Abstract
Endometrial stem/progenitor cells play a role in postpartum uterine tissue regeneration but the underlying mechanisms are poorly understood. While circulating bone marrow (BM)-derived cells (BMDCs) contribute to nonhematopoietic endometrial cells, the contribution of BMDCs to postpartum uterus remodeling is unknown. We investigated the contribution of BMDCs to the postpartum uterus using 5-fluorouracil-based non-gonadotoxic BM transplant from GFP donors into wild-type C57BL/6J female mice. Flow cytometry showed an influx of GFP+ cells to the uterus immediately postpartum accounting for 28.7% of total uterine cells, followed by a rapid decrease to pre-pregnancy levels. The majority of uterine GFP+ cells were CD45+ leukocytes and the proportion of nonhematopoietic CD45-GFP+ cells peaked on postpartum day (PPD) 1 (17.5%). Immunofluorescence colocalization of GFP with CD45 pan-leukocyte and F4/80 macrophage markers corroborated these findings. GFP+ cells were found mostly in subepithelial stromal location. Importantly, GFP+ cytokeratin-positive epithelial cells were found within the luminal epithelium exclusively on PPD1, demonstrating direct contribution to postpartum re-epithelialization. A subset (3.2%) of GFP+ cells were CD31 + CD45− endothelial cells, and found integrated within blood vessel endothelium. Notably, BM-derived GFP+ cells demonstrated preferential proliferation (PCNA+) and apoptosis (TUNEL+) on PPD1 vs resident GFP− cells, suggesting an active role for BMDCs in rapid tissue turnover. Moreover, GFP+ cells gradually acquired cell senescence together with decreased proliferation throughout the postpartum. In conclusion, BM-derived progenitors were found to have a novel nonhematopoietic cellular contribution to postpartum uterus remodeling. This contribution may have an important functional role in physiological as well as pathological postpartum endometrial regeneration.
Keywords: Bone marrow, Postpartum, Remodeling, Stem cells, Uterus
Graphical Abstract
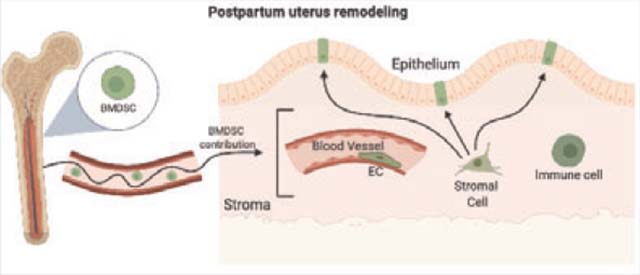
The contents of this page will be used as part of the graphical abstract of html only. It will not be published as part of main.
Bone marrow-derived stem cells (BMDSC) are mobilized to the circulation and get recruited to the uterus in the postpartum period where they contribute to various nonhematopoietic endometrial cell populations as part of the process of cellular turnover and regeneration. They differentiate into stromal cells, endothelial cells (EC) and epithelial cells, actively participating in uterine tissue remodeling
1 |. INTRODUCTION
The adult human uterus is a highly dynamic tissue that undergoes regular cycles of growth, differentiation, breakdown and regeneration with each of the ~400–500 menstrual cycles during a woman’s reproductive lifespan. In humans and other menstruating mammals, cyclic shedding of the functionalis layer of the endometrium is followed by regeneration of endometrial stroma, glands, and vasculature in a predictable fashion driven by the underlying basalis layer. In non-menstruating mammals such as mice, cyclic uterus regeneration is less extensive than in humans. However, in both species the uterus similarly undergoes dramatic remodeling during pregnancy and postpartum (1). The human uterus increases up to 20-fold in weight and 500-fold in volume over the course of pregnancy, with both myometrial hyperplasia (increase in cell number) and hypertrophy (increase in cell size) contributing to its profound growth (2). In both humans and rodents, myometrial hyperplasia is prominent in the initial stages of gestation, while hypertrophy is modest at the beginning of pregnancy but increases as gestation progresses (3). Immediately after parturition, the uterus undergoes a process termed involution by which it returns to its nongravid state. While knowledge regarding the cellular mechanisms underlying this dynamic tissue repair is limited, processes thought to be involved in this remodeling include apoptosis, proliferation, extracellular matrix degradation, and autophagy (4,5). In addition, there is a robust increase in gene expression of inflammatory cytokines and chemokines that is associated with immune cell infiltration in decidua during labor and the early postpartum period, which are thought to play important roles in the uterine involution and remodeling process (6–10). Moreover, to achieve this remarkable cellular turnover and regeneration, endometrial somatic stem/progenitor cells have been proposed to play a role (11–13). Increased understanding of postpartum remodeling events may be crucial for developing better prevention strategies and interventions to combat uterine adhesions and scarring to which the uterus is especially susceptible in the postpartum period (14).
Adult stem cells are rare specialized cells that can self-renew and maintain tissue homeostasis by serving as a cell reservoir for tissue repair and regeneration (15). Endometrial stem cells (ESCs) were first reported and characterized in adult human endometrial tissue in 2004 (16,17). ESCs are multipotent progenitor cells characteristically similar to other classic mesenchymal stem cells (MSCs) through their abilities to self-renew, clonogenicity, plastic adherence, multilineage differentiation, and expression of MSC surface markers (18). Animal studies using label retention (LR) techniques have similarly identified epithelial and stromal ESC populations residing in the endometrium (19,20). Stem/progenitor cells have been shown to play a role in cyclic endometrial regeneration as well as in the dynamic uterine tissue repair and remodeling occurring postpartum. Label-retaining ESCs were found to undergo rapid proliferation immediately after parturition in mice and contribute functionally to the regenerating luminal epithelial layer (13). Other studies have found that progenitor cells located in the endometrial stroma (21) and the epithelium (22,23) participate in endometrial re-epithelialization of the involuting uterus in the mouse. In addition, label-retaining stem/progenitor cells found in the myometrium were shown to express mesenchymal lineage markers, and similarly exhibited marked proliferation in a mouse model of postpartum involution (24), further suggesting the role of stem cells in postpartum uterine remodeling.
Aside from ESCs that are endogenous to the uterus, evidence in both humans and mice suggests that adult bone marrow-derived cells (BMDCs) are an exogenous source of ESCs. Adult BMDCs travel in the circulation and contribute to tissue repair and regeneration of various organs (25). In the uterus, adult BMDCs have been detected in both human (16,26,27) and mouse (26,28–33) endometrium, and demonstrated to give rise to various nonhematopoietic endometrial cell populations including epithelial, stromal and endothelial cells in the nonpregnant state, suggesting that BMDCs may serve as a source of progenitor cells for endometrial regeneration. Using the 5-fluorouracil-based non-gonadotoxic bone marrow transplant (BMT) mouse model (32), we recently showed that BM progenitors are mobilized to the circulation in pregnancy and recruited to the pregnant uterus where they give rise to decidual stromal cells, playing an important functional role in decidualization and pregnancy maintenance (34). However, it is still unknown whether BM progenitors participate in remodeling of the uterus postpartum, and if so what is the nature of their contribution. Therefore, our objective was to characterize the temporal changes and investigate the contribution of BMDCs to postpartum uterine remodeling using our previously described non-gonadotoxic BMT mouse model.
2 |. MATERIALS AND METHODS
2.1 |. Animals and Experimental Model
C57BL/6J wild-type mice, transgenic ubiquitin-GFP mice C57BL/6-Tg (UBC-GFP)30Scha/J (Stock no. 004353), transgenic Tie2-GFP mice (Tg[Tie2-GFP]287Sato/J), mTmG mice B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (Stock no. 007676), and β-actin-Cre mice B6N.FVB-Tmem163Tg(ACTB-cre)2Mrt/CjDswJ (Stock no. 019099) were ordered from Jackson Laboratories (Bar Harbor, ME). Mice were maintained and treated under an approved Yale University Institutional Animal Care and Use Committee protocol (2017-07113) in the Yale School of Medicine Animal Facility. Mice were housed 4–5 per cage in a room with a 12-hour light, 12-hour dark cycle (7 AM to 7 PM) with ad libitum access to food and water.
Six-week-old female wild-type mice (n = 36) underwent non-gonadotoxic submyeloablation followed by bone marrow transplantation (BMT) from syngeneic female UBC-GFP donors (n = 12) as previously described (32). A schematic of the experimental design is shown in Figure 1. Following a 3-week recovery period, BM engraftment was checked in peripheral blood, and only mice with >30% chimerism were used for subsequent experiments. Female BMT recipient mice were then bred for up to 1 week with wild-type fertile C57BL/6J males (in a 1:2 male to female ratio) and checked at 7 AM daily for vaginal plugs. Upon plug detection the female was separated from the male and the morning of plug detection was considered embryonic day 0.5 (E0.5). Mice were euthanized at the following 4 time points: Day 18.5 (E18.5) (n = 6), postpartum day 1 (PPD1) (n = 6), postpartum day 5 (PPD5) (n = 5) and postpartum day 10 (PPD10) (n = 6). In addition, virgin mice that underwent BMT from GFP donors served as the nonpregnant group (n = 6). A negative control sham group consisted of PPD1 mice that were injected with saline instead of BMT (n = 4). The engraftment of GFP-positive BMDCs and their characterization in the uterus and uterine implantation sites was performed by a fluorescent camera, flow cytometry, immunohistochemistry and immunofluorescence.
Figure 1. A schematic of the experimental model.
