Abstract
Pesticides are widely employed as a cost‐effective means of reducing the impacts of undesirable plants and animals. The aim of this paper is to develop a risk ranking of transmission of key pesticides through soil to waterways, taking into account physico‐chemical properties of the pesticides (soil half‐life and water solubility), soil permeability, and the relationship between adsorption of pesticides and soil texture. This may be used as a screening tool for land managers, as it allows assessment of the potential transmission risks associated with the use of specified pesticides across a spectrum of soil textures. The twenty‐eight pesticides examined were differentiated into three groups: herbicides, fungicides and insecticides. The highest risk of pesticide transmission through soils to waterways is associated with soils containing <20% clay or >45% sand. In a small number of cases, the resulting transmission risk is not influenced by soil texture alone. For example, for Phenmedipham, the transmission risk is higher for clay soils than for silt loam. The data generated in this paper may also be used in the identification of critical area sources, which have a high likelihood of pesticide transmission to waterways. Furthermore, they have the potential to be applied to GIS mapping, where the potential transmission risk values of the pesticides can be layered directly onto various soil textures.
Keywords: adsorption, Freundlich, half‐life, pesticides, soil texture
1. INTRODUCTION
A pesticide is any substance, plant protection product or biocide, that is used to repel, control or kill organisms that are considered to be pests (DAFM, 2017). The umbrella term “pesticides” includes herbicides, fungicides, insecticides, molluscicides, bactericides and rodenticides (Mojiri et al., 2020). In Europe, total annual pesticide sales during the period 2011 to 2016 rose from 386,400 to 439,400 tonnes of active ingredients, with France, Spain, Italy and Germany collectively accounting for 80% of the European market (Peña, 2020). In line with increases in global population, the use of pesticides in agriculture has increased to improve crop yields and production rates (Gavrilescu, 2005; Morillo & Villaverde, 2017). While this intensified pesticide application has been beneficial in preventing hazardous diseases in agricultural crops (Maggi et al., 2020), it has also amplified the contact of these compounds with soil (Morillo & Villaverde, 2017), air (Raherison et al., 2019) and aquatic environments (Burri et al., 2019) and increased the risk of subsequent human exposure. This has resulted in human health issues, such as neurological, respiratory and carcinogenic effects (Pouchieu et al., 2018; Van Maele‐Fabry et al., 2017; Ye et al., 2017). In 2007, globally, there was an estimated 258,000 deaths from pesticide self‐poisoning (WHO, 2016).
It has been suggested that, under “worst case” scenarios, such as improper handling and unfavourable weather condition, as little as 1% of applied pesticides may reach their target organism, with the remainder entering soil and water environments (Ali et al., 2019), via direct losses, runoff, spray drift or leaching (Álvarez‐Martín et al., 2017; Cosgrove et al., 2019; Haddad et al., 2019; Mojiri et al., 2020), resulting in contamination of surface water or groundwater (Rojas et al., 2014). The European Environment Agency (EEA) reported that, of the 73,510 natural water bodies with known chemical and ecological status in the European Union (EU), 25,108 failed to achieve good chemical status (EEA, 2018), due to hydromorphological pressures, diffuse water pollution from agricultural practices, waste water treatment plants and sewage systems, as well as high inflow of nutrients and chemical contaminants including pesticides leading to accelerated loss of biodiversity (EEA, 2016).
Mathematical models are now widely used to predict the fate and transport of pesticides in the environment (Bach et al., 2017; D’Andrea et al., 2020; Hartz et al., 2017; Rumschlag et al., 2019). Modelling presents an appealing alternative to environmental monitoring, which is costly and time‐consuming. Modelling is fast, cost‐effective and can predict how soil and climate conditions may affect, for example the environmental fate of pesticides (Bach et al., 2017; McGrath et al., 2019). The main factors influencing the transport of pesticides to receptors are soil half‐life (DT50; Fantke et al., 2014), adsorption and desorption to and from soil particles (Paszko & Jankowska, 2018), and physico‐chemical properties of soil (Boivin et al., 2005). The adsorption of pesticides on the soil surface determines how pesticides are either transported or degraded, which ultimately determines the concentration of pesticides in both soil and soil solution (Gondar et al., 2013). Adsorption is predominantly influenced by the properties and chemical composition of the soil, which is a complex mixture of inorganic materials and organic matter (Leovac et al., 2015), and the physicochemical properties of the pesticide (Kodešová et al., 2011). The relationship between the organic content of the soil and pesticide adsorption has been well examined in the literature (Rojas et al., 2013; Wei et al., 2015; Wu et al., 2018). However, the organic content of soil changes with time (Smith, 2004), meaning that it may not be a reliable metric for determining areas of high risk of pesticide loss in agricultural land management. The organic content of soil is also difficult to map, as it depends on soil and crop management practices. Conversely, the texture of the soil will remain more or less constant over time (Brouwer et al., 1985). A database of existing studies quantifying the relationship between adsorption of pesticides and the texture of the soil, using adsorption isotherm coefficients as a metric, could be a valuable tool in screening and in decision management protocols for the safe use of pesticides on certain soil textures. Although many soil factors have been investigated with regard to pesticide adsorption, including pH (Gondar et al., 2013; Kodešová et al., 2011), organic content (Boivin et al., 2005; Conde‐Cid et al., 2019), pore size (Siek & Paszko, 2019) and cation exchange capacity (Kodešová et al., 2011), to date no study has conducted a meta‐analysis of the literature that investigates the relationship between pesticide adsorption and soil texture.
