Abstract
As consumer needs change, innovative food processing techniques are being developed that have minimal impact on food quality and ensure its microbiological safety. Sous vide (SV) is an emerging technology of cooking foods in vacuum pouches at specific temperatures, which results in even heat distribution. Presented here is an overview of the current state of the art in the application of SV techniques for processing and preserving foods. Unlike the conventional thermal food processing approach, the precise nature of the SV method improves food quality, nutrition and shelf‐life while destroying microorganisms. Foods processed by SV are usually subjected to temperatures between 50 and 100 °C. Although sufficient for food preparation/processing, its effectiveness in eliminating microbial pathogens, including viruses, parasites, vegetative and spore forms of bacteria, is limited. However, the inactivation of spore‐forming microbes can be enhanced by combining the technique with other non‐thermal methods that exert negligible impact on the nutritional, flavour and sensory characteristics of foods. In addition to exploring the mechanism of action of SV technology, the challenges related to its implementation in the food industry are also discussed. SV method potential, applications, and impacts on spore‐forming microbes and spore inactivation are explored in this review. Through the debate and discussion presented, further research and industrial applications of this food processing method could be guided. © 2022 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: food safety, sous vide, shelf‐life, spore‐forming microbes, spores
INTRODUCTION
In order to meet the changing taste of the consumer, new food processing technologies need to be developed. Several novel, emerging and green technologies are being developed and refined with the aim of preserving the sensory and nutritional qualities of food, its safety and shelf‐life. Unfortunately, most of these technologies are limited due to high energy demand, cost or long‐time input which may adversely affect food properties. Sous vide (SV) is one of the emerging food technologies that focuses on minimal heat treatment of foods, involving intermediate cooking at precise temperatures and times using heat‐stable vacuum pouches. 1 The technique is typically employed by professional chefs at low temperatures of 50–65 °C to ensure optimal sensory and nutritional quality are developed and preserved. 2 Other temperatures employed in SV ranges from 50 to 75 °C for fish, seafood and meat processing, maintained for several hours or even days, while high temperatures of 90–100 °C are used for vegetable processing for a few minutes. 3 Recently, the potency of the technique has become evident from the effective results achieved in the processing of meat, 4 , 5 vegetable and plant‐based food, 6 , 7 , 8 , 9 and fish treatment. 10 , 11 , 12
Due to the low and precise temperatures used, and the minimal impact on food's organoleptic and nutritional properties, SV technology is gaining popularity. It is well known that sensory and flavour properties are important criteria from the consumer perception and acceptance of the organoleptic attributes of food and the physiochemical changes which occur in food during processing. 13 Although the culinary aspect of the SV technique has been well established, scientific research on the microbiological safety aspect is still to be fully considered and remains a concern. 14 The evaluation of this aspect of the technology by reviewing and analysing literature would provide bases and insight that will guide further research. Currently, in contrast to its previous position as a fancy technique for individual caterers, SV is increasingly accepted by the mass production sector as a method of processing food.
The major benefit derived from the application of SV technology is optimal quality preservation without alterations of the organoleptic properties of the food. Compared to traditional cooking methods, the precise temperature control employed in the technique gives more choice over doneness and texture. Additionally, the use of heat‐stable vacuum pouches improves shelf‐life and can enhance taste and nutrition. 2 However, this technique's low cooking temperatures could provoke microbiological concerns on the safety of the food products due to non‐conformation to pasteurization standards/processes. 2 It follows that the survival of injured foodborne pathogenic microorganisms post‐treatment may pose a threat to food consumers or result in microbial‐mediated degradation of the product and a shortening of its shelf‐life.
In recent years, ready‐to‐eat meals without extensive thermal processing have become more popular. As a result, the food industry could adapt this technique to meet this need. In the SV technique, foods undergo minimal heat treatment, retaining their freshness, flavour, texture and even colour, and maintaining their microbial safety at the same time. 15 A variety of foods are regularly subjected to this treatment, including fish fillets and beef. However, it is important to fully understand the microbiological implications of this technique with respect to the control of pathogenic organisms in foods and elongation of the shelf‐life of foods. 16 The present review analysed the heat treatment intensity (major factor for SV operation) and the effect on food quality and composition, food‐contaminating organisms and bacterial spores, viruses and parasites. The study also explores and highlights the advantages and disadvantages of the technology. By in highlighting mechanisms of action and impact on spore formers, future research perspectives can be informed.
HEAT TREATMENT INTENSITY
Heat treatment intensity is regarded as one of the determinant factors in SV operation. However, heat treatment intensity is essential, especially in meats, to obtain a tasty and safe product. Depending on the exposure time, heat intensity indicates the extent to which the food can be cooked, and impact on the essential characteristics which the consumer prefers, such as taste, delicacy, colour and appearance. 17 On the other hand, heat treatment can contribute to loss of nutritional changes in meat quality due to lipid oxidation and changes in several segments of protein fraction. Evidently, the SV method is considered to be one of the techniques of heat treatment of food products. The characteristics of this method are pronounced when it is used to vacuum pack products and pasteurize foods in order to prolong their shelf‐life. It is well known that heat treatment intensity is associated with the temperature of exposure, which is also expected to exert some impact on spore‐forming bacteria. Even if the temperature is high enough to cook the food, could it be high enough to inactivate spore‐forming microbes? Although precise temperature control aids to control and promote the cooking process and texture of the product compared with the application of traditional cooking, 2 , 12 the temperature should be sufficient to inactivate spore‐forming bacteria.
Generally, it has been established that SV uses low temperatures of 50–80 °C with longer time, depending on the type of food. In other words, when SV is applied, each food product has a specific temperature range; for instance, meat is cooked at a temperature between 55 and 80 °C. At these temperatures, myoglobin can be denatured entirely and, similarly, the colour of the meat changes. However, the change depends on the type of meat and could be a result of the evolution of flavour and texture. At low temperature, the juiciness of meat is maintained, thereby improving its flavour, 18 but in the case of poultry meat cooked at low temperature there is a tendency for pink colour defection, which affects the appearance and causes consumer complaints concerning the impression of uncooked or bloody meat. 19
The heat treatment of meat conducted by Jaworska et al. 20 was carried out in two stages: the process of boiling in water, followed by SV cooking. In the former stage, chicken meat was cooked in a pot, which was heated on a 3.5 kW induction cooker. The temperature of the cooking was about 100 °C in 75 mL unsalted water. However, the latter stage, involving low‐temperature SV, was conducted using a Hendi low‐temperature cooking unit (Hendi, Gądki, Poland). In this system, 20 L water was used, and the meat sample was cooked at 76 °C for 60 min; then the equilibrium temperature–time was measured. Interestingly, the breast meat was cooled to room temperature (20 °C) and weighed to determine the efficiency of the cooking process, which was done after the heat treatment. The obtained result of 88.5 and 71.0 g kg−1 was reported for the cooking yield using the SV method and traditional method of cooking in water, respectively. This shows that the cooking yield using the SV method for processing poultry meat is higher than that of the traditional method of cooking in water. In addition, from the result, it was observed that sensory quality by the SV method was higher in terms of colour tone, tenderness, juiciness and overall quality. On the other hand, the SV method of processing poultry meat was lower in terms of odour and flavour as compared to meat subjected to traditional cooking. In conclusion, the study recommends further research on the use of spices to improve the flavour characteristics of SV‐treated meat.
In another study, reported by Duma‐Kocan et al., 21 cooking and baking at 80–90 °C and 150–175 °C, respectively, decreases the water content in the dorsi muscle of wild boars as a result of the density of the tissue structure and the fat content in the meat after an increase in the heat treatment. This development contributes more to a favourable texture parameter such as hardness and and gumminess. Interestingly, heat treatment intensity influences the increase in yellow colour and the brightness of meat. After different heat treatments, the average protein content in dorsal muscles was at a similar level. A statistically significant relationship was also found between water content and adhesiveness and resilience, as well as a negative relationship between fat content and adhesiveness in cooked meat at 90–100 °C.
