Abstract
Background
The popularity of the gluten‐free diet and sales of gluten‐free products have increased immensely.
Aims
To investigate whether gluten induces gastrointestinal symptoms, measured by self‐reported questionnaires, as well as mental health symptoms in adolescents from a population‐based cohort.
Methods
The eligible participants (n = 273) were recruited from a population‐based cohort of 1266 adolescents and had at least four different gastrointestinal symptoms. Phase one (n = 54) was a run‐in phase where the participants lived gluten‐free for 2 weeks. If they improved they continued to phase 2 (n = 33), a blinded randomised cross‐over trial. Participants were blindly randomised either to start with 7 days of gluten, eating two granola bars containing 10 g of gluten or to 7 days on placebo, eating two granola bars without gluten, followed by the reverse and separated by a 7‐day washout period. The effects of the intervention on gastrointestinal symptoms and mental health symptoms were assessed.
Results
In total, 54/273 participants entered the run‐in phase and 35 were eligible for randomization. A total of 33 were randomised and 32 completed the trial. The median age was 20.3 (IQR 19.2–20.9) and 32/33 participants were females. Compared with a placebo, gluten did not induce gastrointestinal symptoms. The difference in the average VAS was −0.01 (95% confidence interval −2.07 to 2.05). Nor did we find a difference in the outcomes measuring mental health.
Conclusion
Compared with placebo, adding gluten to the diet did not induce gastrointestinal symptoms or worsened mental health in adolescents recruited from a population‐based cohort. The trial registration number is NCT04639921.
Keywords: gluten, gluten sensitivity enteropathy, gluten‐free diet
1. INTRODUCTION
The gluten‐free diet (GFD) has gained increasing popularity among healthy people without the coeliac disease (CeD) or wheat allergy. Survey‐based studies show that those following a GFD are more likely to be female, well‐educated, and younger (<50 years old). 1 , 2 The main reasons for following a GFD as explored in these surveys are weight control, the perception that a GFD is healthier, and the presence of symptoms after gluten ingestion. 3 , 4 , 5
Concurrently, the global consumption of gluten‐free products has increased and is expected to reach a value of 8.5 billion USD by 2025. 6 However, following a GFD is often expensive and inconvenient and carries an inherent risk of inducing macro‐ and micronutrient deficiencies. 7
Non‐coeliac gluten sensitivity (NCGS) is a newly described disease entity, where the individual shows signs of sensitivity to gluten, but with no evidence of IgE‐mediated wheat allergy or coeliac disease (CeD). 8 The diagnosis is based on a food challenge, preferably blinded, with relief of symptoms on a GFD and a worsening of symptoms on a gluten‐containing diet. 8 The clinical picture of NCGS is broad and can resemble the clinical presentation of CeD, with both gastrointestinal and extra‐gastrointestinal symptoms, and irritable bowel syndrome (IBS) but without growth insufficiency and biochemical abnormalities as seen in CeD. 9 , 10 The reported prevalence of self‐diagnosed NCGS shows wide variation, from 0.9% to 14% in survey‐based studies, 5 , 11 possibly due to varying perceptions of symptoms in different populations. In general, the number is even higher for gluten‐avoidance. 12
Only a limited number of studies have explored the effect of gluten on gastrointestinal symptoms, and none in adolescents, and so far the results have been inconclusive. 13 This may be a result of heterogeneous study designs or the inclusion of highly selective participants with self‐diagnosed NCGS, which carries the risk of a nocebo effect. 10 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24
The present study aimed to address the hypothesis that adding gluten to the diet results in a self‐reported worsening of gastrointestinal symptoms (primary outcome) and mental health (key secondary outcomes) in a well‐characterised group of adolescents.
To this end, we performed a randomised cross‐over trial comparing the effects of a gluten‐containing diet to an equivalent control diet (placebo) by having participants eat two granola bars with or without gluten per day for 1 week, separated by a 1‐week washout period.
2. MATERIAL AND METHODS
2.1. Participants
We recruited the adolescents from the GlutenFunen cohort, which is a cohort based on an unselected subsample of the Danish National Birth Cohort comprising approximately 96,000 children. 25 The GlutenFunen cohort included 1266 out of the 7431 eligible participants (17%) age 15–21 y who were examined for CeD. The participants in the GlutenFunen cohort answered a questionnaire about their gastrointestinal symptoms, and a score based on the number of gastrointestinal symptoms was calculated. The maximum symptom score was 10. The criterion for inclusion in the present study was more than three gastrointestinal symptoms and normal values of tissue transglutaminase and total IgA to exclude CeD, a total of 273 participants. All participants were contacted by phone by the author (NS or CC) and invited to an introductory meeting where the study design was further explained. Exclusion criteria were known wheat allergy or CeD, transglutaminase IgA higher than reference range as an indicator of CeD, known IBD, and current antibiotic treatment.
2.2. Study design
This was a double‐blinded randomised cross‐over trial that took place from September to October 2020 at Hans Christian Andersen Children’s Hospital at Odense University Hospital, Denmark. The study was arranged in two phases. The first phase began with 2 weeks of a GFD. If the participants responded to the GFD, defined as at least a 25% reduction in symptoms, they proceeded to phase 2. Phase 2 was a double‐blinded randomised trial with cross‐over (Figure 1) and consisted of three periods, each lasting 7 days: the first period was a challenge with gluten/placebo, then a wash‐out phase, and finally the second challenge with placebo/gluten. The participants followed a strict GFD throughout the entire trial. At the introductory meeting, participants were informed by a trained dietician experienced in instructing adolescents in following a GFD. Throughout the entire study, participants could obtain help with regard to following the GFD by contacting the dietician by telephone or email. Adherence was not evaluated during the trial, but participants were asked to note whether they had accidentally eaten gluten and were sent a daily text message encouraging them to stick to their GFD.
FIGURE 1.