Pesticide transport models used for national pesticide registration and licensing in the European Union, such as the FOCUS group's PRZM modelling approach, are highly complex models which take hours to run for a single pesticide (European Soil Data Centre, 2022a, 2022b). Complex and data‐hungry pesticide transport modelling software, as is used for pesticide licensing and registration in the EU, is not realistic or suitable for use by small‐scale pesticide users or localized pesticide management projects. Instead a quick and easily applied screening tool, such as that which is outlined in this paper, is proposed as a more practical tool for pesticide users in this case.
Therefore, the aim of this paper is to conduct a meta‐analysis of literature that has assessed pesticide adsorption and soil texture data and integrate this with pesticide properties such as soil half‐life and solubility, in order to determine if a relationship exists that could guide future modelling and decision‐making protocols regarding the safe use of pesticides. This information may be used in the identification of critical source areas, which would have a high likelihood of pesticide transmission to groundwater, or as an application in GIS mapping where the potential groundwater transmission risk values of the pesticides can be layered directly onto the various soil textures.
2. MATERIALS AND METHODS
2.1. Literature review methodology, pesticide selection and grouping
A detailed literature search was undertaken by searching keywords including the following: pesticide, soil, adsorption, sorption, adsorption isotherm and soil texture triangle. The search was limited to peer‐reviewed papers published, in English, since 2000 that included data on adsorption isotherm parameters and soil texture. Several reports were found in languages other than English (see, e.g. Regitano et al., 2002; Rocha et al., 2013) but, as these did not met the criteria outlined above, they were not included. No geographical limitations were employed. Search engines used included databases such as Scopus, as well as publisher‐specific search engines including ScienceDirect, the American Chemical Society and the Royal Society of Chemistry. References from several papers found in these searches were also examined for relevant information. Research papers were selected based on the relevance to the review, with a target on the most commonly used pesticides in articles. A total of 1212 articles and a small number of book chapters and reports were reviewed.
Following this, the pesticides were ranked according to the number of studies in which they were investigated and they also had to be currently approved for use by the EU. This resulted in a short‐list of 54 publications, reporting on the 28 most commonly studied pesticides, which are still available for use and are not banned in the EU or elsewhere. These 28 pesticides were grouped into herbicides, fungicides and insecticides, with no molluscicides, bactericides or rodenticides present in that group.
2.1.1. Herbicide group
Herbicides are chemical agents which are used to kill or inhibit unwanted plants or weeds (Oliveira et al., 2020; Thiour‐Mauprivez et al., 2019). They can act as contact herbicides, which kill only the plant parts contacted by the chemical agent, or as systemic herbicides, which are absorbed through the roots or leaves of the plant and then moved to a different location within the plant. Furthermore, herbicide activity can be selective or non‐selective. Selective herbicides kill unwanted plants without critical damage to the preferred plants. On the other hand, non‐selective herbicides kill or injure all plants present. This study assessed seventeen different herbicides, employed to protect a range of crops, by targeting different weed species (Table 1).
TABLE 1.
Applications, target pests and physicochemical properties of selected pesticides a
| Pesticide | Crop/Site | Target pest | MW | SW | Log KOW | DT50 lab |
|---|---|---|---|---|---|---|
| Herbicide | ||||||
| 2,4‐D | Cereals, grass, amenity use | Broad‐leaved weeds | 221.04 | 24,300 | −0.82 | 4.4 |
| Bensulfuron‐methyl | Cereals | Weeds, sedges | 410.4 | 67 | 0.79 | 77 |
| Bentazone | Cereals, vegetables | Annual weeds | 240.3 | 7112 | −0.46 | 20 |
| Chlorotoluron | Cereals, vegetables, fruit | Broad‐leaved weeds, grasses | 212.68 | 74 | 2.5 | 45 |
| Dimethenamid‐P | Vegetables, vineyards | Broad‐leaved weeds, grasses | 275.8 | 1499 | 1.89 | 12.1 |
| Ethofumesate | Beet, vegetables | Broad‐leaved weeds, grasses | 286.34 | 50 | 2.7 | 21.6 |
| Glyphosate | Agriculture, horticulture, amenity use | Broad‐leaved weeds, grasses | 169.1 | 10,500 | −3.2 | 15 |
| Isoxaflutole | Crops | Broad‐leaved weeds, grasses | 359.32 | 6.2 | 2.34 | 0.9 |
| Lenacil | Beet, vegetables, fruit | Broad‐leaved weeds, grasses | 234.29 | 2.9 | 1.69 | 49.7 |
| MCPA | Cereals, grass | Broad‐leaved weeds, rushes | 200.62 | 29,390 | −0.81 | 24 |
| Mecoprop‐P | Cereals, grass, amenity use | Broad‐leaved weeds | 214.65 | 250,000 | −0.19 | 5.24 |
| Metamitron | Beet crops | Broad‐leaved weeds, grasses | 202.21 | 1770 | 0.85 | 19 |
| Metribuzin | Cereals, vegetables | Broad‐leaved weeds, grasses | 214.29 | 10,700 | 1.75 | 7.03 |
| Metsulfuron‐methyl | Cereals, land removed from production | Broad‐leaved weeds | 381.36 | 2790 | −1.87 | 23.2 |
| Pendimethalin | Cereals, vegetables, vineyards | Broad‐leaved weeds, grasses | 281.31 | 0.33 | 5.4 | 182.3 |
| Phenmedipham | Beet, vegetables | Broad‐leaved weeds | 300.31 | 1.8 | 2.7 | 12 |
| Terbuthylazine | Cereals, vegetables, non‐crop sites | Broad‐leaved weeds, grasses, slime‐forming algae | 229.71 | 6.6 | 3.4 | 72 |
| Fungicide | ||||||
| Azoxystrobin | Cereals, vegetables | Broad‐spectrum | 403.4 | 6.7 | 2.5 | 84.5 |
| Metalaxyl | Many agricultural crops | Air‐ and soil‐borne Peronosporales | 279.33 | 8400 | 1.75 | 7.1 |
| Metalaxyl‐M | Potatoes, vegetables | Air‐ and soil‐borne pathogens | 279.33 | 26,000 | 1.71 | 6.5 |
| Myclobutanil | Perennial and annual crops, fruit, vines | Ascomycetes, Fungi Imperfecti and Basidiomycetes | 288.78 | 132 | 2.89 | 365 |
| Penconazole | Vines, fruit, vegetables | Fungal pathogens | 284.18 | 73 | 3.72 | 117.2 |
