparing a mixed-effects model to a logistic regression model without country-random effects. ICC=intraclass correlation coefficient.
Discussion
To our knowledge, this is the first study that combines individual-level and regional-level data to investigate the independent association between regional parameters, such as a country's climatic, societal, and economic factors, burden on health-care resources, or pandemic policies, and an individual's odds of death attributed to COVID-19. We found that various factors related to geographical residence affected COVID-19 outcomes in people with rheumatic disease. Low country socioeconomic status, environmental exposures, high demands on or reduced capacity of health resources, and few government-imposed containment measures were independently associated with higher odds of death attributed to COVID-19 in this patient group. In particular, specific regional factors associated with death included exposure to air pollutants, proportion of the population aged 65 years and older, and population mobility (as proxied by visits to workplaces and to grocery and pharmacy stores). Factors associated with fewer deaths attributed to COVID-19 included human development index, number of hospital beds, and containment index (reflecting strictness of government response). These identified factors indicate some potential mechanisms of the observed disparities in COVID-19 outcomes among people with rheumatic disease globally.
Poverty, scarcity of health resources, and challenges in coordination of health and other social policies are existing obstacles to achieving global health equity in the burden of and outcomes from rheumatic disease.13 In low-income and middle-income countries, rheumatic conditions can cause considerable morbidity and mortality due to physician shortages, poor health literacy, and reduced access to health care and mental, social, and emotional support systems.14, 15, 16 Treatment of patients with rheumatic disease in low-income countries is limited by the cost of immunosuppressive drugs, and the unavailability and unaffordability of health insurance.17 Additionally, high costs and scarce availability of laboratory and diagnostic tests result in delayed diagnoses that contribute to worse prognoses of rheumatic disease.18 Nevertheless, our ICC analyses add to previous research into global health disparities by showing that differences in the distribution of characteristics of rheumatic disease and comorbidities associated with poor COVID-19 outcomes (eg, high rheumatic disease activity, use of high-dose glucocorticoids, and pre-existing lung disease), accounted for a relatively small proportion (approximately 29%, as measured by a reduction in ICC from 14·2% to 10·1%) of the country-level variation in deaths attributed to COVID-19. Our findings highlight the importance of environmental and societal policy changes in mitigating risk for severe COVID-19 among people with rheumatic disease during the pandemic. These findings provide powerful motivation and lay the foundation for initiatives that seek to address global disparities in COVID-19 outcomes, such as the American College of Rheumatology's Global Health Task Force.
Although various studies have investigated sources of regional variation in adverse COVID-19 outcomes using national4, 5, 19, 20, 21, 22, 23 and subnational24, 25, 26 data, results have been inconclusive, largely due to limitations and methodological inconsistencies in study design. One study examined regional variation in temporal trajectories of COVID-19 case fatality rate, using country as the unit of analysis.4 The study found that this rate was positively associated with proportion of the population aged 70 years or older, and negatively associated with number of hospital beds per 1000 people. However, in contrast to our findings, the study reported increased case fatality rates with increasing levels of government response stringency, gross domestic product per capita, and total health expenditure as share of gross domestic product. Despite controlling for differences in testing strategies among countries, the study also found that death due to lower respiratory infections was negatively associated with COVID-19 case fatality rates. These findings might be biased from not accounting for individual-level risk factors. Another study, which used US states and boroughs as the unit of analysis, identified population density and population mobility as important factors contributing to COVID-19 outcomes and related mortality, although this study did not account for measures of population health or burden of comorbidities.25 The inclusion of individuals as units of analysis in our study allowed for more reliable inferences about individual-level risk than those obtained from ecological study designs, providing insight into inconsistencies observed in previously published research. Our findings consolidate the need for policy changes that ensure equitable access to COVID-19 testing, treatment, and effective vaccination or pre-exposure prophylaxis in patients at high risk; timely and effective government response on face coverings, closures, travel controls, and restrictions on public gatherings; policies on air pollution and clean energy production; and action to alleviate global poverty.