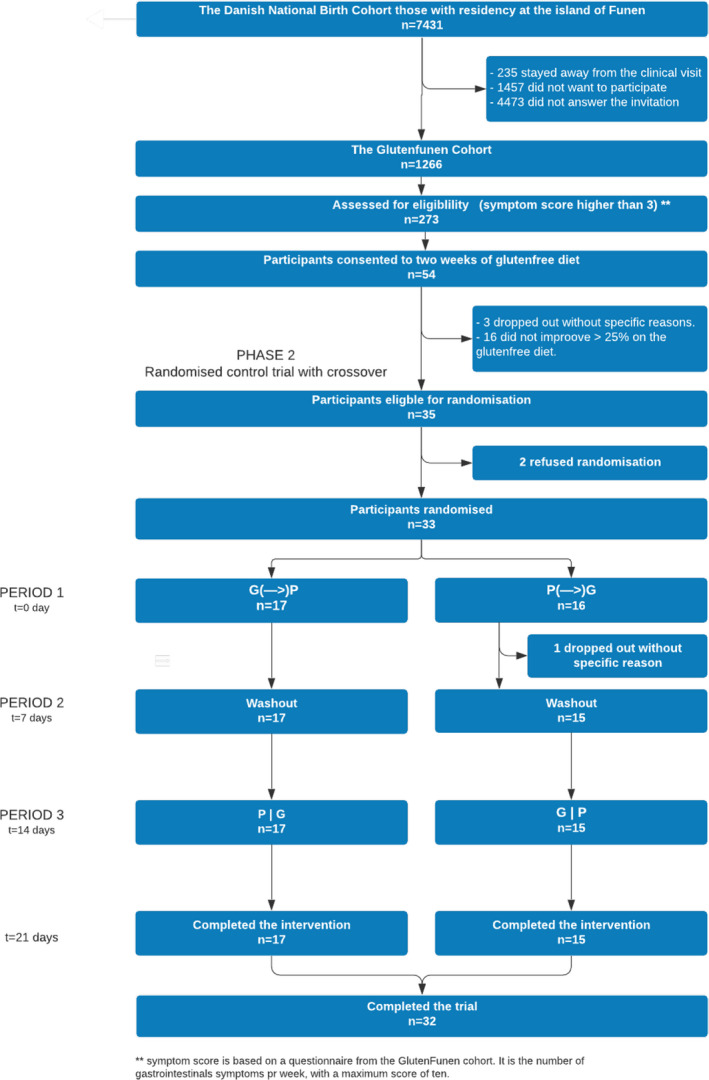
A flow chart illustrating the trial profile
To ensure compliance with the study and to overcome COVID‐19 restrictions, the study was designed with only one clinical visit on day 1 in the randomised trial (phase 2), where all the participants came at different time slots to collect their granola bars and had a blood sample taken, testing for IgE specific to wheat.
2.3. Questionnaires
Gastrointestinal symptoms were recorded on day 1 and 13 in phase 1 and daily during the randomised trial. Gastrointestinal symptoms were measured with a 10‐item self‐administered questionnaire that represented a modified gastrointestinal symptom rating score (Supplementary information). Symptoms were assessed on a 100‐mm visual analogue scale (VAS) (1–100), where 1 indicated no symptoms and 100 represented the worst symptoms ever experienced. The questionnaire included 10 questions about abdominal pain, bloating, borborygmi, diarrhoea, flatulence, constipation, nausea, dyspepsia, incomplete evacuation after a toilet visit, and burping. The symptom scale has been used previously in a similar trial. 17 Mental health was measured using the Short Form 36 (SF36) 26 and Warwick‐Edinburgh Mental Well‐being Scale (WEMWBS). 27 In phase 1, we also asked 18 questions about body perception. The questions were identical to questions from the 11 years follow up in the Danish National Birth Cohort. 25 The symptom questionnaire was sent as a link to the participants in a daily text message.
2.4. The granola bars
The participants had to eat two granola bars every day except during the washout period. The granola bar was lactose‐free and low in FODMAPs, and the gluten‐containing granola bar contained 5.0 g of gluten. The recipe was kindly provided by Professor Knut Lundin, University of Oslo. 22 The source of gluten was “Beneo Vital Wheat Gluten (Südzucker Zeitz)” without FODMAPs. A triangle test with 12 volunteers showed no difference regarding taste, look, or consistency between the gluten and the placebo granola bar.
2.5. Outcome measures
The primary outcome was the average modified VAS score after gluten compared to placebo. Key secondary outcomes were the SF‐36 mental component score (mcs), SF‐36 physical component score (pcs), both scored from 0 to 100 (100 being the best), and WEMWBS which ranged from 14 to 70 (70 being the best). Other secondary outcomes were every individual symptom score (scored from VAS 0 to 100 mm). Gluten responder status was defined as a 30% increase in median VAS score compared to placebo and likewise, placebo responder status was defined as a 30% increase in median VAS score compared to gluten.
2.6. Randomisation and blinding
The participants were randomised to placebo‐gluten or gluten‐placebo in blocks of four by the randomization module in the electronic database REDCap 28 administrated by the data manager of the study. The kitchen facility at Odense University Hospital baked the granola bars and packed them in boxes with either red or white colour to ensure the blinding of the study for the investigators and participants. The randomization code was not revealed before the end of the trial.
2.7. Statistical analysis
Descriptive statistics are described as means (SD), frequencies (%), or medians and interquartile ranges, depending on the empirical data distribution. The difference between groups was estimated based on the intention‐to‐treat population using a repeated‐measures linear mixed‐effects model, with participants modelled as random effects, whereas period, group, and the interaction period × group acted as fixed effect factors. Primary and sensitivity analyses were performed as suggested by White et al. 29 with the main analysis based on the plausible Missing At Random (MAR) assumption about missing data followed by sensitivity analyses (incl. Non‐responder imputation). The analyses were carried out using STATA version 15 and SAS Studio (version 9.4). Results are reported for each group as Least Squares Means with standard errors, while the differences between groups are reported with corresponding 95% confidence intervals (95% CI). All 95% confidence intervals and P values were two‐sided; no explicit adjustments for multiplicity were applied. Instead, we analysed and interpreted the primary and secondary outcomes in prioritised order (as described in the Statistical Analysis Plan; see Supplemental material): The analyses of the secondary outcomes were performed in sequence until one of the analyses failed to show a difference, at a statistical significance level of 0.05.
Primary sample size calculation was based on McNemar’s test for comparison of two related proportions, specifying discordant proportion. The sample size was calculated to 29 participants assuming that 30% would be positive (a 30% increase in mean VAS score compared to placebo) to the challenge with gluten and 1% to the challenge with placebo. The level of significance was set to 0.05, the power to 80% and an expected drop‐out rate of 5%. However, while preparing the statistical analysis plan (before looking at the actual data) it was decided to use the difference in the average VAS as the primary endpoint. A sample size of 30 participants (i.e. 30 paired samples) would correspond to a statistical power of 88.8% to detect (p < 0.05) a difference of 15 VAS units assuming a standard deviation of 25, with a correlation between measures of 0.5.
2.8. Ethics and approvals
The study was approved by The Regional Committees on Health Research Ethics for Southern Denmark (project no S‐20160061) and the Danish Data Protection Agency (2008‐58‐0018). The manuscript was prepared in compliance with the CONSORT statement. 30 The trial was registered at clinicaltrial.gov NCT04639921. All authors had access to the study data and reviewed and approved the final manuscript.