It is said that SV heat treatment intensity is convenient and offers storage stability, especially when used in vacuum‐packed food products (broiler and hen fillets). A study conducted by Ramane et al. 22 aimed to evaluate the effect of a fruit–vegetable additive on the chemical and sensory parameters of heat‐treated vacuum‐packaged poultry meats (broiler and hen fillets). The evaluation of heat treatment intensity showed that there was no significant difference in aroma, colour, flavour or aftertaste of heat‐treated vacuum‐packaged hen and broiler fillet, but the texture of the broiler fillet product was said to be more tender than that of the sample from the hen fillet. 22 Considering the effect of heat intensity on microbes and food quality, this depends on the type of microorganism. 23 Li and Gänzle 24 reported that the reduction in the number of vegetative cells and elimination of pathogenic microbes in food was the result of heat treatment and cooking. According to the researchers, a heat treatment temperature of 71 °C may not be enough to effectively decontaminate pathogenic E. coli strains in meat; hence, there is need to optimize the conditions of heat treatment for effectiveness inactivation. In spite of this, some pathogenic Escherichia coli, such as the E. coli AW 1.7 isolate from beef, have been found to be heat resistant, raising questions about the effectiveness of the method toward inactivating pathogens in beef processing.However, the effect of heat on E. coli depends on the variability of the strain and properties of food formulations, including salt and water activity. It is worth knowing that heat induces the alteration of E. coli cells including membrane, cytoplasm and ribosomes, as well as DNA, specifically on protein misfolding and aggregation. Similarly, in a study by Rodrigo et al., 23 most vegetative cells in food are killed at a sterilization temperature above 110 °C in the food industry. Examples of such vegetative cells pertaining to the safety of heat‐treated foods include Salmonella, Listeria, Campylobacter and E. coli. These microorganisms contaminate foods such as meat, milk, vegetables, fish and eggs. Therefore, if these food products are not properly stored and heat treated, this becomes a problem. From the literature, microorganisms are said to be more resistant to dry heat than wet heat. For this reason, it is recommended that wet heat be used in food preparation to enhance microorganism inactivation. 23 In most cases, heat can activate, deactivate, damage, mutate or cause complete inactivation of bacterial spores, depending on its intensity. In the case of increased temperatures above the optimum for growth, this would result in microbial inactivation, inability to initiate germination and possible deterioration. Additionally, bacteria that are damaged by heat are more susceptible to antibiotics that under normal circumstances would not be able to destroy them. As a result, the membrane is affected by heat, which alters the activity of the antibiotics at the surface. Different factors influence the amount of heat that bacterial endospores absorb. The factors are classified as follows: microorganisms during sporulation; microorganisms during storage of the spores that are produced; consequence of treatment given before, during and after treatment and nature of the medium; and microorganisms during the recovery of survivors. 23 An SV cooker (or cooking under vacuum) uses advanced packaging and processing techniques to cook food inside a vacuum. Figure 1 illustrates the sequential steps involved in preparing food with the SV method using specially designed equipment. With this technique, raw, minimally processed or precooked foods are vacuum‐packed in laminated plastic pouches or containers, and heated at precisely controlled temperatures in a convection steam oven or water bath. SV cooking method takes two forms: cook‐hold and cook‐chill. Cook‐hold involves packaging, vacuum packaging, heating or pasteurizing, finishing and serving; whereas cook‐chill involves packaging, vacuum packaging, pasteurizing, rapid chilling, refrigerating or freezing, reheating or rethermalizing, finishing and serving. A detailed review of this has been reported elsewhere. 2
Figure 1.
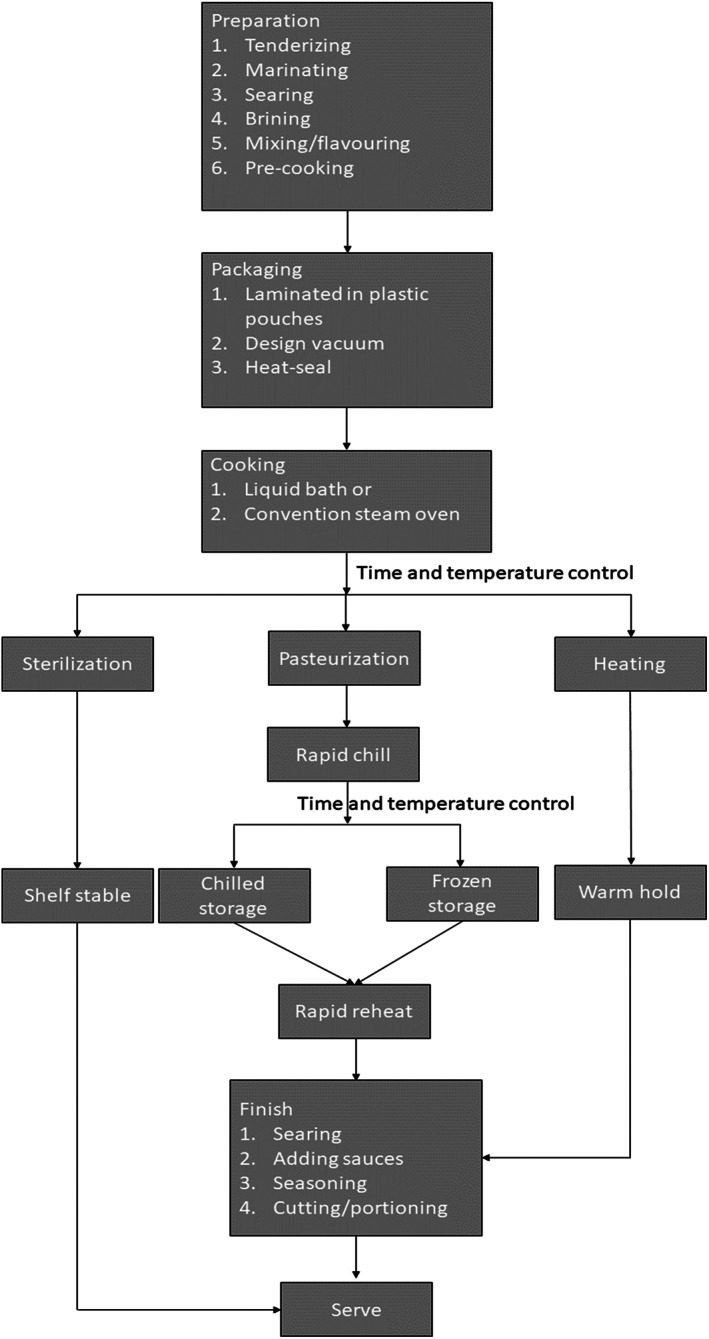
A typical flow diagram of the SV technology cooking process.