| Pyrimethanil | Fruit, vegetables, nuts | Fungal pathogens | 199.28 | 110 | 2.84 | 50.9 |
| Tebuconazole | Cereals, vegetables, vines | Foliar diseases | 307.82 | 36 | 3.7 | 365 |
| Thiabendazole | Cereals, fruit, vegetables | Post‐harvest fungicide | 201.25 | 30 | 2.39 | 1000 |
| Insecticide | ||||||
| Abamectin | Fruit, vegetables | Selective acaricide, nematicide and insecticide | 866.6 | 0.02 | 4.4 | 25.3 |
| α‐Cypermethrin | Cereals, vegetables, beet, fruit, grassland | Broad spectrum | 416.3 | 0.009 | 5.55 | 22.1 |
| Deltamethrin | Cereals, fruit, vegetables, public and industrial buildings | Wide range of sucking and chewing pests | 505.2 | 0.0002 | 4.6 | 28.2 |
Pesticide properties database online (http://sitem.herts.ac.uk/areu/ppdb/en/index.htm). MW, Molecular weight (g mol−1); SW, water solubility (20°C, mg l−1); KOW, Octanol‐water partition coefficient at pH 7, 20°C; DT50 lab, 50% dissipation time under laboratory conditions (days).
2.1.2. Fungicide group
Fungicides can work preventatively or curatively, by either preventing the fungus from infecting the plant, or by partially or entirely treating an existing fungal infestation (Tleuova et al., 2020; Zhang et al., 2020). Like herbicides, they can act as contact fungicides, preventing the fungus from entering the plant, or as systemic fungicides, which are internalized by the plant and are then moved to a different site within the plant. This study assessed the transmission risk of eight different fungicides (Table 1).
2.1.3. Insecticide group
Chemical insecticides are employed to control harmful insects, as a result of either killing the insect or preventing it from doing destructive damage to plants. During the 1950s, the majority of insecticides operated from four different chemical groups (DDT and analogues, Organophosphates, Carbamates and Cyclodienes) using three modes of action (Sparks et al., 2019). These modes of action were inhibition of the acetylcholinesterase, modulation of the voltage‐gated sodium channel and blockage of the gamma‐aminobutyric acid‐gated chloride channel (Sparks et al., 2019). By 2019, this number had increased to 25 different modes of action based on 55 different chemical classes (Swale, 2019). The current study assessed the transmission risk of three insecticides (Table 1). The number of insecticide studies included in our meta‐analysis is low due to the small number of studies that fulfilled our criteria of (i) including an approved insecticide and (ii) reporting soil texture data.
2.2. Adsorption modelling
The manuscripts that fulfilled the selection criteria of this study (Supporting information Excel file) modelled their experimental data using the Freundlich adsorption isotherm, with some also reporting the parameters of the Langmuir adsorption isotherm. The main assumption of the Langmuir adsorption isotherm model is monolayer adsorption, so all potential adsorption sites are treated equivalently (Langmuir, 1918). The Freundlich adsorption model can better describe adsorption on a heterogeneous surface (Freundlich, 1907) and is commonly used to describe pesticide adsorption in soil (Hiller et al., 2012; Papadopoulou et al., 2016; Wang et al., 2020), implying that monolayer adsorption is not representative of pesticide adsorption in soil. To facilitate comparative analysis within this paper, only the Freundlich model was used for determination of the adsorption isotherm coefficients. The Freundlich isotherm model is:
| (1) |
where q e is the amount of adsorbate adsorbed at the equilibrium (mg g−1) and C e is the concentration of the adsorbate at the equilibrium (mg L−1); K F is the Freundlich sorption capacity coefficient (mg g−1 (mg L−1)−1/n) and the exponent n is the Freundlich exponent (dimensionless) (Lima et al., 2015). The adsorption of pesticides on soils can be described using the linear form of the Freundlich equation (Papazlatani et al., 2019):
| (2) |
The Freundlich sorption capacity coefficient K F (mg g−1 (mg L−1)−1/n) represents the pesticide affinity for soil, with a high K F value indicating a stronger adsorption for the pesticide and also suggesting a lower mobility of the pesticide in the soil (Wang et al., 2020).
2.3. Pesticide transport potential ranking
The movement of pesticides from the target crop through the soil and to the water receptor is a function of soil permeability (m s−1), the adsorption capacity of each soil texture for the investigated pesticide (g m−3), soil half‐life of the pesticide (DT50, days) and the pesticide solubility in water (Sw; mg L−1). In order to establish a soil texture‐specific transport potential risk ranking for each of the pesticide groups examined in this study, a ranking system incorporating each of these parameters was developed with the highest value indicative of the greatest risk of transmission to receiving waters. The permeability of soils is well documented and was ranked according to soil texture (USDA, 2022). Soil adsorption values were generated from the median value for each pesticide/soil texture association reported in the literature (Supplementary Information, Excel file and Table S1). The water solubility and soil half‐life values were obtained from the Pesticide Properties DataBase (Tables S2 and S3, respectively; Lewis et al., 2016). Using this rubric, each parameter was independently ranked from one to twelve, where twelve was considered to be the highest risk for pesticide mobility through soil to surface and groundwater bodies, that is high permeability soils, low pesticide adsorption capacity, high soil half‐life and high water solubility. In this study, high permeability soils were considered to be most at risk for surface and groundwater pollution. If surface water processes were only considered, low permeability soils, which would have large surface runoff potential relative to surface flow, would be considered to be most at risk. Finally, these independent risk values were combined (with equal weighting) to give a final risk ranking for each pesticide across all soil textures, but also for all of the pesticides within an individual soil texture classification.
