The endoplasmic reticulum (ER) is the organelle that produces functional proteins in eukaryotes. However, increased protein synthesis often causes protein misfolding, leading to ER stress and reciprocal activation of the unfolded protein response (UPR). The ubiquitin-proteasome system (UPS) and ER stress-associated protein degradation (ERAD) pathways remove immature proteins.
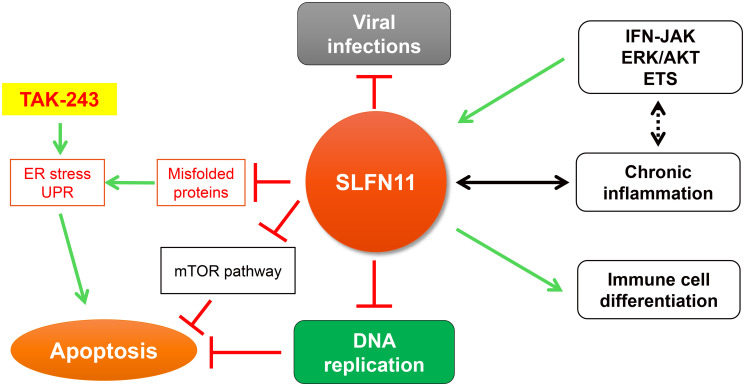
Recently, we demonstrated that Schlafen11 (SLFN11) acts as a surveillance factor for protein homeostasis by alleviating the proteotoxic stress derived from protein synthesis and maturation [1]. Schlafen (“to sleep” in German) is the name of a family of genes encompassing SLFN5, SLFN11, SLFN12, SLFN12L, SLFN13, and SLFN14 in human cells. Among the SLFN family, SLFN11 has been identified as a critical determinant for the cytotoxicity of anticancer agents targeting DNA replication across multiple cancer types. SLFN11 is recruited to damaged replication forks under replication stress. It irreversibly inhibits replication by promoting the destabilization of Cdc45-Mcm2-7-GINS (CMG) helicase complex, degrading the Chromatin Licensing and DNA Replication Factor 1 (CDT1), remodeling chromatin, and inducing immediate early genes [2, 3]. Its lack of expression in ~50% of cancer cells leads to chemoresistance. SLFN11 also plays a pivotal role inhibiting viral infection and tumorigenesis [4, 5] (Figure 1).
Figure 1. The multiple functions and interactions of SLFN11.
Red bars indicate inhibition and green arrows show stimulation.
By screening the NCATS drug library, containing 1978 compounds, we recently reported that TAK-243 (MLN7243), a first-in-class inhibitor of the ubiquitin-activating enzyme UBA1 (also known as UBE1) preferentially suppresses cell proliferation of SLFN11-deficient cancer cells [1]. TAK-243 binds free ubiquitin to form irreversible ubiquitin adducts and induces ER and proteotoxic stress [6], thereby leading to cancer cell death. We also found that cancer cells that do not express SLFN11 exhibit increased global protein ubiquitylation, ER stress and UPR compared to SLFN11-proficient cells. The increased susceptibility of SLFN11-deficient cells to TAK-243 was associated with an enhanced activation of the UPR transducers PERK, phosphorylated eIF2α, phosphorylated IRE1 and ATF6.
Given that phase 1 clinical trials with TAK-243 are underway in patients with advanced solid tumors and blood cancers (NCT02045095 and NCT03816319), our results imply that the expression status of SLFN11 might be utilized to predict therapeutic benefit of TAK-243 in cancer treatment. Regarding the mechanism of action, we identified that TAK-243-induced proteotoxic stress inhibits DNA replication by promoting Claspin-dependent CHK1 phosphorylation independently from ATR, RPA, and γ-H2AX activation. We also found interactions between SLFN11 and the protein synthesis machinery, including translation initiation factors (EIF3A, EIF3B, EIF3D, EIF3E, EIF3F, EIF3H, EIF3L, EIF3M, EIF4B, and EIF4G1) and protein folding related molecules (TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT7, and CCT8). Taken together, our findings suggest that SLFN11 plays a role in protein homeostasis and that lack of SLFN11 expression makes cells vulnerable to anticancer drugs inducing ER and proteotoxic stress (Figure 1).
A detailed immunohistochemistry and RNA expression study recently showed that SLFN11 is expressed in normal human brain and immune cells, not only cancer cells [7, 8]. Uncontrolled ER stress can be causative of multiple human diseases such as atherosclerosis, diabetes, Alzheimer’s, and Parkinson’s diseases [9]. Given that SLFN11 is involved in protein homeostasis through the regulation of cellular protein-ubiquitin adducts, SLFN11 malfunctions may be associated with human diseases beyond cancer.