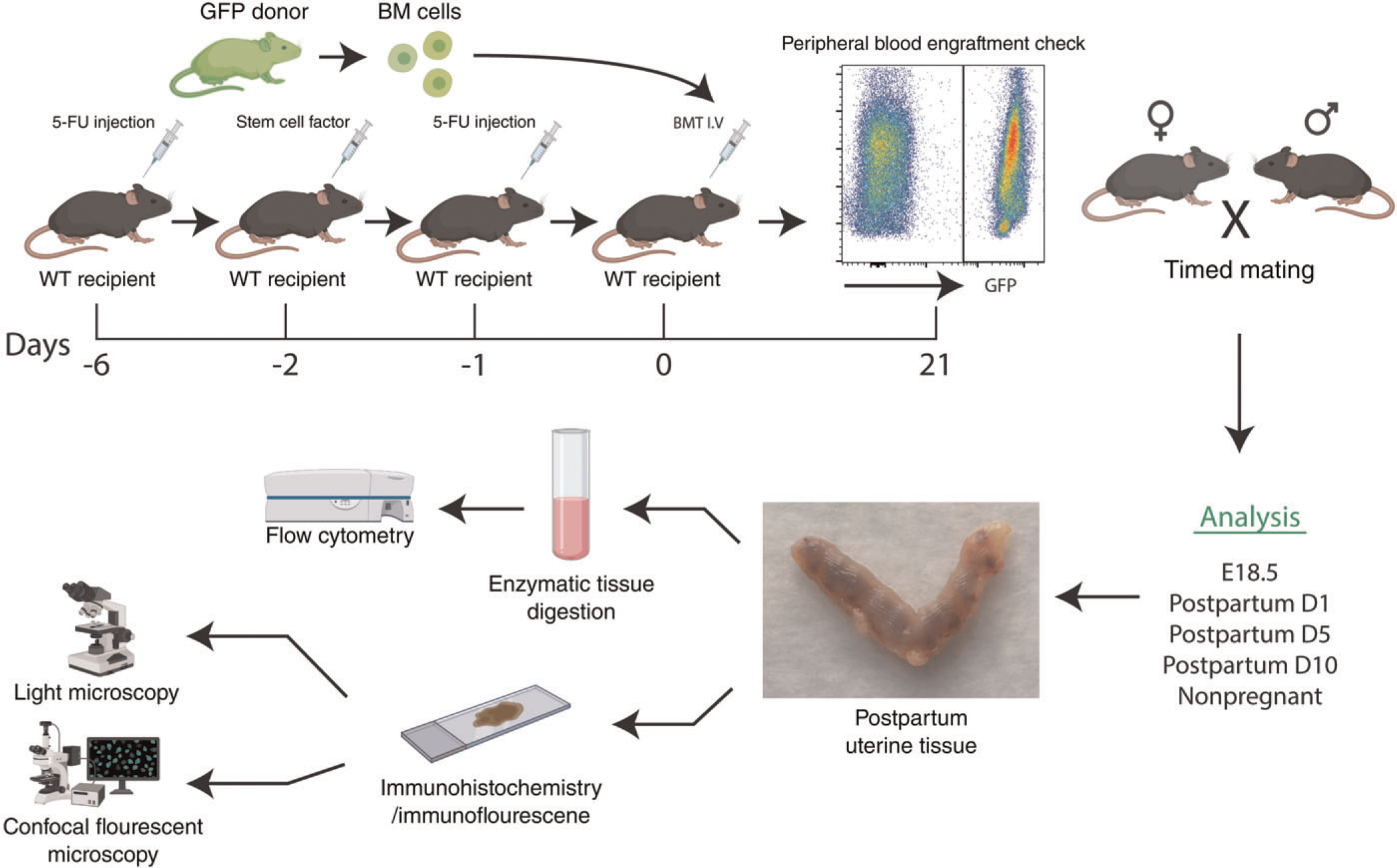
Wild-type (WT) recipient female mice (n = 36) received non-gonadotoxic submyeloablative bone marrow transplant (BMT) protocol based on 5-fluorouracil injection on day −6 and day −1 and stem cell factor (SCF) injections on day −2 prior to BMT. BM engraftment was assayed by flow cytometry of peripheral blood on day 21 post-BMT. Subsequently, engrafted female mice were mated with proven males and timed pregnancies were established. Mice were euthanized for analysis at specified time points including nonpregnant, peripartum (E18.5), postpartum days 1, 5 or 10 (n = 5–6 animals/group)
2.2 |. Bone Marrow Cell Isolation and Non-Gonadotoxic Bone Marrow Transplantation
A 5-fluorouracil (5-FU) based non-gonadotoxic submyeloablation protocol was used as previously described (32). Briefly, WT female recipient mice were given two intraperitoneal (i.p.) injections of 125 mg/Kg 5-FU (days −6 and −1 before BMT), and three i.p. injections of 50 μg/Kg stem cell factor (−21 h, −9 h before and + 3 h after the second 5FU injection) prior to receiving BMT from UBC-GFP mice donors (day 0). Toxicity was monitored daily with weight measurements and general wellbeing assessment. For BMT, bone marrow cells were obtained from 6–10-week-old UBC-GFP donor mice as previously described (32). Briefly, bone marrow was flushed from femurs and tibias into cold sterile DMEM-F12 media and then filtered through a 70 μM filter mesh to remove any bone spicules or clumps of muscle tissue. Following filtration, the cells were washed by centrifugation and resuspended in phosphate-buffered saline (PBS) twice prior to injection. A total of 20 × 106 unfractionated BM cells were injected intravenously via retro-orbital route into 6- to 7-week-old female recipients on day 0 after conditioning with the submyeloablative regimen described above.
2.3 |. Fluorescent Imaging of Uterus
Following organ extraction, the uteri were imaged with Carestream In-Vivo MS Fx Pro (Carestream Health, USA) fluorescent camera to capture GFP fluorescence images from the various peripartum and postpartum time points (E18.5, PPD1, PPD5, PPD10, nonpregnant).
2.4 |. Flow Cytometry Analysis
Following extraction of the uterus it was immediately placed in Hank’s Balanced Salt Solution (HBSS) media on ice. For pregnant uterine implantation sites (E18.5), the implantation site was dissected with careful removal of the embryonic/placental parts of the uterus, leaving only the desired uterine and decidual tissue. The uterine tissues were subsequently finely minced and digested in a solution of HBSS (Life Technologies San Francisco, CA) with 25 mM HEPES, 1 mg/mL Collagenase B (Roche Diagnostics, Indianapolis, IN), and 0.1 mg/mL deoxyribonuclease I (Sigma-Aldrich, St. Louis, MO) for 30 minutes at 37°C with thorough mixing every ten minutes. The cell suspension was filtered through a 70uM mesh and centrifuged at 2000 rpm for 8 minutes at 4°C before being resuspended in FACS Buffer (2% FBS in 1X PBS). Peripheral blood was collected via the retro-orbital method into EDTA-covered tubes and then mixed with 1 mL PBS before centrifugation at 2000 rpm for 8 minutes at 4°C. The uterine and peripheral blood cell suspensions were then resuspended in 5 mL RBC lysis buffer (Miltenyi Biotec, Auburn, CA) and incubated for 10 minutes at room temperature, with frequent mixing throughout. Following incubation, cell suspensions were diluted with 8 mL PBS and centrifuged at 1500 rpm for 5 minutes at 4°C followed by washing with FACS Buffer. The cells were counted using a hemocytometer and then resuspended in appropriate volume to prepare the cells for antibody staining (2 ×106 cells in 100 μL/tube). The cells were then blocked for 10 minutes using 2 μL TruStain fcX anti-mouse CD16/32 (#101320, Biolegend, San Diego, CA) followed by staining for 30 minutes on ice using APC-Cy7 anti-mouse CD45 (#103116, Biolegend, San Diego, CA). Following antibody staining, the cells were washed with FACS buffer twice, resuspended in PBS, and then analyzed on a fluorescence-activated cell sorting MoFlo machine (Beckman Coulter). Live gates were applied appropriately to forward and side scatter dot plots to exclude debris and nonviable cells. Appropriate unstained and antibody IgG isotype controls were used for setting compensation and appropriate gate determinations. The data was analyzed using the FlowJo V10 software (FlowJo).
2.5 |. Immunohistochemistry
Uterine tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and mounted on slides in 5 μm tissue sections. The tissues were then deparaffinized and rehydrated before heat-mediated antigen retrieval in sodium citrate (pH 6.0). The slides were washed in PBS and incubated in H2O2 before blocking with 5% goat serum for 1 hour at room temperature. The slides were then incubated overnight with rabbit anti-GFP primary antibody (1:1000, #ab290, Abcam, Cambridge, MA) at 4°C. The sections were washed, then incubated with goat anti-rabbit biotinylated secondary antibody (1:200, #BA-1000 Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Detection was performed with ABC Vectastain Elite reagents with DAB plus H2O2 (Vector Laboratories, Burlingame, CA). Tissue sections were counterstained with hematoxylin (Sigma Aldrich, St. Louis, MO), and then imaged using an Olympus BX-51 microscope (Olympus).
2.6 |. Immunofluorescence and TUNEL Apoptosis Assay
Uterine tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and mounted on slides in 5 μm tissue sections. The tissues were then deparaffinized and rehydrated. Once washed, the slides were incubated in PBS-T 0.5% for 10 minutes at room temperature to permeabilize the cells, and then heat-mediated antigen retrieval was performed in sodium citrate (pH 6.0) before blocking in 10% donkey serum for 1 hour at room temperature. The slides were then incubated in the appropriate concentration of primary antibody overnight at 4°C. The next morning the slides were washed and incubated with Alexa Fluor 647 donkey anti-rabbit, Alexa Fluor 568-conjugated donkey anti-goat, Alexa Fluor 568-conjugated donkey anti-rabbit, Alexa Fluor 488-conjugated donkey anti-rabbit and/or Alexa Fluor 488-conjugated donkey anti-rat (Life Technologies, San Francisco, CA) at room temperature for one hour. Afterwards, Vectashield fluorescent mounting media with 4’,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories) was applied for mounting and visualization of nuclear staining. Immunoreaction with amplification but without primary and/or secondary antibodies served as negative controls. Images were then taken using laser scanning confocal microscope (LSM 710, Carl Zeiss) and analyzed using ZEN software. Primary and secondary antibodies and their respective concentrations used are listed in Supplementary Table S1.
For TUNEL apoptosis fluorescent staining of apoptotic cells, the TUNEL apoptosis assay was performed according to the manufacturer’s protocol (kit #C10618, Invitrogen, Carlsbad, CA) prior to the immunofluorescence protocol described above. The slides were rehydrated, washed in PBS, and fixed in 4% paraformaldehyde for 15 minutes at 37°C. Proteinase K was added to the sections and the slides incubated for 15 minutes at room temperature before again incubating in 4% paraformaldehyde. Following another wash, some slides were treated with either DNase I (#10104159001, Roche, Basel, Switzerland) as positive apoptotic control or DNase reaction buffer. Next, sections were incubated for 10 minutes in Tdt Reaction buffer at 37°C, followed by incubation in the Reaction mixture for 60 minutes at 37°C. The slides were washed in 3% BSA and 0.1% Triton in PBS for 5 minutes and then incubated with 12X iT TUNEL Cocktail for 30 minutes at 37°C in the dark. After incubation, the slides were again washed in 3% BSA in PBS, and then washed with NH4Cl for 10 minutes before continuing with the immunofluorescence portion of the protocol as described above.
2.7 |. Image Quantification and Analysis
For quantification of GFP-positive and GFP-negative cells in the endometrial stromal compartment, 12 high-power confocal microscopy fields (4 high-power fields [HPFs] from each of 3 uterine sections per animal) were assessed for each time point. HPF images from each section were acquired in random and cell counting was carried out by two independent observers blinded to the experimental group. The total number of DAPI-positive cell nuclei, and GFP-positive cells were counted in each HPF. For quantitation of proliferating cells, the number of GFP+/PCNA+ and GFP−/PCNA+ were counted and expressed as a percentage of the total GFP+ or GFP− cells counted per animal, respectively. Similarly, for quantitation of apoptotic cells, the number of GFP−/TUNEL+ and GFP−/TUNEL+ cells were counted. For quantitation of %CD45+ immune cells and %F4/80+ macrophage cells out of BMDCs, GFP+/CD45+ and GFP+/F4/80+ cells were counted out of total GFP+ cells. For each quantification, at least 1000 cells were counted per animal.
2.8 |. Statistical Analysis
Data were assessed for normal distribution with a Shapiro-Wilk normality test using Graph-Pad Prism 8 software (GraphPad Software, La Jolla, CA). Normally distributed data were analyzed using the Student unpaired two-tailed t test for the comparison of two groups, and oneway ANOVA with Tukey multiple comparison test for multiple group comparison. If data were not normally distributed, or if distribution could not be determined due to small sample size, data were analyzed using a Mann-Whitney U test. P < 0.05 was considered statistically significant.