EFFECT OF SV PROCESSING ON FOOD QUALITY AND COMPOSITION
In hotels and restaurants, SV technology has traditionally been used by chefs. However, the technique is gaining a more significant interest, with various researchers evaluating its success in food processing. It has been reported that temperature and time are parameters that determine the outcome of the process, 4 , 25 and these parameters are believed to be helpful in retaining desirable attributes in foods. However, it was discovered in the treatment of mackerel fillets by the SV processing method that the variations in time and temperature did not influence the formation of primary and secondary products of lipid oxidation, nor increase the yellowness of the fish. 26 On the other hand, it was found that bolognaise meat sauce and chicken tikka masala processed at 70 °C for 90 min, and at 90 °C for 45 min, provided convenient, high‐quality refrigerated foods with extended durability. 16 Notwithstanding, SV‐cooked chicken breast and sliced potatoes treated at 80 °C for 10 and 30 min showed significantly increased flavour intensity, juiciness and moistness of the chicken and potato, respectively, compared to non‐vacuum‐packed freshly cooked products. 27
According to the study, the technique could potentially enhance the organoleptic quality of food. This is evident from the application of the technique in cooking fish portions for 20 min at 90 °C, which resulted in a final product with improved colour and high sensory acceptance. 28 In comparison with traditional cooking, it has been reported that SV (low temperature, long time) cooking of lean tuna resulted in better preservation of its quality. 29 Similarly, there was an improvement in the lipid profile and sensory attributes of salmon cooked using the SV process. 30 To assess the suitability of fresh vegetables such as potatoes for minimal processing, Rizzo et al. 31 conducted a study using the SV packaging method in association with rosemary essential oil (REO). This was used to evaluate the quality preservation of sliced potatoes. The findings from the study demonstrated that the use of REO and SV packaging had a positive effect on the texture of the product and also limited the growth of mesophilic bacteria and Enterobacteriaceae. The authors recommend SV as a valid and promising technology for the preservation of sliced potatoes. In a similar study carried out by Amoroso et al., 32 the benefit of SV cooking on the nutritional value of sliced potato was considered. The study involved the use of SV packaging method with REO as a good strategy for the preservation of sliced potatoes over refrigerated storage up to 11 days. It was reported that the presence of REO had no direct effect on the nutritional content of the cooked potato. Evidently, potato slices treated with SV and REO at 105 ± 5 °C/15 min cooking temperature and time retained all the nutritive compounds (ascorbic acid and total polyphenol content) of the potato sample considered in the study. In another study, which evaluated the quality of chicory stems cooked using conventional and innovative (SV) methods, it was found that the SV technique had a minimal impact on lightness and total colour difference quality parameters. Based on the results of the sensory analysis, SV products were rated higher on nutritional attributes than other cooking methods, since the SV process condition had no impact on total phenol content or antioxidant activity. 33
Consequently, ham prepared using the technique at 61 °C had a higher moisture content and redness, whereas that cooked at 71 °C showed higher cooking loss rate, lightness and volatile basic nitrogen values, with texture analysis indicating more tender meat for the treatment group compared to the control. 34 Subsequently, the examination of SV‐treated largemouth bass (Micropterus salmoides) revealed stable protein secondary structure and lower lipid oxidation. 35 This suggests less impact on food quality, flavour and nutritional characteristics. Similarly, SV‐processed beef subjected to in vitro simulated gastrointestinal protein digestion revealed increased protein digestibility, solubility and release of free amino acids and minerals. 36 These studies reaffirmed that the technique could potentially extend food shelf‐life with little or no impact on quality and sensory attributes. Table 1 presents possible temperature–time combinations that could be utilized in SV cooking for the inactivation of foodborne microorganisms for shelf‐life extension. The two main process variables controlled were temperature and processing time (Table 1). Nevertheless, depending on the type of food, the shelf‐life of the food products increases from 5 to 42 days. As expected, the longer the processing time, the longer is the food shelf‐life (Table 1).
Table 1.
Selected thermal processing time and temperature for SV
| Temperature and time | Type of food | Shelf‐life | Type of bacteria | Reference |
|---|---|---|---|---|
| 70 °C for 40 min | Eggnog, crab cake and breast meat | 6 days shelf‐life | Enterococcus faecalis | 37 |
| 70 °C for 100 min | Raw sausage, raw ham | 21 days shelf‐life | Enterococcus faecalis | 37 |
| 70 °C for 1000 min | Thigh, wings and legs | 42 days shelf‐life | Enterococcus faecalis | 38 |
| 70 °C for 2 min | Canned food, juice, beer | 5 days shelf‐life | Listeria monocytogenes | 39 |
| 80 °C for 26 min | Sweet potatoes, chicken breast | Maximum of 8 days shelf‐life | Clostridium botulinum type E | |
| 70 °C for 2 min | Milk, low‐alcoholic beverages | Short shelf‐life reliable storage temperature | Listeria monocytogenes | 40 |
| 80 °C for 4.6 min | Cup cakes | Maximum 10 days shelf‐life | Clostridium botulinum. | 40 |
The magnitude of heat treatment a food receives depends on the specific risk factors associated with the food, including possible foodborne pathogens. This is the usual guide for conventional cooking approaches to classify foods into lightly processed with minimal heat treatment and dwell time, pasteurized, and botulinum cook at the extreme. This classification scheme can also be applied in SV cooking as advisory heat treatment protocols for foods. 41 The selection of a specific pasteurization or sterilization protocol for a food during SV cooking is usually based on the heat resistance (D‐value; decimal reduction time) of the possible contaminating organisms. Also, consideration is made for the thermal death time (Z‐value), which is utilized in the selection of an appropriate time–temperature relationship to inactivate possible contaminating bacteria. Overall, models can be utilized to extrapolate the bacterial kinetics in relation to the processing conditions under SV treatment, in order to avoid over‐processing, accounting for the survival of pathogens in such treated foods and resuscitation during hold time. Thus, for the safety assurance of SV‐processed foods, predictive microbiology and modelling are important in the selection of treatment protocols applied to food for the removal of possible pathogens and the extension of shelf‐life of a food using multifactorial predictive models, with data extrapolated from published articles in journals which are organized and categorized using online databases, including the COMBASE, for easy retrieval, analysis and collation. 41
EFFECTS OF SV PROCESSING ON FOOD‐CONTAMINATING ORGANISMS
The ability of processing technology to inactivate spores and spore‐forming microbes determines the safety of food. Therefore, inactivation of spoilage and foodborne microorganism remains a fundamental goal of food preservation and shelf‐life extension. Microbial contamination of food is a leading cause of food spoilage, foodborne disease outbreaks and food product recalls. Globally, the contamination of foods by microorganisms has resulted in food product recalls with an economic cost running into millions of dollars. Thus food preservatives and preservation techniques are required to control microorganisms and their effects on foods effectively and efficiently. In this context, it is imperative to analyse and evaluate the ability of the SV technique to inactivate spore‐forming microbes and spores, improve food safety and extend shelf‐life. The low‐temperature range applied in SV technology has attracted several criticisms, as it could be insufficient to assure microbiological safety of food as a preservation technique.
In light of this, research on the shelf‐life extension effects of the technique has demonstrated that microbial growth is reduced after treatment. Hence a synergistic effect has been proposed; for instance, SV combined with other preservation treatments such as high‐pressure processing (HPP) for beef steak 42 could prove more effective for extending the shelf‐life of food products. The treatment of ham with SV alone brought about the inactivation of contaminating microorganisms, 34 and when used in combination with modified atmosphere or soluble gas stabilization packaging of salmon loins was effective in inhibiting the proliferation of Listeria spp. 43
In another study, evaluation of the microbiological quality of SV‐treated muscle cuts of pirarucu showed the absence of Salmonella spp. and sulfite‐reducing clostridia, and a maximum count of 3.5 log CFU g−1 for mesophilic and 2.67 log MPN g−1 coliforms. 44 Furthermore, a decrease in total mesophilic aerobic bacteria count of ~2 log CFU g−1 was observed for turkey cutlet samples prepared at 65, 70, 75 °C × 20, 40, 60 min cooking temperature–time combinations. 25 Similarly, the extension of the shelf‐life of crab lump meat, resulting in a final product that was microbiologically safe without any organisms present in all treatment settings, has been reported. 45 It is clear that the technique possesses the capability of reducing microbial load during food processing. Is this technique effective by itself in inactivating spore‐forming microbes responsible for food spoilage? Analysing published articles will help answer this question.