3. PATIENT AND PUBLIC INVOLVEMENT
The participants were recruited from the Glutenfunen cohort, which was established to explore CeD in adolescents. In the process of patient involvement in designing the Glutenfunen cohort, participants expressed a desire for non‐coeliac gluten sensitivity to be investigated and were thus involved in designing the research question and the study. In October 2019, a pilot project for the conduction of the randomised trial with cross‐over was undertaken. The first author performed semi‐structured interviews with one mother and six participants to improve the study design, which included how the participants interpreted the questionnaires and the burden of the trial.
4. RESULTS
4.1. Recruitment
A total of 273 participants (22%) from the GlutenFunen cohort had more than three gastrointestinal symptoms in the questionnaire and were considered for eligibility (Figure 1). Of these 54 (20%) consented to a 2‐week gluten‐free diet (phase 1). Three dropped out for unknown reasons and 16 (30%) failed to reach an improvement of 25% in their average VAS score. Thus, 35 participants were eligible for randomization. Two participants refused randomization, leaving 33 randomised participants, 16 to placebo‐gluten and 17 to gluten‐placebo. In the first period, one participant dropped out from the placebo‐gluten group for unspecific reasons, and thus 17 in the gluten‐placebo group and 15 in the placebo‐gluten group completed the intervention, totalling 32. None of the participants experienced severe adverse events during the intervention period. The participants self‐reported that all granola bars were consumed during the trial period.
4.2. Recruitment and baseline data
Baseline characteristics of the participants are presented in Table 1. Out of the 33 participants, 32 were females with a median age of 20 years. Four participants were positive for IgE wheat, two of which had a cross‐reaction to IgE grass, and they were all randomised to the placebo‐gluten group. Very few (2/33) had autoimmune disease among their first‐degree relatives. From the questions about body perception before the trial, we found that most participants were afraid of weight gain or wanted to be slimmer and that most of them did exercise to lose weight despite the fact that the median BMI was 22.0 (interquartile range (IQR) 20.6;24.4). At the beginning of phase 1, the median average VAS was 55 (IQR 43–61). At the beginning of phase 2, following 2 weeks of a GFD, the baseline median average VAS was 21 (IQR 9–29). The drop in the median average VAS was significant (p < 0.0000). The SF‐36 mcs was 47.0 (SD 11.2), which was slightly lower compared to the reference value 53.0 (SD 7.5) for females. The SF‐36 pcs was 52.4 (SD 8.0), almost identical to reference value 54.3 (SD 5.8). 31 WEMWBS baseline score after 2 weeks of a GFD was 51.1 (SD 6.8) compared to a norm value of 51.3 (95% confidence interval 50.9 to 51.7). 27 The three most dominating symptoms at baseline were flatulence, borborygmi, and the feeling of incomplete evacuation after toilet visits, with median average VAS values of 27, 26, and 23, respectively. Determination of HLA status revealed that 55% (18/33) were HLA DQ2 and/or HLA DQ8 positive.
TABLE 1.
Baseline demographic and clinical characteristics
|
Period 1 Gluten N = 17 |
Period 1 Placebo N = 16 |
Baseline total combined | |
|---|---|---|---|
| Basic characteristics | |||
| Age, years a | 19.8 [18.9;20.8] | 20.5 [20.1;20;9] | 20.3 [19.2;20;9] |
| Sex, females (n%) b | 17 (100%) | 16 (94%) | 32 (97%) |
| Height, SD‐score c | −0.7 [−1.0;0.7] | −0.5 [−1.1;0.1] | −0.4 [−1.1;0.5] |
| Weight, SD‐score c | 0.0 [−0.4;0.9] | −0.5 [−1.1;0.3] | −0.1 [−0.7;0.6] |
| Body‐Mass‐Index (BMI) kg/m2, d | 22.7 [21.0;24.4] | 21.6 [20.6;24.4] | 22.0 [20.6;24.4] |
| BMI, SD‐score c | 0.4 [0.0;1.3] | 0.1 [−0.7;1.1] | 0.3 [−0.1;1.2] |
| Number of gastrointestinal symptoms (at least 4 out of 10) e | 5 [4;6] | 5 [4;6] | 5 [4;6] |
| IgE Wheat positive (n%) f | 0 (0%) | 4 (25%)* | 4 (12%) |
| On diet to obtain weight loss (n%) g | 5 (29%) | 0 (0%)* | 5 (15%) |
| Medication, fasting, or vomiting to obtain weight loss (n%) h | 2 (12%) | 0 (0%) | 2 (6%) |
| Wanting to be thin or afraid of weight gain (n%) i | 12 (71%) | 14 (88%) | 26 (79%) |
| Exercising to obtain weight loss (n%) j | 11 (65%) | 10 (63%) | 21 (64%) |
| Autoimmune disease among first‐degree relatives (n%) j | 0 (0%) | 2 (13%) | 2 (6%) |
| HLA status, positive HLA DQ2 / DQ8 (n%) | 11 (65%) | 7 (47%) | 18 (55%) |
| Primary outcome | |||
| VAS Symptom Score at baseline, 0‐100 mm | 19 [6–26] | 21 [14–29] | 21 [9–29] |
| VAS symptom Score at the beginning at Phase 1, 0‐100 mm | 55 [50–61] | 54 [43–60] | 55 [43–61] |
| Key secondary outcomes | |||
| SF‐36 Mental Component Score, score: 0‐100 k | 48.1 (10.9) | 45.9 (11.8) | 47.0 (11.2) |
| SF‐36 Physical Component Score, score: 0‐100 k | 53.3 (8.2) | 51.5 (8.0) | 52.4 (8.0) |
| WEMWBS: 14‐70 k | 52.1 (5.2) | 50.1 (8.0) | 51.1 (6.8) |
| Other secondary outcomes | |||
| VAS1, / Abdominal pain, 0‐100 mm | 4 [0–38] | 18 [3–23] | 16.5 [0–28] |
| VAS2, / Bloating, 0‐100 mm | 19 [9–29] | 22 [10–35] | 19 [10–31] |
| VAS3 / Flatulence, 0‐100 mm | 24 [16–17] | 38 [24–44] | 27 [19–44] |
| VAS4, / Borborygmi, 0‐100 mm | 20 [11–49] | 28 [15–75] | 26 [11–57] |
| VAS5, / Diarrhoea, 0‐100 mm | 0 [0–10] | 10 [0–29] | 2 [0–23] |
| VAS6, / Constipation, 0‐100 mm | 11 [0–34] | 18 [0–43] | 17 [0–39] |
| VAS7, / Incomplete evacuation after toilet visit, 0‐100 mm | 11 [0–33] | 31 [17–56] | 23 [0–42] |
| VAS8, / Nausea, 0‐100 mm | 14 [0–28] | 8 [0–14] | 10 [0–26] |
| VAS9, / Burping, 0‐100 mm | 1 [0–17] | 4 [0–14] | 0 [0–16] |
| VAS10, / dyspepsia, 0‐100 mm | 0 [0–4] | 2 [0–13] | 0 [0–13] |
Note: Values are means ± SDs, or median and interquartile range (depending on the empirical data distribution); unless otherwise stated. Significant differences (p < 0.05) are marked with an asterisk (*).
