Abstract
Background and aims
Smoking is extremely common among adults experiencing homelessness, but there is lack of evidence for treatment efficacy. E‐cigarettes are an effective quitting aid, but they have not been widely tested in smokers with complex health and social needs. Here we build upon our cluster feasibility trial and evaluate the offer of an e‐cigarette or usual care to smokers accessing a homeless centre.
Design, Setting and Participants
Multi‐centre two‐arm cluster‐randomized controlled trial with mixed‐method embedded process and economic evaluation in homeless centres in England, Scotland and Wales. Adult smokers (18+ years; n = 480) accessing homeless centres and who are known to centre staff and willing to consent.
Intervention and Comparator
Clusters (n = 32) will be randomized to either an e‐cigarette starter pack with weekly allocations of nicotine containing e‐liquid for 4 weeks [choice of flavours (menthol, fruit and tobacco) and strengths 12 mg/ml and 18 mg/ml] or the usual care intervention, which comprises very brief advice and a leaflet signposting to the local stop smoking service.
Measurements
The primary outcome is 24‐week sustained carbon monoxide‐validated smoking cessation (Russell Standard defined, intention‐to‐treat analysis). Secondary outcomes: (i) 50% smoking reduction (cigarettes per day) from baseline to 24 weeks; (ii) 7‐day point prevalence quit rates at 4‐, 12‐ and 24‐week follow‐up; (iii) changes in risky smoking practices (e.g. sharing cigarettes, smoking discarded cigarettes) from baseline to 4, 12 and 24 weeks; (iv) cost‐effectiveness of the intervention; and (v) fidelity of intervention implementation; mechanisms of change; contextual influences and sustainability.
Conclusions
This is the first study, to our knowledge, to randomly assign smokers experiencing homelessness to an e‐cigarette and usual care intervention to measure smoking abstinence with embedded process and economic evaluations. If effective, its results will be used to inform the larger‐scale implementation of offering e‐cigarettes throughout homeless centres to aid smoking cessation.
Keywords: Cessation, e‐cigarettes, ENDS, harm reduction, homelessness, smoking, tobacco, usual care, vaping, very brief advice
INTRODUCTION
The health of people experiencing homelessness is extremely poor compared with the housed population, and smoking is a significant contributor to this [1, 2, 3]. Smoking prevalence rates among people experiencing homelessness range between 57 and 82% [4], making it three to four times higher than the national UK average of 14.1% [5]. Smoking is a leading cause of death in people aged 45 years and over who are homeless, and the second leading cause of death in adults under this age [2]. There is an urgent public health need to improve the lives of people experiencing homelessness, and reducing the burden of smoking would significantly advance this need.
Against a backdrop of research focused upon reducing tobacco‐related health inequalities, there is a growing evidence base on smoking cessation and homelessness [4, 6]. We conducted a systematic review of studies on smoking prevalence, interventions and facilitators and barriers to quitting in people who are homeless [4]; of 53 studies identified, only two had been conducted in the United Kingdom (one of which was by our group [7]). Studies from the United States and Australia have explored a range of interventions for smoking cessation among people experiencing homelessness, including motivational interviewing, cognitive behavioural therapy, quit lines, nicotine replacement therapy (NRT) and/or other pharmacotherapies (e.g. [7, 8, 9, 10]). From our review, the reported point prevalence (24‐hour or 7‐day) abstinence rates at 6 months were modest, ranging between 4 and 13.6%. One small study in the United States showed that, for veterans experiencing homelessness, a much higher 26‐week past 7‐day point prevalence abstinence rate of 45% (nine of 20) was reported when using contingency management; participants could earn up to $815 for carbon monoxide (CO) verified abstinence alongside use of NRT, bupropion and a smartphone application (app) [8]. In the only study reporting 6‐month sustained abstinence, no one quit [9]. A recent Cochrane Review [6] of the 10 intervention studies to reduce smoking concluded that there was insufficient evidence to assess the effects of any intervention, although there may be modest improvements when offering more intensive interventions. The included studies were deemed to be of low or very low quality; issues with design (e.g. lack of randomization) as well as substantial imprecision owing to the small number of events and small sample sizes (ranging from 11 to 645) resulted in insufficient statistical power in some studies. Follow‐up times varied, but seven of the included 10 studies assessed outcomes at 6 or 12 months (usually point‐prevalence) and these were CO‐validated where possible; however, dropout among studies was high. The authors concluded that more high‐quality randomized controlled trials (RCTs) investigating ways to support people experiencing homelessness to quit smoking are urgently needed. These should be sufficiently powered, retain participants for at least 6 months and work to retain participants until these end‐points.
Feelings of guilt, shame, stigmatization and undesirable or unhelpful past experiences with treatment services have been reported to contribute to reduced quitting success and an impediment to accessing cessation support [10]. There are also studies which highlight the negative views of, and a lack of interest in using, established cessation approaches such as NRT with a preference to engage in self‐defined, alternative tobacco harm‐reduction (THR) interventions such as e‐cigarettes (EC) [7, 10]. As well as being perceived more positively, EC may also offer a cost–benefit for those on a low or no income, as EC can be cheaper than smoking. However, the initial start‐up cost may be a barrier to use. In our survey of 283 smokers accessing homeless services across Great Britain we found that, although willingness to use EC was high, only 34% reported that they were willing or able to spend £20 or more on a starter kit [7].