3 |. RESULTS
3.1 |. Bone Marrow-Derived Cells are Recruited to the Uterus Postpartum and Exhibit Dynamic Temporal Changes
To investigate the contribution of adult BMDCs to the uterus during postpartum remodeling we utilized our previously described submyeloablative non-gonadotoxic BMT model (32) (Figure 1). Using this regimen, stable GFP+ BM chimerism is achieved for a period of at least 6 months (Figure S1). To characterize the spatial and temporal distribution of BMDCs in the uterus during the postpartum period, a time course experiment was performed and mice were euthanized peripartum (E18.5), on postpartum day 1 (PPD1), postpartum day 5 (PPD5), postpartum day 10 (PPD10), or as nonpregnant virgin mice (n = 5–6/group). The percentage GFP+ chimerism was assessed by flow cytometry of peripheral blood at the time of euthanasia and was comparable between all experimental groups (Figure S2). Female mice injected with saline served as nontransplanted (sham) controls. Imaging of the intact uterus using fluorescent GFP camera demonstrated areas of signal enhancement around implantation sites peripartum on E18.5 (Figure 2A). On PPD1, the GFP signal in the uterus was very strong indicating significant recruitment of BMDCs in the immediate postpartum period. The GFP signal gradually diminished on PPD5 and PPD10, when it became similar to the nongravid uterus (Figure 2A).
Figure 2. Temporal contribution of bone marrow-derived cells (BMDCs) to the uterus in peripartum and postpartum period.
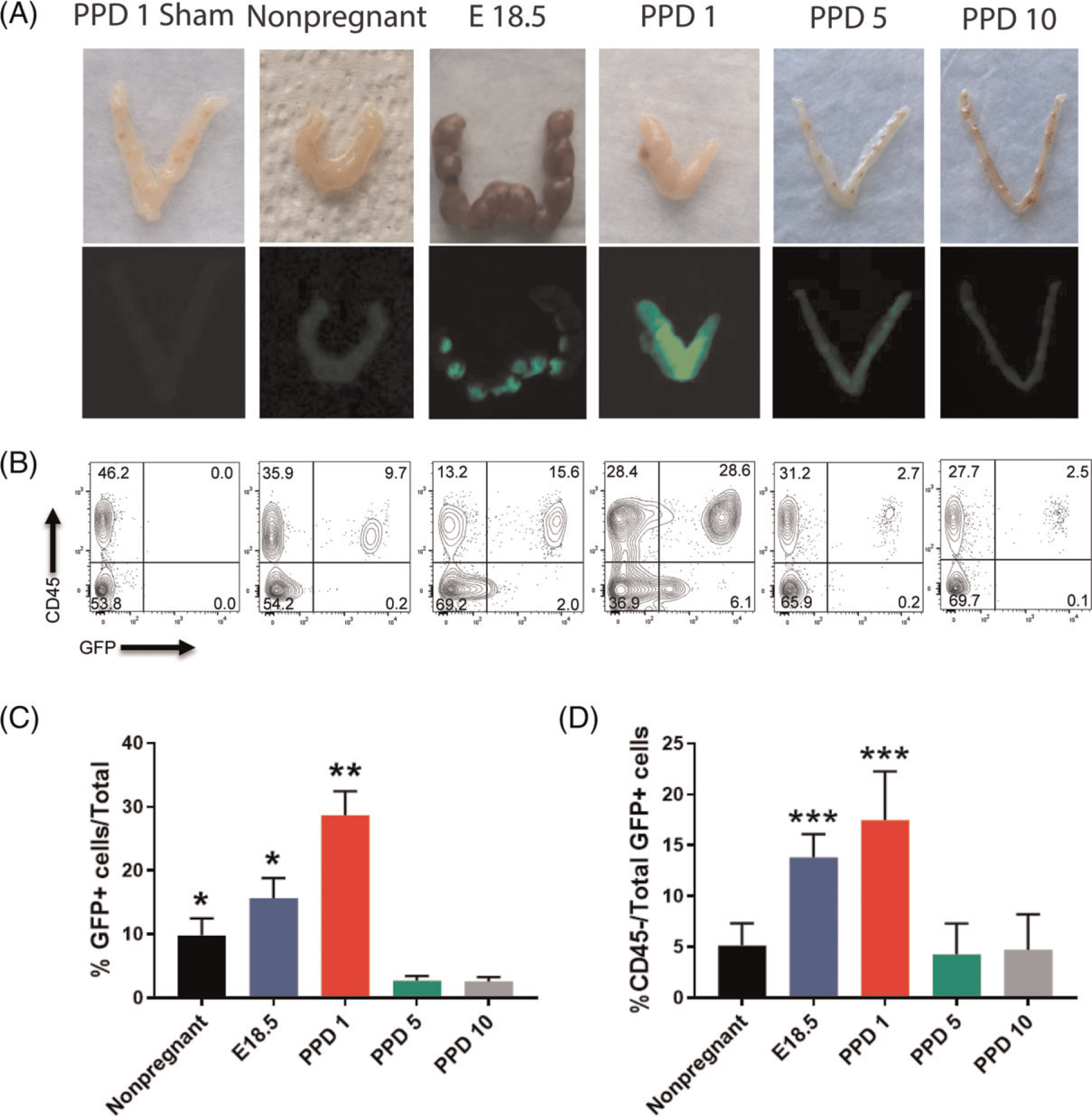
(A) Photographs of uteri (top panel) and fluorescent GFP images obtained by fluorescent camera (bottom panel) from various peripartum and postpartum time points (E18.5, PPD1, 5, 10 and nonpregnant) of mice engrafted with BM from GFP donors. The negative control is PPD1 uterus from a mouse injected with PBS (sham). The white arrows point to the areas of preferential BMDCs localization within E18.5 implantation sites. (B) Representative flow cytometry plots of single cells from uterine tissue gated according to CD45 and GFP positivity showing the changes in CD45+ (hematopoietic) and CD45-GFP+(nonhematopoietic) BMDC populations throughout peripartum and postpartum period. Flow cytometry values shown on plots are representative from one experiment. (C) Quantitative summary of flow cytometry analysis showing %GFP+ cells out of total uterine cells throughout peripartum/postpartum period. (D) Quantitative summary of flow cytometry analysis showing %CD45-nonhematopoietic cells out of total uterine GFP+ BMDC cell population throughout peripartum/postpartum period. Nonpregnant (n = 6), E18.5 (n = 6), PPD1 (n = 6), PPD5 (n = 5), PPD10 (n = 6), PPD1 sham (n = 4). *P < 0.05 vs PPD5 and PPD10 groups; **P < 0.01 vs all other groups; ***P < 0.05 vs nonpregnant, PPD5 and PPD10 groups
This temporal pattern of BMDCs dynamics in the postpartum period was consistent with our flow cytometry data. Following dissociation of uterine cells by enzymatic digestion, cells were treated with CD45 antibody and subjected to flow cytometry. The proportion of GFP+ BMDCs out of total uterine cells significantly increased from nonpregnant (9.7%) and E18.5 (15.6%) to PPD1 (28.6%) (Figure 2B and C). Following the large increase in uterine BMDCs in the immediate postpartum period, the percentage of BMDCs quickly declined on PPD5 (2.7%) and PPD10 (2.5%) (Figure 2B and C). Among uterine BMDCs (GFP+), the proportion of nonhematopoietic BMDCs (CD45−) significantly increased from 5.1% in nonpregnant to 13.8% on E18.5 and 17.5% on PPD1, followed by a precipitous decline subsequently (4.2% on PPD5 and 4.7% on PPD10) (Figure 2B and D). The hematopoietic BMDCs (CD45 + GFP+) were further characterized by flow cytometry on PPD1, timing of peak BMDC recruitment, showing that F4/80+ macrophages were the most abundant immune cell population in the uterus followed by monocytes (Supplementary Figure S3). Flow cytometry showed that the nonhematopoietic BMDCs (GFP + CD45−CD31−) were characterized by high expression of stem cell antigen-1 (Sca-1) (78%) and CD29 (87.2%) (Figure S4). Immunohistochemical analysis of uterine sections using GFP antibody corroborated these findings, demonstrating the large abundance of GFP+ BMDCs in the involuting uterus immediately postpartum (PPD1) (24.5% GFP+ cells out of total endometrial stromal cells) followed by their rapid decline subsequently on PPD5 (2.4%) and PPD10 (2.3%) (Figure 3A and B). These BMDCs localized primarily in the stromal compartment of the endometrium and showed particular concentration in subepithelial stromal location on PPD1 (Figure 3A).
Figure 3. Localization of bone marrow-derived cells (BMDCs) in the postpartum uterus.