A more recent study showed that supplementation of the SV processing method with lauric arginate increased shelf‐life and beef quality and reduced the Listeria monocytogenes population. 46 Similarly, a rainbow trout processed by this method (90 °C for 3.3 min) and stored at 2 °C showed absence of Staphylococcus aureus, Bacillus cereus, Clostridium perfringens and L. monocytogenes. 45 Additionally, neither aerobic nor anaerobic spores were detected in the trout samples. 47 An SV‐cooked salmon slice at 90 °C for 15 min and stored at 2 °C showed lower growth rates of mesophiles and psychrotrophs. S. aureus, B. cereus, C. perfringens, L. monocytogenes and aerobic/anaerobic spore‐forming bacteria were detected in the samples after 45 days of storage. 48 In this way, vegetative cells processed by the SV method are likely to germinate. While some of these studies showed and confirmed that the technique destroyed spore‐forming microbes, reducing food spoilage due to microbes and extending shelf‐life, it was less effective when it came to mesophiles and psychrotrophs. To validate these studies, an evaluation study on SV‐cooked and processed mussels revealed that, with the addition of brine products, the shelf‐life was extended by an additional 30 days. 49 Hence it also important to examine the impact of the technique on the activities of bacterial spores, viruses and parasites, which is presented in the following sections.
EFFECTS OF SV PROCESSING ON BACTERIAL SPORES
Unlike vegetative bacterial cells, bacterial spores such as those of Bacillus and Clostridium are often resistant to food preservatives and preservation techniques, presenting unique safety concerns to food producers and processing industries. They are sensitive to pH > 4.4 and are considered to be an important pathogen in heat‐treated foods as a result of their ability to produce spores and toxins. Interestingly enough, the recommended shelf‐life is limited to 10 days unless the storage temperature is below 2.5 °C, when the shelf‐life should not exceed 90 days. Therefore, unlike other technologies, the application of SV technology can prevent spores of non‐proteolytic bacteria from outgrowing and producing neurotoxins without altering the nutritional value or organoleptic properties of the food. 3 This is attributed to the shelf‐life and storage temperature of SV. It has been reported that SV vacuum packs contain some degree of residual oxygen, which is not sufficient to inhibit the growth and reproduction of Bacillus and Clostridium usually found in food products. 50 Hence the high temperature posed by SV can injure bacterial spores (Bacillus and Clostridium), which may or may not be able to recover and grow. The germination of microbial spores in food under favourable conditions might lead to food contamination, spoilage and disease outbreaks. 51 Spores are a protective form of microorganisms and have an inherently distinct conformation that gives them the ability to withstand and to resist unfavourable environmental conditions such as high temperature, radiation and toxic compounds. 51 , 52 By definition, bacterial spores are said to be the structure that is produced by stressed bacterial cells. They are known to cause infection as a result of their intrinsic resistance. The optimal temperature for growth of most pathogenic bacteria is between 30 and 50 °C, where growth and reproduction of bacteria are initiated. In order to inactivate food pathogens such as Salmonella species, L. monocytogenes and E. coli, the core temperature of food during processing should not drop below 54.4 °C and cooking should be held for up to 6 h. 53 Cooking foods with SV at 70 °C for 2 min can achieve a 6‐log reduction of the most heat‐resistant vegetative pathogen (L. monocytogenes) for foods with a shelf‐life of less than 10 days. For foods with a shelf‐life of more than 10 days, cooking at 90 °C for 10 min (or equivalent) will result in a 6‐log reduction of C. botulinum spores. 53 Studies have demonstrated that the presence of pathogens in cooked SV foods originates from the raw materials as they survive cooking. 3 The storage of SV‐processed food in vacuum pouches, however, is a safe and effective method for preventing recontamination. In order to inactivate bacterial spores effectively, the SV method should be used in conjunction with other non‐thermal methods, such as ultrasound and supercritical carbon dioxide. A variety of bacterial spores can cause diseases such as tetanus, anthrax and botulism, while others can be used in biotechnological applications such as probiotics and biocides. 54 They possess protective layers such as coat and cortex in their resting state, which allow them to endure adverse conditions. 51 Hence, when conditions improve, the spores become active, germinating into the vegetative form of the bacteria; 51 examples of bacteria producing spores are B. cereus, C. botulinum and C. perfringens. The question is whether the temperature range applied in the SV technique is sufficient to inactivate spores. They are heat resistant and usually cannot be killed during processing without compromising the nutritional attributes. Depending on the temperature applied, spores inactivated by heat could germinate and grow if the process of cooling the food to ≤5 °C is prolonged. 55
SV, known for its minimal heat treatment nature, could be adequate for vegetative cells but ineffective in inactivating bacterial spores such as those of C. botulinum and B. cereus and also L. monocytogenes, which are considered to be major microbial hazards. 56 In an SV‐processed food at 77 °C and 94 °C, it was found that there was a 3‐log reduction in the population of B. cereus, from 0.5‐ to 1.0‐log. This suggests spores regerminated within 1 day at 10 °C. 57 However, SV has been shown to be effective in inactivating microbial spores when combined with other methods or used as an adjuvant method and inhibit spore germination/outgrowth. 58 , 59 , 60 Table 2 presents some of the substances and processes which, in combination with SV, can enhance its efficacy in the prevention of microbial growth and enzyme activity of food products, referred to as hurdles.
Table 2.
| Physical hurdle | Physiochemical hurdle | Microbiological hurdle |
|---|---|---|
| Heat processing | Water activity | Competitive microflora |
| Storage temperature | pH | Starter culture/outlook |
| Packaging | Redox potential | Bacteriocins |
| Photodynamic inactivation | Salt | Mould and yeast |
| Ultrahigh‐pressure processing | CO2, O2 | Enterbacteriaceae and antibiotic |
| Ultrasonification | Organic acids, spices and herbs | Antibiotic |
In Table 2, it can be observed that there are three types of hurdles present for SV operation. Hurdle technology is employed in the preservation of meat and seasonal or regional fruits and vegetables. It can provide variable results depending on the microbial stress reactions in food preservation. The aforementioned hurdles include physical, physiochemical and microbiological hurdles (See Table 2). It is obvious from this that basic determining factors are mainly related to the physiochemical hurdles involved in food preservation. This is because they are combined to achieve certain food quality and stability in terms of temperature, pH, redox potential, water activity, etc.
Numerous studies have focused on the effect of SV cooking based on the storage stability of meat. 1 , 65 , 66 It has been reported that anaerobic conditions present inside the SV package allows the growth of C. botulinum, which, if it is a toxigenic strain, can result in cases of botulism. 1 Further studies on the production of toxins by C. botulinum spores have shown that the addition of sodium lactate to low‐temperature‐processed SV beef, chicken breast and salmon resulted in a delay of toxigenesis in all three products, as shown in Table 3. 67
Table 3.