Evidence for the efficacy of EC for smoking cessation is accumulating; in a Cochrane Review published in 2021 [11], among three RCTs with 1498 smokers there was moderate certainty that EC were almost 70% more effective than NRT for long‐term (defined as 12 months) smoking cessation [relative risk (RR) = 1.69; confidence interval (CI) = 1.25–2.27]. Higher quit rates were also found with EC compared with behavioural support among four studies (n = 2312; RR = 2.50; CI = 1.24–5.04), although the certainty here was low due to imprecision and risk of bias. EC may therefore be a viable alternative to traditional pharmacotherapies for smoking cessation in this population, especially if offered free of charge at homeless centres where relationships with staff are already established.
To explore the feasibility of offering EC to adult smokers accessing homeless services we conducted a cluster feasibility trial [12] in four centres; three were in England and one was in Scotland [12, 13]. In this trial, two clusters were assigned to offer participants usual care (UC), which consisted of the standard offer of referral to the local stop smoking service (SSS), and two clusters offered participants a free EC starter pack, which consisted of one refillable battery‐operated EC device, and e‐liquid was provided once per week for 4 weeks. The results showed the intervention was acceptable to both staff and participants. We were able to meet our progression criteria as more than half of all participants invited were recruited to the study (n = 80 in a 5‐month period) and we exceeded 50% retention at each follow‐up point. We were also able to collect the majority of the information needed for an economic evaluation and reports of unintended consequences (e.g. adverse effects, trading the device) were very low. The 24‐week sustained biochemically validated abstinence (ITT) rates were 6.25 (EC) versus 0% (UC)].
Building upon our feasibility study, here we aim to conduct a two‐arm multi‐centre cluster‐randomized controlled trial (cRCT).
Objectives
Primary
To determine the 24‐week sustained, biochemically validated abstinence rates in smokers offered EC compared to UC.
Secondary
Among those who have not achieved full abstinence, to compare the number reporting at least 50% smoking reduction at 24 weeks in the EC versus the UC arm.
To compare the number achieving 7‐day point prevalence quit rates at 4‐, 12‐ and 24‐week follow‐up in the EC versus the UC arm.
To document changes in risky smoking practices (e.g. sharing cigarettes, smoking discarded cigarettes) from baseline to 4, 12 and 24 weeks in both EC and UC arm.
To determine the cost‐effectiveness of the intervention.
To document fidelity of intervention implementation; mechanisms of change; contextual influences and sustainability.
METHODS
Design
A multi‐centre cRCT with internal pilot, with 1:1 cluster randomization to either an offer of an EC starter kit (including e‐liquid) (intervention, n = 16 clusters) or UC comprising very brief advice to quit and signposting to the local SSS (control, n = 16 clusters).
In‐built pilot
To assess the sustainability of the trial, a 6‐month internal pilot with the first 120 participants (eight centres) is included to monitor recruitment within the given time‐frame. After 6 months, based on individual level recruitment rates from the first eight centres, the decision to proceed is based upon the following progression criteria: green: 90% recruitment achieved = go; amber: 60–89% recruitment achieved = present action plan to Trial Steering Committee (TSC) with strategies for overcoming identified recruitment barriers (TSC to manage this plan with involvement of the study funder and formally assess recruitment again at 12 months); and red: < 60% = rescue plan considered by TSC and funder and joint decision on whether the study should continue.
Setting
The study will take place in 32 homeless day centres across five areas of Great Britain: London (n = 8), South East England (n = 6), East Anglia (n = 6), Wales and South West (n = 6) and Scotland (n = 6). The centres will be homeless centres offering a range of support during daytime hours, but do not offer sleeping accommodation or residency as their exclusive provision.
Centres will be recruited into the trial during a 16‐month period. Centres within a 100‐mile radius of the collaborating universities have been identified and those which meet the criteria (i.e. day care /drop in provision; primarily targeted at the homeless; not already providing EC to service users; within 2 hours’ travelling distance from each university) are being invited to participate. The first 32 centres that agree to work with us will be recruited.
Participants
Participants will be people who smoke who are experiencing homelessness in Great Britain, defined here as adults accessing one of the homeless centres in this study.
Inclusion criteria
The inclusion criteria included participants aged 18+, self‐reported daily smoking as verified by staff working at the homeless centres, known to centre staff and willing and able to provide written informed consent. A translator can be provided for those participants who are not able to read English. To represent this population of smokers as accurately as possible, we will not exclude participants based on physical or mental health diagnoses or other substance‐use disorders. Participants must indicate that they are willing to try an e‐cigarette or any other method of quitting smoking, but do not need to be motivated to quit (i.e. willing to engage with the study but make no commitment to quitting).
Exclusion criteria
Exclusion criteria included pregnant or breastfeeding, being a never or former smoker, allergies to any of the e‐liquid ingredients, engaged in an active quit attempt and currently using a smoking cessation aid (i.e. at baseline, although the use of another smoking cessation aid is permitted at follow‐up). While pregnancy is currently an exclusion criterion, this will be reviewed as safety data from ongoing RCTs reporting becomes available up to the point of the first data collection.
Sixteen members of staff in the EC arm, purposively sampled from eight centres (four in England, two in Scotland and two in Wales) will also be recruited for process evaluation interviews.
Recruitment
Recruitment will commence from February 2022 and is planned to continue until June 2023.
Recruitment is managed by centre staff and is restricted to an upper limit of 18 per centre due to cluster size. Participants who meet the inclusion criteria will be identified by staff and asked for expressions of interest before the centre is informed of their allocated condition (EC versus UC). The first 15 expressing an interest will be invited to an appointment with the researcher to complete informed consent. If 15 eligible participants have not been identified by this stage, staff in centres will continue to gain expressions of interest.