3. RESULTS AND DISCUSSION
3.1. Variances in adsorption as a function of soil texture
Table 2 shows the potential pesticide transmission risks as a function of water solubility, soil half‐life, adsorption by soil of the pesticide and also soil texture. The potential transmission risk can be quantified either on the basis of soil texture or pesticide type, with the highest score in each case being the most transmissible.
TABLE 2.
Pesticide transmission risk rankings a
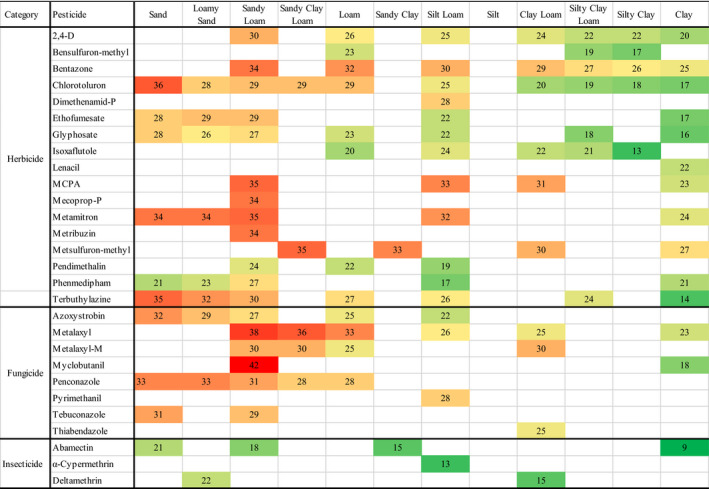
Total transmission risk ranking = Risk rankings for Permeability +Adsorbency + Solubility +Half‐life (Table S5). The higher the score, the higher the risk for transmission through soil to waterways. The colour of the ranking value indicates the likelihood of potential transmission risk, with red being most likely and green being least likely.
It is unfortunate that there are not complete adsorption isotherm data studies across the soil texture triangle for each of the selected herbicides, fungicides and insecticides. These data would facilitate a better understanding of the potential pesticide transmission risk across all soil textures. Given the current findings, it is impossible to assess the potential transmission risk of pesticides in silt or sandy clay, as no data are available for silt and only limited data are available for silty clay textures.
The highest potential transmission risk ranking for each individual pesticide across all herbicides, fungicides and insecticides shows that the soil textures resulting in highest transmission risks are sandy loam and sand, with nineteen of the highest rankings being in one of these two soil textures (Table 2). These two soil textures have low clay content (<20%), implying that a high clay content is important in the retention of pesticides within the soil, as previously reported (García‐Delgado et al., 2020; Ren et al., 2018; Vitoratos et al., 2016). This is in agreement with Komárek et al. (2010), who highlighted that the possible factors influencing pesticide adsorption were physico‐chemical properties of the pesticides and soil properties, such as particle size, soil organic matter and clay content. Komárek et al. (2010) also state that generalizing the behaviour of fungicides in soil is difficult to predict, given the different sorption, mobility and toxicity properties each will have, which is inferred from their different chemical structures. ElGouzi et al. (2012) showed, in their work on adsorption of phenylurea pesticides by Mediterranean soils, that soils with relatively high clay content were better at pesticide retention. García‐Delgado et al., (2020) suggest that the addition of organic amendments to soils, such as spent mushroom substrate, compost, manure or sewage sludge, is an effective method of immobilizing pesticides in the soil as a result of increasing the organic content of the soil. Furthermore, both of these soil textures have a high sand content (>45%), which would suggest that soil textures having a high sand content are also susceptible to high potential transmission risk of pesticides.
The potential risk ranking values (Table 2) for the herbicide group range from 36 (for Chlorotoluron in sand) to 13 (for Isoxaflutole in silty clay). The majority of high values (>30), shown in red and orange, reside in the left hand side of Table 2. The soil textures in this group of sand, loamy sand, sandy loam, sandy clay loam, loam and sandy clay all have a sand content of ≥50%, except for the loam texture where the sand content is 25%. This would imply that there is a high risk of herbicide transmission if the soil contains a high sand content. Although limited adsorption data are available in the literature for the three herbicides with the highest solubility (Mecoprop‐P, MCPA and 2,4‐D), the trends observed for other pesticides indicate that it is likely that these herbicides would pose a high transmission risk in either sand or loamy sand textured soils.
There are two different ways that the data in Table 2 can be interpreted. The data can be viewed from the point of view of the pesticide. Considering the herbicide chlorotoluron, for example the potential risk ranking varies from 36 in sand to 17 in clay. Therefore, the soil textures most likely to transmit chlorotoluron may be identified. Alternatively, the data may be examined considering only soil texture. Within sandy loam soils, for example MCPA, Mecoprop‐P, Bentazone, Metamitron and Metribuzin are some of the highest risk herbicides, with ranking values of 35, 34, 34, 35 and 34, respectively (Table 2). As Pendimethalin, also used for the removal of broad‐leaved weeds from cereals (Table 1), has a much lower transmission ranking value in sandy loam soils (24, Table 2), it might be more appropriate for selection when applying to this soil texture. In a similar manner, the choice of Terbuthylazine (14, Table 2) would be appropriate, when considering removing broad‐leaved weeds and grasses from cereal and vegetable crops in clay soil, than any of the other herbicides in this study (16–27, Table 2).