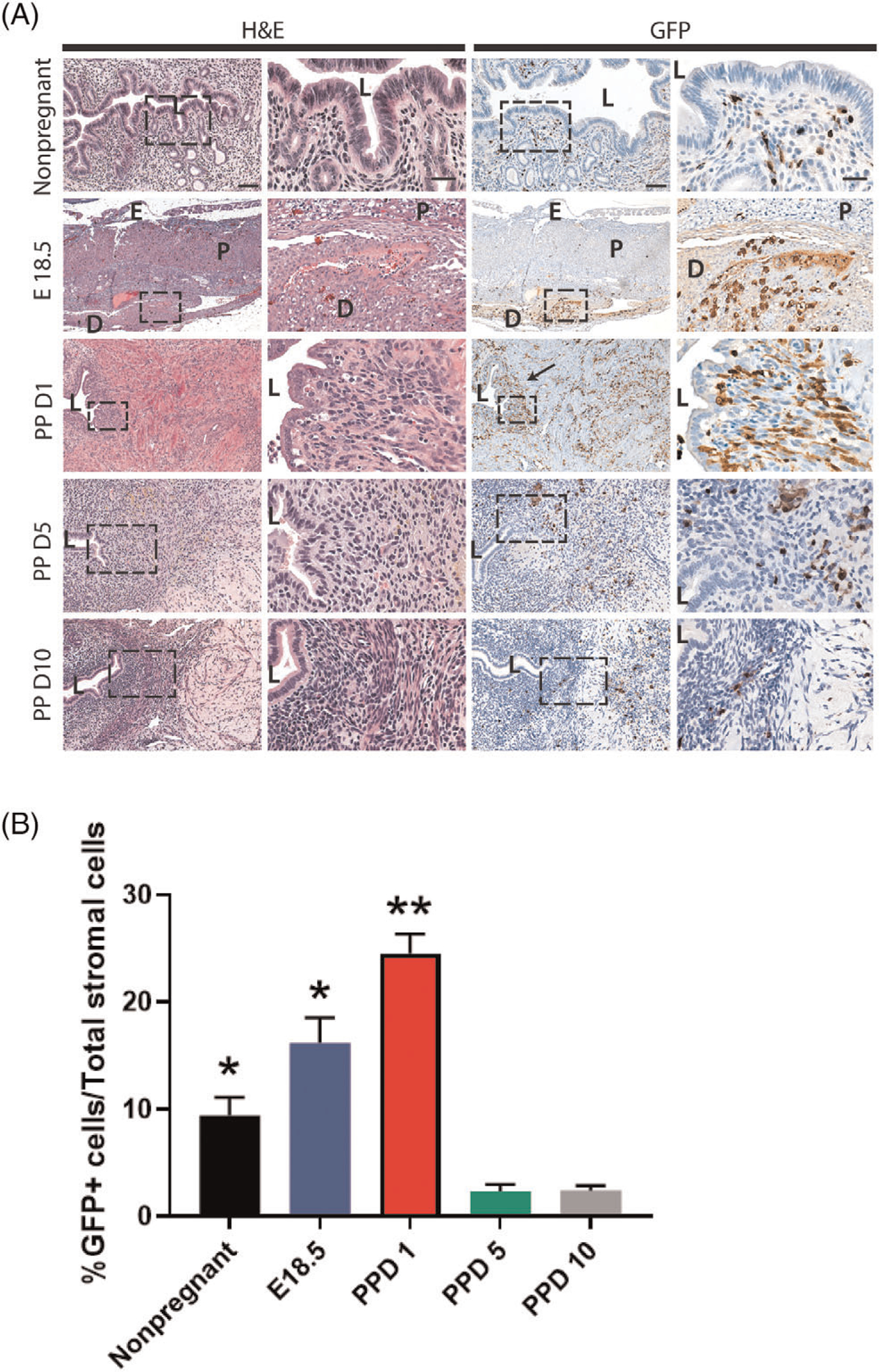
(A) Histological uterine tissue sections of different gestational and postpartum timepoints. Left panel shows H&E staining while right panel shows immunostaining with GFP antibody (brown). Low magnification (10x) and high magnification (20x) images are shown. The right column in each panel is a magnification of the corresponding rectangular area marked by the dashed line. The size bars in the left and right columns correspond to 100 μm and 50 μm, respectively. Black arrow is pointing to subepithelial area where GFP+ BMDCs (brown) are particularly abundant. (B) Quantitative summary of percentage of GFP+ cells out of total stromal cells across different time points. PPD1 (n = 5 animals); n = 4 in all other groups. *P < 0.05 vs PPD5 and PPD10, **P < 0.01 vs all other groups. L, lumen; D, decidua; P, placenta; E, Embryo
3.2 |. Nonhematopoietic BM Progenitor Cells Give Rise to Endometrial Stromal, Endothelial and Epithelial Compartments after Parturition
To further characterize the BM-derived cell types in the endometrium during the postpartum period, immunofluorescence colocalization was performed. Immunostaining with CD45 (pan-leukocyte marker) and F4/80 (macrophage marker) showed that the majority of endometrial GFP+ BMDCs are macrophages (CD45 + F4/80+) at all postpartum time points as well as in the nonpregnant uterus (Figure 4, A and C). A small fraction of stromal GFP+ BMDCs (14.2%) were found to be CD45-F4/80− on PPD1 (Figure 4, A and B), indicating that these were nonhematopoietic stromal cells. Notably, these CD45-F4/80− GFP+ cells were very rare in nonpregnant and PPD10 uteri, accounting for <2% of uterine BM-derived cells. Notably, co-immunostaining with antibody to CD31, an endothelial cell marker, demonstrated that some GFP+ BMDCs were found to colocalize with CD31 and be integrated into the endometrial vasculature as endothelial cells (Figure 5A). This was observed only in the immediate postpartum period (PPD1), indicating a direct cellular contribution of BM-derived progenitor cells to newly forming blood vessels as part of the uterine remodeling process. Flow cytometry analysis of uterine cells from PPD1 revealed that 3.2% of total GFP+ cells are endothelial cells (CD31 + CD45−) (Figure S4). To further confirm this finding, we analyzed uterine sections from mice that underwent BMT from transgenic Tie-2-GFP donors, in which GFP reporter is expressed under the endothelial-specific Tie-2 promoter. Uterine sections from PPD1, but not nonpregnant mice, demonstrated blood vessels that had GFP + endothelial cells (CD31+) (Figure S5). Moreover, we discovered GFP expression in some luminal epithelial cells on PPD1 (Figure 5B), suggesting that a subset of BM progenitor cells differentiated into epithelial cells. Immunostaining with antibody to cytokeratin, an epithelial cell marker, showed that the GFP+ BMDCs that incorporated into the luminal epithelial cell layer were positive for cytokeratin and exhibited typical epithelial cell morphology (Figure 5B). These epithelial BMDCs were found only on PPD1 and not at any of the other time points or nonpregnant endometrium.
Figure 4. Co-immunolocalization of GFP+ BMDCs with CD45 and F4/80 markers.
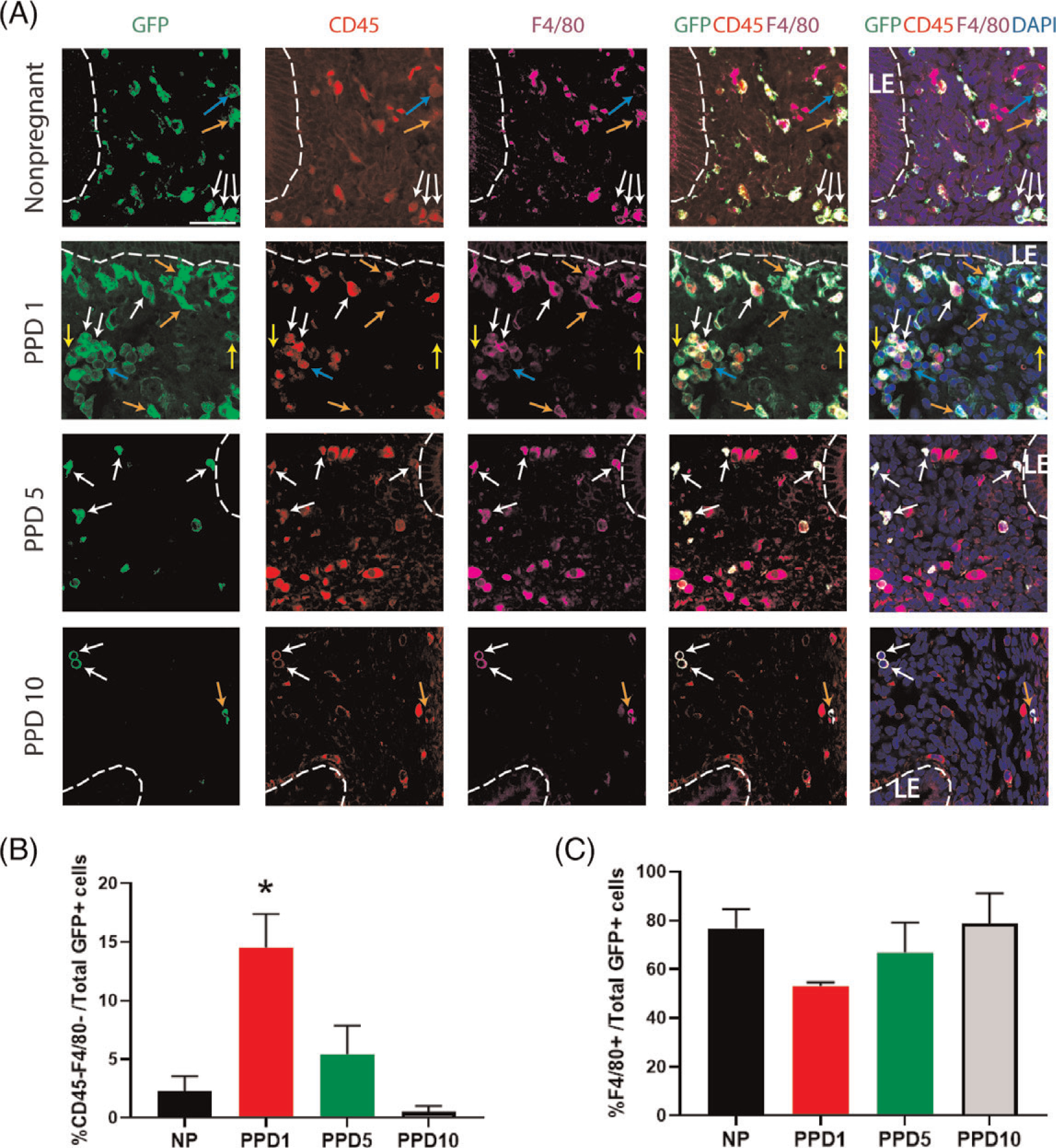
(A) Immunofluorescence of uterine tissue sections showing colocalization of GFP-positive BMDCs (green), with CD45 pan leukocyte marker (red), and F4/80 macrophage marker (purple) across nonpregnant and postpartum day (PPD) 1, 5 and 10. Sections were counterstained with DAPI (blue) for nuclear staining. The dashed white line indicates the border of the luminal epithelium (LE). Blue arrows point to BMDCs (GFP-positive cells) that are positive for CD45 and negative for F4/80. Orange arrows point to cells that are GFP-positive, weakly positive for CD45, and F4/80-positive. White arrows point to cells that are GFP-positive, CD45-positive, and F4/80-positive. Yellow arrows point to nonhematopoietic cells that are GFP-positive but negative for either CD45 or F4/80 markers. (B) Quantitative summary of percentage of CD45-negative and F4/80-negative cells out of total GFP+ cells across different time points. (C) Quantitative summary of %F4/80+ cells out of total GFP+ cells. PPD1 (n = 5 animals); n = 4 in all other groups *P < 0.05 vs all other groups. Images were obtained with 40x lens. Size bar = 50 μm.
Figure 5. Co-immunolocalization of GFP+ BMDCs with CD31 and cytokeratin markers.
