Abstract
Purpose of review
Endometrial stem cells (ESCs) are multipotent cells that are thought to originate locally in the endometrium as well as in the bone marrow (BM). They have remarkable plasticity and hold promise as an autologous source for regenerative medicine. This review focuses on recent studies that have advanced our understanding of the biology and function of ESCs and BM-derived stem cells (BMDSCs) as related to physiological reproductive processes and pathologies. Moreover, it reviews recent data on potential therapeutic applications of stem cells to endometrial disorders that lead to reproductive failure.
Recent findings
Growing evidence from basic and preclinical studies suggests that ESCs participate in endometrial tissue regeneration and repair. Recent evidence also suggests that ESCs and BMDSCs play important roles in physiological reproductive functions including decidualization, implantation, pregnancy maintenance, and postpartum uterine remodeling. Initial preclinical and clinical studies with ESCs and BMDSCs suggest they have the potential to provide new therapies for various endometrial disorders associated with reproductive failure.
Summary
Uterine ESCs and BMDSCs appear to play an important biological role in reproductive success and failure, and have the potential to become treatment targets for reproductive diseases including recurrent implantation failure, thin endometrium, Asherman, and recurrent pregnancy loss.
Keywords: Endometrium, Stem cells, Bone marrow-derived stem cells, Decidualization, Reproductive failure, Implantation failure, Pregnancy loss, Therapy
Introduction
Stem cell research has greatly expanded its reach in the past decade to optimize medical treatments and identify new, cutting-edge therapies to a diverse range of disease conditions. In just the past year, over 35,000 scientific articles have been published (in Pubmed) about the use of stem cells in treating cancer, chronic degenerative diseases associated with aging, neurological illnesses, heart disease, and other regenerative medicine applications. Thousands of stem cell-based clinical trials are ongoing.
Since the first reports of stem/progenitor cells in the adult human endometrium in 2004 [1, 2], we have learned much regarding the biology, typical markers and incredible differentiation capabilities of endometrial stem cell (ESC) populations as well as the potential for prevention and treatment of uterine pathologies [3]. ESCs are adult stem cells (ASCs) that are thought to originate locally in the endometrium but also in the bone marrow [4]. An especially attractive avenue of ESC research is its applications to therapeutic interventions for reproductive failure [5]. On the other hand, aberrations in ESCs are currently thought to contribute to the pathogenesis of proliferative diseases of the uterine lining including endometriosis and myometrial tumors [3]. Moreover, ESCs are a promising autologous source of ASCs due to their abundance, easy accessibility, differentiation potential, and ethical acceptance [4]. As such, ESCs have the potential to expand to regenerative medicine applications outside of the reproductive system (reviewed in detail in [6] and [7]).
This review aims to focus on recent advances in the field of ESCs as related to reproductive failure. We will highlight recent studies published in the past year that further our understanding of the biology and function of ESCs and bone-marrow derived stem cells as well as their potential therapeutic application to endometrial disorders that lead to reproductive failure.
Endometrial stem cells
The human endometrium is a highly dynamic tissue that undergoes regular physiological cycles of proliferation, differentiation, breakdown and regeneration with each of the ~500 menstrual cycles during a female’s lifetime. To accomplish this remarkable cellular turnover and tissue regeneration, endometrial stem/progenitor cells (ESCs) are thought to play a major role [6]. ESCs have been identified in human endometrial tissue in the form of both epithelial and stromal cell populations [1, 2, 6, 8]. ESCs are characteristically similar to other classic mesenchymal stem cells (MSCs) due to their abilities to self-renew, clonogenicity, plastic adherence, multilineage differentiation, and expression of typical MSC surface markers [9]. MSCs have been identified in many adult tissues, including bone marrow, periosteum, skeletal muscle, adipose, umbilical cord, placenta, and endometrium [6, 10]. Of the two subsets of ESCs, epithelial and stromal, the former is more rarely present in human adult tissue. As a result, current uterine stem cells research is predominantly focused on stromal populations of ESCs of several origins, including endometrium, menstrual blood, and bone marrow.
Phenotypic markers specific to ESCs have not yet been fully characterized [6]. Several studies have identified specific markers expressed on ESC populations, utilizing known properties of progenitor cells such as plasticity and clonogenicity. Mesenchymal ESCs (mESCs) have been isolated and characterized through the surface markers CD146 and platelet-derived growth factor-receptor beta (PDGF-Rβ/CD140b) due to their co-expression in cells with high clonogenic ability in vitro [11]. This same study identified that these stem-cell like populations were located in perivascular location in both the functionalis and basalis layers of the human endometrial tissue. This cell population also expressed additional mesenchymal cell surface markers such as CD29, CD44, CD73, CD90, and CD105 and did not express classic hematopoietic makers such as CD34 and CD45 or endothelial marker CD31. Notably, the use of this combination of markers CD140b and CD146 has been used for identification of MSCs in multiple organs, and colocalization studies suggested that they are subpopulations of pericytes surrounding blood vessels [10]. CD146 (MCAM) is broadly expressed by cells that are components of the blood vessel wall, including vascular endothelial cells, smooth muscle cells (SMCs), and pericytes [12]. Another study by the same group identified sushi domain containing-2+ (SUSD2+), also known as W5C5, as a single marker to purify the same CD14b+/CD146+ population [13]. Cells expressing SUSD2+ exhibited high clonogenic capacity, co-expressed typical mesenchymal markers including CD44, CD73, CD90, CD105 and CD140b and were able to produce endometrial stromal-like tissue when transplanted in vivo, highlighting the sufficiency of this marker in identifying ESCs. Notably, similar to the CD140b+/CD146+ subpopulation, they were also found in a perivascular location characteristic of MSCs. However, while 85% of W5C5+ cells expressed CD140b, only 28.3% of W5C5+ cells co-expressed CD146, suggesting that the W5C5+ subpopulation may be more heterogenous than the CD140b+/CD146+ subpopulation.