The age was calculated as the age of the participants on September 1, 2020.
Based on the Danish personal identification number.
Height, weight, and BMI were reported as SD scores to allow for age and sex using the Danish references for growth. 49 The values are obtained from the Glutenfunen cohort, including participants from 2018 to 2020.
Based on the questionnaire to the participants in the Glutenfunen cohort. The score reflects the number of gastrointestinal symptoms, with the maximum being 10. To be included in this study, a symptom score higher than 3 was necessary.
The values are obtained from the Glutenfunen cohort, including participants from 2018 to 2020.
IgE wheat was measured at day 0. Higher than 0.35 kU/l was considered positive
Was considered positive if the participants answered “every day,” “often,” or “several times” and negative if participants answered “a couple of times,” “never,” or “do not know.” The time scale was the last year.
Was considered positive if the participants answered “every day,” “several times per week,” “once a week,” or “1‐3 times per month,” and negative if they answered “never,” “do not know,” or “less than once per month.”
Was considered positive if the participants answered “every day,” “often,” or “sometimes,” and it was considered negative if they answered, “rarely,” “never,” or “do not know.”
Based on the questionnaire to the participants in the GlutenFunen cohort.
Measured at day 0.
4.3. Primary outcome
During the first period, the gluten group had a higher average VAS of 24.4 (SE 2.2) although the difference from the placebo group with an average VAS of 21.8 (SE 2.2) was minor. In the third period, the gluten group had a lower average VAS of 19.6 (SE 2.2) compared to placebo, which was 22.1 (SE 2.2). There was no effect of period × group, and therefore no carry‐over effect, but an expected effect of period (see Table 2). Overall, there was no difference, −0.01 (95% confidence interval −2.07 to 2.05), in the average VAS between eating gluten or placebo (Table 3) (Figure 2). This was confirmed in the non‐responder imputation sensitivity analysis with a difference between gluten and placebo of −0.1 (95% confidence interval −2.0 to 1.8).
TABLE 2.
Summary results for each study group and period
| Period 1 | Period 2 | Period 3 | Statistical tests: Fixed effects | |||||
|---|---|---|---|---|---|---|---|---|
| Gluten | Control | Washout | Gluten | Control | Period*group | Period | Group | |
| Primary outcome | p‐values | p‐values | p‐values | |||||
| Average VAS, 0‐100 mm | 24.4 (2.2) | 21.8 (2.2) | 17.5 (1.9) | 19.6 (2.2) | 22.1 (2.2) | 0.15 | 0.03 | 0.99 |
| Key secondary outcomes | ||||||||
| SF‐36 Mental Component Score, score: 0–100 | 48.3 (1.7) | 48.7 (1.6) | 48.5 (1.3) | 51.4 (1.6) | 49.5 (1.8) | 0.52 | 0.10 | 0.54 |
| SF‐36 Physical Component Score, score: 0‐100g | 51.0 (0.8) | 51.5 (0.8) | 51.8 (0.6) | 50.7 (0.8) | 52.2 (0.8) | 0.59 | 0.73 | 0.69 |
| WEMWBS: 14–70 | 50.4 (1.3) | 51.9 (1.1) | 51.9 (0.8) | 51.9 (1.1) | 52.3 (1.2) | 0.67 | 0.28 | 0.28 |
| Other secondary outcomes | ||||||||
| VAS1 / Abdominal pain, 0‐100 mm | 29.1 (3.5) | 18.4 (3.3) | 17.7 (2.9) | 19.0 (3.4) | 28.0 (3.5) | 0.00 | 0.90 | 0.65 |
| VAS2 / Bloating, 0‐100 mm | 37.4 (4.1) | 30.7 (4.0) | 25.8 (3.6) | 29.2 (4.0) | 34.5 (4.1) | 0.07 | 0.27 | 0.73 |
| VAS3 / Flatulence, 0‐100 mm | 34.9 (3.8) | 32.6 (3.7) | 26.3 (3.3) | 30.6 (3.7) | 32.7 (3.9) | 0.51 | 0.31 | 0.97 |
| VAS4 / Borborygmi 0‐100 mm | 23.2 (3.4) | 27.1 (3.4) | 18.6 (3.0) | 23.7 (3.4) | 19.5 (3.4) | 0.15 | 0.04 | 0.92 |
| VAS5 / Diarrhoea 0‐100 mm | 15.9 (2.9) | 13.5 (2.9) | 11.9 (2.5) | 14.6 (3.0) | 15.8 (3.1) | 0.53 | 0.77 | 0.72 |
| VAS6 / Constipation 0‐100 mm | 20.6 (3.2) | 20.0 (3.1) | 14.8 (2.7) | 13.8 (3.2) | 16.1 (3.2) | 0.62 | 0.00 | 0.64 |
| VAS7 / Incomplete evacuation after toilet visit, 0‐100 mm | 29.4 (3.9) | 28.0 (3.8) | 26.2 (3.3) | 28.7 (3.8) | 38.3 (3.9) | 0.09 | 0.02 | 0.04 |
| VAS8 / Nausea, 0‐100 mm | 29.0 (3.7) | 20.5 (3.6) | 16.2 (3.1) | 17.9 (3.6) | 22.5 (3.7) | 0.05 | 0.02 | 0.34 |
| VAS9 / Burping, 0‐100 mm | 12.8 (2.5) | 18.4 (2.5) | 10.9 (2.2) | 12.0 (2.5) | 6.3 (2.6) | 0.01 | <0.00 | 0.97 |
| VAS10 / dyspepsia, 0‐100 mm | 11.0 (2.1) | 12.2 (2.0) | 6.8 (1.7) | 8.3 (2.0) | 6.7 (2.1) | 0.47 | 0.00 | 0.87 |
Notes: Values are Least‐Squares Means (SE) unless otherwise stated.
Statistical tests are based on a repeated‐measures linear mixed‐effects model (participants modelled as a random effects variable).
TABLE 3.