Staff will discuss the project with potential participants, and for those who express an interest the staff member will make an appointment with the research team for the baseline assessment (including consent).
The research team will introduce themselves, and the study, to service users at each centre. Interviews with participants in our feasibility study [12] revealed high levels of suspicion regarding research and distrust of external visitors. A candid and open discussion with potential participants should help to alleviate these concerns, build rapport and increase recruitment and retention.
Sample size
Assuming 0.05 alpha (two‐tailed), 90% power and cluster size of 15 participants (the feasibility study average in day centres [12]), this trial requires 240 participants per arm and 16 clusters per arm (480 participants and 32 clusters in total) to detect a difference of 5.75% between arms (i.e. 6.25 versus 0.5%, respectively, in the EC versus UC arms) using the power command in Stata version 15. The intraclass correlation coefficient (ICC) was set at 0.01, assuming equal cluster sizes. A final sample of 480 provides at least 90% power if the cluster size was smaller (n = 12) or greater (n = 18) than the planned 15 participants per cluster. No adjustment for attrition is applied to the sample size calculations, as participants lost to follow‐up will be classified as smokers as per Russell Standard.
There is sufficient power to detect more modest differences with smaller cessation rates in the EC arm; for example, with 5% cessation rate in the EC arm (versus 0.5% UC), allowing 81% power with an ICC of 0.01 and 74% with an ICC of 0.025.
Sensitivity sample size calculations will be conducted for the secondary outcome measuring 50% CO reduction, as previous studies have shown that CO reduction is a good predictor of future successful smoking quit attempts [14]. If we assumed a minimally clinical important difference (MCID) to be 10% (i.e. 13% EC versus 3% NRT), for 90% power, ICC = 0.01, alpha = 0.05 (two‐tailed) and cluster size 15, we would need 360 participants among 24 clusters in total. If abstinence rates are higher than observed in the pilot (e.g. 1%) the trial would still have 82% power, assuming all other assumptions are the same.
Intervention
Delivery of the EC intervention will be as per our feasibility study [12]. Centre staff will provide EC arm participants with a tank‐style refillable EC starter kit (the PockeX device used in our feasibility study and re‐confirmed through recent public and patient involvement (PPI), a choice of nicotine‐strength e‐liquids (12 and 18 mg/ml) and flavours (tobacco, menthol or fruit) and an EC fact‐sheet (developed for, and used in, our feasibility study). E‐liquids (five 10‐ml| bottles) will be supplied weekly for 4 weeks by centre staff; five bottles are always provided regardless of levels of use. Participants will be given time to try different flavours and nicotine strengths at baseline and be permitted to switch between flavours in accordance with documented vaping practices [15]. EC charging will be available at homeless centres. Although signposting and the provision of local SSS details do not form part of the EC intervention (as above), if participants make enquiries regarding their local SSS (we believe this would be rare) they can be signposted in the usual way as per homeless centre protocol. This information would be recorded as part of the standard health care utilization questionnaires administered at each follow‐up point (see economic evaluation section).
Comparator
The control intervention will form UC, defined here as very brief advice (VBA) about smoking cessation [in the form of a ‘National Health Services (NHS) choices’ leaflet adapted for this population, as used in our feasibility study] and signposting to the local SSS with information about their local service. Although some homeless centres offer more than this, this is not standard practice. Any centres with an established EC ‘in house’ provision or EC funding stream will be excluded. However, support or provision of EC from local SSS will be permitted, as this constitutes part of UC. SSS vary widely in terms of services they offer; although all SSS offer NRT and behavioural support, in 2019 only 11% of local authority‐funded SSS in England offered EC as part of their service [16], whereas others who consider themselves ‘e‐cigarette‐friendly’ offer support and advice concerning EC use. In cases where homeless centres do not have established links with their local SSS (the majority), we will facilitate these links and liaise with the relevant SSS.
Procedure
Figure 1 presents the study flow diagram and data collection at each time‐point. Table 1 presents the schedule of assessment at baseline and follow‐up.
FIGURE 1.
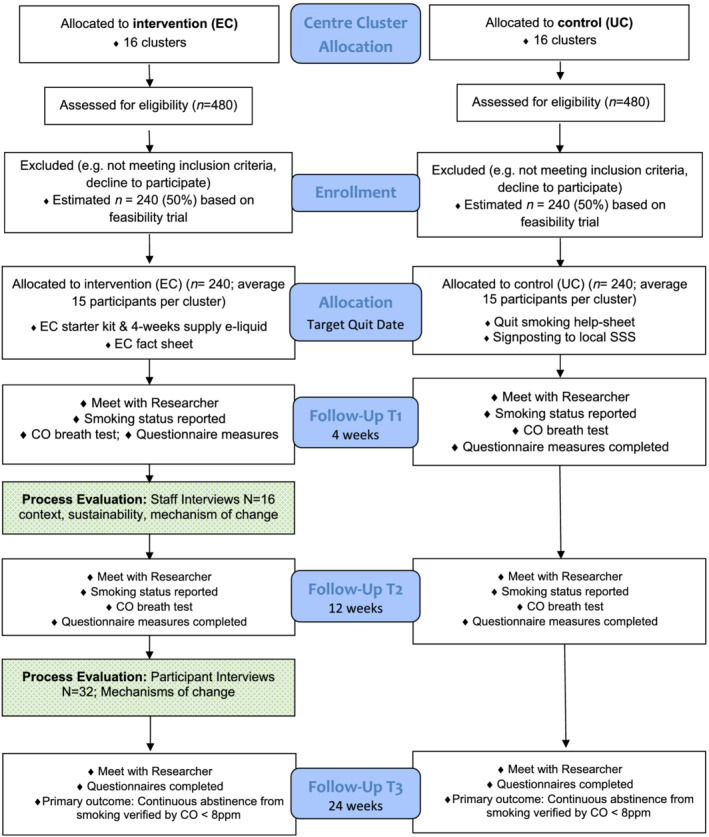
Flow diagram. Effects of e‐cigarettes (EC) versus usual care (UC) for smoking cessation when offered at homeless centres: a cluster‐randomized controlled trial (cRCT)
TABLE 1.