The label-retaining cell (LRC) approach which consists of bromodeoxyuridine (BrdU) pulse administration, relies on the relative quiescence of stem/progenitor cells, and has also been used to identify putative adult stem cells in the endometrium. Chan et al. [14] found that the majority of endometrial LRCs in adult mice were found to be stromal while a minority were epithelial, whereas Cervello et al. [15] showed that LRCs were found to be only stromal and not epithelial after 8 week follow-up period. Endometrial LRCs are found in three main stromal areas; near the luminal epithelium, in the endometrial-myometrial junction, and in perivascular location [14]. About a third of LRCs are located perivascular and found to express α-smooth muscle actin (SMA) suggesting that they are pericytes or vascular smooth muscle cells [14]. These perivascular LRCs have also been found to express CD140b and CD146 and are thought to be the mouse counterparts of the human CD140b+CD146+ perivascular pericyte mesenchymal stem/progenitor cell population [16]. The other subpopulation of stromal LRCs near the luminal epithelium may be related to the mesenchymal-epithelial transition (MET), that has been described in the endometrium, and contributes to the regenerating luminal epithelium during postpartum uterine involution [17]. A subpopulation of LRCs found in the lower region of the stromal compartment were found to express the stem cell markers c-kit and POU5F1 (Oct-4) and are likely phenotypically different than the perivascular LRCs [15]. LRCs appear to play a functional role in endometrial regeneration as they have been shown to proliferate in response to endometrial repair, postpartum uterine remodeling, and pseudopregnancy [16–18].
Other studies have suggested that human ESCs are positive for the hematopoietic markers c-kit (CD117) and CD34 and are found in the basalis of the endometrium [19]. Interestingly, CD45+ hematopoietic progenitors have been shown to colonize the uterus and give rise to as much as 80% of the epithelium in mice [20]. In addition, a recent mouse study characterized a population of stromal cells expressing the hematopoietic marker CD34 and also positive for KLF4+ function as endometrial stem cells, being present in the stroma at an early phase but localize and incorporate into the regenerating epithelial layer during repair [21]. Thus, it is apparent that there are likely multiple subpopulations of ESCs of different origins in the uterus and further studies are warranted to determine whether they are functionally equivalent or perhaps have different roles in various reproductive processes.
Bone marrow-derived stem cells
While many ESCs are thought to be endogenous to the uterus, there is evidence from both humans and animal models that bone marrow is an exogenous source of ESCs. Bone-marrow derived stem cells (BMDSCs) have been identified in the human endometrium in several studies. In 2004, when ESCs were first reported and characterized in adult human endometrial tissue [1], a study by Taylor showed bone marrow as an origin of endometrial stromal and epithelial cells in patients of an HLA-mismatched allogenic bone marrow transplant (BMT) [2]. These patients were reported to have non-hematopoietic bone marrow-derived cells (BMDCs) that accounted for up to 48% of epithelial endometrial cells [2], and 52% of stromal endometrial cells. Subsequent studies of patients who received a BMT similarly showed the presence of BMDSCs in human endometrial tissue, albeit to a lesser extent [22–24]. One such study noted that approximately 8% of uterine epithelial cells and 9% of uterine stromal cells were exogenously derived from bone marrow. In addition, it was shown that BM-derived cells gave rise to endothelial cells within endometrial vasculature [22]. Consistent with evidence in humans, mouse models using BMT to track labeled BM cells following myeloablative radiation showed that BMDSCs contribute to various nonhematopoietic cell populations of the nonpregnant endometrium including epithelial, stromal, and endothelial [20, 22, 25–28].