One potential explanation for the inverse association between number of visits to transit stations and risk of death is that travel restrictions were more frequently in place during peak periods of COVID-19-related lockdowns than were restrictions that governed visits to grocery and pharmacy stores or workplaces. Another potential explanation might be residual confounding by country-level socioeconomic status—ie, populations from countries with higher socioeconomic status were more likely to travel nationally and internationally, but less likely to experience adverse COVID-19 outcomes. The estimated associations in this study between individual-level characteristics and mortality attributed to COVID-19 were consistent with previously published reports (appendix p 7).3, 27, 28, 29, 30, 31, 32
Strengths of this study include the large size of the study population, with individuals from 23 countries across four continents. This population captured a wide diversity in the distribution of regional covariates and powered the study to detect associations between regional characteristics and death attributed to COVID-19. Furthermore, our methodological approach enabled us to show that the identified regional characteristics almost entirely explained the observed country-level variations in death.
Limitations of this study include the potential of provider reporting bias, given that the GRA registry used convenience sampling. However, results from the registry have suggested that reporting bias did not substantially affect previously reported estimates of association.33 Nonetheless, reporting biases could partially explain the country-level case fatality rates reported in this study; importantly, these rates should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19. Although all-cause mortality rates facilitate a more accurate assessment of global disparities by additionally capturing the indirect effects of COVID-19 during the pandemic, we were not able to study all-cause death because the GRA registry did not include individuals without a diagnosis of COVID-19. Further studies should establish whether the findings from this study remain consistent for all-cause death during the pandemic. The GRA registry included academic centres and community-based practices and reported both hospitalised deaths and out-of-hospital deaths. However, death from undiagnosed COVID-19 would not have been captured in the registry. There is the potential for misclassification of death attributed to COVID-19. However, a mean time of 19 days from date of COVID-19 diagnosis to death in this study was consistent with results from published research investigating COVID-19 fatality.34 Misclassification of regional covariates, particularly mobility trends, is plausible given that individuals can opt not to use location services, and are likely to differentiate with respect to region. Although we suspect this misclassification to be non-differential with respect to an individual's risk of death attributed to COVID-19, it remains a potential source of bias. We relied on geocoding of the reporting clinician to identify US state, which might not match that of the patient, particularly for those who live on the borders or in areas where regions are closely aggregated (eg, in the northeast of the USA). This study might have limited generalisability because many countries and US states were not included in the analyses. Regional variations generally increase with number, size, and granularity of the regions. Therefore, with large study populations (eg, with the inclusion of more countries and more patients from each country) and more granular data (eg, data collected at the county or health system level), additional regional parameters and further considerations of their complex high-dimensional relationships will be needed to account for regional variations in COVID-19 outcomes. Furthermore, it was not possible to account for additional important individual-level risk factors, such as socioeconomic status and COVID-19 vaccination status, severity, and treatment.
In conclusion, our results suggest that, among people with rheumatic disease, time period of the pandemic wave, exposure to air pollutants, regional socioeconomic factors, availability of and burden on health resources, stringency of government response, and population mobility are associated with death attributed to COVID-19, independent of individual-level demographics, characteristics of rheumatic disease, and comorbidities. These findings highlight the importance of environmental and societal factors as potential explanations of the observed regional disparities in COVID-19 outcomes among people with rheumatic disease and lay foundation for a new research agenda to address these disparities.
Data sharing
Researchers interested in performing additional analyses from the registry are invited to submit proposals through the COVID-19 Global Rheumatology Alliance. For approved projects, we will be able to provide summary tables and data analyses as requested. We do not currently have approval from an Institutional Review Board to make the raw data available to other researchers.
