Abstract
Aim
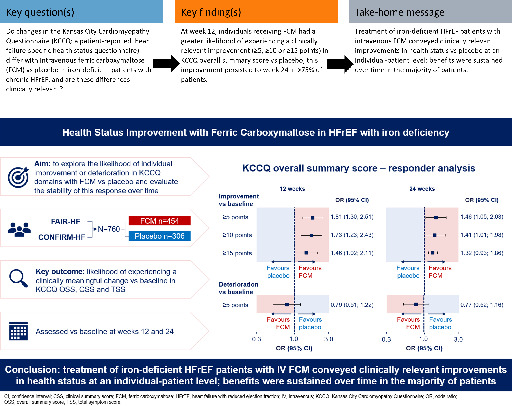
Intravenous ferric carboxymaltose (FCM) has been shown to improve overall quality of life in iron‐deficient heart failure with reduced ejection fraction (HFrEF) patients at a trial population level. This FAIR‐HF and CONFIRM‐HF pooled analysis explored the likelihood of individual improvement or deterioration in Kansas City Cardiomyopathy Questionnaire (KCCQ) domains with FCM versus placebo and evaluated the stability of this response over time.
Methods and results
Changes versus baseline in KCCQ overall summary score (OSS), clinical summary score (CSS) and total symptom score (TSS) were assessed at weeks 12 and 24 in FCM and placebo groups. Mean between‐group differences were estimated and individual responder analyses and analyses of response stability were performed. Overall, 760 (FCM, n = 454) patients were studied. At week 12, the mean improvement in KCCQ OSS was 10.6 points with FCM versus 4.8 points with placebo (least‐square mean difference [95% confidence interval, CI] 4.36 [2.14; 6.59] points). A higher proportion of patients on FCM versus placebo experienced a KCCQ OSS improvement of ≥5 (58.3% vs. 43.5%; odds ratio [95% CI] 1.81 [1.30; 2.51]), ≥10 (42.4% vs. 29.3%; 1.73 [1.23; 2.43]) or ≥15 (32.1% vs. 22.6%; 1.46 [1.02; 2.11]) points. Differences were similar at week 24 and for CSS and TSS domains. Of FCM patients with a ≥5‐, ≥10‐ or ≥15‐point improvement in KCCQ OSS at week 12, >75% sustained this improvement at week 24.
Conclusion
Treatment of iron‐deficient HFrEF patients with intravenous FCM conveyed clinically relevant improvements in health status at an individual‐patient level; benefits were sustained over time in most patients.
Keywords: Ferric carboxymaltose, Health status, Heart failure with reduced ejection fraction, Iron deficiency, Kansas City Cardiomyopathy Questionnaire, Minimal clinically important difference, Quality of life
This analysis shows that treating iron‐deficient heart failure with reduced ejection fraction patients with ferric carboxymaltose, an intravenous iron preparation, resulted in a clinically meaningful improvement in health status for individual patients, many of whom experienced a sustained improvement over time.
Introduction
Iron deficiency is present in almost half of all patients with heart failure (HF). 1 In this context, it confers a high mortality and hospitalization risk and is associated with reduced exercise capacity and impaired quality of life. 2 , 3 , 4 , 5 Several randomized trials have shown that intravenous ferric carboxymaltose (FCM), a nanoparticulate iron‐carbohydrate complex engineered to be taken up and processed by macrophages in order to release iron, 6 , 7 improves physical functioning and quality of life in patients with HF with reduced ejection fraction (HFrEF) and iron deficiency, both with and without anaemia. 3 , 8 , 9 , 10 , 11 , 12 This is important because one of the major treatment goals in HFrEF is to improve quality of life. 13 The United States Food and Drug Administration recognizes the Kansas City Cardiomyopathy Questionnaire (KCCQ) as a qualified assessment instrument for this endpoint. 14 , 15
Interpretation of quality of life requires a clinically interpretable framework that evaluates the magnitude of change perceived to be important by patients, known as the minimal clinically important difference (MCID) 16 ; this measure facilitates both clinical interpretation of trial results and communication of potential treatment benefits with patients. The MCIDs of various KCCQ domains have been established previously using FAIR‐HF (Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure) trial data. 16 Although the overall population‐based differences in KCCQ health‐status scores with FCM versus placebo therapy have been assessed, responder analyses to evaluate the proportion of individual patients who achieved a clinically relevant improvement or deterioration in KCCQ score have not been performed; these data can also be used to determine the number needed to treat (NNT) to achieve these MCIDs. In addition, the extent to which these responses remain stable over multiple measurements is not known.
In this pooled analysis of FAIR‐HF and CONFIRM‐HF (Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure) trials, we sought to assess the likelihood of improvement or deterioration in KCCQ domains with FCM versus placebo and to evaluate the stability of the response over time.
Methods
Study design
Two double‐blind, randomized controlled trials (RCTs: FAIR‐HF and CONFIRM‐HF) that evaluated the effects of intravenous FCM versus placebo on health status in ambulatory systolic HF patients with iron deficiency were included in this study. The main study design features of each RCT are shown in online supplementary Table S1 . The primary results of these studies have been previously reported, alongside safety outcomes and dosing information. 11 , 12 The studies were approved by the appropriate regulatory authorities and ethics committees, and all patients who participated in the individual RCTs provided written informed consent. The RCTs were conducted in strict compliance with the guidelines for Good Clinical Practice of the International Council for Harmonization and with the Declaration of Helsinki.