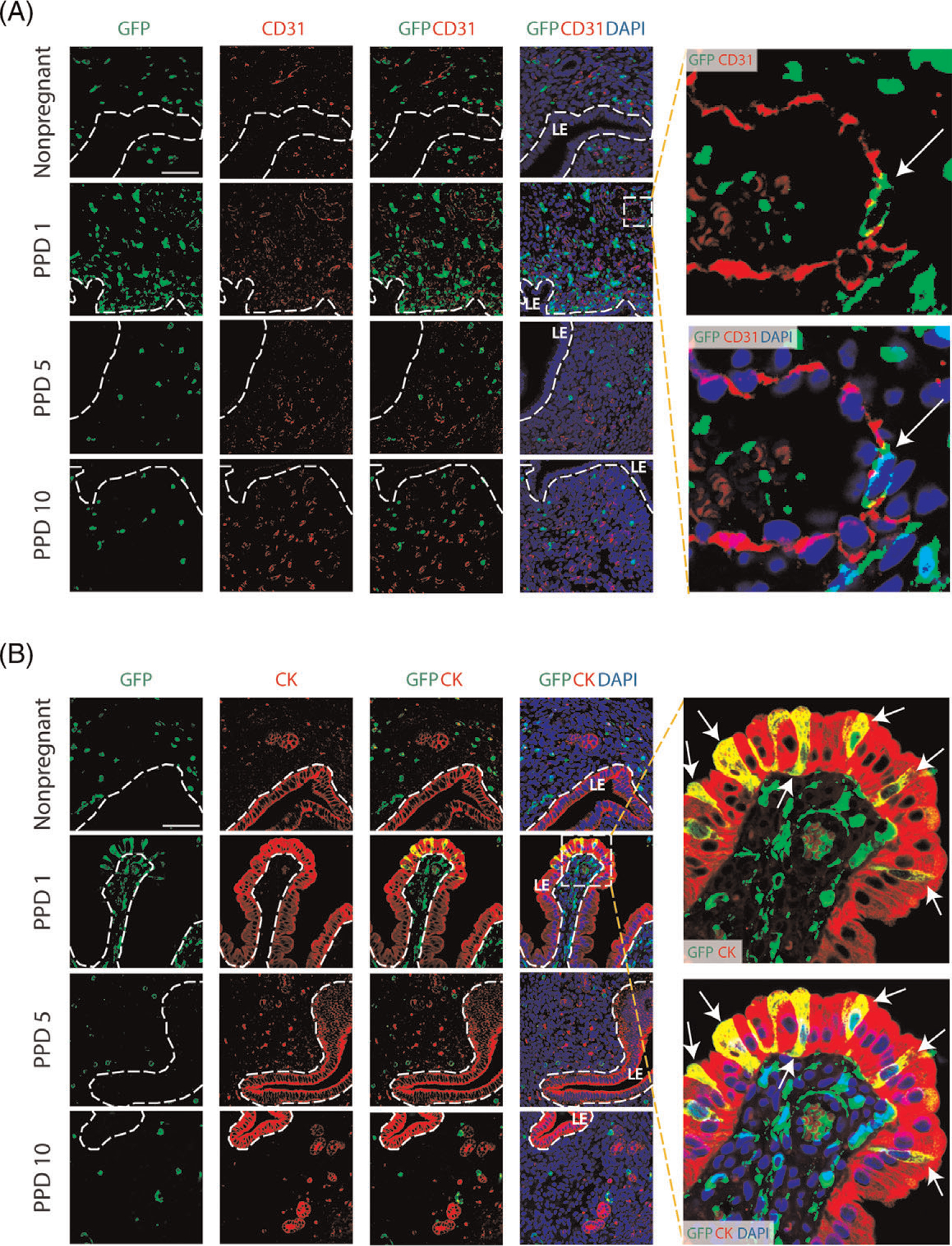
(A) Immunofluorescence of uterine tissue sections showing colocalization of GFP-positive BMDCs (green), with CD31 endothelial marker (red) across nonpregnant and postpartum day (PPD) 1, 5 and 10. Sections were counterstained with DAPI (blue) for nuclear staining. The dashed white line indicates the border of the luminal epithelium (LE). Images on the right are magnified images of the dashed square area. They show a bone-marrow derived endothelial cell integrated into a blood vessel on postpartum day 1 (white arrow), as visualized with GFP-positive cell colocalized with CD31 staining. (B) Immunofluorescence of uterine tissue sections showing colocalization of GFP-positive BMDCs (green) with the epithelial marker cytokeratin (CK) (red) across nonpregnant and postpartum day (PPD) 1, 5 and 10. Sections were counterstained with DAPI (blue) for nuclear staining. The dashed white line indicates the border of the luminal epithelium (LE). Magnified images to the right show GFP+ bone-marrow derived epithelial cells (white arrows) exhibiting typical epithelial morphology and integrated into the luminal epithelium on PPD1. Images were obtained with 40x lens. Size bar = 50 μm.
To exclude the possibility that cell fusion between BMDCs and uterine resident cells is responsible for our observations, we utilized the well-established Cre-Lox P recombination approach (35,36) with a dual color fluorescent membrane tomato/membrane EGFP (mT/mG) system. In this system, upon Cre recombinase expression in the cell, membrane EGFP (mG) fluorescence expression replaces the membrane tomato (mT expression). mT/mG mice co-expressing Cre recombinase transgene ubiquitously under β-actin-Cre promoter were used as positive controls, confirming the efficiency of Cre-mediated conversion from mT to mG in the blood as well as uterus in this system (Figure S6). mT/mG transgenic mice were used as BM donors. Bone marrow transplant was performed into β-actin-Cre mice following the same 5-FU-based BMT regimen. Flow cytometry demonstrated that only BMDCs expressing mT and not mG were found in the uterus on PPD1 (Figure S6), timing of peak BMDCs recruitment. This indicates that nonhematopoietic phenotype of uterine BMDCs is not explained by cell fusion with resident uterine cells.
3.3 |. BM-Derived Cells Undergo Rapid Cellular Turnover after Parturition
Our observations of a sharp rise in GFP+ BMDCs in the endometrium in the immediate postpartum period followed by a rapid subsequent decline in the abundance of these cells suggests that endometrial BMDCs undergo rapid cellular turnover. To gain further insight into these processes, we looked at proliferation and apoptosis markers in the endometrium across the various time points. Co-immunostaining of GFP with PCNA, a cell proliferation marker, revealed that endometrial GFP+ BMDCs were highly proliferative in the immediate postpartum period (28.4% proliferating PCNA+ cells, P < 0.01) and significantly more than at later postpartum time points (1.4% and 1.2% PCNA+ on PPD5 and PPD10, respectively) or nonpregnant (1.5% PCNA+) (Figure 6A and B). Moreover, GFP+ BMDCs were significantly more proliferative than tissue-resident (GFP−) cells on PPD1 (28.4% vs 16.7%, P < 0.05) (Figure 6 A and B). In addition, co-immunostaining of GFP with TUNEL apoptosis assay demonstrated that there were numerous TUNEL+ cells in the endometrium on PPD1 indicating profound apoptosis (Figure 7A). This was in sharp contrast to later in the postpartum period and the nonpregnant uterus when generally minimal TUNEL+ apoptotic cells were found (Figure 7A and B). Notably, the proportion of apoptotic cells within the GFP+ subpopulation on PPD1 was greater than that of GFP− cells (53.7% vs 34.3%, P < 0.05) (Figure 7B), consistent with their rapid numerical decline after PPD1. Positive and negative control images of immunofluorescent stains are provided in Figure S7.
Figure 6. Proliferation and apoptosis of BMDCs in the postpartum period.
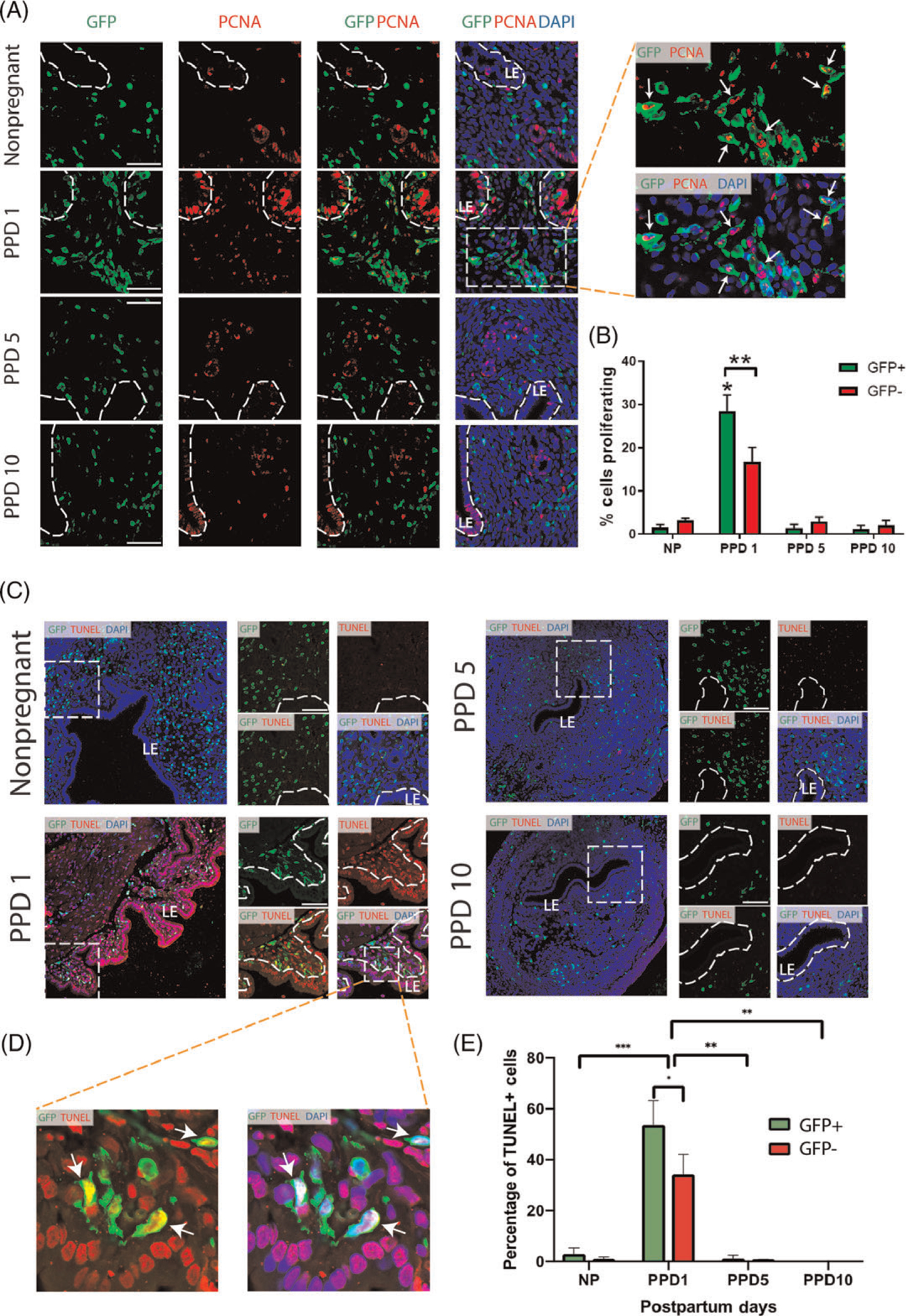
(A) Immunofluorescence of uterine tissue sections showing colocalization of GFP-positive BMDCs (green), with the proliferation marker PCNA (red) across nonpregnant and postpartum day (PPD) 1, 5 and 10. Sections were counterstained with DAPI (blue) for nuclear staining. The dashed white line indicates the border of the luminal epithelium (LE). Magnified images to the right have white arrows pointing to numerous proliferative (PCNA+) GFP+ bone-marrow derived cells on PPD1. (B) Quantitative summary of percentage of cells proliferating among GFP+ and GFP− cell populations across different time points. PPD1 (n = 5 animals); n = 4 in all other groups. * P < 0.01 vs all other groups; ** P < 0.05. Images were obtained with 40x lens. Scale bar = 50 μm. (C) Immunofluorescence of uterine tissue sections showing colocalization of GFP-positive BMDCs (green), with TUNEL apoptosis staining (red) across nonpregnant and postpartum day (PPD) 1, 5 and 10. Sections were counterstained with DAPI (blue) for nuclear staining. Smaller images on the right are a magnification of the respective white dashed square area on the left. The dashed white line indicates the border of the luminal epithelium (LE). White arrows point to GFP-positive BMDCs that are positive for TUNEL apoptosis marker. (D) Magnified images of dashed white area from PPD1 have white arrows pointing to apoptotic (TUENL+) GFP+ bone-marrow derived cells. (E) Quantitative summary of percentage of apoptotic (TUNEL+) cells within GFP+ and GFP− cell populations across different time points. PPD1 (n = 5 animals); n = 4 in all other groups. *P < 0.05, **P < 0.01, ***P < 0.001. Images were obtained with 40x lens. Scale bar = 50 μm.