Schedule of enrolment, interventions and assessments
| Activities | Study period | |||||
|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post‐allocation | Close‐out | |||
| Baseline | 0 | 4‐week | 12‐week | 24‐week | t x | |
| Enrolment | ||||||
| Eligibility screen | X | |||||
| Informed consent | X | |||||
| Allocation | X | |||||
| Interventions | ||||||
| Usual care | X | |||||
| EC | X | |||||
| Assessments | ||||||
| Socio‐demographic characteristics | X | |||||
| Mental health status | X | |||||
| CO breath sample | X | X | X | X | ||
| Smoking behaviour (incl. risky smoking practices) | X | X | X | X | ||
| Motivation to stop smoking (MTSS) | X | X | X | X | ||
| Fagerström test of cigarette dependence (FTCD) | X | X | X | X | ||
| 7‐day point prevalence and 50% smoking reduction | X | X | X | |||
| Thoughts about EC | X | X | X | X | ||
| Adverse effects | X | X | X | X | ||
| Use of EC and unintended consequences (EC arm) | X | X | X | |||
| EC positive effects and EC support (EC arm) | X | X | X | |||
| Smoking cessation support received | X | X | X | X | ||
| Health‐care service use | X | X | X | X | ||
| Health‐related quality of life | X | X | X | X | ||
| Substance use | X | |||||
| AUDIT‐C | X | |||||
| Assessment of main effectiveness outcome | X | |||||
| Debrief | X | X | ||||
EC = electronic cigarettes; CO = carbon monoxide; AUDIT‐C = Alcohol Use Disorders Identification Test–Consumption.
Eligibility assessment
Participants will be screened for eligibility by the centre staff; they must be both known to the centre staff and a known current smoker. Details of other inclusion criteria are presented above.
Consenting
At the baseline appointment participant consent will be obtained by the researcher to: (a) take part in the study, (b) be contacted regarding participation in qualitative process evaluation interviews, (c) the sharing and appropriate linkage of anonymized data in accordance with the London South Bank University and European Social Research Council research ethics and government policies and (d) long‐term (up to 2 years) follow‐up (beyond the outcomes to be collected in the funded study). Individuals (participants and staff) agreeing to take part in the qualitative process evaluation interviews will provide further written consent prior to interviews and will consent to (a) recording the interviews and (b) the use of anonymized quotes in reports and publications.
Baseline assessment
Table 1 presents the schedule of assessments. At baseline, participants will be asked to complete both the consent form and the baseline assessment measures (see Measures, below). If participants cannot complete both on the same day, consent will be taken and another appointment to complete the baseline assessment will be arranged.
Randomization
Participating centres will be randomly allocated to the EC intervention (n = 16) or UC (n = 16). The intervention the participant receives will be based on their centre's allocation. The trial statistician will create the randomization list, which will be embedded/read in REDCap, which will be hosted by King's College London (KCL).
Intervention and comparator delivery
As per our feasibility study protocol, the intervention will be delivered by centre staff (Figure 1). The researchers will be involved in the data collection only. Training for staff will commence before recruitment (within 2 weeks). Staff training will focus upon (a) delivery of the study and (b) smoking cessation and tobacco harm reduction. Training will be to the standard by National Centre for Smoking Cessation and Training (NCSCT) recommendations [17]: information on smoking prevalence and patterns in the general population and in disadvantaged groups; health effects of smoking and benefits of cessation; evidence‐based smoking cessation treatment; misperceptions around smoking cessation in the context of other addictions and mental illness and study importance. Additionally, staff in the EC arm will be provided with information on the evidence base of EC use and effectiveness and information about how to deliver correct advice about EC to participants, together with a practical hands‐on demonstration and practice of EC (full details can be found in [Cox et al., 12]).
Recruitment will begin within 2 weeks of staff training. The researcher is responsible for gaining consent and will conduct the baseline assessment; centre staff will then deliver the intervention—either an EC or UC as per cluster assignment above (see above, ‘Intervention and comparator delivery’).
Follow‐up data collection
Follow‐up participant data will be collected in person by the research team at weeks 4, 12 and 24 after baseline assessment. Qualitative interviews (to address secondary outcome 5) will be conducted by the research team in four centres in the EC arm, with staff (n = 16) between weeks 4 and 8 and with service users/participants (n = 32) between weeks 12 and 24. Researcher observations around week 4 will also capture information on the fidelity of intervention implementation and contextual influences.
Staff are responsible for offering the 4 weeks’ provision of e‐liquid, and are required to monitor uptake of the liquids and report this to the research team.
Debriefing
All participants who attend the 24‐week follow‐up appointment or withdraw from the study will be debriefed (where possible). At this point participants will be given further information concerning the trial, including overall aims and expected outputs; this will be accompanied by information on the NHS SSS.
MEASURES
Table 1 presents the following assessments at the point of use throughout the trial.