Toxicity time of Clostridium botulinum in SV product with respect to storage temperature, sodium lactate and product type effects 67
| Sous vide product | Sodium lactate (g kg−1) | Rate of production of toxin (days) | |||
|---|---|---|---|---|---|
| 4 °C | 8 °C | 12 °C | 30 °C | ||
| Beef (65–70 °C; 22–25 min) | 0 | 90 | 8 | 4 | 1 |
| 2.4 | >90 | 90 | >40 | 3 | |
| 4.8 | >90 | >90 | >40 | 6 | |
| Chicken (65–75 °C; 25–30 min) | 0 | 90 | 16 | 12 | 2 |
| 1.8 | >90 | 60 | >40 | 4 | |
| 3.6 | >90 | >90 | >40 | 6 | |
| Salmon (65–70 °C; 22–25 min) | 0 | 60 | 8 | 4 | 1 |
| 2.4 | 90 | 12 | 6 | 2 | |
| 4.8 | >90 | >90 | >40 | 4 | |
It is clear that at low storage temperature there is an enhancement of the ability of SV treatment to inhibit toxigenesis of C. botulinum in beef, chicken and salmon products. Considering the low levels of bacterial spores in meat used in the study and thermal processing, the use of ≥2.4 g kg−1 sodium lactate at a storage temperature of ≤12 °C inhibits toxigenesis for a period of 3–6 weeks, which is their anticipated shelf‐life. 67 Similarly, a study evaluating the effect of SV treatment on chicken products infused with sodium lactate on bacterial spore C. perfringens outgrowth in vacuum packaging 68 showed that temperature abuse of products for a period of 24 h or longer in the absence of sodium lactate results in the growth of C. perfringens from a spore inoculum. The findings demonstrated that the C. perfringens might germinate and grow to unsafe levels if the SV products are poorly handled and temperature abused for a relatively long period. It has also been reported that co‐inoculation with a protective culture such as Pediococcus spp. could not inhibit toxin production in SV inoculated with C. botulinum. 69
Experimental work was conducted by Farkas et al. 70 in 2002 on smoked–cured pork in stewed bean sauce inoculated with psychrotrophic B. cereus, which is more heat and radiation resistant than spores of non‐proteolytic C. botulinum. The meals were treated with combinations of pasteurizing heat treatments and γ‐irradiation of 5 kGy following vacuum packaging. 70 In combination with medium‐dose γ‐irradiation and/or nisin addition, SV cooking significantly improved microbiological safety and keeping quality of the meals. Recent work has suggested that the use of a non‐thermal food processing technique coupled with an antimicrobial peptide like nisin will significantly increase the inactivation of bacterial spores, 71 though the result showed that heat sensitization of the bacterial spores could result in survival after irradiation. Conclusively, adding nisin may increase the antimicrobial effectiveness of physical preservation treatments, but adverse sensory effects limit the amount of radiation or concentration that can be used. 70
A study by Miguel‐Garcia et al. 72 on C. perfringens spore outgrowth from pre‐inoculated SV‐processed pork revealed a significant impact of the treatment against C. perfringens that provides a degree of protection against the pathogen under mild temperature abuse of ≤15 °C conditions. The study advocated the maintenance of a good cold chain to guard against C. perfringens in SV‐processed pork meat marinated with tomato sauce. At a temperature of 15 °C, the combination of nisin and pediocin prevented the outgrowth of Bacillus subtilis (SV product in mushroom) and Bacillus licheniformis (SV product in shellfish salad). 59 Both nisin and pediocin are well‐known biopeptides that are effective against Gram‐positive organisms and the spore‐forming bacilli and clostridia. 73 , 74 By combining SV and these biopeptides, package swelling due to bacterial growth can be avoided, as well as ensuring safety when it comes to heat‐resistant bacterial strains. According to this study, nisin was the most effective for reducing thermal resistance of B. subtilis, whereas pediocin was more effective for reducing thermal resistance of B. licheniformis. 59
EFFECTS OF SV PROCESSING ON VIRUSES AND PARASITES
Noroviruses and hepatitis A virus (HAV) are the two most common human viruses that cause foodborne infections. Hepatitis E virus (HEV) is considered an emerging foodborne virus. It has been reported that evaluation of the heat inactivation of norovirus and hepatitis E virus is limited due to the lack of culture methods that help to determine viable virus particles in food products. 75 Hence, viruses do not grow on foods, but with a low infectious dose of 10–100 particles there is a tendency and the possibility for low‐level contamination to readily result in infection due to exposure.
A study conducted by Greening et al. 76 showed that viruses were relatively stable at 37 °C and could retain infectivity for days or weeks at 4 °C as well as remain infectious following freezing. It has been shown that feline calicivirus (FCV‐F9) and murine norovirus (MNV‐1) inoculated into spinach were inactivated at 56 °C with decimal reduction time (D‐values) of 0.16 (72 °C) to 14.57 (50 °C) and 0.15 (72 °C) to 17.39 (50 °C), respectively. 77 In another study with oysters, both MNV‐1 and Tulane virus (TV) were inactivated relatively faster when SV treatment was applied above 58 °C. After 1 m of thermal processing at 67 °C for MNV‐1 and 63 °C for TV, the viral load was below the detection limit. 78 Similarly, in non‐vacuum‐packed dried mussels, there was a 3.16‐log reduction of HAV at 60 °C.
The inactivation of parasites in the food industry is commonly processed by thermal control, such as heating or freezing. 79 One of the most common foodborne parasite infections is toxoplasmosis, caused by Toxoplasma gondii. Generally, the tissue cysts of T. gondii in meat are inactivated at a minimum cooking temperature of 67 °C. 80 However, this parasite has been shown to be inactivated by even lower heat treatment of 49 °C for 5.6 min, 55 °C for 44 s and 61 °C for 6 s using pork meat. 81 Similarly, in a non‐vacuum‐packed thermal inactivation test of the infectivity of Trichinella spiralis‐contaminated pork, it was shown that the viability of the parasite declined in <2 min at 60 °C 82 while the oocysts of Cryptosporidium parvum and Cryptosporidium hominis in milk and water were inactivated at 71.7 °C for 15 s 83 and 64.2 °C for 2 min. 84 These treatments are effective in the elimination of parasites, below the standard temperature requirements of 75 °C for 20s and 60 °C for 45 s, to lose the infectivity of Cryptosporidium parvum. 85
ADVANTAGES AND DISADVANTAGES OF SV COOKING
Although the focus of the review is on food safety, it will be of interest to highlight the advantages and disadvantages of SV cooking. This section is of essence as it is dedicated to the arguments or consideration for and against the technique within and outside the context of food safety. The technique has been proven to preserve the mineral content of food products, 86 improve protein digestibility and solubility, 36 and preserve the methylglyoxal scavenging potential of meat. 87 Other advantages of SV technology include prevention of aerobic bacterial growth, minimal loss of volatile flavour compounds and moisture, unaltered sensory qualities, preservation of the nutritional value of food, minimization of the generation of chemical species known for their deleterious effects on human health, such as heterocyclic amines and polycyclic aromatic hydrocarbons, juiciness, tenderness of meat and prevention of oxidation of plant pigments. 3 The advantages and disadvantages of the SV are summarized in Table 4.
Table 4.
| Advantages of sous vide | Disadvantages of sous vide |
|---|---|
| Presence of centralized production | — |
| Decreases the cost of raw materials | — |
| Production range is enlargeable | — |
| Prolonged shelf‐life at 0–3 °C | Requires staff education cost |
| Maximum keeping of aroma, texture, flavour and nutrients | Cost of equipment is high |
| Diminished post‐process cross‐contamination risk | High psychotropic Clostridium botulinum spore risk if product is undercooked or temperature abuse exists |
Although SV technology possesses several advantages, one major limitation and drawback relates to the microbiological safety of SV‐processed food when treatment is undertaken alone. SV technology has been reported to effectively inactivate aerobic and vegetative cells of bacteria such as Bacillus and Clostridium spp. In addition, SV cooking involves specialized equipment and training. Overall, the main problem associated with SV is mild heat treatment and anaerobic conditions, as well as decrease in the admissibility of the method by food processors and regulators. 90
CONCLUSION AND FUTURE PERSPECTIVES
The use of SV cooking methods satisfies current consumer taste and demand for fresh food that has not been subjected to high‐temperature treatments. However, even though this technology improves food organoleptic and nutritional attribute, concerns about the microbiological safety of processed foods still remain. This challenge can be addressed by supplementing SV cooking with other hurdle technologies for the improved elimination of microbial pathogens in foods. Although this could alter the concept of SV cooking, such treatments are able to achieve commercially sterile foods using temperatures below those obtainable during conventional cooking. Future applications of SV technology should explore the development of standardized equipment that can be precisely controlled to obtain even cooking. Also, treatment parameters such as temperatures and cooking time should be optimized for different food types and classes. In the context of food safety and spore inactivation, future perspectives must, however, be directed towards synergistic effects in combination with non‐thermal food processing technologies.