Declaration of interests
MID reports research support from Pfizer for unrelated work. AS reports grants from a consortium of 13 companies (AbbVie, Bristol Myers Squibb, Celltrion, Fresenius Kabi, Lilly, Mylan, Hexal, Merck, Pfizer, Roche, Samsung, Sanofi-Aventis, and UCB) supporting the German RABBIT register, and personal fees from lectures for AbbVie, Merck, Roche, Bristol Myers Squibb, and Pfizer, outside of the submitted work. EFM reports that the Portuguese League Against Rheumatic Diseases received support for specific activities: grants from Abbvie, Novartis, Janssen-Cilag, Lilly Portugal, Sanofi, Grünenthal SA, Merck, Celgene, Medac, Pharmakern, the Global Alliance for Patient Access; grants and non-financial support from Pfizer; and non-financial support from Grünenthal GmbH, outside of the submitted work. KLH reports receiving speaker fees from Abbvie and grant income from Bristol Myers Squibb, UCB, and Pfizer, unrelated to this work. KLH is also supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre. LG reports research grants from Amgen, Galapagos, Janssen, Lilly, Pfizer, Sandoz, and Sanofi; and consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Biogen, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis, and UCB, all unrelated to this work. LC has not received fees or personal grants from any laboratory, but her institute works by contract for laboratories among other institutions, such as Abbvie Spain, Eisai, Gebro Pharma, Merck Sharp & Dohme España, SA Pharma, Novartis Farmaceutica, Pfizer, Roche Farma, Sanofi, Aventis, Astellas Pharma, Actelion Pharmaceuticals España, Grünenthal GmbH, and UCB Pharma. JAS has performed consultancy for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, and Optum, unrelated to this work. LW has received consulting or speaking fees from Aurinia Pharma, outside of the submitted work. MFU-G reports grant or research support from Jannsen and Pfizer, unrelated to this work. The Swedish Rheumatology Quality Register, with LL as register holder, has agreements with Abbvie, Amgen, Eli Lilly, Gilead, Novartis, Pfizer, Sanofi, Sobi, and UCB for register data analyses, unrelated to this work. CR has received consulting or speaker fees from Abbvie, Amgen, AstraZeneca, BMS, Biogen, Eli Lilly, Glenmark, GlaxoSmithKline, Merck, Mylan, and Pfizer; and grants from Biogen, Lilly, and Nordic Pharma, all unrelated to this work. MJS has received speaker fees from Abbvie, AstraZeneca, Novartis, and Pfizer. AR has received speaker fees from Janssen, Pfizer, and Novartis. GP-E reports reports personal consulting fees, speaking fees, or both from Pfizer, GlaxoSmithKline, Janssen, Sandoz, and Sanofi, outside of the submitted work. PCR reports personal consulting fees, speaking fees, or both from Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and travel assistance from Roche. PMM has received consulting fees, speaker fees, or both from Abbvie, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Roche, and UCB, unrelated to this work. PMM is supported by the NIHR University College London Hospitals Biomedical Research Centre. ES is a Board Member of the Canadian Arthritis Patient Alliance, a patient-run, volunteer-based organisation whose activities are largely supported by independent grants from pharmaceutical companies. JWL has received research funding from Pfizer, outside of the submitted work. JSH is supported by grants from the Rheumatology Research Foundation and has salary support from the Childhood Arthritis and Rheumatology Research Alliance. JSH has performed consulting for Novartis, Sobi, and Biogen, unrelated to this work. PS reports honorarium for doing social media for American College of Rheumatology journals. RG reports personal fees, speaking fees, or both from Abbvie, Janssen, Novartis, Pfizer, and Cornerstones; and travel assistance from Pfizer. SB reports non-branded consulting fees for AbbVie, Horizon, and Novartis; and is employed by Pfizer. ZSW reports grant support from Bristol Myers Squibb and Principia–Sanofi; and performed consultancy for Viela Bio and MedPace, outside of the submitted work. ZSW's work is supported by grants from the National Institutes of Health. JY has performed consulting for Eli Lilly, Pfizer, Aurinia, and AstraZeneca, outside of the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge financial support from the American College of Rheumatology and European Alliance of Associations for Rheumatology. The authors were not paid by a pharmaceutical company or other agency to write this article. MAG is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers K01 AR070585 and K24 AR074534 [JY]). KDW is supported by the Department of Veterans Affairs and the Rheumatology Research Foundation Scientist Development award. JAS is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers K23 AR069688, R03 AR075886, L30 AR066953, P30 AR070253, and P30 AR072577), the Rheumatology Research Foundation (K Supplement Award and R Bridge Award), the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. NJP is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (T32-AR-007258). AD-G is supported by grants from the Centers for Disease Control and Prevention and the Rheumatology Research Foundation. RH was supported by the Justus-Liebig University Giessen Clinician Scientist Program in Biomedical Research to work on this registry. JY is supported by grants from the National Institutes of Health (K24 AR074534 and P30 AR070155). The views expressed in this Article are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance, and do not necessarily represent the views of the American College of Rheumatology, the European League Against Rheumatism, the UK National Health Service, the NIHR, the UK Department of Health, or any other organisation.