In the case of the selected fungicides, the majority of high values (>30) reside in the left hand side of Table 2, Indeed, Metalaxyl‐M has equally high potential transmission risk rankings across the range of soil textures. Furthermore, transmission risks are available for most fungicides for sandy loam soils (Table 2). As the transmission risk of Azoxystrobin was deemed to be the lowest of the eight fungicides (Table 2), then the selection of Azoxystrobin for application on sandy loam soils could be proposed as a management tool to minimize the risk of fungicide transmission through soil to waterways. Specifically, Tebuconazole (27, Table 2) could be a suitable alternative to Metalaxyl or Metalaxyl‐M (38 and 30, Table 2) for the control of air‐borne pathogens of vegetables grown in sandy loam soil (Table 1).
Of the three insecticides, Deltamethrin has the higher transmission risk rankings across all textures (Table 2). The transmission risk for Abamectin was much greater in sandy soils (21, Table 2) than in clay soils (9, Table 2), again demonstrating the potential for applying the proposed transmission risk ranking scheme to pesticide selection and management. Consideration of the reported transmission risk ranking, based on soil texture, crop and target pest, will contribute to decision‐making practices for safer pesticide use.
4. CONCLUSIONS
Using soil texture‐specific adsorption isotherm data for several groups of pesticides, their solubility in water, soil half‐life and soil permeability, a transmission risk ranking was developed in this study. This is designed as a decision‐making support tool for agricultural land management, as it allows the agricultural sector to assess, either by soil texture or pesticide type, the risk of loss of pesticides to receptors. While this is a simple decision making support tool, rather than the more complicated and complex PRZM modelling approach (European Soil Data Centre, 2022b), it offers a manageable choice for the end user. It is also useful for modelling the loss of pesticides to water and for identification of critical source areas for better land management. The risk ranking index demonstrated specific examples of support for decision making, such as that pendimethalin is a lower transmission risk option than MCPA, Mecoprop‐P, Bentazone, Metamitron and Metribuzin in the removal of broad‐leaved weeds from cereal crops. It has also illustrated that the fungicide, Azoxystrobin, is a lower transmission risk alternative to either Metalaxyl or Metalaxyl‐M in sandy loam soil.
The risk ranking index indicated that there is a high risk of transmission of pesticides from soils containing <20% clay. Furthermore, the data suggest that, if the soil content contains more than 45% sand, then there is a much higher risk of potential pesticide transmission. There are several reports in the literature discussing the movement of pesticides through soil. However, the aim of this paper was to develop a tool that the farmer could easily access to see if the pesticide of choice for the required job was environmentally friendly or if there was a potential threat to the environment through its use. Further analysis should be undertaken to examine potential transmission risk rankings of pesticides not selected in this review, across all soil textures.
DISCLAIMER
Although every effort has been made to ensure the accuracy of the material contained in this journal paper, complete accuracy cannot be guaranteed. Neither the Environmental Protection Agency nor the authors accept any responsibility whatsoever for loss or damage occasioned or claimed to have been occasioned, in part or in full, as a consequence of any person acting or refraining from acting, as a result of a matter contained in this journal paper.
Supporting information
Table S1‐S5
ACKNOWLEDGEMENTS
This project is funded under the Irish EPA Research Programme 2014‐2020 (Project reference number 2019‐HW‐LS‐3). The EPA Research Programme is a Government of Ireland initiative funded by the Department of Communications, Climate Action and Environment. It is administered by the Environmental Protection Agency, which has the statutory function of co‐ordinating and promoting environmental research. Open access funding enabled and organized by IRel.
McGinley, J. , Harmon O’Driscoll, J. , Healy, M. G. , Ryan, P. C. , Mellander, P. E. , Morrison, L. , Callery, O. , & Siggins, A. (2022). An assessment of potential pesticide transmission, considering the combined impact of soil texture and pesticide properties: A meta‐analysis. Soil Use and Management, 38, 1162–1171. 10.1111/sum.12794
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- Ali, I. , Alharbi, O. M. L. , Othman, Z. A. , Al‐Mohaimeed, A. M. , & Alwarthan, A. (2019). Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water. Chemosphere, 170, 389–397. 10.1016/j.envres.2018.12.066 [DOI] [PubMed] [Google Scholar]
- Álvarez‐Martín, A. , Sánchez‐Martín, M. J. , Ordax, J. M. , Marín‐Benito, J. M. , & Rodríguez‐Cruz, M. S. (2017). Leaching of two fungicides in spent mushroom substrate amended soil: Influence of amendment rate, fungicide ageing and flow condition. Science of the Total Environment, 584–585, 828–837. 10.1016/j.scitotenv.2017.01.126 [DOI] [PubMed] [Google Scholar]