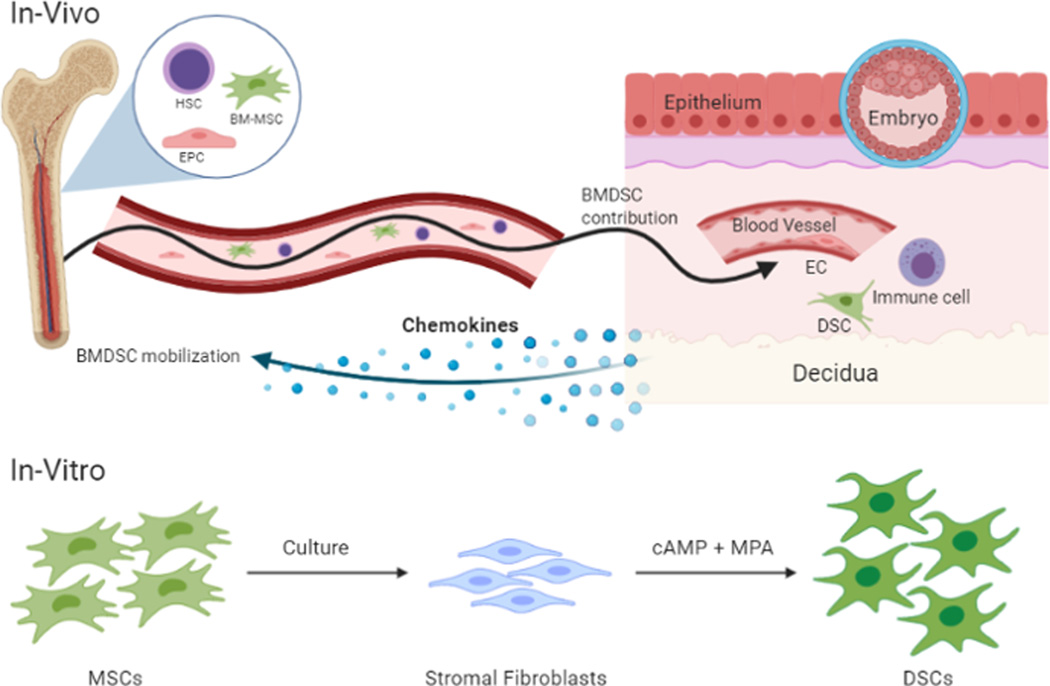
Various factors have been shown to affect recruitment of BMDSCs to the uterine lining. Estrogen, secreted during the follicular phase of the menstrual cycle, has been shown to promote incorporation of endothelial progenitor cells into the uterine vasculature [29]. Recruitment can also occur due to pathological stimuli, such as uterine injury or ischemia [26, 30]. CXCL12 ligand secreted by stromal cells has been shown to induce chemotaxis of BM-derived cells in-vitro [31], while inhibition of its CXCR4 receptor leads to decreased migration of BMDCs to the injured uterus in Asherman mouse model [32] and to endometriosis lesions in-vivo [33], suggesting that the CXCL12-CXCR4 axis plays an important role in recruitment of these cells to the uterus. Using a non-gonadotoxic BMT mouse model with 5-fluorouracil-based sub-myeloablation which allows tracking of donor BMDCs in reproduction [28], we have shown that implantation and pregnancy provide a strong stimulus for mobilization of BM-MSCs to the circulation and their recruitment to the decidua (approximately 4-fold) where these cells can differentiate into populations of nonhematopoietic, decidual stromal cells [34]. Moreover, pregnancy provides a stimulus for incorporation of endothelial progenitor cells into decidual vasculature in a process termed vasculogenesis [35] (Figure 1). While various markers have been found on BMDSCs, there are no known markers that can distinguish them from endogenous ESCs.
Figure 1.
Bone marrow-derived stem cell contribution to the decidua in pregnancy. (In-Vivo) The implantation of an embryo into the endometrium provides a chemokine gradient stimulating mobilization of bone-marrow derived stem cells (BMDSCs) from the bone marrow and their recruitment to the decidua. Circulating hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and endothelial progenitor cells (EPCs) are recruited to the pregnant uterus. BM-derived HSCs differentiate into immune cell populations, MSCs into decidual stromal cells (DSCs), and EPCs into endothelial cells (ECs) that get integrated into newly forming decidual blood vessels in a process termed vasculogenesis. (In-Vitro) MSCs extracted from bone marrow are grown in culture where they can differentiate into stromal fibroblasts. When treated with a combination of cyclic adenosine monophosphate (cAMP) and the medroxyprogesterone acetate (MPA), these MSCs differentiate into DSCs undergoing characteristic morphological changes and secrete decidualization markers including prolactin and IGFBP-1.
HSC, Hematopoietic Stem Cell; BM-MSC, Bone Marrow-Mesenchymal Stem Cell; EPC, Endothelial Progenitor Cell; BMDSC, Bone Marrow Derived Stem Cell; EC, Endothelial Cell; DSC, Decidual Stromal Cell; MSC Mesenchymal, Stem Cell; cAMP, Cyclic adenosine monophosphate; MPA, Medroxyprogesterone acetate; IGFBP-1, insulin-like growth factor binding protein-1.
The stem cell origin of decidual stromal cells and contribution to decidualization
It is well known that decidualization, the process of transformation of endometrial stromal cells into specialized epitheloid (decidual) cells, is induced by progesterone and cAMP and is crucial for implantation and pregnancy maintenance [36, 37]. Perturbations in decidualization can have negative effects on trophoblast invasion and implantation, placental development and, ultimately maternal and fetal well-being. Endometrial stromal fibroblasts (ESFs) are phylogenetic and ontogenic precursors of DSCs [38], but the exact stem cell type/s that gives rise to ESFs/DSCs remains to be determined. This insight may have important implications for better understanding of the biology of the decidualization process as well as implantation failure and pregnancy pathologies.