Health‐related quality‐of‐life measurements
The KCCQ is a validated tool to measure disease‐related health status in patients with HF. The instrument has a 2‐week recall period (given the day‐to‐day variability in symptoms) and includes 23 items that map to seven domains: symptom frequency; symptom burden; symptom stability; physical limitations; social limitations; quality of life; and self‐efficacy. The KCCQ scores are summarized as: (i) a total symptom score (TSS), which consists of symptom frequency and symptom burden domains; (ii) a clinical summary score (CSS), consisting of physical limitations and TSS; and (iii) an overall summary score (OSS), which is formed by combining physical limitation, TSS, quality of life and social limitations domains. The scores range from 0 to 100, where 100 is the best possible score.
Outcomes
Mean KCCQ scores characterize a population average effect, yet it is important to describe the proportion of patients with clinically important changes in health status. Therefore, the key outcome for this exploratory analysis was the likelihood (odds) of an individual achieving a clinically important change in health status with FCM versus placebo. Clinically important changes were defined using both conventional thresholds (improvement of ≥5, ≥10, or ≥15 points or deterioration of ≥5 points in KCCQ OSS, CSS or TSS) 17 and previously published MCID thresholds based on the FAIR‐HF cohort (improvement of ≥4.3, ≥4.5, and ≥4.9 points for KCCQ OSS, CSS and TSS, respectively [based on assessments at week 24]). 16 The ‘stability’ of the response over time was also assessed as the proportion of patients experiencing a change versus baseline that was ≥ the threshold for clinical relevance at week 12 and in whom this change was sustained at week 24.
Statistical analysis
Baseline demographic and clinical data were summarized as mean (standard deviation [SD]) for continuous variables and n (%) for categorical variables. Least‐square (LS) changes from baseline in KCCQ domains at week 12 and 24 were reported per treatment group and the corresponding LS mean treatment differences with 95% confidence intervals (CIs) and two‐sided p‐values were calculated using a mixed‐effects model for repeated measures (MMRM), adjusted for study and baseline KCCQ score, age, estimated glomerular filtration rate, diabetes status, sex and left ventricular ejection fraction. To investigate between‐study heterogeneity in the treatment effect, the MMRM was also expanded by including random treatment‐by‐study interactions.
For the responder analyses, the number and proportion of patients experiencing a clinically meaningful change in KCCQ score versus baseline (responders) at weeks 12 and 24 was reported. For the MCID‐based analysis, additional responder definitions were used, including a change in KCCQ score of at least twice the magnitude of the MCID. A correction for ceiling effect was used in which patients with a baseline value within the range of 100 minus the MCID for each of the KCCQ domains were considered to have improved if the values remained within that range at each follow‐up. Similarly, patients with a KCCQ score below the MCID at baseline were categorized as ‘deteriorated’ if their score remained below the MCID. This correction was similarly applied when using multiples of the MCID or conventional thresholds for the responder definition. Patients who died before assessment were recorded as ‘not improved’ in the analysis of improvement and ‘deteriorated’ in the deterioration analysis. The treatment effect was assessed using logistic regression models, with results reported as odds ratios (ORs) with 95% CIs and two‐sided p‐values. Because the pooled studies were similar in terms of design, patient populations, and endpoint assessments up to week 24, a fixed‐effects model was considered appropriate for this exploratory analysis; however, a random‐effects model including random treatment‐by‐study interactions was also used to account for the effect of between‐trial heterogeneity. The logistic regression models were adjusted for treatment group, study, and the following baseline factors: KCCQ score, age, estimated glomerular filtration rate, diabetes status, sex and left ventricular ejection fraction. ORs were converted into NNT values using the formula described by Hutton 18 and the placebo control response/deterioration proportion.
The ‘stability’ of the response status was also assessed. To evaluate how many patients remained stable in their response, the proportions of patients that were categorized as having the same response (improved, not improved, deteriorated, not deteriorated) versus baseline at both week 12 and week 24 were descriptively summarized per KCCQ domain. For this purpose, a flow chart detailing the proportion of patients for each permutation and combination at each time point was generated.
While the follow‐up period was 24 weeks in FAIR‐HF 12 and 1 year in CONFIRM‐HF, 11 patient follow‐up was restricted to 24 weeks for this pooled analysis (in which the data set was derived from both studies). SAS® Version 9.4 or later (SAS Institute, Inc., Cary, NC, USA) or R version 3.6.3 or later (R Foundation for Statistical Computing, Vienna, Austria) were used for the analyses.
Results
Patient characteristics
Of the 760 patients included in the pooled full analysis sets from the two studies, 454 (60%) were receiving FCM while 306 (40%) were receiving placebo. Table 1 shows the baseline demographics and clinical characteristics of the assigned patients by treatment group. The mean (SD) age of the patients was 68.0 (10.2) years, 50.7% were female and 44.6% had haemoglobin levels ≤12 g/dl. At week 12 there had been a total of 10 deaths in 760 (1.3%) patients in the two trials: 5 in 454 (1.1%) patients receiving FCM and 5 in 306 (1.6%) patients receiving placebo. At week 24 there had been a total of 21 deaths in 760 (2.8%) patients in the two trials: 12 in 454 (2.6%) patients receiving FCM and 9 in 306 (2.9%) patients receiving placebo. Ninety percent of patients had KCCQ OSS, CSS and TSS follow‐up data available at weeks 12 and 24. Mean scores at baseline for KCCQ OSS, CSS and TSS in the FCM group (54.2, 57.5, and 60.5, respectively) were similar to those in the placebo group (55.2, 57.6, and 60.7, respectively).