Summary of results for each intervention group derived from the cross‐over design (i.e. all periods included)
| Gluten (active) | Control (placebo) | Difference between groups (95% confidence interval) | p‐value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Average VAS, 0‐100 mm | 22.0 (2.2) | 22.0 (2.2) | −0.01 [−2.1 to 2.1] | 0.99 |
| Key secondary outcomes | ||||
| SF‐36 Mental Component Score, score: 0–100 | 49.8 (1.7) | 49.1 (1.7) | −0.7 [−3.1 to 1.6] | 0.54 |
| SF‐36 Physical Component Score, score: 0‐100 | 50.8 (0.8) | 51.9 (0.8) | 1.0 [−0.2 to 2.2] | 0.10 |
| WEMWBS: 14–70 | 51.1 (1.1) | 52.1 (1.1) | 1.0 [−0.8 to 2.7] | 0.28 |
| Other secondary outcomes | ||||
| VAS1, /abdominal pain, 0‐100 mm | 24.0 (3.4) | 23.2 (3.4) | −0.9 [−4.6 to 2.9] | |
| VAS2 / Bloating, 0‐100 mm | 33.3 (4.1) | 32.6 (4.1) | −0.7 [−4.6 to 3.2] | 0.72 |
| VAS3 / Flatulence, 0‐100 mm | 32.7 (3.8) | 32.7 (3.8) | −0.1 [−4.1 to 4.0] | 0.97 |
| VAS4, / Borborygmi, 0‐100 mm | 23.5 (3.4) | 23.3 (3.4) | −0.2 [−3.5 to 3.1] | 0.92 |
| VAS5, / Diarrhoea, 0‐100 mm | 15.3 (3.0) | 14.6 (3.0) | −0.6 [−4.2 to 2.9] | 0.72 |
| VAS6, / Constipation, 0‐100 mm | 17.2 (3.2) | 18.0 (3.2) | 0.9 [−2.8 to 4.5] | 0.64 |
| VAS7, / Incomplete evacuation after toilet visit, 0‐100 mm | 29.1 (3.8) | 33.2 (3.8) | 4.1 [0.2 to 8.0] | 0.04* |
| VAS8 / Nausea, 0‐100 mm | 23.4 (3.6) | 21.5 (3.6) | −1.9 [−5.9 to 2.1] | 0.34 |
| VAS9 / Burping, 0‐100 mm | 12.4 (2.5) | 12.3 (2.5) | −0.1 [−2.8 to 2.7] | 0.97 |
| VAS10 / dyspepsia, 0‐100 mm | 9.7 (2.0) | 9.5 (2.0) | −0.2 [−2.6 to 2.2] | 0.87 |
Notes: Values are Least Squares Means (SE) unless otherwise stated. Statistical tests are based on Repeated‐Measures Linear Mixed‐Effects Model (participants modelled as a random effects variable). Significant differences (p < 0.05) are marked with an asterisk (*).
FIGURE 2.
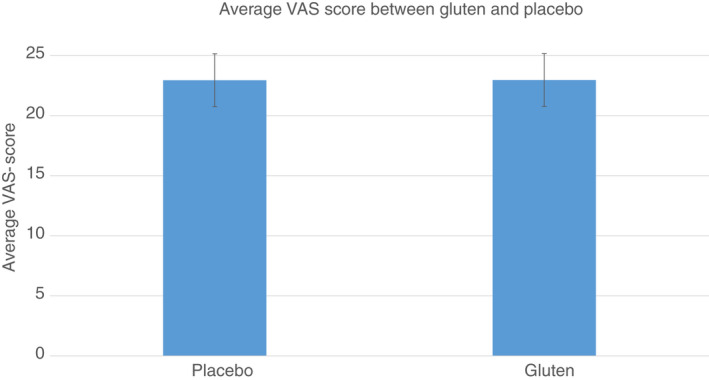
Average vas score (95% confidence intervals) between gluten and placebo. Values are least squares means (SE). Statistical tests are based on repeated‐measures linear mixed‐effects model (participants modelled as a random effects variable)
By categorising the participants who had experienced a “worsening” effect of either gluten or placebo, defined as a 30% increase in the median VAS for gluten compared to placebo, or vice versa, 12 participants were defined as gluten responders, and 13 as placebo responders. Among the gluten responders, 64% were HLA DQ2 and/or DQ8 positive. Likewise, 54% of the placebo‐responders were HLA DQ2 and/or DQ8 positive. None of the participants with positive IgE wheat was gluten or placebo responders. Based on the Salerno criteria, 36% could be diagnosed with NCGS, and 36% with placebo‐sensitivity, whereas 24% did not experience a difference in their gastrointestinal symptoms between placebo and gluten.
4.4. Secondary outcomes
In the outcomes measuring mental health (SF‐36 mcs, SF‐36 pcs and WEMWBS), no difference was observed between periods and groups. The differences were −0.7 (95% confidence interval −3.1 to 1.6) for SF‐36mcs, 1.0 (95% confidence interval −0.19 to 2.2) for SF‐36pcs, and 1.0 (95% confidence interval −0.8 to 2.7) for WEMWBS. See Tables 2 and 3.
4.5. Other secondary outcomes
To investigate whether specific symptoms were dominant, we evaluated each specific symptom. Overall, we could not detect a difference in any of the specific symptoms during the gluten and placebo periods. However, there was a significantly higher difference in the placebo group on the VAS score for feeling incomplete evacuation after a toilet visit, 4.1 (95% confidence interval 0.2 to 8.0).
5. DISCUSSION
In this double‐blinded randomised cross‐over trial with the aim of investigating the potential detrimental effect of gluten on gastrointestinal symptoms in young adolescents recruited from a population‐based cohort, it was not possible to detect a difference in symptoms between gluten and placebo at a group level. The very narrow 95% confidence interval for the difference between gluten and placebo demonstrates that the negative finding cannot be attributed to statistical error. On an individual level, we found a comparable number of gluten responders and placebo responders, underscoring the insignificant difference between gluten and placebo.