Initial studies in mice in which BM was reconstituted in the fetal period suggested that hematopoietic precursors give rise to DSCs [39]. Moreover, studies of human DSCs, both freshly isolated and cultured, have shown that they express antigens typical of hematopoietic cells such as CD34 [40–42], further pointing to a hematopoietic origin of DSCs. More recent studies, however, have suggested that the ESF, the precursor of DSC, originates in MSCs. MSCs are able to suppress the innate and adaptive immune response and display niche-dependent pro- and anti-inflammatory functions via cytokine and chemokine secretion [43, 44]. Thus, interactions of MSCs with DSCs and with immune cells at the maternal-fetal interface may play important roles in pregnancy. In a study that examined the relationship between perivascular mesenchymal ESCs (mESCs) and ESFs, endometrial cells were sorted into CD140b+/CD146+ (mESCs), CD140b+CD146- (ESFs), and CD140b-CD146+ (endothelial) cells and transcriptional analysis was performed by microarray. mESCs expressed many pericyte markers but clustered closer to ESFs than endothelial cells by unsupervised hierarchical clustering [45]. In a study on W5C5+ mESCs, it was shown by RNA sequencing that these cells retain phenotypic features of perivascular niche cells in culture, being enriched for many of the genes (30%) also enriched in CD140b+CD146+ mESCs, indicating a close lineage between these subpopulations. Notably, W5C5+ mESCs mount a decidual response that is highly divergent from ESFs [46]. Other types of human MSCs derived from amnion and from menstrual blood also showed decidualization potential characteristic of ESFs in vitro [47]. Another study showed that human BM-MSCs can be differentiated down the ESF pathway when treated with estradiol and progesterone, and undergo decidualization in-vitro in response to cAMP, expressing common decidualization markers [48]. The decidualized BM-MSCs also shared a similar transcriptional signature with decidualized ESFs, supporting BM-MSCs as a source of DSCs [48]. Consistent with this concept, experimental studies in pregnant mice demonstrated that BM-MSCs get mobilized to the circulation in pregnancy, and are recruited to the decidua where they give rise to differentiated functional DSCs with characteristic decidual stromal transcriptional signature [34]. In an interesting study that compared BM-MSCs and mESCs with ESFs, both BM-MSCs and mESCs displayed similar surface marker profiles and high proliferation and migration potential compared to ESFs but had a different cytokine profile [49]. Whether such different secretory profile may be explained by niche differences (i.e. BM vs. endometrium) or whether BM-MSCs have different functional properties that are retained in the endometrial environment is still unknown. The origin of DSCs has also been examined by a phylogenetic approach using RNA sequencing to infer lineage relations between different human endometrial cell types based on gene expression patterns. As expected, DSCs clustered closely with ESFs, but interestingly DSCs clustered closer to immune follicular dendritic cells than to classic cells of mesenchymal lineage such as chondrocyte and myofibroblast [50]. Interestingly, both DSCs and follicular dendritic cells are stromal cells with hematopoietic supportive activity that have been shown to be closely related to BM-MSCs [51]. Taken together, these data may lend further support to the idea that bone marrow is an origin of DSCs, that is consistent with these cells having immunoregulatory roles at the maternal-fetal interface [52]. While it is now clear that both BMDSCs and resident ESCs can give rise to DSCs, further studies are needed to characterize the types of stem cells (mesenchymal and/or hematopoietic) that give rise to DSCs in vivo, their functional properties in the decidual niche, and elucidate the mechanisms governing the differentiation of stem cells to DSCs within the decidua.
Potential therapeutic applications of endometrial and bone marrow-derived stem cells for endometrial disorders
Accumulating evidence from preclinical studies and initial clinical trials suggests there could be many therapeutic applications for stem cells in reproductive medicine, specifically for treating endometrial conditions underlying reproductive failure including recurrent implantation failure, thin endometrium, recurrent pregnancy loss and Asherman syndrome.
Recurrent implantation failure and thin endometrium
While embryo quality is a central determinant of implantation and pregnancy success, temporally coordinated differentiation of endometrial stromal cells into DSCs to attain uterine receptivity, and a synchronized cross-talk between maternal and embryonic tissues are crucial for successful implantation [53, 54]. These specialized DSCs play essential roles in endocrine regulation, nutritional sensing, immune tolerance and evaluation of embryo quality [55, 56]. Recurrent implantation failure (RIF) is estimated to affect 5–10% of women seeking IVF treatment. It refers to the failure to achieve a clinical pregnancy after the transfer of at least four good quality embryos in a minimum of three IVF treatment attempts into a normal, functioning uterus [57, 58]. Although multiple causes have been proposed, no clear cause can be identified in the vast majority of patients [59]. Currently, it is thought that an endometrial factor is most likely underlying RIF pathogenesis. Thin endometrium is another related condition that is associated with implantation failure and poor reproductive outcome in which the endometrium does not grow above 5mm, affecting about 0.5% of infertile women undergoing IVF [60]. Several studies have reported differences in endometrial transcriptional profile between RIF patients and control subjects [61–63]. Using a genetic knockout mouse model harboring a deletion in the transcription factor Homeobox a11 (Hoxa11) gene, we explored the functional importance of nonhematopoietic BMDSCs to implantation [34]. Hoxa11−/− mice are infertile due to endometrial-specific defects; they have abnormal endometrial stromal cells and lack endometrial glands, leading to decidualization defects and early implantation failure [64]. Hoxa11 was an ideal candidate since its expression in the bone marrow is restricted to MSCs [65], allowing us to study the specific contribution of BM-MSCs to implantation. BMT from wild-type (Hoxa11+/+) mice into Hoxa11−/− recipients led to significant stromal cell expansion, gland formation, and marked decidualization of the endometrium which was otherwise absent from Hoxa11−/− mice [34]. These results indicated that Hoxa11+ BMDSCs can affect gene expression at the uterine implantation site leading to profound effects on decidualization. In a murine experimental model of thin endometrium, intravenous injection of unfractionated BM cells resulted in endometrial regeneration and improvement in endometrial receptivity markers and was associated with better reproductive outcomes [66]. In a recent clinical trial, 29 women with history of thin endometrium and RIF had an endometrial biopsy followed by processing of the endometrial tissue and culture to obtain MSCs [67]. These autologous eMSCs were later administered to the patients together with platelet rich plasma (PRP) by subendometrial injection. Following treatment, the patients had an increase in endometrial thickness associated with 79.3% clinical pregnancy rate and 24% live birth rate. The study is limited by lack of a control group, and the concomitant endometrial biopsy and use of PRP, both of which have been suggested to have beneficial effects on endometrial receptivity [68, 69]. Further preclinical studies and carefully designed clinical trials are needed to investigate the potential of MSC therapy for RIF.