Figure 7. Senescence of BMDCs in the postpartum uterus.
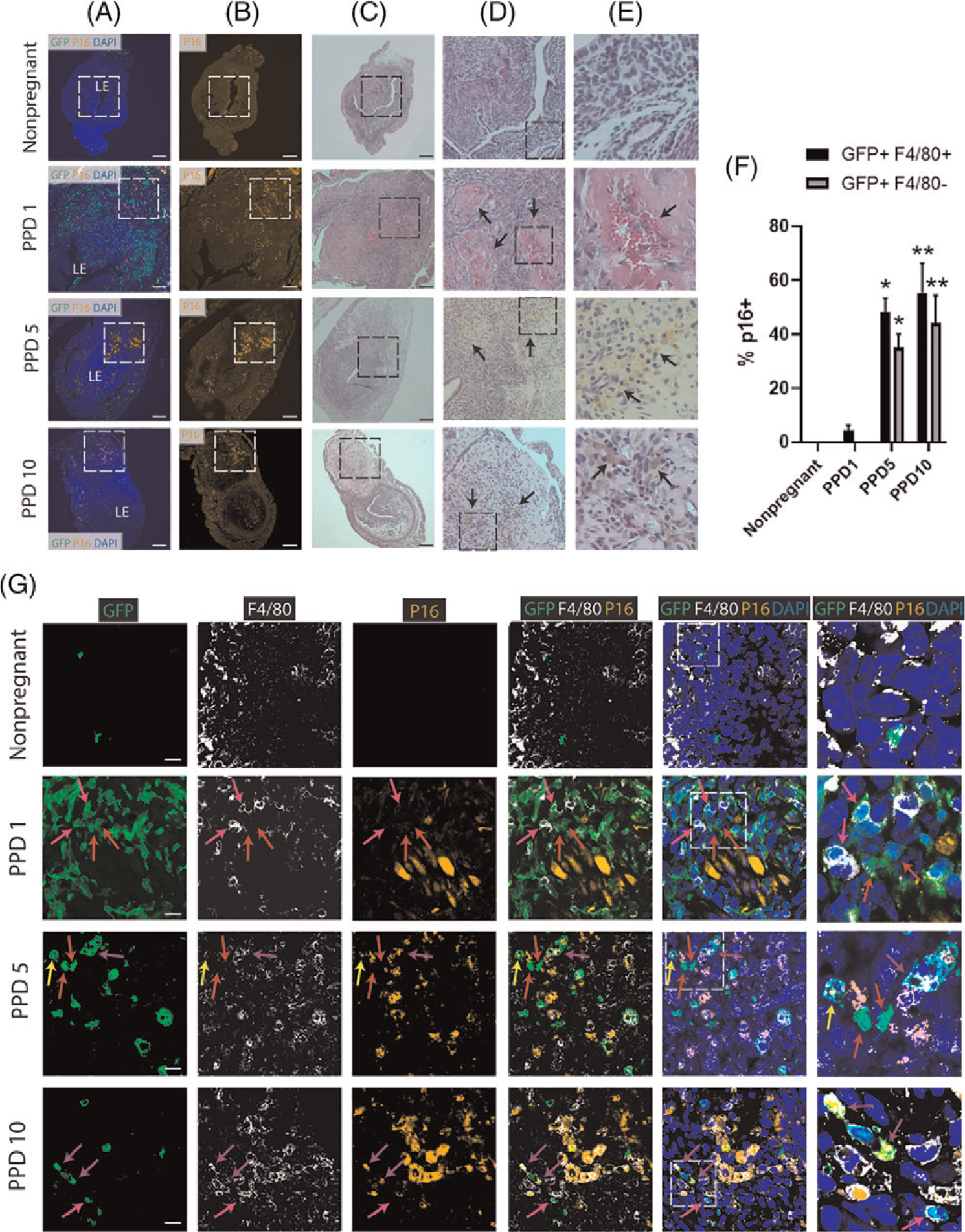
(A-B) Immunofluorescence of uterine tissue sections showing colocalization of GFP-positive BMDCs (green) and P16-positive senescent cells (orange) across postpartum time points and nonpregnant uterus; sections were counterstained with DAPI for nuclear staining (blue). ‘LE’ indicates the luminal epithelium. Note the white dashed squares on PPD1, PPD5 and PPD10 that are rich in p16-positive senescent areas within the implantation scar. The white dashed area in (B) is corresponding to the black dashed area in (C). (C-E) H&E staining of uterine tissue sections across postpartum time points and nonpregnant uterus. (C) 10x magnification showing black dashed square area which corresponds to the white dashed area in (A-B). (D-E) Column D are magnified (20x) H&E images of the black square in (C), and column E are magnified (40x) images of the black square area in (D). Black arrows on PPD1 in (D) are pointing to areas of fibrin deposition to the extracellular matrix and hemorrhage from blood vessel in (E). On PPD5 and PPD10, black arrows in (D) are pointing to areas rich in hemosiderin, a red blood cell product, indicating active areas of tissue organization and repair. Higher magnification images in (E) from PPD5 and PPD10 show black arrows pointing to hemosiderin-laden macrophages. Scale bar, 200 μm. (F) Quantitative summary of immunostaining from (G) showing percentage of GFP+/F4/80+ cells and GFP+/F4/80− cells that express p16 senescence marker across postpartum time points and nonpregnant state. n = 3 in all groups. * P < 0.05, and ** P < 0.01 vs nonpregnant and PPD1 timepoints.
(G) Immunofluorescence of uterine tissue sections from nonpregnant and across postpartum time points showing colocalization of GFP-positive BMDCs (green), F4/80 macrophage marker (white), and p16-positive senescent cells (orange). Sections were counterstained with DAPI for nuclear staining (blue). Red arrows point to nonhematopoietic GFP+ BMDCs that are negative for p16. Pink arrows point to GFP+/F4/80+ BM-derived macrophages that are p16-negative. Yellow arrows point to nonhematopoietic GFP+/P16+ senescent cells. Purple arrows point to GFP +/F4/80+/P16+ BM-derived senescent macrophages. Dashed white squares in merged images show tissue area that is magnified in the column to the right. Note the near absence of senescent (p16+) BMDCs on PPD1, and gradual appearance of p16+ BMDCs on PPD5 and PPD10. Scale bar, 20 μm
3.4 |. BM-Derived Nonhematopoietic Cells and BM-Derived Macrophages are found in Areas of Cell Senescence and Gradually Acquire Senescence as Part of Postpartum Uterine Remodeling
Cellular senescence, defined as an irreversible cell cycle arrest, is typically found in aging tissues and tumors but is also an important process that is an integral part of normal development and proper tissue regeneration and repair (37–39). It has been shown that senescent cells become abundant in the postpartum uterus and that specific immune cells concentrate around areas rich in senescent cells and are identified as F4/80+ macrophages (10). These macrophages play a key role in senescent cells clearance as part of physiological postpartum uterine remodeling (10). Given the important role of cell senescence in tissue repair and the rapid decline in BMDC cell proliferation that we noted in the postpartum uterus, we wished to investigate the relationship between BMDCs, macrophages and cell senescence during the postpartum period. We used p16 (p16INK4a), a cyclin-dependent kinase inhibitor, to identify senescent cells as it is considered a specific biomarker of cell senescence (40). We found p16-positive areas of cell senescence in the postpartum uterus on PPD1, PPD5 and PPD10 but not in the nonpregnant uterus (Figure 7A and B). Histologically, these senescent areas were found predominantly in the postpartum implantation scar as active areas of tissue repair. On PPD1, the senescent areas demonstrated features of hemorrhage to the extracellular matrix with fibrin deposition, which subsequently organized on PPD5 and PPD10 with considerable uptake of hemosiderin, a red blood product (Figure 7C–E), indicating that senescent areas were active areas of tissue repair. Immunofluorescence colocalization demonstrated that numerous GFP + BMDCs were found in the senescent areas and consisted of mostly F4/80+ macrophages as well as nonhematopoietic cells (Figure 7G). Notably, the vast majority of GFP+ BMDCs on PPD1 did not express p16 senescent marker (<5%), while cell senescence (p16+) gradually increased on PPD5 and PPD10 in BM-derived F4/80+ macrophages (48.1% and 55.3% on PPD5 and PPD10, respectively) as well as BM-derived nonhematopoietic cells (35% and 44.1% on PPD5 and PPD10, respectively) (Figure 7F and G).
4 |. DISCUSSION
Adult BM-derived progenitor cells have been detected in both human (16,26,27) and mouse (26,28–33) uterine endometrium. Despite their identification in endometrial homeostasis, the role of these cells in highly dynamic physiological reproductive processes has remained unknown until recently (34). Herein, we describe a novel finding that BM-derived progenitor cells can respond to the tissue damage associated with parturition and actively participate in endometrial tissue regeneration postpartum by contributing to nonhematopoietic cell lineages in the remodeling mouse uterus.
Parturition and the postpartum period are unique physiological states characterized by significant endometrial tissue injury with rapid cellular turnover and regeneration. In this study we found that BMDCs undergo dramatic changes during the postpartum period with a significant increase in their numbers immediately postpartum, comprising ~30% of endometrial cells, followed by a rapid decline. While the majority of these BMDCs are immune (CD45+) cells, this immediate postpartum BMDCs expansion also corresponds to a peak in nonhematopoietic (CD45−) BMDCs subpopulation accounting for ~17% of total uterine BMDCs on PPD1. Previously, utilizing the same 5-FU-based non-gonadotoxic BMT model, we have shown that during mouse pregnancy BMDCs numbers increase in the uterine decidua at the beginning of gestation peaking at mid-gestation around E9.5 followed by a gradual decline towards peripartum (34). Similarly to the postpartum observation, that peak in BMDCs at mid-gestation also corresponds to a peak in uterine nonhematopoietic (CD45−) BMDC subpopulation (34), suggesting that nonhematopoietic BMDC progenitors play an active role in these physiological conditions of tissue turnover. The nonhematopoietic BMDCs on PPD1 exhibit high expression of Sca-1 and CD29, similar to previously reported surface marker expression of nonhematopoietic BMDCs in decidua (34).