To date, 12 studies with blinded placebo‐controlled cross‐over trials with gluten have been published and have shown divergent outcomes. 10 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Five studies found an effect of gluten on gastrointestinal symptoms in adults, 10 , 14 , 16 , 17 , 21 and one study found an effect of gluten in children with gastrointestinal symptoms. 18 Six studies observed no effect or demonstrated a larger effect of placebo or fructan. 15 , 19 , 20 , 22 , 23 , 24 In general, the studies are characterised by patient recruitment from gastroenterological clinics or individuals self‐diagnosed with NCGS. 32 The recruitment of participants with self‐diagnosed NCGS carries a high risk of a nocebo effect since it is probable that a diagnosis of NCGS is expected. 33 In this trial, the adolescents were recruited based on the presence of gastrointestinal symptoms rather than a diagnosis to reduce the nocebo effect. Despite this precaution, we found a substantial nocebo effect. Furthermore, all previous studies except for one, have been limited to adults. 13 Our study stands out because we investigated the effect of gluten in adolescents, which according to a survey among adolescents might be the age of onset of symptoms. 34 The heterogeneous study results reflect the different study designs, such as the varying amounts of gluten ranging from 2 to 16 g daily. 15 , 19 It is plausible to expect that challenging with too little amount of gluten results in negative results. 15 , 19 Thus, in the present trial we challenged with 10 g of gluten, which is the standard daily gluten intake in the Danish population. 35 Furthermore, some of the placebo vehicles, especially among studies that found no result, contained FODMAPs, which might have blurred the results. 19 , 24 Therefore, the placebo vehicle in this trial was low in FODMAPs and free of lactose. Another variation is the discrepancy in the primary outcome—only five studies 15 , 16 , 20 , 22 , 23 reported an overall symptom score of gluten compared to placebo, even though it is recommended by the CONSORT guidelines. 30
5.1. Gluten‐free diet in the general population
During the last decade, adhering to a GFD, without having CeD or any other medical reason, has gained popularity. 36 Concurrently, there has been a tremendous increase in the market for gluten‐free products. 6
The exact prevalence of gluten avoidance in the population is unknown. Previous trial participants were typically recruited from tertiary centres or were self‐diagnosed with NCGS, resulting in a highly selected population. 16 , 17 In this study, the adolescents were recruited from a population‐based cohort, which may better reflect the general population despite the possibility of selection bias. Our baseline data support that our participants, in general, were healthy, had normal BMI and had mental health compared to the reference population. However, the majority of participants were concerned about keeping their weight low, but such behaviour could very well be normal for the age group. It is possible that NCGS does exist in highly selected patient groups, but our trial demonstrates that a GFD in the general population has no beneficial effect on gastrointestinal symptoms. Nor did we observe any differences in outcomes measuring mental health, although this could have been hampered by the short duration of the time periods. Looking on an individual level, 36% (12/36) of the participants could be defined as having NCGS according to the Salerno criteria 8 in accordance with previous studies 10 , 17 , 24 but the same number could be defined as placebo‐responders. We found that the proportion of HLA DQ2 and/or DQ8 among the participants was comparable to the known frequency of HLA DQ2 and/or DQ8 in the Danish adult population. 37 Furthermore, we found no significant difference in the frequency of HLA DQ2 and/or DQ8 among either the gluten or placebo‐responders. These data suggest that HLA DQ2 and/or DQ8 does not play an important role in adolescents with gastrointestinal symptoms.
5.2. The health effects of the gluten‐free diet
Presumably, people adhere to the GFD because they perceive it as healthier. 38 In a randomised controlled study with cross‐over but no blinding which compared a high gluten diet to a low gluten diet in healthy adults, a moderate change of the intestinal microbiota was associated with a drop in self‐reported bloating. However, the change was probably due to a qualitative change in fibre and not gluten itself, 39 which may explain the beneficial effect of a GFD. Still, in this study, we were not able to detect a difference when looking specifically at the symptom of bloating. In phase 1, the non‐blinded part, most of the participants had an improvement of gastrointestinal symptoms on a GFD. However, in phase 2, when the study was blinded, we were not able to reproduce these findings.
The participants in our study had at least a 25% decrease in their gastrointestinal symptoms when adhering to the GFD in phase 1. If the improvement was due to the GFD, one would expect a difference between gluten compared to placebo in phase 2. This suggests a substantial nocebo effect of gluten in phase 1. Another possibility is that, rather than the GFD, the decrease in symptoms could be due to a healthier lifestyle in general. In addition, a general awareness of their lifestyle could be expected solely due to the health‐related questions being asked. A third possibility is a reduction of the intake of FODMAPs which happens in some cases when people change to GFD. It would have strengthened our study to have kept food frequency records, especially in phase 1, but this was counterbalanced by the risk of further complicating the study.
IBS has many of the characteristics of NCGS, and there has been considerable interest in the potential beneficial effects of a GFD for IBS patients. In two prospective studies, a GFD reduced gastrointestinal symptoms, particularly diarrhoea, in patients with IBS. 40 , 41 However, these studies were not blinded, and further research is needed before drawing any conclusions, especially due to the large placebo effect demonstrated in food trials. 13 In our trial, IBS was not an exclusion criterion and a potential effect of GFD in patients with IBS may blur the results. However, when looking at the symptom of diarrhoea, we detected no significant difference. Furthermore, the aim was to investigate the effect of gluten on gastrointestinal symptoms in a population mirroring the general population, and not under specific conditions.
5.3. The adverse effects of a GFD
In our study, there was an equal number of placebo responders compared to gluten responders, and this raises an important question: Why not follow a GFD if it results in better well‐being? There are several adverse consequences of following a GFD, the two most serious being the possible micro‐ and macronutrient deficiencies 42 and the psychosocial consequences. A recent study following 35 participants without CeD on a GFD demonstrated that the mean intake of fibre, calcium, and iron was lower than recommended. 43 Additionally, some studies have shown that a GFD in CeD is associated with increased weight 44 and non‐alcoholic fatty liver disease. However, the results are conflicting 45 and in contrast, people adhering to the GFD may obtain a better cardiovascular risk profile. 1 The psychosocial consequences of the GFD is illustrated in a study of adolescents with CeD where half of the participants managed the GFD with maladaptive eating behaviours leading to diminished quality of life. 46 It may be concluded that the long‐term consequences of a GFD in a healthy population are unknown.
5.4. Factors other than gluten
The pathogenesis of NCGS is still unexplained and thus, there are no established NCGS biomarkers. 36 Furthermore, some authors claim that the reaction to gluten is instead due to other components of wheat such as fructans 22 and wheat amylase trypsin inhibitors. 47 In IBS, a well‐defined low FODMAP diet is now recommended in the clinical guidelines from the American College of Gastroenterology. 48 In this trial, we did not control for FODMAP content and although we asked the participants not to change their diet between phase 1 and phase 2 (the randomised trial), we cannot rule out a possible effect of FODMAPs. So far, two studies have investigated the association between NCGS and FODMAPs. In a Norwegian study, the authors concluded that fructan rather than gluten induces gastrointestinal symptoms, but they found no significant difference between fructan and placebo in their primary outcome. However, in their secondary outcome, measuring gastrointestinal symptoms on a VAS score, a difference between placebo and FODMAPs occurred, still with a significant nocebo response. 22 The effect of FODMAPs was also demonstrated in the study where a reduced FODMAP diet diminished the gastrointestinal symptoms induced by gluten observed in patients with NCGS. 15
5.5. Strengths
The major strengths of this study are the trial design and setting, with a blinded randomised controlled trial with cross‐over and repeated measurements and the recruitment of participants from a population‐based cohort. These characteristics are contrary to several of the studies that found an effect of gluten, where the participants were only asked about symptoms at the end of each period, possibly inducing recall‐bias or not allowing for correlation between participants. 17 , 18 , 24
Another major strength is that the study was easy to complete. The communication, including the questionnaires to the participants, was done via text messages and the authors were able to provide immediate answers when there were doubts, which resulted in high compliance. Only a single clinical visit at the hospital was planned, where the granola bars were distributed and blood samples were drawn to determine S‐IgE for wheat, with the latter resulting in four participants being randomised even though they had elevated IgE for wheat. However, during the trial, the results were blinded for the participants as well as for the authors.