Table 1.
Pooled baseline characteristics of iron‐deficient heart failure with reduced ejection fraction patients in FAIR‐HF and CONFIRM‐HF trials
| Variable | FCM pool (n = 454) | Placebo pool (n = 306) | Total (n = 760) |
|---|---|---|---|
| Age, years, mean (SD) | 67.8 (10.1) | 68.2 (10.4) | 68.0 (10.2) |
| Female sex, n (%) | 226 (49.8) | 159 (52.0) | 385 (50.7) |
| White European ethnicity, n (%) | 452 (99.6) | 305 (99.7) | 757 (99.6) |
| NYHA class III, n (%) | 321 (70.7) | 186 (60.8) | 507 (66.7) |
| LVEF, %, mean (SD) | 33.6 (6.7) | 34.7 (6.9) | 34.1 (6.8) |
| BMI, kg/m2, mean (SD) | 28.1 (4.7) | 28.6 (5.4) | 28.3 (5.0) |
| 6MWT distance, m, mean (SD) | 278.6 (102.8) | 285.1 (104.2) | 281.2 (103.3) |
| Hypertension, n (%) | 373 (82.2) | 259 (84.6) | 632 (83.2) |
| Diabetes mellitus, n (%) | 131 (28.9) | 82 (26.8) | 213 (28.0) |
| Smoking, n (%) | 133 (29.3) | 82 (26.8) | 215 (28.3) |
| Atrial fibrillation, n (%) | 493 (53.9) | 431 (57.7) | 924 (55.6) |
| Myocardial infarction, n (%) | 500 (54.7) | 395 (52.9) | 895 (53.9) |
| Stroke, n (%) | 99 (10.8) | 103 (13.8) | 202 (12.2) |
| Coronary revascularization, n (%) | 312 (34.1) | 278 (37.2) | 590 (35.5) |
| Ischaemic HF etiology, n (%) | 370 (81.5) | 249 (81.4) | 619 (81.4) |
| KCCQ score, mean (SD) a | |||
| OSS | 54.2 (19.0) | 55.2 (18.2) | 54.6 (18.7) |
| CSS | 57.5 (19.4) | 57.6 (18.1) | 57.5 (18.9) |
| TSS | 60.5 (20.7) | 60.7 (19.4) | 60.6 (20.2) |
| Laboratory test results | |||
| Hb, g/dl, mean (SD) | 12.1 (1.3) | 12.2 (1.4) | 12.1 (1.3) |
| Hb <10 g/dl, n (%) | 26 (5.7) | 12 (3.9) | 38 (5.0) |
| Hb ≥10 and <12 g/dl, n (%) | 181 (39.9) | 120 (39.2) | 301 (39.6) |
| Hb ≥12 g/dl, n (%) | 247 (54.4) | 174 (56.9) | 421 (55.4) |
| Ferritin, ng/ml, mean (SD) | 54.0 (52.6) | 58.6 (55.6) | 55.9 (53.8) |
| Ferritin <50 ng/ml, n (%) | 266 (58.6) | 172 (56.2) | 438 (57.6) |
| Ferritin ≥50 and <100 ng/ml, n (%) | 138 (30.4) | 95 (31.1) | 233 (30.7) |
| Ferritin ≥100 ng/ml, n (%) | 50 (11.0) | 39 (12.8) | 89 (11.7) |
| TSAT, %, mean (SD) | 18.5 (14.5) | 17.4 (8.3) | 18.1 (12.4) |
| TSAT ≥0% and ≤10%, n (%) | 94 (20.7) | 61 (19.9) | 155 (20.4) |
| TSAT >10% and ≤20%, n (%) | 213 (46.9) | 140 (45.8) | 353 (46.5) |
| TSAT >20%, n (%) | 147 (32.4) | 105 (34.3) | 252 (33.2) |
| eGFR (CKD‐EPI), ml/min/1.73 m2, mean (SD) | 64.4 (20.8) | 64.2 (22.5) | 64.3 (21.5) |
| eGFR <60 ml/min/1.73 m2, n (%) | 179 (39.4) | 137 (44.8) | 316 (41.6) |
| Concomitant medications, n (%) | |||
| ARNI or SGLT2 inhibitor | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ACEI or ARB or ARNI | 423 (93.2) | 283 (92.5) | 706 (92.9) |
| Beta blocker | 393 (86.6) | 267 (87.3) | 660 (86.8) |
| Aldosterone antagonists | 237 (52.2) | 147 (48.0) | 384 (50.5) |
| Triple therapy | 194 (42.7) | 122 (39.9) | 316 (41.6) |
6MWT, six‐minute walk test; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CSS, clinical summary score; eGFR, estimated glomerular filtration rate; FCM, ferric carboxymaltose; Hb, haemoglobin; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; NYHA, New York heart Association; OSS, overall summary score; SD, standard deviation; SGLT2, sodium–glucose cotransporter‐2; TSAT, transferrin saturation; TSS, total symptom score.
N‐numbers for the KCCQ scores at baseline were 447 for FCM and 302 for placebo.