In addition to physiological stimuli of pregnancy and postpartum, there are pathological factors that drive recruitment of nonhematopoietic BMDCs to the endometrium, including uterine ischemia and injury (30,41). The molecular signal/s mediating the recruitment of BMDCs to the early postpartum uterus is unknown. Several chemokines have been implicated in regulating infiltration of immune cells into the decidua during labor and early postpartum period and may also play a role in mediating recruitment of nonhematopoietic BMDCs. It was demonstrated in humans that the choriodecidua but not amnion was responsible for the increased chemotactic activity through expression of CCL2, CXCL10 (monocytes/macrophages attractants) and CXCL8 and CCL3 (neutrophil recruiter/activators) (42). In mice, it was shown that mRNA and protein expression of CXCL1 and CCL2 is increased in decidua during labor and early postpartum period (8). The chemokine CXCL12 (stromal-derived factor 1), is well established as one of the most important chemoattractants for BM-derive stem cells (43,44). The expression of CXCL12 increases in response to tissue injury in various organs where it recruits mesenchymal stem cells to the damaged areas facilitating tissue repair (45–47). Previously, CXCL12 ligand and its CXCR4 receptor have been shown to be important in mediating migration of BMDCs towards endometrial stromal cells, myometrial stromal cells and BMDCs differentiation in vitro (48–50). In addition, the CXCL12-CXCR4 axis is important in mediating recruitment of nonhematopoietic BMDCs to the injured uterus in vivo in a murine model of Asherman syndrome (51). In addition to chemokines, it is possible that sex-steroid hormones regulate the recruitment of BMDCs to the postpartum uterus. Indeed, it was shown that the administration of estrogen and/or progesterone to ovariectomized mice restores recruitment of macrophages into the uterus (52). The exact mechanisms and chemokines involved in regulating the recruitment of BMDCs to the uterus in the postpartum period remain to be determined.
Our observations of simultaneous increase of both cell proliferation and cell death in the endometrium in the immediate postpartum period suggests that there is rapid cellular turnover, and is consistent with prior reports in rodents (5). Interestingly, the endometrial BMDCs population exhibits a profound change in numbers from an initial increase immediately after parturition to a rapid decline throughout the postpartum period. We observed preferential proliferation of BMDCs on PPD1 compared to resident GFP-negative cells. This is consistent with other studies showing increased proliferation of stem/progenitor cells in the endometrium and myometrium in the setting of postpartum uterine involution (13,24). Moreover, the precipitous decline in BMDCs in the subsequent postpartum period can be explained by the higher rates of apoptosis (TUNEL+) in BMDCs relative to cell proliferation observed on PPD1, which is also considerably greater than in the resident non-BM-derived cells. Moreover, since apoptosis is a relatively fast process it is only detected in a fraction of the apoptotic cells, and thus the number of apoptotic cells is likely to be underestimated with the TUNEL method. Further studies are warranted to elucidate the mechanisms responsible for triggering cell proliferation and apoptosis in BMDCs after parturition.
Cellular senescence, defined as an irreversible cell cycle arrest, is typically found in aging tissues and tumors but is also an important process that is an integral part of physiological development and proper tissue regeneration and repair (37–39). Our results demonstrate that the postpartum uterus has specific areas of cell senescence found in the implantation scar but not in the nonpregnant uterus, consistent with the physiological role of cell senescence in postpartum tissue repair. Moreover, we found numerous GFP+ F4/80+ BM-derived macrophages in areas of cell senescence in the postpartum uterus, consistent with a prior study that showed that F4/80+ macrophages specifically concentrate around areas rich in senescent cells and play a key role in senescent cells clearance as part of physiological postpartum uterine remodeling (10). In addition to F4/80+ macrophages, nonhematopoietic BMDCs were also found in areas of cell senescence and may also play an important role in facilitating the clearance of senescent cells as part of tissue remodeling. Ultimately, this process may be essential for scarless uterus healing since abnormal accumulation and lack of clearance of senescent cells has been previously linked to various pathologies of tissue fibrosis as well as preterm birth (53,54). Interestingly, we found that while initially in the immediate postpartum period (PPD1) BMDCs are not senescent, BM-derived F4/80+ macrophages as well as nonhematopoietic BMDCs gradually acquire cellular senescence subsequently in the postpartum period. This is also consistent and may partly explain our observation that the proliferation of BMDCs rapidly declines after its PPD1 peak. Our finding that over half of the macrophages in senescent areas at later postpartum time points also express markers of cellular senescence (p16) is intriguing since macrophages are traditionally thought as the immune cells that eliminate senescent cells. It was shown by Hall et al. that macrophages can acquire p16 expression in fat tissue of older mice as part of the aging process (55), but that induction of p16 in macrophages is reversible and can be part of physiological programming towards an M2-like phenotype (56). Moreover, p16 was shown to be associated with an anti-inflammatory response, suppressing the secretion of IL-6 following LPS stimulation in macrophages, but not in synovial fibroblasts (57). Thus, the accumulation of p16 expression in uterine macrophages in the postpartum period would be consistent with their known in M2 phenotype in the postpartum period (58), and may have an important role in regulating macrophage inflammatory function as part of normal postpartum tissue repair.
Importantly, the nonhematopoietic contribution of BMDCs to the uterus in the immediate postpartum period is very different than the nonpregnant state as well as later postpartum time points both qualitatively and quantitatively. Epithelial cells and endothelial cells that were BM-derived were observed only in the immediate postpartum period (PPD1) indicating that the cellular contribution of BM progenitors to these lineages is transient and serves a specific role in postpartum remodeling. Moreover, the nonpregnant uterus and later postpartum timepoints had a very modest number of BMDCs that were nonhematopoietic (<5%) compared to the PPD1 peak, suggesting that the uterine BM progenitor population is active in the immediate postpartum period but quickly regains quiescence after the first few days of uterine involution. In accordance with our findings, a prior study by Ong et al. using an irradiated BMT mouse model reported that all BMDCs in the nonpregnant uterus were immune cells (59). They found that BM-derived cells were either positive for the pan-leukocyte marker CD45 and/or positive for the macrophage marker F4/80 (59). Similar to these findings, in our study the vast majority of BMDCs in the nonpregnant uterus were CD45+ and/or F4/80+ immune cells (>95%).
The large infiltration of CD45+ BM-derived leukocytes, predominantly F4/80+ macrophages, in the immediate postpartum period observed in our study is consistent with prior studies showing infiltration of the decidua by leukocytes during labor that remains elevated in the early postpartum period (6,7). This infiltration is thought to play an important role in the inflammatory changes that are part of normal labor. In our study, over 50% of the endometrial BM-derived (GFP+) leukocytes were F4/80+ macrophages. In accordance, Shynlova et al. showed that myeloid cells, predominantly macrophages, account for the majority of leukocytes infiltrating the decidua at labor into the early postpartum period, contributing to uterine involution (8). The origin of the endometrial macrophage expansion in the postpartum period has been unclear and it is debatable whether they are recruited from exogenous source or arise from local proliferation of uterine resident macrophages. In other tissues including the liver, brain, skin and lung, it was suggested that tissue macrophages are derived from embryonic yolk sac erythro-myeloid precursors that persist in adult mice and are distinct from hematopoietic stem cells (HSCs) (60,61). In our study, the uterine macrophages appear to be largely GFP+ (BM-derived) indicating that they originate, at least in part, in exogenous BM HSCs.
After birth and placental expulsion, the uterine epithelial lining is completely lost by PPD1. The endometrium then undergoes rapid cell turnover and regeneration as an incomplete layer of epithelial cells and stroma appears by PPD2. In this study, we found evidence that BM-derived progenitor cells contribute to the re-epithelialization process and give rise to epithelial cells lining the regenerating epithelium on PPD1. These GFP+ cells were incorporated into the luminal epithelium showing typical epithelial morphology and were positive for cytokeratin. This phenomenon was only observed immediately postpartum on PPD1 and not in the later postpartum period or in nonpregnant uteri, and was overall a rare event, albeit one that was observed in all PPD1 mice examined. Other studies have found that progenitor cells located in the endometrial stroma (13,21) as well as those in the epithelium itself (22,23) participate in the re-epithelialization process of the endometrium after parturition in the mouse. Cao et al. found stromal progenitor cells, marked by their label-retaining (LR) property, to be increased near the luminal epithelium with some LR cells incorporated as epithelial cells (13). Similar to our study, they found this epithelial incorporation to be transient and occur only on PPD1 and not in nonpregnant uteri. In another study using lineage tracing with Amhr2-cre mice, Huang et al. showed that a subset of stromal cells of mesenchymal origin differentiate into epithelial cells in a process of mesenchymal-to-epithelial transition (MET) after parturition (21). Notably, cells from the Amhr2 became permanently integrated into the luminal epithelium long after uterine involution was complete (21). Whether the BM progenitors that differentiate into epithelium described in our study are the same as the mesenchymal progenitor cells that reside in the stromal niche and undergo MET or a distinct cell population is unclear. Given that the stromal progenitor cells described in the lineage tracing study by Huang et al. originate from cells expressing Amhr2, a marker of uterine mesenchyme, and also provide a non-transient epithelial contribution, it is likely that the BM progenitors are a different subpopulation. However, the possibility that BM-derived cells acquire Amhr2 expression in the local uterine environment after recruitment to the uterus and upon differentiation into endometrial stromal cells cannot be excluded. A certain degree of overlap may exist and it is likely that multiple subpopulations of endometrial stem/progenitor cells are found in the uterus. Indeed, a recent mouse study characterized a population of Axin2+ epithelial progenitor cells found in glands that was shown to contribute to luminal epithelium only in the long-term setting, leading the authors to suggest that there is likely a separate short-lived progenitor population responsible for epithelial regeneration (23). Future investigations combining lineage tracing with BM transplantation models would help to further characterize the cell origins and relative contributions of these various uterine progenitors. We have previously shown that a population of BM MSCs marked by Hoxa11 expression gets mobilized to the circulation during pregnancy and gives rise to endometrial progenitor cells that differentiate into decidual stromal cells (34). Taken together, these data suggest that as part of the remodeling process of the endometrium after parturition, BM progenitor cells are reacting to an extrinsic signal that induces them to proliferate and take part in epithelial regeneration along with other resident stem/progenitor cells.
In this study, we found that BM-derived progenitor cells contribute to endothelial cells integrated within neovasculature in the immediate postpartum period. Such a phenomenon was only detected after parturition and not in later postpartum periods or in nonpregnant uteri. Prior studies from our laboratory showed that BM-progenitors are mobilized to the circulation and recruited to the uterus in response to pregnancy in the mouse where they contribute to new blood vessels in the developing decidua in a process termed vasculogenesis (33,34). Moreover, in a mouse model of endometrial breakdown and repair it was found that endothelial cells undergo increased proliferation and endometrial stromal progenitor cells become significantly more localized in the perivascular region during endometrial repair (62). Taken together, these studies indicate that BM-derived progenitor cells actively participate in forming endometrial neovasculature in response to specific tissue demands of endometrial regeneration and repair.
Our study has several limitations. First, our flow cytometry analysis of uterine cell suspensions obtained by enzymatic digestion do not allow us to distinguish between intravascular leukocytes and extravascular (tissue) leukocyte subsets. One method to address this could be to label the intravascular cells prior to euthanasia. However, intravascular leukocytes account for a minority (~20%) of total uterine leukocytes analyzed by flow cytometry even in the highly vascularized pregnant decidua and is more negligible (~5%) in less vascularized zones such as the myometrium (63). Moreover, our study is focused on the nonhematopoietic (CD45−) BM-derived cell populations in the uterus and their presence in the intravascular space would be very rare, as also confirmed by immunohistochemical localization of GFP cells within the tissue in our study. Second, the possibility that BM (GFP+) cells seed the uterus directly upon transplantation and populate it afterwards, rather than exogenous BMDC recruitment in the postpartum period, cannot be excluded. However, we have shown that expansion of BMDCs in the uterus from such direct seeding in the uterus is very limited (<0.1% of total uterine cells) following i.v. injection of BM cells without myeloablation and BM reconstitution (32). Moreover, we have shown that the percentage of GFP+ cells in the nonpregnant uterus following 5FU BMT is low (~5%) and rapidly increases to ~30% of total uterine cells within about a week of pregnancy (E9.5) (34), suggesting that the increase is unlikely to be local uterine cells populating the uterus over time. In addition, we have also shown in the same work (34) that Hoxa11+ BM MSCs are not found in the nonpregnant uterus and first appear during pregnancy coinciding with BM MSC mobilization to the circulation, further suggesting that it is more likely that the vast contribution of BM GFP+ cells to the uterus is exogenous in response to physiological stimuli rather than endogenous.