Socio‐demographic and housing characteristics
Sex, age, ethnicity, education, employment and immigration status, access to government benefits (recourse to public funds) and current housing status, including where the participant stayed the night before the assessment, will be recorded.
Mental health status
This will be recorded by asking if the person has a diagnosed mental health condition.
CO breath sample
Participants will be asked to hold their breath for 15 sec and then to blow out slowly into a disposable mouthpiece attached to a Bedfont Pico Smokerlyzer; sample taken by research assistants.
Smoking characteristics and behaviour
Cigarettes smoked per day, smoking history (e.g. length of time smoking, previous quit attempts), severity of tobacco dependence as measured by the Fagerström Test of Cigarette Dependence (FTCD [18], the Motivation to Stop Smoking Scale [19], risky smoking practices (e.g. sharing cigarettes with others ‘going‐twos’ and smoking discarded cigarettes) and CO breath sample will be recorded.
Thoughts about EC
Two questions examining the extent to which participants agree or disagree with the statements ‘e‐cigarettes can help people stop smoking’ and ‘e‐cigarettes can help people reduce their smoking’ (1, strongly agree to 5, strongly disagree). Perceptions of harm of e‐cigarettes compared with cigarettes (as measured elsewhere [20]).
Use of the EC, effects of use, unintended consequences and support of use (EC arm only)
Questions relating to possession of the EC or whether the device has been lost, stolen, sold, exchanged, swapped or given away or broken, use of the device, reasons if stopped using it or if any additional non‐e‐liquid substance have been added to the device. Effects from the EC, including how satisfying and pleasant the EC is, how it tastes, and how helpful it was in reducing craving, and three questions relating to staff and social support around use of the device are included.
Adverse effects
Participants will be asked to indicate on a 5‐point Likert scale (1, not at all to 5, extremely) if they have felt any adverse health effects over the previous week; these include the most commonly reported adverse effects in previous trials [11] and those from the pre‐trial feasibility study [12], e.g. cough, shortness of breath, dizziness, weak and nauseous (full list not included).
Smoking cessation support received
Receipt of support for smoking cessation including advice or medication/products from a health practitioner and receipt, self‐purchase, and use of licensed nicotine replacement therapies.
Use of health‐care services
Travel time and costs to health appointments and health related quality of life as measured by the EQ‐5D‐3L will be recorded.
Substance use
To measure alcohol use the Alcohol Use Disorders Identification Test–Consumption (AUDIT‐C), a brief three‐item questionnaire [21], is included, and for other substances a single question asking if in the last month any illicit substances have been used.
Primary outcome measures
Sustained CO‐validated smoking cessation at 24 weeks using the Russell Standard for cessation trials i.e. no more than five cigarettes since 2 weeks’ post‐target quit date (TQD) validated by expired CO < 8 parts per million (p.p.m.) [22] and intention‐to‐treat analysis; i.e. analysis will be according to treatment allocation, regardless of compliance or cross‐over. All participants will be included in the primary analysis, and those lost to follow‐up who fail CO validation or refuse to provide a CO reading will be treated as non‐abstainers.
Secondary outcomes measures
Fifty per cent smoking reduction [calculated by reduction in self‐reported cigarettes per day (CPD) from baseline] at 24 weeks; 7‐day point prevalence quit rates at 4, 12 and 24 weeks; self‐reported changes in risky smoking practices (e.g. sharing cigarettes, smoking discarded cigarettes) from baseline to 4,12 and 24 weeks; cost‐effectiveness of the intervention; fidelity of intervention implementation; mechanisms of change; contextual influences; and sustainability.
Data management and monitoring
The data will be managed via the REDCap password‐protected system. The research team will enter the data, which will be checked by areas leads and overseen by the clinical trial manager.
Analysis
Participants’ demographic and smoking characteristics at baseline will be presented, broken down by trial arms. We will present means and standard deviations for continuous measures that are approximately symmetrical and median and quartiles if the distribution is skewed. Discrete outcomes will be described using both the number and proportion (percentage). Similarly, we will present summary measures of the primary and secondary outcomes.
The primary analysis will use mixed‐effect models with random effects for clusters and fixed‐effect models for treatment to compare the two arms on quit rates. A logistic mixed‐effect model will be used for binary outcomes. The model will be adjusted for cluster‐level. Sensitivity analyses of the primary outcome will be adjusted for individual‐level variables that differ between arms at baseline and if they are related to the outcome [23]. GEE Logistic modelling will be used as an alternative analysis approach if the mixed model does not converge. The results of the main analysis of the primary outcome will be presented as a difference in proportions (95% CI) and the number needed to treat (95% CI) will also be estimated based on the results of the primary end‐point.
The pattern of missing data by baseline characteristics will be explored. Sensitivity analyses will be conducted to assess the robustness of conclusions to missing outcome data (complete case analysis, multiple imputation) and departures from randomized treatment (per‐protocol analysis).
A detailed statistical analysis plan will be developed by the trial statistician and reviewed by the independent statistician. It will be finalized prior to completion of data collection and agreed with the DM(E)C/TSC.
Economic evaluation
This will be an incremental cost‐effectiveness of the EC intervention over and above the UC intervention.
The costs of providing the EC intervention will be recorded, including the costs of training, staff time and overheads and the EC products. We will collect costs prospectively and apply local unit costs to the quantities of each resource utilized. We will also record the costs of providing UC.