Association between use of ferric carboxymaltose and mean group changes in health status
Least‐square mean changes in KCCQ OSS, CSS, and TSS versus baseline at weeks 12 and 24 are shown in Figure 1 , alongside corresponding treatment differences based on the fixed‐effects model. At week 12, patients receiving FCM experienced a mean KCCQ OSS improvement of 10.6 (17.7) points compared with 4.8 (13.9) points in patients receiving placebo (fixed‐effects model LS mean difference: 4.36 [95% CI 2.14; 6.59] points; p = 0.0001). Similar robust changes in KCCQ OSS were observed at week 24, with a mean improvement of 11.4 (18.7) points in patients receiving FCM compared with 5.7 (15.0) points in those receiving placebo (fixed‐effects model LS mean difference: 4.45 [95% CI 2.19; 6.72] points; p = 0.0001). Results were similar for KCCQ CSS and TSS domains. Mean differences based on the random‐effects model showed similar effect sizes to those based on the fixed‐effects model, with wider confidence intervals (online supplementary Figure S1 ).
Figure 1.
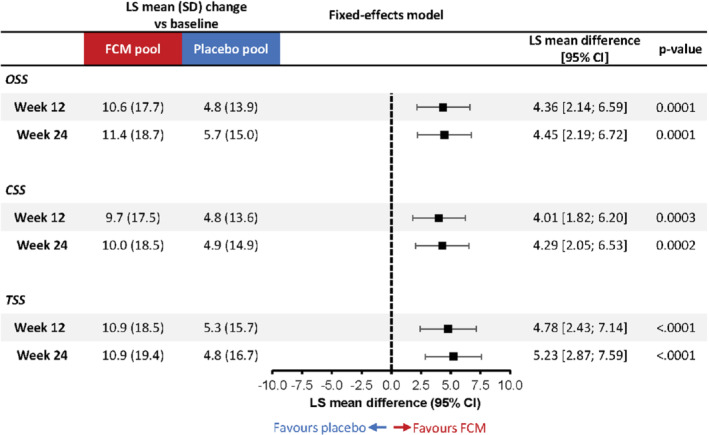
Mean change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score (OSS), clinical summary sore (CSS) and total symptom score (TSS) with ferric carboxymaltose (FCM) versus placebo at weeks 12 and 24 (fixed‐effects model). Fixed‐effects mixed‐model for repeated measures analysis adjusted for study, baseline KCCQ score, age, estimated glomerular filtration rate, diabetes status, sex and left ventricular ejection fraction. CI, confidence interval; LS, least‐square; SD, standard deviation.
Responder analysis: conventional thresholds
Results from the conventional threshold responder analysis for KCCQ OSS, CSS and TSS using the fixed‐effects model are shown in Figure 2 . The proportions of patients on FCM versus placebo who experienced a ≥5‐point improvement in KCCQ OSS at week 12 were 58.3% versus 43.5% (fixed‐effects OR 1.81 [95% CI 1.30; 2.51]; p = 0.0004); corresponding proportions for a ≥10‐point improvement were 42.4% versus 29.3% (1.73 [1.23; 2.43]; p = 0.0017), and for a ≥15‐point improvement were 32.1% versus 22.6% (1.46 [1.02; 2.11]; p = 0.0400); results were similar at week 24. Based on these ORs, the respective NNTs for a ≥5‐, ≥10‐ and ≥15‐point improvement at week 12 were 7, 10, and 17 (Table 2 ). Results based on the random‐effects model were similar (online supplementary Figure S2 and Table S2 ).
Figure 2.

Responder analyses across conventional and minimal clinically important difference thresholds for (A) overall summary score (OSS), (B) clinical summary score (CSS), and (C) total symptom score (TSS) Kansas City Cardiomyopathy Questionnaire (KCCQ) domains (fixed‐effects model). Odds ratios (ORs) were obtained from logistic regression models including treatment group, study, and the following baseline factors: KCCQ score, age, estimated glomerular filtration rate, diabetes status, sex and left ventricular ejection fraction. N = number of patients with KCCQ data available at each time point, plus patients who died before assessment and were recorded as ‘not improved’ in the analysis of improvement and ‘deteriorated’ in the deterioration analysis. CI, confidence interval; FCM, ferric carboxymaltose; PBO, placebo.
Table 2.
Number needed to treat with ferric carboxymaltose to achieve defined change versus baseline in Kansas City Cardiomyopathy Questionnaire overall summary score, clinical summary score or total symptom score at weeks 12 and 24 (fixed‐effects model)
| Week 12 | Week 24 | |
|---|---|---|
| KCCQ OSS | ||
| Improvement | ||
| ≥4.3 points | 8 | 10 |
| ≥8.6 points | 9 | 13 |
| ≥5 points | 7 | 11 |
| ≥10 points | 10 | 14 |
| ≥15 points | 17 | 19 |
| Deterioration | ||
| ≥ 5 points | 26 | 21 |
| KCCQ CSS | ||
| Improvement | ||
| ≥4.5 points | 11 | 10 |
| ≥9 points | 13 | 18 |
| ≥5 points | 13 | 10 |
| ≥10 points | 12 | 18 |
| ≥15 points | 13 | 16 |
| Deterioration | ||
| ≥ 5 points | 254 | 32 |
| KCCQ TSS | ||
| Improvement | ||
| ≥4.9 points | 10 | 13 |
| ≥9.8 points | 9 | 11 |
| ≥5 points | 10 | 13 |
| ≥10 points | 9 | 11 |
| ≥15 points | 11 | 9 |
| Deterioration | ||
| ≥ 5 points | 23 | 21 |
CCS, clinical summary score; KCCQ, Kansas City Cardiomyopathy Questionnaire; NNT, number needed to treat; OSS, overall summary score; TSS, total symptom score.