AUTHOR CONTRIBUTIONS
Conceptualization: HO; writing –original draft: HO, ON; writing – review and editing: AH, CA, TM; data curation: SJ, KO; supervision: HO. All authors were involved in the preparation of the final draft.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
REFERENCES
- 1. Schellekens M, New research issues in sous‐vide cooking. Trends Food Sci Technol 7:256–262 (1996). [Google Scholar]
- 2. Baldwin DE, Sous vide cooking: a review. Int J Gastron Food Sci 1:15–30 (2012). [Google Scholar]
- 3. Kilibarda N, Brdar I, Baltić B, Marković V, Mahmutović H, Karabasil N et al., The safety and quality of sous vide food. Meat Technol 59:38–45 (2018). [Google Scholar]
- 4. Ismail I, Hwang Y‐H and Joo S‐T, Effect of different temperature and time combinations on quality characteristics of sous‐vide cooked goat gluteus medius and biceps femoris. Food Bioproc Tech 12:1000–1009 (2019). [Google Scholar]
- 5. Park C, Lee B, Oh E, Kim Y and Choi Y, Combined effects of sous‐vide cooking conditions on meat and sensory quality characteristics of chicken breast meat. Poult Sci 99:3286–3291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiavaro E, Mazzeo T, Visconti A, Manzi C, Fogliano V and Pellegrini N, Nutritional quality of sous vide cooked carrots and Brussels sprouts. J Agric Food Chem 60:6019–6025 (2012). [DOI] [PubMed] [Google Scholar]
- 7. Guillén S, Mir‐Bel J, Oria R and Salvador ML, Influence of cooking conditions on organoleptic and health‐related properties of artichokes, green beans, broccoli and carrots. Food Chem 217:209–216 (2017). [DOI] [PubMed] [Google Scholar]
- 8. Lafarga T, Bobo G, Viñas I, Zudaire L, Simó J and Aguiló‐Aguayo I, Steaming and sous‐vide: effects on antioxidant activity, vitamin C, and total phenolic content of brassica vegetables. Int J Gastron Food Sci 13:134–139 (2018). [Google Scholar]
- 9. Venzke Klug T, Collado E, Martínez‐Sánchez A, Gómez PA, Aguayo E, Artés F et al., Viability of sous vide, microwave and high pressure processing techniques on quality changes during shelf life of fresh cowpea puree. Food Sci Technol Int 26:706–714 (2020). [DOI] [PubMed] [Google Scholar]
- 10. Cai WQ, Wei JL, Chen YW, Dong XP, Zhang JN, Bai F et al., Effect of low‐temperature vacuum heating on physicochemical properties of sturgeon (Acipenser gueldenstaedti) fillets. J Sci Food Agric 100:4583–4591 (2020). [DOI] [PubMed] [Google Scholar]
- 11. Cropotova J, Mozuraityte R, Standal IB, Aftret KC and Rustad T, The effect of sous‐vide cooking parameters, chilled storage and antioxidants on quality characteristics of Atlantic mackerel (Scomber scombrus) in relation to structural changes in proteins. Food Technol Biotechnol 57:191–199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Głuchowski A, Czarniecka‐Skubina E, Wasiak‐Zys G and Nowak D, Effect of various cooking methods on technological and sensory quality of Atlantic salmon (Salmo salar). Foods 8:323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roldán M, Antequera T, Martín A, Mayoral AI and Ruiz J, Effect of different temperature–time combinations on physicochemical, microbiological, textural and structural features of sous‐vide cooked lamb loins. Meat Sci 93:572–578 (2013). [DOI] [PubMed] [Google Scholar]
- 14. Shamsuzzaman K, Lucht L and Chuaqui‐Ofermanns N, Effects of combined electron‐beam irradiation and sous‐vide treatments on microbiological and other qualities of chicken breast meat. J Food Prot 58:497–501 (1995). [DOI] [PubMed] [Google Scholar]
- 15. Ayub H and Ahmad A, Physiochemical changes in sous‐vide and conventionally cooked meat. Int J Gastron Food Sci 17:100145 (2019). [Google Scholar]
- 16. Armstrong GA and McIlveen H, Effects of prolonged storage on the sensory quality and consumer acceptance of sous vide meat‐based recipe dishes. Food Qual Prefer 11:377–385 (2000). [Google Scholar]
- 17. Modzelewska‐Kapituła M, Dąbrowska E, Jankowska B, Kwiatkowska A and Cierach M, The effect of muscle, cooking method and final internal temperature on quality parameters of beef roast. Meat Sci 91:195–202 (2012). [DOI] [PubMed] [Google Scholar]
- 18. Aguilera JM, Relating food engineering to cooking and gastronomy. Compr Rev Food Sci Food Saf 17:1021–1039 (2018). [DOI] [PubMed] [Google Scholar]
- 19. Kieffer K, Claus J and Wang H, Inhibition of pink color development in cooked, uncured ground turkey by the addition of citric acid. J Muscle Foods 11:235–243 (2000). [Google Scholar]
- 20. Jaworska D, Rosiak E, Kostyra E, Jaszczyk K, Wroniszewska M and Przybylski W, Effect of herbal addition on the microbiological, oxidative stability and sensory quality of minced poultry meat. Foods 10:1537 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duma‐Kocan P, Gil M, Stanisławczyk R and Rudy M, The effect of selected methods of heat treatment on the chemical composition, colour and texture parameters of longissimus dorsi muscle of wild boars. CyTA – J Food 17:472–478 (2019). [Google Scholar]
- 22. Ramane K, Strautniece E and Galoburda R, Chemical and sensory parameters of heat‐treated vacuum‐packaged broiler and hen fillet products. Proc Latv Univ Agric 27:54–58 (2012). [Google Scholar]
- 23. Rodrigo D, Tejedor W and Martínez A, Heat treatment: effect on microbiological changes and shelf life, in Encyclopedia of Food and Health, Vol. 3, ed. by Caballero B, Finglas P and Toldrá F. Elsevier, Amsterdam, pp. 311–315 (2016). [Google Scholar]
- 24. Li H and Gänzle M, Some like it hot: heat resistance of Escherichia coli in food. Front Microbiol 7:1763 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bıyıklı M, Akoğlu A, Kurhan Ş and Akoğlu İT, Effect of different sous vide cooking temperature–time combinations on the physicochemical, microbiological, and sensory properties of turkey cutlet. Int J Gastron Food Sci 20:100204 (2020). [Google Scholar]
- 26. Cropotova J, Mozuraityte R, Standal IB and Rustad T, The influence of cooking parameters and chilled storage time on quality of sous‐vide Atlantic mackerel (Scomber scombrus). J Aquat Food Product Technol 28:505–518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Church IJ and Parsons AL, The sensory quality of chicken and potato products prepared using cook–chill and sous vide methods. Int J Food Sci Technol 35:155–162 (2000). [Google Scholar]
- 28. Fagan J and Gormley T, Effect of sous vide cooking, with freezing, on selected quality parameters of seven fish species in a range of sauces. Eur Food Res Technol 220:299–304 (2005). [Google Scholar]
- 29. Llave Y, Shibata‐Ishiwatari N, Watanabe M, Fukuoka M, Hamada‐Sato N and Sakai N, Analysis of the effects of thermal protein denaturation on the quality attributes of sous‐vide cooked tuna. J Food Process Preserv 42:e13347 (2018). [Google Scholar]