Recurrent pregnancy loss
Recurrent pregnancy loss (RPL) is defined as the loss of 3 or more consecutive pregnancies prior to 20 weeks of gestation [70]. RPL affects 1–2% of couples and its etiology is unexplained in over 50% of cases [70]. Lucas et al. found that endometrial stromal cells isolated from midluteal biopsies of women with RPL showed reduced clonogenic capacity that was associated with a loss of a particular epigenetic stem cell signature [71]. The same Brosens group found decreased endometrial clonogenic cell populations also in women with obesity-associated reproductive failure [72], suggesting that RPL is associated with ESC deficiency and/or dysfunction. Interestingly, they found that midluteal endometrium of RPL women is associated not only with eMSC deficiency but also excessive decidual senescence, and suggested that such an imbalance may predispose to the formation of a proinflammatory decidual-placental interface that is detrimental for pregnancy [73].
Further support to the concept that unexplained RPL may have an underlying stem cell dysfunction that can be ameliorated by stem cell therapy was provided by our group [34]. Hoxa11 heterozygous (+/−) mice are characterized by normal early implantations but increased pregnancy loss (resorption) due to decidualization defects. Hoxa11 expression has also been implicated in human implantation and is decreased in pregnancy loss and other conditions associated with pregnancy failure [74–76]. When Hoxa11+/− mice underwent BMT from WT mice donors, the recipients demonstrated a transformation of their uterine implantation transcriptome towards normalization in favor of decidualization. The Hoxa11+/− mice had a rescue of pregnancy loss and normal litter size after the BMT [34]. Ultimately, this study suggests that BMDSCs have nonhematopoietic physiological contribution to the developing decidual stroma in pregnancy and can influence the decidualization process of the endometrium leading to prevention of pregnancy loss. Although it remains to be established to what extent these findings of bone marrow progenitor contribution translate to human pregnancy, it supports the concept that BMDSCs dysfunction may contribute to implantation failure or pregnancy loss in women, and that BM stem cells may be a potential target for therapeutic intervention for these frustrating conditions. Other studies in abortion-prone mice (CBA/J x DBA/2), in which abortions are immune-mediated, have proposed that MSC therapy could be beneficial through MSCs immunomodulatory properties. In these mice, BM-MSC injection into the uterine horns [77] and intraperitoneal injection of adipose-derived MSCs [78] have been shown to change the pro- and anti-inflammatory balance and was associated with decreased embryo resorption rate.
A randomized placebo controlled clinical trial in women with RPL using sitagliptin, a dipeptidyl-peptidase IV (DPP4) inhibitor that is used for the treatment of diabetes, was recently conducted by Tewary and colleagues [79]. They performed two biopsies, at baseline and 3 menstrual cycles post-treatment, and found that sitagliptin treatment increased the abundance of clonogenic endometrial populations by 51% in the sitagliptin group. This was associated with rebalancing of the relative abundance of decidual cell subpopulations as measured by the ratio of SCARA5/DIO2 mRNA expression, reflecting attenuation of decidual senescence. Notably, the investigators noted large variability in clonogenic efficiency (0.07% to 5.2%) in women with RPL at baseline, suggesting that the severity of RPL is not only due to loss of eMSC numbers. The study does not provide direct evidence that the molecular changes reflective of improvement in decidual senescence in the endometrium of sitagliptin-treated women is due to the increase in eMSC numbers. Although it was a feasibility trial and was not powered to assess pregnancy outcome, the initial results are encouraging and a subsequent large-scale clinical trial is much awaited.
Asherman syndrome
Asherman syndrome is an acquired condition characterized by replacement of the endometrium by fibrotic tissue leading to intrauterine adhesions. It is detrimental to reproduction and manifests with infertility, recurrent miscarriage, placentation defects, menstrual abnormalities and pelvic pain [80].
In a mouse model of Asherman syndrome, based on mechanical damage to the endometrium, several studies have demonstrated that systemic injection of BMDSCs led to improvement in uterine scarring and reproductive outcomes [30, 32, 81, 82]. Interestingly, incorporation of BMDCs into the uterus in all of these models was rare, suggesting that local paracrine mechanisms rather than direct differentiation into uterine cells is likely underlying the observed beneficial effects.
Following the first two case reports of local injection of autologous BMDSCs to treat Asherman in humans [83, 84], a non-controlled Phase I clinical trial was conducted in 11 women with Asherman and 5 with thin endometrium. G-CSF was given for BM mobilization followed by collection of CD133+ circulating BM stem cells. CD133+ BMDSCs are enriched for circulating endothelial progenitor cells (EPCs) and are known to have pro-angiogenic effects [85]. Patients were administered autologous injection of CD133+ BMDSCs via uterine artery catheterization which resulted in increased endometrial thickness, improved angiogenesis indices and improvement in uterine scarring noted on hysteroscopy [86]. The same group evaluated the contribution of human CD133+ BMDSCs in a mouse model of Asherman following systemic or local BMDSCs administration. Labeled human cells engrafted around small endometrial blood vessels of damaged uterine horns and induced local proliferation of surrounding cells regardless of the cell administration route [82]. In another study using a mouse model of uterine injury, BMDSCs were recruited to the uterus more efficiently than endogenous uterine stem cells using systemic administration compared to local intrauterine injection [81], supporting the use of systemic route. While cell-based therapies hold promise in treating these conditions, there is still need for further research to guide optimal choice of stem cell subset, method and timing of administration.