Odds ratios from the fixed‐effects responder analysis were converted into NNT using the formula described in Hutton 18 and the placebo control response/deterioration proportion.
The proportions of patients who experienced a ≥5‐point deterioration in KCCQ OSS were similar in the FCM and placebo groups at week 12 (14.9% and 18.7%, respectively; fixed‐effects OR 0.79 [95% CI 0.51; 1.22]; p = 0.28]). Similarly, at week 24, nominally fewer patients treated with FCM experienced a ≥5‐point deterioration in KCCQ OSS compared with those receiving placebo but the difference was not statistically significant. The random‐effects model showed similar effect sizes to those based on the fixed‐effects model, with wider CIs for the estimated ORs (online supplementary Figure S2 ). Results for the KCCQ CSS and TSS for NNT were similar to those for the KCCQ OSS (Table 2 , online supplementary Table S2 ).
Responder analysis — minimal clinically important difference‐based thresholds
The previously determined MCID thresholds for KCCQ OSS, CSS, and TSS were ≥4.3‐, ≥4.5‐, and ≥4.9‐point improvements, respectively. 16 Results from the MCID‐based threshold responder analysis for KCCQ OSS, CSS, and TSS using the fixed‐effects model are shown in Figure 2 . The proportions of patients in the FCM versus placebo groups who experienced a ≥4.3‐point improvement in KCCQ OSS at week 12 were 60.5% versus 46.6% (fixed‐effects OR 1.75 [95% CI 1.26; 2.44]; p = 0.0008), and the proportions experiencing a ≥8.6‐point improvement were 46.5% versus 31.5% (1.86 [1.33; 2.60]; p = 0.0003), with similar results at week 24. Based on these ORs, the respective NNTs for a ≥4.3‐ and ≥8.6‐point improvement at week 12 were 8 and 9 (Table 2 ). Results for CSS and TSS domains were similar to those for the OSS domain (Figure 2 , Table 2 ). The ORs based on the random‐effects model again showed similar effect sizes with wider CIs (online supplementary Figure S2 ) and similar NNTs (online supplementary Table S2 ).
Response stability analysis
Results of the response stability analysis for the KCCQ OSS are shown in Figure 3 . Of the 244 patients who experienced a ≥4.3‐point improvement in KCCQ OSS with FCM at week 12, this improvement was sustained in 196 patients (80.3%) at week 24. Of the 190 patients who experienced a ≥8.6‐point improvement in KCCQ OSS with FCM at week 12, this improvement was sustained in 145 patients (76.3%) at week 24. Among the patients in the placebo group who experienced an improvement in KCCQ OSS of ≥4.3 and ≥8.6 points at week 12, the proportions experiencing this improvement at week 24 were 73.6% and 76.1%, respectively.
Figure 3.

Response stability analysis – change in Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score (OSS) response between week 12 and week 24. N = number of patients that had non‐missing KCCQ data available at both week 12 and week 24. FCM, ferric carboxymaltose.
Of the 160 patients who did not experience a ≥4.3‐point improvement in KCCQ OSS with FCM at week 12, 43 patients (26.9%) experienced this improvement at week 24. Similarly, of the 214 patients who did not experience a ≥8.6‐point improvement in KCCQ OSS with FCM at week 12, 46 patients (21.5%) experienced this improvement at week 24. Among the patients in the placebo group who did not experience an improvement in KCCQ OSS of ≥4.3 and ≥8.6 points at week 12, the proportions experiencing this improvement at week 24 were 26.5% and 19.1%, respectively.
Of the 61 patients in the FCM group who experienced a ≥5‐point deterioration in KCCQ OSS at week 12, 21 patients (34.4%) were no longer experiencing this deterioration at week 24. Of the 52 patients in the placebo group who experienced a ≥5‐point deterioration in KCCQ OSS at week 12, 21 patients (40.4%) were no longer experiencing this deterioration at week 24.
The response stability analysis yielded similar results for conventional thresholds (Figure 3 ) and across KCCQ CSS and TSS scores (online supplementary Figures S3 and S4 ).
Discussion
Although several previous studies have shown that intravenous iron, 11 , 12 , 19 , 20 , 21 , 22 , 23 particularly FCM, 11 , 12 , 23 improves health status in the iron‐deficient HFrEF population, there are few data regarding the interpretation and clinical significance of these changes. In this combined analysis of the FAIR‐HF and CONFIRM‐HF trials, we report four key findings. Firstly, in the analysis of between‐group differences, patients who received FCM experienced a greater mean improvement in KCCQ OSS than those receiving placebo at week 12 and this benefit was still evident at week 24. Secondly, in the responder analysis performed to assess the within‐patient achievement of clinically relevant improvements in KCCQ, a higher proportion of patients in the FCM group experienced a ≥4.3‐, ≥8.6‐, ≥5‐, ≥10‐, or ≥15‐point improvement in KCCQ OSS at weeks 12 and 24 compared with patients in the placebo group (Graphical Abstract). In addition, nominally fewer patients receiving FCM had a deterioration of ≥5 points in KCCQ OSS at each of these two time points compared with patients receiving placebo, with similar findings for KCCQ CSS and TSS domains. Thirdly, of patients who had experienced a ≥5‐, ≥10‐, or ≥15‐point improvement in KCCQ OSS with FCM at week 12, more than three quarters sustained this improvement at week 24, indicating that the effects of FCM had robust stability. Lastly, those who had not experienced a clinically important improvement by week 12 had an approximately one‐in‐five chance of doing so by week 24 (Graphical Abstract). Considering that only a few therapies have been shown to consistently and reliably improve patient‐reported health status in patients with HFrEF with or without iron deficiency, these findings have important clinical implications.