- 30. Redfern S, Dermiki M, Fox S, Lordan R, Shiels K, Saha SK et al., The effects of cooking salmon sous‐vide on its antithrombotic properties, lipid profile and sensory characteristics. Food Res Int 139:109976 (2021). [DOI] [PubMed] [Google Scholar]
- 31. Rizzo V, Amoroso L, Licciardo F, Mazzaglia A, Muratore G, Restuccia C et al., The effect of sous vide packaging with rosemary essential oil on the storage quality of fresh cut potato. Lebensm Wiss Technol 94:111–118 (2018). [Google Scholar]
- 32. Amoroso L, Rizzo V and Muratore G, Nutritional values of potato slices added with rosemary essential oil in sous vide bag. Int J Gastron Food Sci 15:1–5 (2019). [Google Scholar]
- 33. Renna M, Gonnella M, Giannino D and Santamaria P, Quality evaluation of cook–chilled chicory stems (Cichorium intybus L., Catalogna group) by conventional and sous vide cooking methods. J Sci Food Agric 94:656–665 (2014). [DOI] [PubMed] [Google Scholar]
- 34. Jeong K, Hyeonbin O, Shin SY and Kim Y‐S, Effects of sous‐vide method at different temperatures, times and vacuum degrees on the quality, structural, and microbiological properties of pork ham. Meat Sci 143:1–7 (2018). [DOI] [PubMed] [Google Scholar]
- 35. Wan J, Cao A and Cai L, Effects of vacuum or sous‐vide cooking methods on the quality of largemouth bass (Micropterus salmoides). Int J Gastron Food Sci 18:100181 (2019). [Google Scholar]
- 36. Bhat ZF, Morton JD, Zhang X, Mason SL and Bekhit AE‐DA, Sous‐vide cooking improves the quality and in‐vitro digestibility of semitendinosus from culled dairy cows. Food Res Int 127:108708 (2020). [DOI] [PubMed] [Google Scholar]
- 37. Morandi S, Brasca M, Alfieri P, Lodi R and Tamburini A, Influence of pH and temperature on the growth of Enterococcus faecium and Enterococcus faecalis . Lait 85:181–192 (2005). [Google Scholar]
- 38. Anon , Chilled and Frozen: Guidelines on Cook‐Chill and Cook‐Freeze Catering Systems. HMSO, London: (1989). [Google Scholar]
- 39. Committee SVA, Code of practice for sous vide catering systems. Sous Vide Advisory Committee, Gloucestershire, UK: (1991). [Google Scholar]
- 40. Gould, G. Conclusions of the ECFF botulinum working party, in Proceedings of the Second European Symposium on Sous Vide. ALMA Sous vide Competence Centre, Katholieke Universiteit Leuven. pp. 173–180 (1996).
- 41. Stringer SC and Metris A, Predicting bacterial behaviour in sous vide food. Int J Gastron Food Sci 13:117–128 (2018). [Google Scholar]
- 42. Sun S, Sullivan G, Stratton J, Bower C and Cavender G, Effect of HPP treatment on the safety and quality of beef steak intended for sous vide cooking. LWT – Food Sci Technol 86:185–192 (2017). [Google Scholar]
- 43. Abel N, Rotabakk BT, Rustad T, Ahlsen VB and Lerfall J, Physiochemical and microbiological quality of lightly processed salmon (Salmo salar L.) stored under modified atmosphere. J Food Sci 84:3364–3372 (2019). [DOI] [PubMed] [Google Scholar]
- 44. Pino‐Hernández E, de Carvalho Júnior RN, Alves RCB, Joele MRSP, E Silva NDS, da Silva EVC et al., Evaluation of muscle cuts of pirarucu (Arapaima gigas) and sous vide product characterization and quality parameters. Int J Gastronomy Food Sci 20:100200 (2020). [Google Scholar]
- 45. Olatunde OO and Benjakul S, Sous‐vide cooking as a systematic approach for quality maintenance and shelf‐life extension of crab lump meat. LWT – Food Sci Technol 142:111004 (2021). [Google Scholar]
- 46. Juneja VK, Osoria M, Tiwari U, Xu X, Golden CE, Mukhopadhyay S et al., The effect of lauric arginate on the thermal inactivation of starved Listeria monocytogenes in sous‐vide cooked ground beef. Food Res Int 134:109280 (2020). [DOI] [PubMed] [Google Scholar]
- 47. Gonzalez‐Fandos E, Garcıa‐Linares M, Villarino‐Rodrıguez A, Garcıa‐Arias M and Garcıa‐Fernandez M, Evaluation of the microbiological safety and sensory quality of rainbow trout (Oncorhynchus mykiss) processed by the sous vide method. Food Microbiol 21:193–201 (2004). [Google Scholar]
- 48. González‐Fandos E, Villarino‐Rodrıguez A, Garcıa‐Linares M, Garcıa‐Arias M and Garcıa‐Fernández M, Microbiological safety and sensory characteristics of salmon slices processed by the sous vide method. Food Control 16:77–85 (2005). [Google Scholar]
- 49. Bongiorno T, Tulli F, Comi G, Sensidoni A, Andyanto D and Iacumin L, Sous vide cook‐chill mussel (Mytilus galloprovincialis): evaluation of chemical, microbiological and sensory quality during chilled storage (3 C). LWT – Food Sci Technol 91:117–124 (2018). [Google Scholar]
- 50. Kilibarda N, Comparative study of selected quality parameters during storage of cold smoked trout packed in vacuum and modified atmosphere. PhD thesis, in Faculty of Veterinary Medicine. University of Belgrade, Serbia: (2010). [Google Scholar]
- 51. Hart A, Anumudu C, Onyeaka H and Miri T, Application of supercritical fluid carbon dioxide in improving food shelf‐life and safety by inactivating spores: a review. J Food Sci Technol 59:417–428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tehri N, Kumar N, Raghu H, Shukla R and Vashishth A, Microbial spores: concepts and industrial applications, in Microbial Bioprospecting for Sustainable Development. Springer, Dordrecht, pp. 279–289 (2018). [Google Scholar]
- 53. Zavadlav S, Blažić M, Van de Velde F, Vignatti C, Fenoglio C, Piagentini AM et al., Sous‐vide as a technique for preparing healthy and high‐quality vegetable and seafood products. Foods 9:1537 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abel‐Santos E, Bacetrial Spores. Caister Academic Press, Poole, UK: (2012). [Google Scholar]
- 55. Setlow P and Johnson EA, Spores and their significance, in Food Microbiology: Fundamentals and Frontiers, ed. by Doyle MP, Diez‐Gonzalez F and Hill C. ASM Press, Washington, DC, pp. 45–79 (2012). [Google Scholar]
- 56. Carlin F and Peck M, Growth and toxin production by non‐proteolytic and proteolytic Clostridium botulinum in cooked vegetables. Lett Appl Microbiol 20:152–156 (1995). [DOI] [PubMed] [Google Scholar]
- 57. Turner B, Foegeding P, Larick D and Murphy A, Control of Bacillus cereus spores and spoilage microflora in sous vide chicken breast. J Food Sci 61:217–219 (1996). [Google Scholar]
- 58. Aran N, The effect of calcium and sodium lactates on growth from spores of Bacillus cereus and Clostridium perfringens in a ‘sous‐vide’ beef goulash under temperature abuse. Int J Food Microbiol 63:117–123 (2001). [DOI] [PubMed] [Google Scholar]
- 59. Cabo M, Torres B, Herrera JR, Bernardez M and Pastoriza L, Application of nisin and pediocin against resistance and germination of bacillus spores in sous vide products. J Food Prot 72:515–523 (2009). [DOI] [PubMed] [Google Scholar]