Conclusion and Future Directions
Assisted reproductive technologies have transformed the field of reproductive medicine allowing many infertile couples to achieve their dream of parenthood. However, several conditions of infertility associated with reproductive failure such as RIF and RPL are poorly understood and have remained frustrating and challenging medical problems with significant social and psychological implications. Stem cell therapy is a rapidly expanding field that holds promise for alleviating numerous diseases involving chronic inflammation, fibrosis, and tissue injury. The adult human uterus harbors populations of tissue resident ESCs as well as exogenous BMDSCs in its uterine lining. Growing evidence from basic and preclinical studies suggests that these stem cells participate in endometrial tissue regeneration and repair. Recent evidence also suggests that ESCs and BMDSCs play a role in physiological reproductive functions including decidualization, implantation, pregnancy maintenance, and postpartum uterine remodeling. As such, stem cells have the potential to provide new therapies for various endometrial disorders associated with reproductive failure, including infertility, recurrent implantation failure, thin endometrium, Asherman, and recurrent pregnancy loss.
The initial preclinical and clinical studies with ESCs and BMDSCs in the field of reproduction are promising. However, we still have limited understanding of their therapeutic mechanisms or safety, and several issues are paramount in successful translation of their potential from the bench to the bedside. A key step would be to further characterize the various subpopulations of ESCs and BMDSCs in the human endometrium in terms of their characteristic cell markers and phenotype as well as specific roles in endometrial physiology and disease. Future research should also focus on increasing our understanding of the underlying molecular mechanisms of action and regulation of uterine BMDSCs as well as factors contributing to their propagation and recruitment to the uterus. Such understanding would allow appropriate selection and manipulation of stem cells that is tailored for treating specific reproductive conditions.
Acknowledgements
This study was supported in part by the US National Institutes of Health NICHD grant 5K12HD047018 (to RT).
References and Recommended Reading
- 1.Chan RW, Schwab KE, and Gargett CE, Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod, 2004. 70(6): p. 1738–50. [DOI] [PubMed] [Google Scholar]
- 2.Taylor HS, Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA, 2004. 292(1): p. 81–5. [DOI] [PubMed] [Google Scholar]
- 3.Zhu X, et al. , Stem Cells and Endometrial Regeneration: From Basic Research to Clinical Trial. Curr Stem Cell Res Ther, 2019. 14(4): p. 293–304. [DOI] [PubMed] [Google Scholar]
- 4.Santamaria X, et al. , Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update, 2018. 24(6): p. 673–693. [DOI] [PubMed] [Google Scholar]
- 5.Tal R. and Kisa J, Uterine stem cells: potential and pitfalls. Maturitas, 2020. 134: p. 54–55. [DOI] [PubMed] [Google Scholar]
- 6.Gargett C, Schwab K, and Deane J, Endometrial stem/progenitor cells: The first 10 years. Human reproduction update, 2015. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutlu L, Hufnagel D, and Taylor HS, The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol Reprod, 2015. 92(6): p. 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zakrzewski W, et al. , Stem cells: past, present, and future. Stem Cell Res Ther, 2019. 10(1): p. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tempest N, Maclean A, and Hapangama DK, Endometrial Stem Cell Markers: Current Concepts and Unresolved Questions. Int J Mol Sci, 2018. 19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crisan M, et al. , A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell, 2008. 3(3): p. 301–13. [DOI] [PubMed] [Google Scholar]
- 11.Schwab KE and Gargett CE, Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod, 2007. 22(11): p. 2903–11. [DOI] [PubMed] [Google Scholar]
- 12.Valenzuela JI, Hasan SJ, and Steeves JD, Stimulation of the brainstem reticular formation evokes locomotor activity in embryonic chicken (in ovo). Brain Res Dev Brain Res, 1990. 56(1): p. 13–8. [DOI] [PubMed] [Google Scholar]
- 13.Masuda H, et al. , A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant, 2012. 21(10): p. 2201–14. [DOI] [PubMed] [Google Scholar]
- 14.Chan RW and Gargett CE, Identification of label-retaining cells in mouse endometrium. Stem Cells, 2006. 24(6): p. 1529–38. [DOI] [PubMed] [Google Scholar]
- 15.Cervello I, et al. , Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod, 2007. 22(1): p. 45–51. [DOI] [PubMed] [Google Scholar]
- 16.Patterson AL, et al. , Label-Retaining, Putative Mesenchymal Stem Cells Contribute to Murine Myometrial Repair During Uterine Involution. Stem Cells Dev, 2018. 27(24): p. 1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao M, Chan RW, and Yeung WS, Label-retaining stromal cells in mouse endometrium awaken for expansion and repair after parturition. Stem Cells Dev, 2015. 24(6): p. 768–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaitu’u-Lino TJ, et al. , Identification of label-retaining perivascular cells in a mouse model of endometrial decidualization, breakdown, and repair. Biol Reprod, 2012. 86(6): p. 184. [DOI] [PubMed] [Google Scholar]
- 19.Cho NH, et al. , Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril, 2004. 81(2): p. 403–7. [DOI] [PubMed] [Google Scholar]
- 20.Bratincsák A, et al. , CD45-positive blood cells give rise to uterine epithelial cells in mice. Stem Cells, 2007. 25(11): p. 2820–6. [DOI] [PubMed] [Google Scholar]
- 21.Yin M, et al. , CD34(+)KLF4(+) Stromal Stem Cells Contribute to Endometrial Regeneration and Repair. Cell Rep, 2019. 27(9): p. 2709–2724.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mints M, et al. , Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod, 2008. 23(1): p. 139–43. [DOI] [PubMed] [Google Scholar]