To aid the interpretation of clinical trial results as a whole, it is useful to define a clinically meaningful difference in KCCQ mean change versus baseline between treatment groups. 17 It has been shown that an estimated mean difference of ≥2–3 points translates into a clinically relevant improvement in subjective patient wellbeing. 24 , 25 , 26 In the current analysis, the estimated mean difference between FCM and placebo groups exceeded this threshold, ranging from 4.0 to 4.8 points across the different scores at 12 months. These numbers compare favourably to other interventions that have previously been shown to improve health status in HF: in SHIFT (Systolic Heart Failure Treatment with the I f inhibitor ivabradine Trial), the estimated between‐group difference in the change from baseline in KCCQ OSS at 12 months with ivabradine versus placebo was 2.4 points 27 ; in the HF‐ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial, exercise therapy resulted in a 1.9‐point greater improvement in KCCQ OSS at 3 months, compared with usual care only 26 ; in the EMPEROR‐Reduced (EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction) trial, empagliflozin improved KCCQ OSS by 1.77 points versus placebo at 3 months 24 ; and in the PARADIGM‐HF (Prospective Comparison of Angiotensin Receptor–Neprilysin Inhibitor With Angiotensin‐Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial, sacubitril/valsartan improved KCCQ OSS by an additional 1.27 points at 8 months, compared with enalapril. 25 Collectively, these findings indicate that FCM may confer similar, if not greater, health status benefits in patients with HF; however, it is important to note that directly comparing changes in KCCQ scores across studies can be challenging because of the differing patient populations, time points assessed, KCCQ domains investigated, and methods of handling deaths and missing data.
It is also important to recognize clinically relevant changes in KCCQ scores for individual patients, as these are easier to interpret and more directly applicable in clinical practice. 28 Improvements in KCCQ scores of ≥5, ≥10, or ≥15 points have been suggested to correlate with small, moderate‐to‐large, or large clinically significant improvements in health status, respectively, whereas a ≥5‐point deterioration is considered a clinically significant worsening. 17 In addition, a prior study suggested that changes in KCCQ that are even smaller than the lowest conventional threshold of ≥5 points may be clinically meaningful for individuals such as those included in the FAIR‐HF and CONFIRM‐HF trial populations. 16 In this pooled analysis, we showed that FCM increased the likelihood of achieving clinically meaningful improvements in health status versus placebo at an individual patient level; ORs for improvements in KCCQ OSS of ≥5, ≥10, or ≥15 points with FCM versus placebo were 1.81, 1.73, and 1.46, respectively, and the effect size changed only slightly when adjusted for between‐study heterogeneity. The ORs observed can also be translated into NNT, which may increase ease of clinical interpretation. Based on our analysis, only 7 and 11 patients, respectively, needed to be treated with FCM for one patient to experience a clinically relevant (≥5‐point) improvement in KCCQ OSS at weeks 12 and 24. Again, these values compare favourably to the benefits observed with other foundational HF therapies, such as sodium–glucose cotransporter‐2 inhibitors, 24 , 29 although the aforementioned differences in study and analysis designs limit direct comparisons. Notably, FCM is a nanomedicine, for which the manufacturing process defines the product and therefore its outcomes in patients 7 , 30 , 31 ; these clinically meaningful effects have thus far only been investigated with FCM and, until and unless proven otherwise, other intravenous iron complexes may not render the same benefits.
In a 2021 prespecified analysis of AFFIRM‐AHF data, it was also shown that patients receiving intravenous FCM versus placebo had significantly greater improvements in health status, beginning at week 4 and continuing up to week 24. 23 It is important to emphasize that the patient population in AFFIRM‐AHF consisted of patients with acute HF, and that this FAIR‐HF and CONFIRM‐HF pooled analysis is the first to report a large treatment benefit with FCM in stable, iron‐deficient chronic HFrEF patients. Nevertheless, iron deficiency is a common and important comorbidity in HF, 1 and periodic screening of all HF patients for iron deficiency is now recommended in European Society of Cardiology guidelines, along with consideration of iron supplementation with FCM in iron‐deficient symptomatic HF patients with a left ventricular ejection fraction <45%. 32 It will be important to improve the awareness of these recommendations among clinicians to ensure their routine implementation as standard of care in real‐world clinical practice.
To our knowledge at the time of writing, no previous study has yet determined the proportion of patients with a sustained health status improvement over time with a particular intervention. The concept of ‘stability’ is particularly relevant to eliminate the interference of chance factors due to KCCQ‐ and intra‐patient variability on a day‐to‐day basis; a high proportion of patients with a sustained improvement would suggest that any benefit observed is robust. Although thresholds for MCID have been developed for various populations, no specific threshold for ‘stability’ (i.e. the percentage of patients with a sustained improvement needed for the treatment effect to be considered stable and robust) has yet been defined and future studies should investigate this. Moreover, the time frame over which these repeated measurements should be assessed needs further exploration.