- Bach, M. , Diesner, M. , Großmann, D. , Guerniche, D. , Hommen, U. , Klein, M. , Kubiak, R. , Müller, A. , Preuss, T. G. , Priegnitz, J. , Reichenberger, S. , Kai, T. , & Trapp, M. (2017). Pesticide exposure assessment for surface waters in the EU. Part 2: Determination of statistically based run‐off and drainage scenarios for Germany. Pest Management Science, 73, 852–861. 10.1002/ps.4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin, A. , Cherrier, R. , & Schiavon, M. (2005). A comparison of five pesticides adsorption and desorption processes in thirteen contrasting field soils. Chemosphere, 61, 668–676. 10.1016/j.chemosphere.2005.03.024 [DOI] [PubMed] [Google Scholar]
- Brouwer, C. , Goffeau, A. , & Heibloem, M. (1985). Irrigation water management – training manual no. 1, introduction to irrigation, chapter 2 soil and water. Food and Agricultural Organisation of the United Nations. [Google Scholar]
- Burri, N. M. , Weatherl, R. , Moeck, C. , & Schirmer, M. (2019). A review of threats to groundwater quality in the anthropocene. Science of the Total Environment, 684, 136–154. 10.1016/j.scitotenv.2019.05.236 [DOI] [PubMed] [Google Scholar]
- Conde‐Cid, M. , Santás‐Miguel, V. , Campillo‐Cora, C. , Pérez‐Novo, C. , & Fernández‐Calviño, D. (2019). Retention of propiconazole and terbutryn on acid sandy‐loam soils with different organic matter and Cu concentrations. Journal of Environmental Management, 248, 109346–109353. 10.1016/j.jenvman.2019.109346 [DOI] [PubMed] [Google Scholar]
- Cosgrove, S. , Jefferson, B. , & Jarvis, P. (2019). Pesticide removal from drinking water sources by adsorption: A review. Environmental Technology Reviews, 8, 1–24. 10.1080/21622515.2019.1593514 [DOI] [Google Scholar]
- D’Andrea, M. F. , Letourneau, G. , Rousseau, A. N. , & Brodeur, J. C. (2020). Sensitivity analysis of the pesticide in water calculator model for applications in the Pampa region of Argentina. Science of the Total Environment, 698, 134232–134244. 10.1016/j.scitotenv.2019.134232 [DOI] [PubMed] [Google Scholar]
- Department of Agriculture, Food and the Marine (DAFM) . (2017). Pesticide usage in Ireland, grassland and fodder crops survey report. [Google Scholar]
- ElGouzi, S. , Mingorance, M. D. , Draoui, K. , Chtoun, E. H. , & Peña, A. (2012). Assessment of phenylurea herbicides sorption on various Mediterranean soils affected by irrigation with wastewater. Chemosphere, 89, 334–339. 10.1016/j.chemosphere.2012.04.051 [DOI] [PubMed] [Google Scholar]
- European Environment Agency (EEA) . (2016). European water policies and human health. European Environment Agency (EEA). Combining reported environmental information. 10.2800/821091 [DOI] [Google Scholar]
- European Environment Agency (EEA) . (2018). Chemicals in european waters. Knowledge Developments. European Environment Agency (EEA). 10.2800/265080 [DOI] [Google Scholar]
- European Soil Data Centre . (2022a). FOCUS DG SANTE. European Soil Data Centre. Retrieved from https://esdac.jrc.ec.europa.eu/projects/focus‐dg‐sante [Google Scholar]
- European Soil Data Centre . (2022b). PRZM_SW. European Soil Data Centre. Retrieved from <https://esdac.jrc.ec.europa.eu/projects/przmsw> [Google Scholar]
- Fantke, P. , Gillespie, B. W. , Juraske, R. , & Jolliet, O. (2014). Estimating half‐lives for pesticide dissipation from plants. Environmental Science and Technology, 48, 8588‐8602. 10.1021/es500434p [DOI] [PubMed] [Google Scholar]
- Freundlich, H. (1907). Over the adsorption in solution. Zeitschrift für Physikalische Chemie, 57, 385–470. [Google Scholar]
- García‐Delgado, C. , Marín‐Benito, J. M. , Sánchez‐Martín, M. J. , & Rodríguez‐Cruz, M. S. (2020). Organic carbon nature determines the capacity of organic amendments to adsorb pesticides in soil. Journal of Hazardous Materials, 390, 122162–122170. 10.1016/j.jhazmat.2020.122162 [DOI] [PubMed] [Google Scholar]
- Gavrilescu, M. (2005). Fate of pesticides in the environment and its bioremediation. Engineering in Life Sciences, 5, 497–526. 10.1002/elsc.200520098 [DOI] [Google Scholar]
- Gondar, D. , López, R. , Antelo, J. , Fiol, S. , & Arce, F. (2013). Effect of organic matter and pH on the adsorption of metalaxyl and penconazole by soils. Journal of Hazardous Materials, 260, 627–633. 10.1016/j.jhazmat.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Haddad, K. , Gheid, A. , Haddad, D. , & Oulmi, K. (2019). Experimental and numerical study on the leaching of pesticides into the groundwater through a porous medium: Effects of transport parameters. Environmental Technology & Innovation, 13, 244–256. 10.1016/j.eti.2018.12.009 [DOI] [Google Scholar]
- Hartz, K. E. H. , Edwards, T. M. , & Lydy, M. J. (2017). Fate and transport of furrow‐applied granular tefluthrin and seed‐coated clothianidin insecticides: Comparison of field‐scale observations and model estimates. Ecotoxicology, 26, 876–888. 10.1007/s10646-017-1818-z [DOI] [PubMed] [Google Scholar]
- Hiller, E. , Tatarková, V. , Šimonovičová, A. , & Bartal, M. (2012). Sorption, desorption, and degradation of (4‐chloro‐2‐methylphenoxy)acetic acid in representative soils of the Danubian Lowland, Slovakia. Chemosphere, 87, 437–444. 10.1016/j.chemosphere.2011.12.021 [DOI] [PubMed] [Google Scholar]