- 23.Ikoma T, et al. , Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol, 2009. 201(6): p. 608 e1–8. [DOI] [PubMed] [Google Scholar]
- 24.Cervello I, et al. , Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One, 2012. 7(1): p. e30260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du H. and Taylor HS, Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells, 2007. 25(8): p. 2082–6. [DOI] [PubMed] [Google Scholar]
- 26.Du H, Naqvi H, and Taylor HS, Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev, 2012. 21(18): p. 3324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morelli SS, Rameshwar P, and Goldsmith LT, Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol Reprod, 2013. 89(1): p. 7. [DOI] [PubMed] [Google Scholar]
- 28.Tal R, et al. , A Murine 5-Fluorouracil-Based Submyeloablation Model for the Study of Bone Marrow-Derived Cell Trafficking in Reproduction. Endocrinology, 2016. 157(10): p. 3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda H, et al. , Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res, 2007. 101(6): p. 598–606. [DOI] [PubMed] [Google Scholar]
- 30.Alawadhi F, et al. , Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS One, 2014. 9(5): p. e96662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, et al. , Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res, 2015. 15(1): p. 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahin Ersoy G, et al. , CXCL12 Promotes Stem Cell Recruitment and Uterine Repair after Injury in Asherman’s Syndrome. Mol Ther Methods Clin Dev, 2017. 4: p. 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pluchino N, et al. , Effect of local aromatase inhibition in endometriosis using a new chick embryo chorioallantoic membrane model. J Cell Mol Med, 2019. 23(8): p. 5808–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tal R, et al. , Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol, 2019. 17(9): p. e3000421. ** This article is the first to demonstrate that adult BM-derived progenitor cells become decidual stromal cells and to describe a functional role for BM-derived progenitors in implantation and pregnancy maintenance.
- 35. Tal R, et al. , Bone-marrow-derived endothelial progenitor cells contribute to vasculogenesis of pregnant mouse uterus†. Biol Reprod, 2019. 100(5): p. 1228–1237. * This is a recent study that describes a direct contribution of BM-derived endothelial progenitor cells as endothelial cells incorporated as part of decidual vasculature in pregnnacy.
- 36.Cakmak H. and Taylor HS, Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update, 2011. 17(2): p. 242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dey SK, et al. , Molecular cues to implantation. Endocr Rev, 2004. 25(3): p. 341–73. [DOI] [PubMed] [Google Scholar]
- 38.Erkenbrack EM, et al. , The mammalian decidual cell evolved from a cellular stress response. PLoS Biol, 2018. 16(8): p. e2005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kearns M. and Lala PK, Bone marrow origin of decidual cell precursors in the pseudopregnant mouse uterus. J Exp Med, 1982. 155(5): p. 1537–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montes MJ, et al. , Cultured human decidual stromal cells express antigens associated with hematopoietic cells. J Reprod Immunol, 1996. 30(1): p. 53–66. [DOI] [PubMed] [Google Scholar]
- 41.Oliver C, et al. , Antigen phenotype of cultured decidual stromal cells of human term decidua. J Reprod Immunol, 1999. 45(1): p. 19–30. [DOI] [PubMed] [Google Scholar]
- 42.García-Pacheco JM, et al. , Human decidual stromal cells express CD34 and STRO-1 and are related to bone marrow stromal precursors. Mol Hum Reprod, 2001. 7(12): p. 1151–7. [DOI] [PubMed] [Google Scholar]
- 43.Kim N. and Cho SG, Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol, 2016. 103(2): p. 129–37. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, et al. , Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol, 2014. 15(11): p. 1009–16. [DOI] [PubMed] [Google Scholar]
- 45.Spitzer TL, et al. , Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod, 2012. 86(2): p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami K, et al. , Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology, 2014. 155(11): p. 4542–53. [DOI] [PubMed] [Google Scholar]
- 47.Sugawara K, et al. , Derivation of human decidua-like cells from amnion and menstrual blood. Sci Rep, 2014. 4: p. 4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aghajanova L, et al. , The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod, 2010. 82(6): p. 1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khatun M, et al. , Niche matters: The comparison between bone marrow stem cells and endometrial stem cells and stromal fibroblasts reveal distinct migration and cytokine profiles in response to inflammatory stimulus. PLoS One, 2017. 12(4): p. e0175986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kin K, et al. , Cell-type phylogenetics and the origin of endometrial stromal cells. Cell Rep, 2015. 10(8): p. 1398–409. [DOI] [PubMed] [Google Scholar]
- 51.Munoz-Fernandez R, et al. , Human decidual stromal cells secrete C-X-C motif chemokine 13, express B cell-activating factor and rescue B lymphocytes from apoptosis: distinctive characteristics of follicular dendritic cells. Hum Reprod, 2012. 27(9): p. 2775–84. [DOI] [PubMed] [Google Scholar]
- 52.Erlebacher A, Immunology of the maternal-fetal interface. Annu Rev Immunol, 2013. 31: p. 387–411. [DOI] [PubMed] [Google Scholar]
- 53.Salker M, et al. , Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One, 2010. 5(4): p. e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weimar CH, et al. , The motile and invasive capacity of human endometrial stromal cells: implications for normal and impaired reproductive function. Hum Reprod Update, 2013. 19(5): p. 542–57. [DOI] [PubMed] [Google Scholar]