In conclusion, we report that BM-derived progenitor cells actively participate in postpartum uterine remodeling, undergoing dynamic changes and contributing to various endometrial cell populations as part of the process of cellular turnover and regeneration. Our observations that a subpopulation of BM-derived progenitor cells can respond to the tissue damage associated with parturition to differentiate into epithelial cells and contribute to neovasculature in the remodeling uterus may have important implications for human uterine physiology and pathological conditions such as postpartum uterine scarring. Increased understanding of the stem/progenitor cell molecular pathways and cellular processes associated with uterine involution may be crucial in developing future interventions to promote scarless uterine wound healing.
Supplementary Material
Significance Statement.
The uterus undergoes significant tissue regeneration in the postpartum period following parturition where endometrial stem cells are thought to play an important role. Here we show that bone marrow-derived progenitor cells actively participate in postpartum uterus remodeling in the mouse, undergoing dynamic changes and contributing to various non-immune endometrial cell populations as part of the process of cellular turnover and regeneration. These observations may have important implications for human uterine physiology and pathological conditions such as postpartum uterine scarring
ACKNOWLEDGEMENTS
Grant support: This work was supported by the funds from NICHD grant 5K12HD047018 (to HST and RT), Ferring/New England Fertility Society grant (to RT), American Society for Reproductive Medicine (ASRM) grant (to RT), the Robert E. Leet and Clara Guthrie Patterson Fellowship award (to RT), and the Albert McKern award (to RT).
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
R.T. disclosed research funding from Celmatix. All of the other authors declared no potential conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Emera D, Romero R, Wagner G. The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness. Bioessays. 2012;34(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsey EM. Anatomy of the Human Uterus. Cambridge, UK: Cambridge Univ Press; 1994. [Google Scholar]
- 3.Shynlova O, Oldenhof A, Dorogin A, et al. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod. 2006;74(5):839–849. [DOI] [PubMed] [Google Scholar]
- 4.Hsu KF, Pan HA, Hsu YY, Wu CM, Chung WJ, Huang SC. Enhanced myometrial autophagy in postpartum uterine involution. Taiwan J Obstet Gynecol. 2014;53(3):293–302. [DOI] [PubMed] [Google Scholar]
- 5.Takamoto N, Leppert PC, Yu SY. Cell death and proliferation and its relation to collagen degradation in uterine involution of rat. Connect Tissue Res. 1998;37(3–4):163–175. [DOI] [PubMed] [Google Scholar]
- 6.Osman I, Young A, Ledingham MA, et al. Leukocyte density and proinflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton S, Oomomian Y, Stephen G, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86 (2):39. [DOI] [PubMed] [Google Scholar]
- 8.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T, Lye SJ. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med. 2013;17(2):311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapwanya A, Meade KG, Foley C, et al. The postpartum endometrial inflammatory response: a normal physiological event with potential implications for bovine fertility. Reprod Fertil Dev. 2012;24(8):1028–1039. [DOI] [PubMed] [Google Scholar]
- 10.Egashira M, Hirota Y, Shimizu-Hirota R, et al. F4/80+ Macrophages Contribute to Clearance of Senescent Cells in the Mouse Postpartum Uterus. Endocrinology. 2017;158(7):2344–2353. [DOI] [PubMed] [Google Scholar]
- 11.Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padykula HA. Regeneration in the primate uterus: the role of stem cells. Ann N Y Acad Sci. 1991;622:47–56. [DOI] [PubMed] [Google Scholar]
- 13.Cao M, Chan RW, Yeung WS. Label-retaining stromal cells in mouse endometrium awaken for expansion and repair after parturition. Stem Cells Dev. 2015;24(6):768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20(2):262–278. [DOI] [PubMed] [Google Scholar]
- 15.Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7(6): 656–670. [DOI] [PubMed] [Google Scholar]
- 16.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. [DOI] [PubMed] [Google Scholar]
- 17.Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738–1750. [DOI] [PubMed] [Google Scholar]
- 18.Tal R, Kisa J. Uterine stem cells: potential and pitfalls. Maturitas. 2020;134:54–55. [DOI] [PubMed] [Google Scholar]
- 19.Cervello I, Martinez-Conejero JA, Horcajadas JA, Pellicer A, Simon C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod. 2007;22(1):45–51. [DOI] [PubMed] [Google Scholar]
- 20.Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. STEM CELLS. 2006;24(6):1529–1538. [DOI] [PubMed] [Google Scholar]
- 21.Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7(8):e44285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin S Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc Natl Acad Sci U S A. 2019;116(14):6848–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali A, Syed SM, Jamaluddin MFB, Colino-Sanguino Y, Gallego-Ortega D, Tanwar PS. Cell Lineage Tracing Identifies Hormone-Regulated and Wnt-Responsive Vaginal Epithelial Stem Cells. Cell Rep. 2020;30(5):1463–1477. e7. [DOI] [PubMed] [Google Scholar]
- 24.Patterson AL, George JW, Chatterjee A, et al. Label-Retaining, Putative Mesenchymal Stem Cells Contribute to Murine Myometrial Repair During Uterine Involution. Stem Cells Dev. 2018;27(24):1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001; 105(3):369–377. [DOI] [PubMed] [Google Scholar]
- 26.Mints M, Jansson M, Sadeghi B, et al. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod. 2008;23(1):139–143. [DOI] [PubMed] [Google Scholar]
- 27.Ikoma T, Kyo S, Maida Y, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. American journal of obstetrics and gynecology. 2009;201(6):608 e1–608 e8. [DOI] [PubMed] [Google Scholar]
- 28.Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. STEM CELLS. 2007;25(8):2082–2086. [DOI] [PubMed] [Google Scholar]
- 29.Bratincsak A, Brownstein MJ, Cassiani-Ingoni R, et al. CD45-positive blood cells give rise to uterine epithelial cells in mice. STEM CELLS. 2007;25(11):2820–2826. [DOI] [PubMed] [Google Scholar]
- 30.Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21 (18):3324–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morelli SS, Rameshwar P, Goldsmith LT. Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol Reprod. 2013;89(1):7. [DOI] [PubMed] [Google Scholar]
- 32.Tal R, Liu Y, Pluchino N, Shaikh S, Mamillapalli R, Taylor HS. A Murine 5-Fluorouracil-Based Submyeloablation Model for the Study of Bone Marrow-Derived Cell Trafficking in Reproduction. Endocrinology. 2016;157(10):3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tal R, Dong D, Shaikh S, Mamillapalli R, Taylor HS. Bone-marrow-derived endothelial progenitor cells contribute to vasculogenesis of pregnant mouse uterusdagger. Biol Reprod. 2019;100(5):1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tal R, Shaikh S, Pallavi P, et al. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol. 2019;17(9):e3000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprangers AJ, Freeman BT, Kouris NA, Ogle BM. A Cre-Lox P recombination approach for the detection of cell fusion in vivo. J Vis Exp. 2012;59:e3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305(5680):90–93. [DOI] [PubMed] [Google Scholar]
- 37.Munoz-Espin D, Canamero M, Maraver A, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013; 155(5):1104–1118. [DOI] [PubMed] [Google Scholar]
- 38.Storer M, Mas A, Robert-Moreno A, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155(5):1119–1130. [DOI] [PubMed] [Google Scholar]
- 39.Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular Senescence: Aging, Cancer, and Injury. Physiol Rev. 2019;99 (2):1047–1078. [DOI] [PubMed] [Google Scholar]
- 40.Liu JY, Souroullas GP, Diekman BO, et al. Cells exhibiting strong p16 (INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci U S A. 2019;116(7):2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alawadhi F, Du H, Cakmak H, Taylor HS. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PloS one. 2014;9(5):e96662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009; 80(1–2):122–131. [DOI] [PubMed] [Google Scholar]
- 43.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. [DOI] [PubMed] [Google Scholar]
- 44.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185(1):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21): 3300–3305. [DOI] [PubMed] [Google Scholar]
- 46.Hill WD, Hess DC, Martin-Studdard A, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63(1):84–96. [DOI] [PubMed] [Google Scholar]
- 47.Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16(9):879–886. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Mamillapalli R, Mutlu L, Du H, Taylor HS. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moridi I, Mamillapalli R, Kodaman PH, Habata S, Dang T, Taylor HS. CXCL12 Attracts Bone Marrow-Derived Cells to Uterine Leiomyomas. Reprod Sci. 2020;27(9):1724–1730. [DOI] [PubMed] [Google Scholar]
- 50.Chen P, Mamillapalli R, Habata S, Taylor HS. Endometriosis stromal cells induce bone marrow mesenchymal stem cell differentiation and PD-1 expression through paracrine signaling. Mol Cell Biochem. 2021; 476(4):1717–1727. [DOI] [PubMed] [Google Scholar]
- 51.Sahin Ersoy G, Zolbin MM, Cosar E, Moridi I, Mamillapalli R, Taylor HS. CXCL12 Promotes Stem Cell Recruitment and Uterine Repair after Injury in Asherman’s Syndrome. Mol Ther Methods Clin Dev. 2017;4:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De M, Wood GW. Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol. 1990;126(3): 417–424. [DOI] [PubMed] [Google Scholar]
- 53.Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest. 2010;120(3): 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schafer MJ, White TA, Iijima K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall BM, Balan V, Gleiberman AS, et al. Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY). 2016;8(7):1294–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall BM, Balan V, Gleiberman AS, et al. p16(Ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY). 2017;9(8):1867–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murakami Y, Mizoguchi F, Saito T, Miyasaka N, Kohsaka H. p16 (INK4a) exerts an anti-inflammatory effect through accelerated IRAK1 degradation in macrophages. J Immunol. 2012;189(10):5066–5072. [DOI] [PubMed] [Google Scholar]
- 58.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182(5):2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong YR, Cousins FL, Yang X, et al. Bone Marrow Stem Cells Do Not Contribute to Endometrial Cell Lineages in Chimeric Mouse Models. STEM CELLS. 2018;36(1):91–102. [DOI] [PubMed] [Google Scholar]
- 60.Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. [DOI] [PubMed] [Google Scholar]
- 61.Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaitu’u-Lino TJ, Ye L, Salamonsen LA, Girling JE, Gargett CE. Identification of label-retaining perivascular cells in a mouse model of endometrial decidualization, breakdown, and repair. Biol Reprod. 2012;86 (6):184. [DOI] [PubMed] [Google Scholar]
- 63.Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med. 2011;208(9):1901–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