Contributors
ZI and MAG directly accessed and verified the underlying data reported in the manuscript. ZI designed the data analysis, performed the literature search and the statistical analyses, developed the figures and tables, and takes responsibility for the data and analyses. ZI, MAG, SL-T, and JY contributed to data quality control. ZI, MAG, GS, PK, MID, KDW, AS, EFM, KLH, LG, LC, SL-T, MS, JAS, NJP, LW, AD-G, LL, MJS, PCR, PMM, JWL, RG, ZSW, and JY contributed to data analysis and interpretation of data and writing of the manuscript. JY supervised the work. PCR, JY, and PMM had final responsibility for the decision to submit for publication. All authors contributed to data collection and to intellectual content during the drafting of the work and approved the final version. All authors had full access to all the data in the study.
Contributor Information
COVID-19 Global Rheumatology Alliance Registry:
Brahim Dahou, Gimena Gómez, Karen Roberts, Roberto M Baez, Vanessa V Castro Coello, María J Haye Salinas, Federico N Maldonado, Alvaro A Reyes, Gelsomina Alle, Romina Tanten, Hernán Maldonado Ficco, Romina Nieto, Carla Gobbi, Yohana Tissera, Cecilia Pisoni, Alba Paula, Juan A Albiero, Maria M Schmid, Micaela Cosatti, Maria J Gamba, Carlevaris Leandro, María A Cusa, Noelia German, Veronica Bellomio, Lorena Takashima, Mariana Pera, Karina Cogo, Maria S Gálvez Elkin, María A Medina, Veronica Savio, Romina Rojas Tessel, Rodolfo P Alamino, Marina L Werner, Sofía Ornella, Luciana Casalla, Maria de la Vega, María Severina, Mercedes García, Luciana Gonzalez Lucero, Cecilia Romeo, Sebastián Moyano, Tatiana Barbich, Ana Bertoli, Andrea Baños, Sandra Petruzzelli, Carla Matellan, Silvana Conti, Maria A Lazaro, Gustavo F Rodriguez Gil, Fabian Risueño, Maria I Quaglia, Julia Scafati, Natalia L Cuchiaro, Jonathan E Rebak, Susana I Pineda, María E Calvo, Eugenia Picco, Josefina G Yanzi, Pablo Maid, Debora Guaglianone, Julieta S Morbiducci, Sabrina Porta, Natalia Herscovich, José L Velasco Zamora, Boris Kisluk, Maria S Castaños Menescardi, Rosana Gallo, María V Martire, Carla Maldini, Cecilia Goizueta, Sabrina S de la Vega Fernandez, Carolina Aeschlimann, Gisela Subils, Eva Rath, Yves Piette, Mieke Devinck, Bea Maeyaert, Francinne Machado Ribeiro, Sandra L Euzebio Ribeiro, Marcelo Pinheiro, Sebastián Ibáñez, Anne-Marie Chassin Trubert, Lingli Dong, Lui Cajas, Marko Barešić, Branimir Anić, Melanie-Ivana Ćulo, Tea A Pavelić, Kristina K Stranski, Boris Karanovic, Jiri Vencovsky, Marta Píchová, Maria Filkova, Hesham Hamoud, Dimitrios Vassilopoulos, Gabriela M Guzman Melgar, Ho So, Márta Király, Mahdi Vojdanian, Alexandra Balbir Gurman, Fatemah Abutiban, Julija Zepa, Inita Bulina, Loreta Bukauskiene, Beatriz E Zazueta Montiel, Angel A Castillo Ortiz, Erick Zamora Tehozol, David Vega Morales, Diana Cervántes Rosete, Eduardo Martín Nares, Tatiana S Rodriguez Reyna, Marina Rull Gabayet, Deshiré Alpízar Rodríguez, Fedra Irazoque, Xochitl Jimenez, Lenny Geurts van Bon, Theo Zijlstra, Monique Hoekstra, Nasra Al Adhoubi, Babur Salim, Enrique Giraldo, Ariel Salinas, Manuel Ugarte Gil, Jarosław Nowakowski, Richard Conway, Rachael Flood, Geraldine McCarthy, Ioana Felea, Ileana Filipescu, Simona Rednic, Laura Groseanu, Maria M Tamas, Vanda Mlynarikova, Martina Skamlova, Martin Zlnay, Dagmar Mičeková, Lubica Capova, Zelmira Macejova, Emőke Šteňová, Helena Raffayova, Gabriela Belakova, Eva Strakova, Marieta Senčarová, Soňa Žlnayová, Anna Sabová, Daniela Spisakova, Mária Oetterová, Olga Lukacova, Martina Bakosova, Alojzija Hocevar, Natalia de la Torre Rubio, Juan J Alegre Sancho, Montserrat Corteguera Coro, Juan C Cobeta Garcia, Maria C Torres Martin, Jose Campos, Jose A Gomez Puerta, Gozd K Yardimci, Servet Akar, Ozan C Icacan, Selda ÇELİK, Viktoriia Vasylets, Su-Ann Yeoh, Claire Vandevelde, Sasha Dunt, Jane Leeder, Elizabeth Macphie, Rosaria Salerno, Christine Graver, Katie Williams, Sheila O'Reilly, Kirsty Devine, Jennifer Tyler, Elizabeth Warner, James Pilcher, Samir Patel, Elena