5.6. Limitations
The major limitation of this study is that we did not clinically control the GFD by measuring gluten peptides in urine or by administering food frequency questionnaires. It was important for us to keep the trial simple to maintain high compliance, which meant that we decided not to use food frequency questionnaires, as we had experienced in our pilot study that the participants were reluctant to complete more questionnaires. Furthermore, we did not assess formally for other gastrointestinal diseases such as a peptic ulcer or IBS since the aim of this study was not a diagnosis but to investigate the effect of gluten in healthy adolescents with gastrointestinal symptoms. We believe that severe gastrointestinal symptoms would have led to a health contact. However, there remains the possibility that IBS is the important driver in the gastrointestinal symptoms, especially since symptoms such as incomplete evacuation, flatulence and constipation were some of the most severe symptoms at baseline.
Indeed, a participation criterion was the presence of more than three complaints, and fewer but more severe symptoms could theoretically qualify for NCGS.
6. CONCLUSION
In this blinded, placebo‐controlled, randomised trial with cross‐over in adolescents with gastrointestinal symptoms, no effect of adding gluten to the diet was detected.
AUTHORSHIP
Guarantor of the article: Caecilie Crawley
Author contributions: SH, JM, CH and CC were involved in the conception and design of the study. CC and SH were the investigators of the study and took part in obtaining the funding and all required permissions. CH and CC conducted the pilot project and NS and CC ran the trial. A‐MNA and JM provided expert advice on the epidemiological aspects. SDS, SH, MA, and JM contributed to the development of methods and data collection. RC and CC developed a statistical analysis plan and RC conducted the statistical analyses and was involved in interpreting data. CC and SH drafted the work. All the authors have critically revised this article and approved the final version to be published.
STATEMENT OF INTEREST
The Danish National Birth cohort was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation, and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation.
Follow‐up of mothers and children has been supported by the Danish Medical Research Council (SSVF 0646, 271–08‐0839/06–066023, O602‐01042B, 0602‐02738B), the Lundbeck Foundation (195/04, R100‐A9193), Innovation Fund Denmark 0603‐00294B (09–067124), the Nordea Foundation (02–2013‐2014), Aarhus Ideas (AU R9‐A959‐13‐S804), University of Copenhagen Strategic Grant (IFSV 2012), and the Danish Council for Independent Research (DFF‐4183‐00594 and DFF‐4183‐00152).
DISCLOSURES
Dr Joseph Murray has received consultancy fees from Bionix, Lilly Research Laboratory, Johnson & Johnson, Dr. Schar USA, UCB Biopharma, Celimmune, Intrexon Corporation, Chugai Pharma, Kanyos, and Boehringer Ingelheim; holds patents licensed to Evelo Biosciences; and receives royalties from Thorax Medical. The other authors have nothing to declare.
DATA TRANSPARENCY STATEMENT
Researchers can apply for de‐identified data and biomaterial by submitting a proposal to the author CC (mail to caecilie.larsen@rsyd.dk), especially in the research areas of gluten sensitivity. Data can be made available immediately after publication for a period of 3 years.
Supporting information
Data S1
ACKNOWLEDGEMENTS
The authors would like to thank the DNBC participants, especially those who entered the GlutenFunen cohort and this trial. We thank Professor Knut Lundin, University of Oslo, Norway, for the recipe for the granola bars (25)
Crawley C, Savino N, Halby C, Sander SD, Andersen A‐M, Arumugam M, et al. The effect of gluten in adolescents and young adults with gastrointestinal symptoms: a blinded randomised cross‐over trial. Aliment Pharmacol Ther. 2022;55:1116–1127. doi: 10.1111/apt.16914
The Handling Editor for this article was Professor Richard Gearry, and it was accepted for publication after full peer‐review.
Funding informationThis project was funded by the A.P. Møller Fund (grant nos.19‐L‐0228 and 18‐L‐0032), Odense University Hospital Research Fund (A3675 and A 2971), Direktør Kurt Bønnelyckes og hustru Fru Grethe Bønnelyckes Fund (100 53030), Torben og Alice Frimodts Fund (n/a), and L.F. Foghts Fund (21.844) to CC and the Augustinus Fund (19–2419) to SH. Thermo Fischer (n/a) donated $70 000 and had no influence on the design of the study. CC holds a PhD grant funded by the University of Southern Denmark, the Region of Southern Denmark, and Hans Christian Andersen Children’s Hospital. The Department of Clinical Immunology, Odense University Hospital covered the cost of the Coeliac screening analysis. Professor Christensen acknowledges that the Section for Biostatistics and Evidence‐Based Research, the Parker Institute is supported by a core grant from the Oak Foundation (OCAY‐18‐774‐OFIL). Novo Nordisk Foundation Center for Basic Metabolic Research is supported by Novo Nordisk Foundation (grant number NNF18CC0034900). Dr. Joseph Murray has received study grants from Nexpep/ImmusanT, National Institutes of Health, Immunogenix, Takeda Pharmaceutical, Allakos, Oberkotter, and Cour.
REFERENCES
- 1. Littlejohns TJ, Chong AY, Allen NE, Arnold M, Bradbury KE, Mentzer AJ, et al. Genetic, lifestyle, and health‐related characteristics of adults without celiac disease who follow a gluten‐free diet: a population‐based study of 124,447 participants. Am J Clin Nutr. 2020;113(3):622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Gils T, Nijeboer P, IJssennagger C, Sanders D, Mulder C, Bouma G. Prevalence and characterization of self‐reported gluten sensitivity in The Netherlands. Nutrients. 2016;8(11):714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ontiveros N, Real‐Delor RE, Mora‐Melgem JA, Beltrán‐Cárdenas CE, Figueroa‐Salcido OG, Vergara‐Jiménez MJ, et al. Prevalence of wheat/gluten‐related disorders and gluten‐free diet in Paraguay: an online survey‐based study. Nutrients. 2021;13(2):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perrin L, Allès B, Buscail C, Ravel C, Hercberg S, Julia C, et al. Gluten‐free diet in French adults without coeliac disease: sociodemographic characteristics, motives and dietary profile. Br J Nutr. 2019;122(2):231–9. [DOI] [PubMed] [Google Scholar]
- 5. Potter MD, Jones MP, Walker MM, Koloski NA, Keely S, Holtmann G, et al. Incidence and prevalence of self‐reported non‐coeliac wheat sensitivity and gluten avoidance in Australia. Med J Australia. 2020;212(3):126–31. [DOI] [PubMed] [Google Scholar]
- 6. Marketsandmarkets . Gluten‐free products market by type (bakery products, snacks & RTE products, condiments & dressings, pizzas & pastas), distribution channel (conventional stores, specialty stores and Drugstores & Pharmacies), Form & Region ‐ Global Forecast to 2025. 2020.