Following National Institute for Health and Care Excellence (NICE) guidance [24], we will collect health care utilization data for contacts with the NHS and personal and social services (PSS) using a bespoke service use questionnaire. This includes the use of primary and secondary health‐care services and social care. Quantities recorded are multiplied by national average unit costs [25, 26] to derive a cost profile for each patient. The service use questionnaire includes patients’ out‐of‐pocket expenditure on cessation aids, costs of travel to health services and lost productivity.
EQ‐5D‐5L [27] will be administered at baseline and each follow‐up. The UK social tariff is applied to derive quality‐adjusted life years (QALYs). We will use the tariff recommended by NICE at the time of analysis to calculate QALYs as the primary outcome for the economic evaluation [26, 28]. We will present a secondary analysis using the cost per quitter from an NHS/PSS perspective and a societal perspective (including patient cost of buying cessation aids, travel and productivity).
We will calculate QALYs using the QALY profiles as plotted at baseline and each follow‐up point using the standard area under the curve method [28]. Patient costs are combined with QALYs to estimate the incremental cost per QALY. The health economic analysis will use an existing model to extrapolate the longer‐term cost‐effectiveness [29]. Uncertainty around the decision to adopt the intervention is assessed using non‐parametric bootstrap re‐sampling. Bootstrapping is an efficient method for calculating the confidence limits for the incremental cost‐effectiveness ratio (ICER), as its validity does not depend upon any specific form of underlying distribution. The process for the bootstrapping uses 5000 replications of sampling with replacement to create a distribution for the ICER. The 95% CIs for the ICERs based on the bootstrapping results are derived from using the 2.5th and 97.5th percentiles. Cost‐effectiveness acceptability curves (CEAC) will be constructed based on the bootstrap iterations, as outlined above, [30] to estimate the probability that the intervention is cost‐effective at different threshold values for one QALY.
In addition to addressing the uncertainty surrounding the point estimate of the ICER, sensitivity analysis is undertaken to account for missing data.
Process evaluation
The process evaluation will use both quantitative and qualitative approaches to explore treatment context, fidelity of implementation, mechanisms of change (mediators as per logic model; Figure 2) and sustainability. Methods include observation, checklists, staff evaluation forms, questions within participant baseline and follow‐up questionnaires, in‐depth qualitative interviews and decisionmaker work‐shops. Further details, including methods and data analysis, are available in the Supporting information.
FIGURE 2.

Trial logic model
Ethics statement
Ethical approval has been gained from London South Bank University (Ref: ETH2021–0176). The results from the project will be published as open access and made available to the centres taking part. We will run a series of free‐to‐access impact events, which will be informed by our TSC with public involvement. We will adhere to our funders’ (NIHR) guidelines for publishing. The anonymized data will be made available on the LSBU (study sponsor) open research repository.
DISCUSSION
This trial will be the first cRCT of e‐cigarettes offered to smokers accessing homeless services in GB. The results will have significance for researchers, policymakers and clinicians interested in how to treat tobacco dependence among this population. If shown to be effective and cost‐effective, EC may be a viable alternative to smoking for this population and help to reduce the enormous burden of tobacco‐related death and disease prevalent within this group. Together with the main results, the embedded process evaluation will provide information on mechanisms of change, as well as implementation and scalability.
There are several challenges to this trial, which centre around recruitment and retention. The in‐built pilot will assess early recruitment targets with clear targets and protocols for different outcomes. Given the transitory nature of this group and competing health and social needs we will need to work sensitively and flexibly. In relation to retention, we observed retention rates of 75, 63 and 59%, respectively, at 4, 12 and 24 weeks in our feasibility study. These rates are similar to those of other studies in this population [4], but could be improved. Interviews with participants in our feasibility study revealed that mistrust, suspicion and anxiety around research were key barriers to retention, although some participants could not be followed‐up because they were no longer attending the homeless centre (e.g. due to imprisonment, hospitalization or had moved out of the area). Because retention (as well as recruitment) is key to the success of the trial, we have conducted two focus groups with eight members of staff and service users at homeless centres about ways to maximize retention. Psychological intrusion from questionnaires (personal, seemingly irrelevant questions), length of follow‐up sessions and appointments with different researchers were raised as additional barriers. We have reduced the length of our questionnaire and removed sensitive questions. We will also attempt, as far as possible, to ensure that the same researcher collects baseline and follow‐up data with each participant. Other suggestions from our PPI group were to maintain more regular contact with participants between sessions. We will therefore send regular text messages and make telephone calls between appointments (participants were generally quite willing to provide mobile phone numbers). We will also explore the option of following‐up participants at another mutually convenient location if they are no longer attending homeless centre services.
Another challenge concerns the use of an EC: the highly publicized media stories of EC harms have negatively impacted smokers’ perceptions of EC; indeed, in our feasibility study qualitative interviews highlighted participant uncertainty around EC. Our training with staff who will be delivering the EC intervention will be updated with the most recent safety and efficacy literature and we will continue to respond to all staff queries if these media headlines are released.
This is an important study, but there are some limitations. First, this study is conducted in Great Britain where the homeless population may vary from elsewhere, both in access to health care and specifically smoking cessation support (which is free to access in the United Kingdom); the nature of the homeless sector may also differ. Our findings may therefore have little transferability to low–middle‐income countries or countries without free point‐of‐access health care. E‐cigarettes are also a recommended smoking cessation aid in the United Kingdom, endorsed by leading public health bodies, minimizing the validity of the findings to other countries with more punitive regulations or stricter access.
CONCLUSION
To conclude, this will be the first cRCT in GB of e‐cigarettes versus usual care for people accessing homeless services who smoke. The findings are vital for understanding the impact of ECs in harder‐to‐reach and treat smokers and also for contributing to the evidence base on what works for smokers experiencing homelessness.