- 60. Cosansu S and Juneja VK, Growth of Clostridium perfringens in sous vide cooked ground beef with added grape seed extract. Meat Sci 143:252–256 (2018). [DOI] [PubMed] [Google Scholar]
- 61. Creed PG, Sensory and nutritional aspects of sous vide processed foods, in Sous Vide and Cook–Chill Processing for the Food Industry, ed. by Ghazala S. Aspen, New York, pp. 57–88 (1998). [Google Scholar]
- 62. Akoglu IT, Biyikli M, Akoglu A and Khusan S, Determination of the quality and shelf life of sous vide cooked turkey stored at 4 °C and 12 °C. Rev Bras Cienc Avic 20:1–8 (2018). [Google Scholar]
- 63. Gómez I, Ibañez FC and Beriain M, Physicochemical and sensory properties of sous vide meat and meat analog products marinated and cooked at different temperature–time combinations. Inte J Food Prop 22:1693–1708 (2019). [Google Scholar]
- 64. Nura A, Chukwuma A and Oneh A, Critical review on principles and applications of hurdle technology in food preservation. Ann Food Sci Technol 17:485–491 (2016). [Google Scholar]
- 65. Hansen TB, Knøchel S, Juncher D and Bertelsen G, Storage characteristics of sous vide cooked roast beef. Int J Food Sci Technol 30:365–378 (1995). [Google Scholar]
- 66. Vaudagna SR, Sánchez G, Neira MS, Insani EM, Picallo AB, Gallinger MM et al., Sous vide cooked beef muscles: effects of low temperature–long time (LT–LT) treatments on their quality characteristics and storage stability. Int J Food Sci Technol 37:425–441 (2002). [Google Scholar]
- 67. Meng J and Genigeorgis C, Delaying toxigenesis of Clostridium botulinum by sodium lactate in ‘sous‐vide’ products. Lett Appl Microbiol 19:20–23 (1994). [Google Scholar]
- 68. Juneja VK, Delayed Clostridium perfringens growth from a spore inocula by sodium lactate in sous‐vide chicken products. Food Microbiol 23:105–111 (2006). [DOI] [PubMed] [Google Scholar]
- 69. Crandall AD, Winkowski K and Montville TJ, Inability of Pediococcus pentosaceus to inhibit Clostridium botulinum in sous vide beef with gravy at 4 and 10°C. J Food Prot 57:104–107 (1994). [DOI] [PubMed] [Google Scholar]
- 70. Farkas J, Polyák‐Fehér K, Andrássy É and Mészáros L, Improvement of microbiological safety of sous‐vide meals by gamma radiation. Radiat Phys Chem 63:345–348 (2002). [Google Scholar]
- 71. Anumudu CH, Hart A, Miri T and Onyeaka H, Recent advances in the application of the antimicrobial peptide Nisin in the inactivation of spore‐forming bacteria in foods. Molecules 26:5552 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Miguel‐Garcia DY, Juneja VK, Valenzuela‐Melendrez M, Díaz‐Cinco ME, Thippareddi H and Aida Peña‐Ramos E, Clostridium perfringens growth from spore inocula in sous‐vide processed pork‐based Mexican entrée. J Food Sci 74:M172–M176 (2009). [DOI] [PubMed] [Google Scholar]
- 73. De Vuyst L and Vandamme EJ, Nisin, a lantibiotic produced by Lactococcus lactis subsp. lactis: properties, biosynthesis, fermentation and applications, in Bacteriocins of Lactic Acid Bacteria, ed. by De Vuyst L and Vandamme EJ. Springer, Berlin, pp. 151–221 (1994). [Google Scholar]
- 74. Scott VN and Taylor SL, Effect of nisin on the outgrowth of Clostridium botulinum spores. J Food Sci 46:117–126 (1981). [Google Scholar]
- 75. Horn B, Hewitt J, Withers H, Olsen L, Lymburn J. Review of microbial pathogen inactivation relevant to sous vide cooking at temperatures below 55 °C. Ministry of Primary Industries (MPI), New Zealand Government, (2016). Available: https://www.mpi.govt.nz/dmsdocument/17881‐Review‐of‐microbial‐pathogen‐inactivation‐relevant‐to‐sous‐vide‐cooking‐at‐temperatures‐below‐55C
- 76. Greening GE, Dawson J and Lewis G, Survival of poliovirus in New Zealand green‐lipped mussels, Perna canaliculus, on refrigerated and frozen storage. J Food Prot 64:881–884 (2001). [DOI] [PubMed] [Google Scholar]
- 77. Bozkurt H, D'souza DH and Davidson PM, Thermal inactivation of human norovirus surrogates in spinach and measurement of its uncertainty. J Food Prot 77:276–283 (2014). [DOI] [PubMed] [Google Scholar]
- 78. Shao L, Chen H, Hicks D and Wu C, Thermal inactivation of human norovirus surrogates in oyster homogenate. Int J Food Microbiol 281:47–53 (2018). [DOI] [PubMed] [Google Scholar]
- 79. Kotula A, Dubey J, Sharar A, Andrews C, Shen S and Lindsay D, Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. J Food Prot 54:687–690 (1991). [DOI] [PubMed] [Google Scholar]
- 80. Hill DE and Dubey JP, Toxoplasma gondii as a parasite in food: analysis and control. Preharvest Food Saf 4:227–247 (2018). [DOI] [PubMed] [Google Scholar]
- 81. Mirza Alizadeh A, Jazaeri S, Shemshadi B, Hashempour‐Baltork F, Sarlak Z, Pilevar Z et al., A review on inactivation methods of Toxoplasma gondii in foods. Pathogens Global Hlth 112:306–319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kotula A, Murrell K, Acosta‐Stein L, Lamb L and Douglass L, Trichinella spiralis: effect of high temperature on infectivity in pork. Exp Parasitol 56:15–19 (1983). [DOI] [PubMed] [Google Scholar]
- 83. Harp JA, Fayer R, Pesch BA and Jackson GJ, Effect of pasteurization on infectivity of Cryptosporidium parvum oocysts in water and milk. Appl Environ Microbiol 62:2866–2868 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fayer R, Effect of high temperature on infectivity of Cryptosporidium parvum oocysts in water. Appl Environ Microbiol 60:2732–2735 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Moriarty E, Duffy G, McEvoy J, Caccio S, Sheridan J, McDowell D et al., The effect of thermal treatments on the viability and infectivity of Cryptosporidium parvum on beef surfaces. J Appl Microbiol 98:618–623 (2005). [DOI] [PubMed] [Google Scholar]
- 86. Rondanelli M, Daglia M, Meneghini S, Di Lorenzo A, Peroni G, Faliva MA et al., Nutritional advantages of sous‐vide cooking compared to boiling on cereals and legumes: determination of ashes and metals content in ready‐to‐eat products. Food Sci Nutr 5:827–833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cömert ED and Gökmen V, Effects of different cooking methods on methylglyoxal scavenging potential of meat under simulated gastrointestinal conditions. LWT – Food Sci Technol 132:109833 (2020). [Google Scholar]
- 88. Tansey F, Gormley RT, Carbonell S, Oliveira J, Bourke P and O'Beirne D, Developing sous vide/freezing systems for ready‐meal components. Teagasc, Dublin: (2005). [Google Scholar]
- 89. Yıkmış S, Aksu H, Çöl BG and Demirçakmak İL, Evaluation of sous‐vide technology in gastronomy. Int J Agric Life Sci 4:226–231 (2018). [Google Scholar]
- 90. Öztürk K and Nilüfer‐Erdil APDD, Packaging applications for ready‐to‐eat foods. Istanbul Technical University Faculty of Chemical and Metallurgical Engineering, Istanbul: (2015). [Google Scholar]