There are some limitations that should be considered. Firstly, as with other trials, the generalizability of our results may be limited in real‐world clinical practice because of the prespecified inclusion and exclusion criteria of the trials included. Secondly, the impact of FCM dosing on health status could not be investigated, and also the impact of FCM on long‐term health status for responder stability analysis could not be assessed either. Thirdly, pooling results from different trials may have introduced some heterogeneity; when accounting for this in the random‐effects model responder analyses, the effect sizes changed modestly, with wider CIs. Thus, while this post hoc, exploratory analysis suggests that FCM increases the likelihood of improving the health status of an individual with HFrEF and iron deficiency, a dedicated, prospective study may be of benefit to determine the treatment effect more precisely. Lastly, we did not analyse the association between the degree of health status improvement or worsening with clinical outcomes.
In conclusion, there was a significantly higher likelihood of clinically relevant improvements and a numerically lower likelihood of deterioration across various KCCQ domains with FCM versus placebo in patients with stable, chronic HFrEF and iron deficiency. Of the patients who experienced a significant improvement in KCCQ OSS with FCM at week 12, the improvement was sustained in the majority of patients at week 24, indicating robust stability of the beneficial effects of FCM. These results reinforce the role of FCM in improving patient‐centred outcomes in patients with HFrEF.
Supporting information
Table S1. Key characteristics of the two randomized controlled trials (FAIR‐HF and CONFIRM‐HF) in patients with HFrEF and iron deficiency included in the analysis.
Table S2. Number needed to treat with ferric carboxymaltose to achieve defined change vs. baseline in KCCQ OSS, CSS, or TSS at weeks 12 and 24 (random‐effects model).
Figure S1. Mean change from baseline in KCCQ OSS, CSS, and TSS with ferric carboxymaltose vs. placebo at weeks 12 and 24 (random‐effects model).
Figure S2. Responder analyses for ferric carboxymaltose vs. placebo across conventional and MCID thresholds for (A) OSS, (B) CSS and (C) TSS KCCQ domains (random‐effects model).
Figure S3. Response stability analysis – change in KCCQ CSS response between week 12 and week 24 with ferric carboxymaltose and placebo.
Figure S4. Response stability analysis – change in KCCQ TSS response between week 12 and week 24 with ferric carboxymaltose and placebo.
Acknowledgements
The authors acknowledge assistance with language editing, formatting, preparation of figures and submission provided by Helen Sims (AXON Communications), funded by Vifor Pharma.
Conflict of interest: J.B. reports personal consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma; and payment for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, BI‐Lilly, Janssen, and Novartis. T.F. reports support for statistical consultancies and personal fees from Vifor for the present manuscript; consulting fees for statistical consultancies and personal fees from Bayer, CSL Behring, Galapagos, Minoryx, Vifor, Novartis and LivaNova outside of the current work; payment for educational events from Fresenius Kabi outside of the current work; personal fees from Novartis, Eli Lilly and Co., Bayer, BiosenseWebster, Janssen, Roche, and Enanta for participation in a Data Safety Monitoring Board. E.A.J. has received research grants and personal fees from Vifor Pharma (co‐PI of the AFFIRM trial); personal fees from Bayer, Novartis, Abbott, Boehringer Ingelheim, Pfizer, Servier, AstraZeneca, Berlin Chemie, Cardiac Dimensions, Fresenius, Respicardia, Takeda, Swixx Biopharma and Gedeon Richter; treasurer of the Executive Committee for the Heart Failure Association. V.F., U.M.G. and F.D. are full‐time employees of Vifor Pharma. M.M. has received personal fees from Vifor Pharma (Executive Committee member), Amgen (Executive Committee member and National PI), AstraZeneca, Abbott Vascular, Bayer (participation in Advisory Boards), Boehringer Ingelheim (Advisory Board member), Servier (participation in Advisory Boards and speeches at sponsored symposia), Edwards Therapeutics (speeches at sponsored symposia), Actelion (DMC Member), LivaNova (Executive Committee member), and Windtree Therapeutics (Executive Committee member and Advisory Board). I.L.P. reports personal fees from Boehringer Ingelheim, outside the submitted work. A.J.S.C. reports personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, and Respicardia, outside the submitted work. J.C.C. reports unrestricted grants from Vifor Pharma and Novartis; consulting fees from Vifor Pharma, AstraZeneca and Boehringer Ingelheim; and honoraria for conference activities from Vifor Pharma, AstraZeneca and Boehringer Ingelheim. G.S.F. reports grants from the European Commission; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer and Boehringer Ingelheim; participation in a Data Safety Monitoring Board or Advisory Board from Bayer and Boehringer Ingelheim; leadership or fiduciary role in the Heart Failure Association; and other financial or non‐financial interests as a Committee Member for Medtronic, Vifor Pharma, Amgen, Servier, and Novartis. S.D.A. has received research grants and personal fees from Vifor Int and Abbott Vascular (IIT/Trial Steering Committee work); personal fees from Bayer, Boehringer Ingelheim and Impulse Dynamics (Trial Steering Committee work), Novartis, Cardiac Dimensions and Occlutech (Adivsory Committee work), Servier (Registry Steering Committee). P.P. reports participation in clinical trials for and grants and personal fees from Vifor Pharma during the conduct of the study; participation in clinical trials for and personal fees from Amgen, Bayer, Novartis, Abbott Vascular, Boehringer Ingelheim, Pfizer, Servier, AstraZeneca, Cibiem, BMS, and Impulse Dynamics, outside the submitted work; participation in clinical trials for Cardiac Dimensions, outside the submitted work; and personal fees from Berlin Chemie, outside the submitted work. All other authors have nothing to disclose.