Nikiphorou, Laura Chadwick, Caroline M Jones, Beverley Harrison, Lucy Thornton, Diana O'Kane, Lucia Fusi, Audrey Low, Sarah Horton, Shraddha Jatwani, Sara Baig, Hammad Bajwa, Vernon Berglund, Angela Dahle, Walter Dorman, Jody Hargrove, Maren Hilton, Nicholas Lebedoff, Susan Leonard, Jennifer Morgan, Emily Pfeifer, Archibald Skemp, Jeffrey Wilson, Anne Wolff, Eduardo Cepeda, Derrick Todd, Denise Hare, Cassandra Calabrese, Christopher Adams, Arezou Khosroshahi, Adam Kilian, Douglas White, Melanie Winter, Theodore Fields, Caroline Siegel, Nicole Daver, Melissa Harvey, Neil Kramer, Concetta Lamore, Suneya Hogarty, Karen Yeter, Faizah Siddique, Byung Ban, Tamar Tanner, Eric Ruderman, William Davis, Robert Quinet, Evangeline Scopelitis, Karen Toribio, Tameka Webb Detiege, Jerald Zakem, Khurram Abbass, Gilbert Kepecs, Lilliam Miranda, Michael Guma, Ammar Haikal, Sushama Mody, Daric Mueller, Arundathi Jayatilleke, JoAnn Zell, Alison Bays, Kathryn Dao, Fatemeh Ezzati, Deborah Parks, David Karp, and Guillermo Quiceno
Supplementary Material
References
- 1.Mehta B, Pedro S, Ozen G, et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. 2019;5 doi: 10.1136/rmdopen-2019-000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimentel-Quiroz VR, Ugarte-Gil MF, Harvey GB, et al. Factors predictive of serious infections over time in systemic lupus erythematosus patients: data from a multi-ethnic, multi-national, Latin American lupus cohort. Lupus. 2019;28:1101–1110. doi: 10.1177/0961203319860579. [DOI] [PubMed] [Google Scholar]
- 3.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorci G, Faivre B, Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep. 2020;10 doi: 10.1038/s41598-020-75848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pana TA, Bhattacharya S, Gamble DT, et al. Country-level determinants of the severity of the first global wave of the COVID-19 pandemic: an ecological study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlinsky A, Kobak D. Tracking excess mortality across countries during the COVID-19 pandemic with the World Mortality Dataset. eLife. 2021;10 doi: 10.7554/eLife.69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firebaugh G. In: International Encyclopedia of the Social & Behavioral Sciences. Smelser NJ, Baltes PB, editors. Pergamon; Oxford: 2001. Ecological Fallacy, Statistics of; pp. 4023–4026. [Google Scholar]
- 8.Gianfrancesco MA, Hyrich KL, Gossec L, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin PC, Leckie G. The effect of number of clusters and cluster size on statistical power and Type I error rates when testing random effects variance components in multilevel linear and logistic regression models. J Stat Comput Simul. 2018;88:3151–3163. [Google Scholar]
- 10.Marioli FA, Bullano F, Kucinskas S, Rondón-Moreno C. Tracking R of COVID-19: a new real-time estimation using the Kalman Filter. SSRN 2020. PLoS One. 2021;16 doi: 10.1371/journal.pone.0244474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayward MD, Majmundar MK. The National Academies Press; Washington, DC: 2018. Future directions for the demography of aging: proceedings of a workshop. [PubMed] [Google Scholar]
- 12.Gelman A, Hill J. Cambridge University Press; Cambridge: 2007. Data analysis using regression and multilevel/hierarchical models. [Google Scholar]
- 13.Dey D, Sciascia S, Pons-Estel GJ, Ding H, Shen N. Health disparities in rheumatic diseases: understanding global challenges in Africa, Europe, Latin America, and Asia and proposing strategies for improvement. Rheum Dis Clin North Am. 2021;47:119–132. doi: 10.1016/j.rdc.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Phuti A, Schneider M, Tikly M, Hodkinson B. Living with systemic lupus erythematosus in the developing world. Rheumatol Int. 2018;38:1601–1613. doi: 10.1007/s00296-018-4017-1. [DOI] [PubMed] [Google Scholar]