CLINICAL TRIAL REGISTRATION
ID ISRCTN18566874.
DECLARATION OF INTERESTS
S.C. is a Senior Editor for Addiction; she has no other competing interests. L.B., R.B., M.C., A.F., J.L., C.N., S.P., F.P., D.R., K.S., A.T. and E.W. declare no competing interests. L.D. has provided consultancy to the pharmaceutical industry around the development of reduced risk nicotine containing products. She is also a Senior Editor for Addiction. P.H. received research funding from and provided consultancy to Pfizer.
AUTHOR CONTRIBUTIONS
Sharon Cox: Conceptualization; funding acquisition; methodology; project administration. Linda Bauld: Conceptualization; funding acquisition. Rachel Brown: Methodology; project administration. Matthew Carisle: Writing ‐ review & editing‐Supporting. Allison Ford: Conceptualization; methodology; project administration. Peter Hajek: Conceptualization; methodology. Jinshuo Li: Methodology. Caitlin Notley: Methodology; project administration. Steve Parrott: Funding acquisition; methodology. Francesca Pesola: Funding acquisition; methodology. Deborah Robson: Funding acquisition; methodology; resources. Kirstie Soar: Project administration. Allan Tyler: Funding acquisition; methodology. Emma Ward: Methodology; project administration. Lynne Dawkins: Conceptualization; funding acquisition; investigation; methodology; project administration; supervision.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGEMENTS
This study/project is funded by the National Institute for Health Research, Public Health Research Programme (NIHR132158). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Cox S, Bauld L, Brown R, Carlisle M, Ford A, Hajek P, et al. Evaluating the effectiveness of e‐cigarettes compared with usual care for smoking cessation when offered to smokers at homeless centres: protocol for a multi‐centre cluster‐randomized controlled trial in Great Britain. Addiction. 2022;117:2096–2107. 10.1111/add.15851
Funding information National Institute for Health Research, Public Health Research Programme, Grant/Award Number: NIHR132158
Contributor Information
Sharon Cox, Email: s.cox@ucl.ac.uk.
Kirstie Soar, Email: soark@lsbu.ac.uk.
REFERENCES
- 1. Lewer D, Aldridge RW, Menezes D, Sawyer C, Zaninotto P, Dedicoat M, et al. Health‐related quality of life and prevalence of six chronic diseases in homeless and housed people: a cross‐sectional study in London and Birmingham, England. BMJ Open [internet]. 2019;9:e025192. https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2018-025192. Accessed 17th June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baggett TP, Chang Y, Singer DE, Porneala BC, Gaeta JM, O'Connell JJ, et al. Tobacco‐, alcohol‐, and drug‐attributable deaths and their contribution to mortality disparities in a cohort of homeless adults in Boston. Am J Public Health [internet]. 2015;105:1189–97. [cited 2021 May 24]. Available at: http://ajph.aphapublications.org/doi/10.2105/AJPH.2014.302248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hwang SW, Wilkins R, Tjepkema M, O'Campo PJ, Dunn JR. Mortality among residents of shelters, rooming houses, and hotels in Canada: 11 year follow‐up study. BMJ [internet]. 2009;339:b4036–6. [cited 2021 May 24]. Available at: https://www.bmj.com/lookup/doi/10.1136/bmj.b4036. Accessed 17th June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soar K, Dawkins L, Robson D, Cox S. Smoking amongst adults experiencing homelessness: A systematic review of prevalence rates, interventions and the barriers and facilitators to quitting and staying quit. J Smok Cessat [internet]. 2020;15:94–108. [cited 2020 Oct 6]. Available at: https://www.cambridge.org/core/product/identifier/S1834261220000110/type/journal_article. Accessed 17th June 2021. [Google Scholar]
- 5.Office for National Statistics (ONS). Adult smoking habits in the UK: 2019 [internet]. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2019#strengths-and-limitations. Accessed 17th June 2021.