References
- 1. Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71:782–93. [DOI] [PubMed] [Google Scholar]
- 2. Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, et al. Iron deficiency and health‐related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014;174:268–75. [DOI] [PubMed] [Google Scholar]
- 3. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. [DOI] [PubMed] [Google Scholar]
- 4. Marchi G, Busti F, Vianello A, Girelli D. Anemia and iron deficiency in heart failure: extending evidences from chronic to acute setting. Intern Emerg Med. 2021;16:167–70. [DOI] [PubMed] [Google Scholar]
- 5. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid‐range and preserved ejection fraction. Acta Cardiol. 2018;73:115–23. [DOI] [PubMed] [Google Scholar]
- 6. Bhandari S, Pereira DIA, Chappell HF, Drakesmith H. Intravenous irons: from basic science to clinical practice. Pharmaceuticals (Basel). 2018;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin‐Malo A, Borchard G, Flühmann B, Mori C, Silverberg D, Jankowska EA. Differences between intravenous iron products: focus on treatment of iron deficiency in chronic heart failure patients. ESC Heart Fail. 2019;6:241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail. 2018;20:125–33. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al.; AFFIRM‐AHF Investigators . Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet. 2020;396:1895–904. [DOI] [PubMed] [Google Scholar]
- 10. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, et al.; EFFECT‐HF Investigators . Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, et al.; CONFIRM‐HF Investigators . Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al.; FAIR‐HF Trial Investigators . Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–48. [DOI] [PubMed] [Google Scholar]
- 13. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, et al.; American Heart Association Council on Quality of Care and Outcomes Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Stroke Council . Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–49. [DOI] [PubMed] [Google Scholar]
- 14. Center for Drug Evaluation and Research (CDER) . Qualification of the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score and its Component Scores. A Patient‐Reported Outcome Instrument for Use in Clinical Investigations in Heart Failure. April 9, 2020. https://www.fda.gov/media/136862/download (14 March 2022).
- 15. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 16. Butler J, Khan MS, Mori C, Filippatos GS, Ponikowski P, Comin‐Colet J, et al. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2020;22:999–1005. [DOI] [PubMed] [Google Scholar]
- 17. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. [DOI] [PubMed] [Google Scholar]
- 18. Hutton JL. Number needed to treat: properties and problems. J R Stat Soc A Stat Soc. 2000;163:381–402. [Google Scholar]
- 19. Hildebrandt PR, Bruun NE, Nielsen OW, Pantev E, Shiva F, Videbæk L, et al. Effects of administration of iron isomaltoside 1000 in patients with chronic heart failure. A pilot study. Transfus Altern Transfus Med. 2010;11:131–7. [Google Scholar]
- 20. Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–65. [DOI] [PubMed] [Google Scholar]
- 21. Bolger AP, Bartlett FR, Penston HS, O'Leary J, Pollock N, Kaprielian R, et al. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48:1225–7. [DOI] [PubMed] [Google Scholar]
- 22. Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency: FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol. 2008;51:103–12. [DOI] [PubMed] [Google Scholar]
- 23. Jankowska EA, Kirwan BA, Kosiborod M, Butler J, Anker SD, McDonagh T, et al. The effect of intravenous ferric carboxymaltose on health‐related quality of life in iron‐deficient patients with acute heart failure: the results of the AFFIRM‐AHF study. Eur Heart J. 2021;42:3011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Butler J, Anker SD, Filippatos G, Khan MS, Ferreira JP, Pocock SJ, et al.; EMPEROR‐Reduced Trial Committees and Investigators . Empagliflozin and health‐related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR‐Reduced trial. Eur Heart J. 2021;42:1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, et al. Health‐related quality of life outcomes in PARADIGM‐HF. Circ Heart Fail. 2017;10:e003430. [DOI] [PubMed] [Google Scholar]
- 26. Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al.; HF‐ACTION Investigators . Effects of exercise training on health status in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ekman I, Chassany O, Komajda M, Böhm M, Borer JS, Ford I, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J. 2011;32:2395–404. [DOI] [PubMed] [Google Scholar]
- 28. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;76:2379–90. [DOI] [PubMed] [Google Scholar]
- 29. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation. 2020;141:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crommelin DJA, de Vlieger JSB, Weinstein V, Mühlebach S, Shah VP, Schellekens H. Different pharmaceutical products need similar terminology. AAPS J. 2014;16:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crommelin DJ, Shah VP, Klebovich I, McNeil SE, Weinstein V, Flühmann B, et al. The similarity question for biologicals and non‐biological complex drugs. Eur J Pharm Sci. 2015;76:10–7. [DOI] [PubMed] [Google Scholar]
- 32. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Key characteristics of the two randomized controlled trials (FAIR‐HF and CONFIRM‐HF) in patients with HFrEF and iron deficiency included in the analysis.
Table S2. Number needed to treat with ferric carboxymaltose to achieve defined change vs. baseline in KCCQ OSS, CSS, or TSS at weeks 12 and 24 (random‐effects model).
Figure S1. Mean change from baseline in KCCQ OSS, CSS, and TSS with ferric carboxymaltose vs. placebo at weeks 12 and 24 (random‐effects model).
Figure S2. Responder analyses for ferric carboxymaltose vs. placebo across conventional and MCID thresholds for (A) OSS, (B) CSS and (C) TSS KCCQ domains (random‐effects model).
Figure S3. Response stability analysis – change in KCCQ CSS response between week 12 and week 24 with ferric carboxymaltose and placebo.
Figure S4. Response stability analysis – change in KCCQ TSS response between week 12 and week 24 with ferric carboxymaltose and placebo.