- 15.Al Maini M, Adelowo F, Al Saleh J, et al. The global challenges and opportunities in the practice of rheumatology: white paper by the World Forum on Rheumatic and Musculoskeletal Diseases. Clin Rheumatol. 2015;34:819–829. doi: 10.1007/s10067-014-2841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson T. Rheumatology in India and Pakistan today. Rheumatology (Oxford) 2015;54:753–754. doi: 10.1093/rheumatology/keu306. [DOI] [PubMed] [Google Scholar]
- 17.Aregbeshola BS, Khan SM. Out-of-pocket payments, catastrophic health expenditure and poverty among households in Nigeria 2010. Int J Health Policy Manag. 2018;7:798–806. doi: 10.15171/ijhpm.2018.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dey D. Juggling art: making critical clinical decisions without vital laboratory support in autoimmune rheumatic patients in a resource poor setting. Ghana Med J. 2017;51:47–49. [PMC free article] [PubMed] [Google Scholar]
- 19.Pistor M, Hoepner AGF, Lin Y, et al. Immunotherapies and COVID-19 mortality: a multidisciplinary open data analysis based on FDA's Adverse Event Reporting System. Ann Rheum Dis. 2021;80:1633–1635. doi: 10.1136/annrheumdis-2021-220679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenyon C. Flattening-the-curve associated with reduced COVID-19 case fatality rates- an ecological analysis of 65 countries. J Infect. 2020;81:e98–e99. doi: 10.1016/j.jinf.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhry R, Dranitsaris G, Mubashir T, Bartoszko J, Riazi S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banik A, Nag T, Chowdhury SR, Chatterjee R. Why do COVID-19 fatality rates differ across countries? an explorative cross-country study based on select indicators. Glob Bus Rev. 2020;21:607–625. [Google Scholar]
- 23.Sabawoon W. Differences by country-level income in COVID-19 cases, deaths, case-fatality rates, and rates per million population in the first five months of the pandemic. medRxiv. 2020 doi: 10.1101/2020.07.13.20153064. published online July 15. (preprint). [DOI] [Google Scholar]
- 24.Huang Q, Jackson S, Derakhshan S, et al. Urban-rural differences in COVID-19 exposures and outcomes in the South: a preliminary analysis of South Carolina. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S, Ghosh P. Factors affecting COVID-19 infected and death rates inform lockdown-related policymaking. PLoS One. 2020;15:e0241165. doi: 10.1371/journal.pone.0241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozzer A, Dominici F, Haines A, Witt C, Münzel T, Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc Res. 2020;116:2247–2253. doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bower H, Frisell T, Di Giuseppe D, et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis. 2021;80:1086–1093. doi: 10.1136/annrheumdis-2021-219845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordtz R, Lindhardsen J, Soussi BG, et al. Incidence and severeness of COVID-19 hospitalisation in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology (Oxford) 2021;60:SI59–SI67. doi: 10.1093/rheumatology/keaa897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peach E, Rutter M, Lanyon P, et al. Risk of death among people with rare autoimmune diseases compared with the general population in England during the 2020 COVID-19 pandemic. Rheumatology (Oxford) 2021;60:1902–1909. doi: 10.1093/rheumatology/keaa855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FAI2R /SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2021;80:527–538. doi: 10.1136/annrheumdis-2020-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasseli R, Mueller-Ladner U, Hoyer BF, et al. Older age, comorbidity, glucocorticoid use and disease activity are risk factors for COVID-19 hospitalisation in patients with inflammatory rheumatic and musculoskeletal diseases. RMD Open. 2021;7 doi: 10.1136/rmdopen-2020-001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marschner IC. Estimating age-specific COVID-19 fatality risk and time to death by comparing population diagnosis and death patterns: Australian data. BMC Med Res Methodol. 2021;21:126. doi: 10.1186/s12874-021-01314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.