- 55.Oreshkova T, Dimitrov R, and Mourdjeva M, A cross-talk of decidual stromal cells, trophoblast, and immune cells: a prerequisite for the success of pregnancy. Am J Reprod Immunol, 2012. 68(5): p. 366–73. [DOI] [PubMed] [Google Scholar]
- 56.Brosens JJ, et al. , Uterine selection of human embryos at implantation. Sci Rep, 2014. 4: p. 3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zohni KM, Gat I, and Librach C, Recurrent implantation failure: a comprehensive review. Minerva Ginecol, 2016. 68(6): p. 653–67. [PubMed] [Google Scholar]
- 58.Coughlan C, et al. , Recurrent implantation failure: definition and management. Reprod Biomed Online, 2014. 28(1): p. 14–38. [DOI] [PubMed] [Google Scholar]
- 59.Simon A. and Laufer N, Repeated implantation failure: clinical approach. Fertil Steril, 2012. 97(5): p. 1039–43. [DOI] [PubMed] [Google Scholar]
- 60.Senturk LM and Erel CT, Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol, 2008. 20(3): p. 221–8. [DOI] [PubMed] [Google Scholar]
- 61.Koot YE, et al. , An endometrial gene expression signature accurately predicts recurrent implantation failure after IVF. Sci Rep, 2016. 6: p. 19411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koler M, et al. , Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod, 2009. 24(10): p. 2541–8. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz-Alonso M, et al. , The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril, 2013. 100(3): p. 818–24. [DOI] [PubMed] [Google Scholar]
- 64.Hsieh-Li HM, et al. , Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development, 1995. 121(5): p. 1373–85. [DOI] [PubMed] [Google Scholar]
- 65.Rux DR, et al. , Regionally Restricted Hox Function in Adult Bone Marrow Multipotent Mesenchymal Stem/Stromal Cells. Dev Cell, 2016. 39(6): p. 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yi KW, et al. , Bone marrow-derived cells or C-X-C motif chemokine 12 (CXCL12) treatment improve thin endometrium in a mouse model. Biol Reprod, 2019. 100(1): p. 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tersoglio AE, et al. , Regenerative therapy by endometrial mesenchymal stem cells in thin endometrium with repeated implantation failure. A novel strategy. JBRA Assist Reprod, 2020. 24(2): p. 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Hoogenhuijze NE, et al. , Endometrial scratching prior to IVF; does it help and for whom? A systematic review and meta-analysis. Hum Reprod Open, 2019. 2019(1): p. hoy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maleki-Hajiagha A, et al. , Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: A systematic review and meta-analysis. J Reprod Immunol, 2020. 137: p. 103078. [DOI] [PubMed] [Google Scholar]
- 70.Ford HB and Schust DJ, Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol, 2009. 2(2): p. 76–83. [PMC free article] [PubMed] [Google Scholar]
- 71.Lucas ES, et al. , Loss of Endometrial Plasticity in Recurrent Pregnancy Loss. Stem Cells, 2016. 34(2): p. 346–56. [DOI] [PubMed] [Google Scholar]
- 72.Murakami K, et al. , Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure--a pilot study. PLoS One, 2013. 8(12): p. e82582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lucas ES, et al. , Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun Biol, 2020. 3(1): p. 37. ** Very recent data that characterizes transcriptomic changes in decidual stromal cells throughout decidualization and shows that recurrent pregnancy loss in humans is associated with a pro-senescent decidual response in stromal cells.
- 74.Sarno J, et al. , Thrombin and interleukin-1beta decrease HOX gene expression in human first trimester decidual cells: implications for pregnancy loss. Mol Hum Reprod, 2009. 15(7): p. 451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor HS, et al. , HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod, 1999. 14(5): p. 1328–31. [DOI] [PubMed] [Google Scholar]
- 76.Rackow BW and Taylor HS, Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril, 2010. 93(6): p. 2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng YH, et al. , Bone mesenchymal stem cells improve pregnancy outcome by inducing maternal tolerance to the allogeneic fetus in abortion-prone matings in mouse. Placenta, 2016. 47: p. 29–36. [DOI] [PubMed] [Google Scholar]
- 78.Sadighi-Moghaddam B, et al. , Mesenchymal Stem Cell Therapy Prevents Abortion in CBA/J x DBA/2 Mating. Reprod Sci, 2018. 25(8): p. 1261–1269. [DOI] [PubMed] [Google Scholar]
- 79. Tewary S, et al. , Impact of sitagliptin on endometrial mesenchymal stem-like progenitor cells: A randomised, double-blind placebo-controlled feasibility trial. EBioMedicine, 2020. 51: p. 102597. * Very recent data from a pilot clinical trial showing that the antidiabetic drug sitagliptin increases the numbers of endometrial clonogenic cells in women with recurrent pregnancy loss.
- 80.Di Guardo F, et al. , Evaluation and treatment of infertile women with Asherman syndrome: an updated review focusing on the role of hysteroscopy. Reprod Biomed Online, 2020. 41(1): p. 55–61. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, et al. , Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J Cell Mol Med, 2018. 22(1): p. 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cervello I, et al. , Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril, 2015. 104(6): p. 1552–60 e1–3. [DOI] [PubMed] [Google Scholar]
- 83.Nagori CB, Panchal SY, and Patel H, Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci, 2011. 4(1): p. 43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh N, et al. , Autologous stem cell transplantation in refractory Asherman’s syndrome: A novel cell based therapy. J Hum Reprod Sci, 2014. 7(2): p. 93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urbich C. and Dimmeler S, Endothelial progenitor cells: characterization and role in vascular biology. Circ Res, 2004. 95(4): p. 343–53. [DOI] [PubMed] [Google Scholar]
- 86.Santamaria X, et al. , Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod, 2016. 31(5): p. 1087–96. [DOI] [PubMed] [Google Scholar]