- Kodešová, R. , Kočárek, M. , Kodeš, V. , Drábek, O. , Kozák, J. , & Hejtmánková, K. (2011). Pesticide adsorption in relation to soil properties and soil type distribution in regional scale. Journal of Hazardous Materials, 186, 540–550. 10.1016/j.jhazmat.2010.11.040 [DOI] [PubMed] [Google Scholar]
- Komárek, M. , Čadková, E. , Chrastný, V. , Bordas, F. , & Bollinger, J.‐C. (2010). Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environment International, 36, 138–151. 10.1016/j.envint.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica, and platinum. Journal of the American Chemical Society, 40, 1361–1403. 10.1021/ja02242a004 [DOI] [Google Scholar]
- Leovac, A. , Vasyukova, E. , Ivančev‐Tumbas, I. , Uhl, W. , Kragulj, M. , Tričković, J. , Kerkez, Đ. , & Dalmacija, B. (2015). Sorption of atrazine, alachlor and trifluralin from water onto different geosorbents. RSC Advances, 5, 8122–8133. 10.1039/c4ra03886j [DOI] [Google Scholar]
- Lewis, K. A. , Tzilivakis, J. , Warner, D. , & Green, A. (2016). An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment: an International Journal, 22, 1050–1064. 10.1080/10807039.2015.1133242 [DOI] [Google Scholar]
- Lima, E. C. , Adebayo, M. A. , & Machado, F. M. (2015). Chapter 3 kinetic and equilibrium models of adsorption. In Bergmann C. P., & Machado F. M. (Eds). Carbon nanomaterials as adsorbents for environmental and biological applications (pp. 33‐69). Springer; 10.1007/978-3-319-18875-1_3 [DOI] [Google Scholar]
- Maggi, F. , la Cecilia, D. , Tang, F. H. M. , & McBratney, A. (2020). The global environmental hazard of glyphosate use. Science of the Total Environment, 717, 137167–137178. 10.1016/j.scitotenv.2020.137167 [DOI] [PubMed] [Google Scholar]
- McGrath, G. , Rao, P. S. C. , Mellander, P.‐E. , Kennedy, I. , Rose, M. , & van Zwieten, L. (2019). Real‐time forecasting of pesticide concentrations in soil. Science of the Total Environment, 663, 709–717. 10.1016/j.scitotenv.2019.01.401 [DOI] [PubMed] [Google Scholar]
- Mojiri, A. , Zhou, J. L. , Robinson, B. , Ohashi, A. , Ozaki, N. , Kindaichi, T. , Farraji, H. , & Vakili, M. (2020). Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere, 253, 126646–126669. 10.1016/j.chemosphere.2020.126646 [DOI] [PubMed] [Google Scholar]
- Morillo, E. , & Villaverde, J. (2017). Advanced technologies for the remediation of pesticide‐contaminated soils. Science of the Total Environment, 586, 576–597. 10.1016/j.scitotenv.2017.02.020 [DOI] [PubMed] [Google Scholar]
- Oliveira, M. C. , Osipitan, O. A. , Begcy, K. , & Werle, R. (2020). Cover crops, hormones and herbicides: Priming an integrated weed management strategy. Plant Science, 301, 110550–110554. 10.1016/j.plantsci.2020.110550 [DOI] [PubMed] [Google Scholar]
- Papadopoulou, E. S. , Karas, P. A. , Nikolaki, S. , Storck, V. , Ferrari, F. , Trevisan, M. , Tsiamis, G. , Martin‐Laurent, F. , & Karpouzas, D. G. (2016). Dissipation and adsorption of isoproturon, tebuconazole, chlorpyrifos and their main transformation products under laboratory and field conditions. Science of the Total Environment, 569–570, 86–96. 10.1016/j.scitotenv.2016.06.133 [DOI] [PubMed] [Google Scholar]
- Papazlatani, C. V. , Karas, P. A. , Tucat, G. , & Karpouzas, D. G. (2019). Expanding the use of biobeds: Degradation and adsorption of pesticides contained in effluents from seed‐coating, bulb disinfestation and fruit‐packaging activities. Journal of Environmental Management, 248, 109221–109230. 10.1016/j.jenvman.2019.06.122 [DOI] [PubMed] [Google Scholar]
- Paszko, T. , & Jankowska, M. (2018). Modeling the effect of adsorption on the degradation rate of propiconazole in profiles of Polish Luvisols. Ecotoxicology and Environmental Safety, 161, 584–593. 10.1016/j.ecoenv.2018.05.093 [DOI] [PubMed] [Google Scholar]
- Peña, A. , Delgado‐Moreno, L. , & Rodríguez‐Liébana, J. A. (2020). A review of the impact of wastewater on the fate of pesticides in soils: Effect of some soil and solution properties. Science of the Total Environment, 718, 134468–134488. 10.1016/j.scitotenv.2019.134468 [DOI] [PubMed] [Google Scholar]
- Pouchieu, C. , Piel, C. , Carles, C. , Gruber, A. , Helmer, C. , Tual, S. , Marcotullio, E. , Lebailly, P. , & Baldi, I. (2018). Pesticide use in agriculture and Parkinson's disease in the AGRICAN cohort study. International Journal of Epidemiology, 47, 299–310. 10.1093/ije/dyx225 [DOI] [PubMed] [Google Scholar]
- Raherison, C. , Baldi, I. , Pouquet, M. , Berteaud, E. , Moesch, C. , Bouvier, G. , & Canal‐Raffin, M. (2019). Pesticides exposure by air in vineyard rural area and respiratory health in children: A pilot study. Environmental Research, 169, 189–195. 10.1016/j.envres.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Regitano, J. B. , Prata, F. , Dias, N. M. P. , Lavorenti, A. , & Tornisielo, V. L. (2002). Sorção‐dessorção do fungicida clorotalonil em solos com diferentes teores de matéria orgânica. Revista Brasileira De Ciência do Solo, 26, 267–274. 10.1590/S0100-06832002000100028 [DOI] [Google Scholar]