- 6. Vijayaraghavan M, Elser H, Frazer K, Lindson N, Apollonio D. Interventions to reduce tobacco use in people experiencing homelessness. Cochrane Database Syst Rev [internet]. 2020. [cited 2020 Dec 15]. Available at: http://doi.wiley.com/10.1002/14651858.CD013413.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dawkins L, Ford A, Bauld L, Balaban S, Tyler A, Cox S. A cross sectional survey of smoking characteristics and quitting behaviour from a sample of homeless adults in Great Britain. Addict Behav [internet]. 2019;95:35–40. [cited 2020 Dec 15]. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0306460318312632. Accessed 17th June 2021. [DOI] [PubMed] [Google Scholar]
- 8. Carpenter VL, Hertzberg JS, Kirby AC, Calhoun PS, Moore SD, Dennis MF, et al. Multicomponent smoking cessation treatment including mobile contingency management in homeless veterans. J Clin Psychiatry. 2015;76:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Segan CJ, Maddox S, Borland R. Homeless clients benefit from smoking cessation treatment delivered by a homeless persons’ program. Nicotine Tob Res. 2015;17:996–1001. [DOI] [PubMed] [Google Scholar]
- 10. Collins SE, Orfaly VE, Wu T, Chang S, Hardy RV, Nash A, et al. Content analysis of homeless smokers’ perspectives on established and alternative smoking interventions. Int J Drug Policy [internet]. 2018;51:10–7. [cited 2020 Dec 15]. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0955395917302852 [DOI] [PubMed] [Google Scholar]
- 11. Hartmann‐Boyce J, McRobbie H, Lindson N, Bullen C, Begh R, Theodoulou A, et al. Electronic cigarettes for smoking cessation. Cochrane tobacco addiction group, editor. Cochrane Database Syst Rev [internet]. 2020. [cited 2020 Dec 15]. Available at: http://doi.wiley.com/10.1002/14651858.CD010216.pub4. Accessed 17th June 2021. [Google Scholar]
- 12. Cox S, Ford A, Li J, Best C, Tyler A, Robson DJ, et al. Exploring the uptake and use of electronic cigarettes provided to smokers accessing homeless centres: a four‐centre cluster feasibility trial. Public Health Res [internet]. 2021;9(7). Available at: 10.3310/phr09070. Accessed 17th June 2021. [DOI] [PubMed] [Google Scholar]
- 13. Dawkins L, Bauld L, Ford A, Robson D, Hajek P, Parrott S, et al. A cluster feasibility trial to explore the uptake and use of e‐cigarettes versus usual care offered to smokers attending homeless centres in Great Britain. PLOS ONE [internet]. 2020;15:e0240968. [cited 2020 Nov 26]. Available at: https://dx.plos.org/10.1371/journal.pone.0240968. Accessed 17th June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klemperer EM, Hughes JR. Does the magnitude of reduction in cigarettes per day predict smoking cessation? A qualitative review. Nicotine Tob Res [internet]. 2016;18(1):88–92. [cited 2020 Dec 15]. Available at: https://academic.oup.com/ntr/article-lookup/doi/10.1093/ntr/ntv058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farsalinos K, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health [internet]. 2013;10:7272–82. [cited 2020 Dec 15]. Available at: http://www.mdpi.com/1660-4601/10/12/7272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Action on Smoking and Health and Cancer Research UK. Many ways forward: stop smoking services and tobacco control work in English local authorities [internet] 2019. Available at: https://ash.org.uk/information-and-resources/reports-submissions/reports/many-ways-forward/. Accessed 17th June 2021.
- 17.National Centre for Smoking Cessation Training. Local Stop Smoking Services: Service and Delivery Guidance 2014 [internet] [cited 2020. Aug 1]. Available at: https://www.ncsct.co.uk/publication_service_and_delivery_guidance_2014.php
- 18. Fagerstrom K. Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nicotine Tob Res [internet]. 2012;14:75–78. [cited 2021 May 24]. Available at: https://academic.oup.com/ntr/article-lookup/doi/10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- 19. Kotz D, Brown J, West R. Predictive validity of the motivation to stop scale (MTSS): a single‐item measure of motivation to stop smoking. Drug Alcohol Depend [internet]. 2013;1281:15–9. [cited 2021 May 24]. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0376871612002864 [DOI] [PubMed] [Google Scholar]
- 20. Smith CA, Shahab L, McNeill A, Jackson SE, Brown J, Brose L. Harm perceptions of E‐cigarettes among smokers with and without mental health conditions in England: a cross‐sectional population survey. Nicotine Tob Res [internet]. 2021;23:511–7. [cited 2021 May 24]. Available at: https://academic.oup.com/ntr/article/23/3/511/5714358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction [internet]. 1993;88:791–804. [cited 2021 May 21]. Available at: http://doi.wiley.com/10.1111/j.1360-0443.1993.tb02093.x. Accessed 17th June 2021. [DOI] [PubMed] [Google Scholar]
- 22. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299–303. [DOI] [PubMed] [Google Scholar]
- 23. Bolzern JE, Mitchell A, Torgerson DJ. Baseline testing in cluster randomised controlled trials: should this be done? BMC Med Res Methodol [internet]. 2019;19(106). [cited 2022 Jan 31]. Available at: https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-019-0750-8. Accessed 17th June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence (NICE). Developing NICE Guidelines: the Manual [internet]. London, UK: National Institute for Health and Care Excellence; 2014. Available at: https://www.nice.org.uk/process/pmg20/chapter/introduction. Accessed 17th June 2021.
- 25. Curtis LA & Burns A Unit Costs of Health and Social Care 2019 [internet]. University of Kent; 2019 [cited 2020 Dec 15]. Available at: https://kar.kent.ac.uk/id/eprint/79286. Accessed 17th June 2021.
- 26. NHS England, NHS Improvement. National Cost Collection 2019 [internet]. NHS England; 2020. Available at: https://www.england.nhs.uk/national-cost-collection/#ncc1819. Accessed 17th June 2021.
- 27. van Hout B, Janssen MF, Feng Y‐S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ‐5D‐5L: Mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value Health [internet]. 2012;15:708–15[cited 2020 Dec 15]. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1098301512000587 [DOI] [PubMed] [Google Scholar]
- 28. Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ [internet]. 2004;13:1203–10. [cited 2020 Dec 15]. Available at: http://doi.wiley.com/1210.1002/hec.901 [DOI] [PubMed] [Google Scholar]
- 29. Wu Q, Parrott S, Godfrey C, Gilbert H, Nazareth I, Leurent B, et al. Cost‐effectiveness of computer‐tailored smoking cessation advice in primary care: a randomized trial (ESCAPE). Nicotine Tob Res [internet]. 2014;16:270–8. [cited 2020 Dec 15]. Available at: https://academic.oup.com/ntr/article-lookup/doi/10.1093/ntr/ntt136 [DOI] [PubMed] [Google Scholar]
- 30. Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost‐effectiveness acceptability curves. Health Econ [internet]. 2001;10(8):779–787. Available at: http://doi.wiley.com/10.1002/hec.635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