Furthermore, alteration of SLFN11 could be related to immune deficiency via regulation of immune response and inflammation (Figure 1). We recently reported that SLFN11 expression is regulated by the IFN-JAK pathway and its downstream MAPK(AKT/ERK)-ETS pathway [10]. SLFN11 expression is known to change during the differentiation of B-cell-derived cancers [8] and is associated with chronic intestinal mucosal inflammation and excessive apoptosis in organoid models and patient samples of ulcerative colitis [11]. These observations suggest that SLFN11 works as a co-regulator of immune cells and as an indicator of chronic inflammation. Therefore, further studies are warranted to investigate the role of SLFN11 expression in human autoimmune and inflammatory diseases and as a predictor biomarker of response for patients treated with TAK-243.
ACKNOWLEDGMENTS
This work was supported by the Sakurai Memorial Fund for Medical Research, Hirosaki University (to Yasuhisa Murai), JSPS KAKENHI Grant Number JP21K20887 (to Yasuhisa Murai) and the Intramural Program, Center for Cancer Research of the National Cancer Institute, NIH (to Yves Pommier): Z01-BC006150.
Footnotes
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
REFERENCES
- 1.Murai Y, Jo U, Murai J, Jenkins LM, Huang SN, Chakka S, Chen L, Cheng K, Fukuda S, Takebe N, Pommier Y. SLFN11 Inactivation Induces Proteotoxic Stress and Sensitizes Cancer Cells to Ubiquitin Activating Enzyme Inhibitor TAK-243. Cancer Res. 2021; 81:3067–78. 10.1158/0008-5472.CAN-20-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murai J, Tang SW, Leo E, Baechler SA, Redon CE, Zhang H, Al Abo M, Rajapakse VN, Nakamura E, Jenkins LMM, Aladjem MI, Pommier Y. SLFN11 Blocks Stressed Replication Forks Independently of ATR. Mol Cell. 2018; 69:371–84.e6. 10.1016/j.molcel.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo U, Murai Y, Chakka S, Chen L, Cheng K, Murai J, Saha LK, Miller Jenkins LM, Pommier Y. SLFN11 promotes CDT1 degradation by CUL4 in response to replicative DNA damage, while its absence leads to synthetic lethality with ATR/CHK1 inhibitors. Proc Natl Acad Sci U S A. 2021; 118:e2015654118. 10.1073/pnas.2015654118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Kao E, Gao X, Sandig H, Limmer K, Pavon-Eternod M, Jones TE, Landry S, Pan T, Weitzman MD, David M. Codon-usage-based inhibition of HIV protein synthesis by human schlafen 11. Nature. 2012; 491:125–28. 10.1038/nature11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Liu C, Liu W, Chen W, Yin Y, Li CW, Hsu JL, Sun J, Zhou Q, Li H, Hu B, Fu P, Atyah M, et al. SLFN11 inhibits hepatocellular carcinoma tumorigenesis and metastasis by targeting RPS4X via mTOR pathway. Theranostics. 2020; 10:4627–43. 10.7150/thno.42869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyer ML, Milhollen MA, Ciavarri J, Fleming P, Traore T, Sappal D, Huck J, Shi J, Gavin J, Brownell J, Yang Y, Stringer B, Griffin R, et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat Med. 2018; 24:186–93. 10.1038/nm.4474. [DOI] [PubMed] [Google Scholar]
- 7.Takashima T, Sakamoto N, Murai J, Taniyama D, Honma R, Ukai S, Maruyama R, Kuraoka K, Rajapakse VN, Pommier Y, Yasui W. Immunohistochemical analysis of SLFN11 expression uncovers potential non-responders to DNA-damaging agents overlooked by tissue RNA-seq. Virchows Arch. 2021; 478:569–79. 10.1007/s00428-020-02840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moribe F, Nishikori M, Takashima T, Taniyama D, Onishi N, Arima H, Sasanuma H, Akagawa R, Elloumi F, Takeda S, Pommier Y, Morii E, Takaori-Kondo A, Murai J. Epigenetic suppression of SLFN11 in germinal center B-cells during B-cell development. PLoS One. 2021; 16:e0237554. 10.1371/journal.pone.0237554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol. 2017; 13:477–91. 10.1038/nrneurol.2017.99. [DOI] [PubMed] [Google Scholar]
- 10.Murai Y, Jo U, Murai J, Fukuda S, Takebe N, Pommier Y. Schlafen 11 expression in human acute leukemia cells with gain-of-function mutations in the interferon-JAK signaling pathway. iScience. 2021; 24:103173. 10.1016/j.isci.2021.103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe S, Nishimura R, Shirasaki T, Katsukura N, Hibiya S, Kirimura S, Negi M, Okamoto R, Matsumoto Y, Nakamura T, Watanabe M, Tsuchiya K. Schlafen 11 Is a Novel Target for Mucosal Regeneration in Ulcerative Colitis. J Crohns Colitis. 2021; 15:1558–72. 10.1093/ecco-jcc/jjab032. [DOI] [PubMed] [Google Scholar]