- Ren, X. , Zeng, G. , Tang, L. , Wang, J. , Wan, J. , Liu, Y. , Yu, J. , Yi, H. , Ye, S. , & Deng, R. (2018). Sorption, transport and biodegradation – an insight into bioavailability of persistent organic pollutants in soil. Science of the Total Environment, 610–611, 1154–1163. 10.1016/j.scitotenv.2017.08.089 [DOI] [PubMed] [Google Scholar]
- Rocha, P. R. R. , Faria, A. T. , Borges, L. G. F. C. , Silva, L. O. C. , Silva, A. A. , & Ferreira, E. A. (2013). Sorção e dessorção do diuron em quarto latossolos brasileiros. Planta Daninha, 31, 231–238. [Google Scholar]
- Rojas, R. , Morillo, J. , Usero, J. , Delgado‐Moreno, L. , & Gan, J. (2013). Enhancing soil sorption capacity of an agricultural soil by addition of three different organic wastes. Science of the Total Environment, 458–460, 614–623. 10.1016/j.scitotenv.2013.04.032 [DOI] [PubMed] [Google Scholar]
- Rojas, R. , Vanderlinden, E. , Morillo, J. , Usero, J. , & El Bakouri, H. (2014). Characterization of sorption processes for the development of low‐cost pesticide decontamination techniques. Science of the Total Environment, 488–489, 124–135. 10.1016/j.scitotenv.2014.04.079 [DOI] [PubMed] [Google Scholar]
- Rumschlag, S. L. , Bessler, S. M. , & Rohr, J. R. (2019). Evaluating improvements to exposure estimates from fate and transport models by incorporating environmental sampling effort and contaminant use. Water Research, 156, 372–382. 10.1016/j.watres.2019.03.038 [DOI] [PubMed] [Google Scholar]
- Siek, M. , & Paszko, T. (2019). Factors affecting coupled degradation and time‐dependent sorption processes of tebuconazole in mineral soil profiles. Science of the Total Environment, 690, 1035–1047. 10.1016/j.scitotenv.2019.06.409 [DOI] [PubMed] [Google Scholar]
- Smith, P. (2004). How long before a change in soil organic carbon can be detected? Global Change Biol, 10, 1878–1883. 10.1111/j.1365-2486.2004.00854.x [DOI] [Google Scholar]
- Sparks, T. C. , Wessels, F. J. , Lorsbach, B. A. , Nugent, B. M. , & Watson, G. B. (2019). The new age of insecticide discovery‐the crop protection industry and the impact of natural products. Pesticide Biochemistry and Physiology, 161, 12–22. 10.1016/j.pestbp.2019.09.002 [DOI] [PubMed] [Google Scholar]
- Swale, D. R. (2019). Perspectives on new strategies for the identification and development of insecticide targets. Pesticide Biochemistry and Physiology, 161, 23–32. 10.1016/j.pestbp.2019.07.001 [DOI] [PubMed] [Google Scholar]
- Thiour‐Mauprivez, C. , Martin‐Laurent, F. , Calvayrac, C. , & Barthelmebs, L. (2019). Effects of herbicide on non‐target microorganisms: Towards a new class of biomarkers? Science of the Total Environment, 684, 314–325. 10.1016/j.scitotenv.2019.05.230 [DOI] [PubMed] [Google Scholar]
- Tleuova, A. B. , Wielogorska, E. , Talluri, V. S. S. L. P. , Štěpánek, F. , Elliott, C. T. , & Grigoriev, D. O. (2020). Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. Journal of Controlled Release, 326, 468–481. 10.1016/j.jconrel.2020.07.035 [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture Natural Resources Conservation Service (USDA NRCS) . (2022). Soil Quality Test Kit Guide. USDA NRCS. Retrieved from https://www.nrcs.usda.gov/wps/portal/nrcs/site/soils/home [Google Scholar]
- Van Maele‐Fabry, G. , Gamet‐Payrastre, L. , & Lison, D. (2017). Residential exposure to pesticides as risk factor for childhood and young adult brain tumors: A systematic review and meta‐analysis. Environment International, 106, 69–90. 10.1016/j.envint.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Vitoratos, A. , Fois, C. , Danias, P. , & Likudis, Z. (2016). Investigation of the soil sorption of neutral and basic pesticides. Water, Air, and Soil Pollution, 227, 397. 10.1007/s11270-016-3076-8 [DOI] [Google Scholar]
- Wang, X. Q. , Liu, J. , Zhang, N. , & Yang, H. (2020). Adsorption, mobility, biotic and abiotic metabolism and degradation of pesticide exianliumi in three types of farmland. Chemosphere, 254, 126741–126752. 10.1016/j.chemosphere.2020.126741 [DOI] [PubMed] [Google Scholar]
- Wei, T. , Guizhen, L. , Mei, B. , Min, Y. , Jinhui, P. , Barrow, C. J. , Wenrong, Y. , & Hongbin, W. (2015). The study of adsorption mechanism of mixed pesticides prometryn‐acetochlor in the soil‐water system. International Biodeterioration and Biodegradation, 102, 281–285. 10.1016/j.ibiod.2015.04.005 [DOI] [Google Scholar]
- World Health Organisation . (2016). Safer access to pesticides for suicide prevention. World Health Organisation. [Google Scholar]
- Wu, D. , Yun, Y. , Jiang, L. , & Wu, C. (2018). Influence of dissolved organic matter on sorption and desorption of MCPA in ferralsol. Science of the Total Environment, 616–617, 1449–1456. 10.1016/j.scitotenv.2017.10.169 [DOI] [PubMed] [Google Scholar]
- Ye, M. , Beach, J. , Martin, J. W. , & Senthilselvan, A. (2017). Pesticide exposures and respiratory health in general populations. Journal of Environmental Sciences, 51, 361–370. 10.1016/j.jes.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Zhou, T. , Xu, Y. , Du, Z. , Li, B. , Wang, J. , Wang, J. , & Zhu, L. (2020). Ecotoxicology of strobilurin fungicides. Science of the Total Environment, 742, 140611–140618. 10.1016/j.scitotenv.2020.140611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S5
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