- 7. Kreutz JM, Adriaanse MPM, van der Ploeg EMC, Vreugdenhil ACE. Narrative review: nutrient deficiencies in adults and children with treated and untreated celiac disease. Nutrients. 2020;12(2):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catassi C, Elli L, Bonaz B, Bouma G, Carroccio A, Castillejo G, et al. Diagnosis of non‐celiac gluten sensitivity (NCGS): the Salerno Experts' criteria. Nutrients. 2015;7(6):4966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR, The study Group for non‐Celiac Gluten Sensitivity . An Italian prospective multicenter survey on patients suspected of having non‐celiac gluten sensitivity. BMC Med. 2014;12(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carroccio A, Mansueto P, Iacono G, Soresi M, D'Alcamo A, Cavataio F, et al. Non‐celiac wheat sensitivity diagnosed by double‐blind placebo‐controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107(12):1898–906. quiz 1907. [DOI] [PubMed] [Google Scholar]
- 11. Ontiveros N, Lopez‐Gallardo JA, Vergara‐Jimenez MJ, Cabrera‐Chavez F. Self‐reported prevalence of symptomatic adverse reactions to gluten and adherence to gluten‐free diet in an adult Mexican population. Nutrients. 2015;7(7):6000–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cabrera‐Chávez F, Dezar G, Islas‐Zamorano A, Espinoza‐Alderete J, Vergara‐Jiménez M, Magaña‐Ordorica D, et al. Prevalence of self‐reported gluten sensitivity and adherence to a gluten‐free diet in Argentinian adult population. Nutrients. 2017;9(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molina‐Infante J, Carroccio A. Suspected nonceliac gluten sensitivity confirmed in few patients after gluten challenge in double‐blind, Placebo‐Controlled Trials. Clin Gastroenterol Hepatol. 2017;15(3):339–48. [DOI] [PubMed] [Google Scholar]
- 14. Barone M, Gemello E, Viggiani MT, Cristofori F, Renna C, Iannone A, et al. Evaluation of non‐celiac gluten sensitivity in patients with previous diagnosis of irritable bowel syndrome: a randomized double‐blind placebo‐controlled crossover trial. Nutrients. 2020;12(3):705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self‐reported non‐celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short‐chain carbohydrates. Gastroenterology. 2013;145(2):320–328 e321‐323. [DOI] [PubMed] [Google Scholar]
- 16. Di Sabatino A, Volta U, Salvatore C, Biancheri P, Caio G, De Giorgio R, et al. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: a randomized, double‐blind, placebo‐controlled, cross‐over trial. Clin Gastroenterol Hepatol. 2015;13(9):1604–1612 e1603. [DOI] [PubMed] [Google Scholar]
- 17. Elli L, Tomba C, Branchi F, Roncoroni L, Lombardo V, Bardella M, et al. Evidence for the presence of non‐celiac gluten sensitivity in patients with functional gastrointestinal symptoms: results from a multicenter randomized double‐blind placebo‐controlled gluten challenge. Nutrients. 2016;8(2):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Francavilla R, Cristofori F, Verzillo L, Gentile A, Castellaneta S, Polloni C, et al. Randomized double‐blind placebo‐controlled crossover trial for the diagnosis of non‐celiac gluten sensitivity in children. Am J Gastroenterol. 2018;113(3):421–30. [DOI] [PubMed] [Google Scholar]
- 19. Moleski SM, Shah A, Durney P, Matthews M, Kaushal G, Smith C, et al. Symptoms of gluten ingestion in patients with non‐celiac gluten sensitivity: a randomized clinical trial. Nutrition. 2021;81:110944. [DOI] [PubMed] [Google Scholar]
- 20. Potter MDE, Duncanson K, Jones MP, Walker MM, Keely S, Talley NJ. Wheat sensitivity and functional dyspepsia: a pilot, double‐blind, randomized, placebo‐controlled dietary crossover trial with novel challenge protocol. Nutrients. 2020;12(7):1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shahbazkhani B, Fanaeian MM, Farahvash MJ, Aletaha N, Alborzi F, Elli L, et al. Prevalence of non‐celiac gluten sensitivity in patients with refractory functional dyspepsia: a randomized double‐blind placebo controlled trial. Sci Rep. 2020;10(1):2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, et al. Fructan, rather than gluten, induces symptoms in patients with self‐reported non‐celiac gluten sensitivity. Gastroenterology. 2018;154(3):529–539 e522. [DOI] [PubMed] [Google Scholar]
- 23. Dale HF, Hatlebakk JG, Hovdenak N, Ystad SO, Lied GA. The effect of a controlled gluten challenge in a group of patients with suspected non‐coeliac gluten sensitivity: a randomized, double‐blind placebo‐controlled challenge. Neurogastroenterol Motil. 2018;30(8):e13332. [DOI] [PubMed] [Google Scholar]
- 24. Zanini B, Baschè R, Ferraresi A, Ricci C, Lanzarotto F, Marullo M, et al. Randomised clinical study: gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non‐coeliac gluten sensitivity. Aliment Pharmacol Ther. 2015;42(8):968–76. [DOI] [PubMed] [Google Scholar]
- 25. Olsen J, Melbye M, Olsen SF, Sørensen TIA, Aaby P, Nybo Andersen AM, et al. The Danish National Birth Cohort ‐ its background, structure and aim. Scand J Public Healt. 2001;29(4):300–7. [DOI] [PubMed] [Google Scholar]
- 26. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 27. Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, Parkinson J, Secker J, Stewart‐Brown S The Warwick‐Edinburgh mental well‐being scale (WEMWBS): development and UKvalidation. Health Qual Life Outcomes 2007;5(63). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366:l4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bjorner JB, Damsgaard MT, Watt T, Groenvold M. Tests of data quality, scaling assumptions, and reliability of the Danish SF‐36. J Clin Epidemiol. 1998;51(11):1001–11. [DOI] [PubMed] [Google Scholar]
- 32. Francavilla R, Cristofori F, Castellaneta S, Polloni C, Albano V, Dellatte S, et al. Clinical, serologic, and histologic features of gluten sensitivity in children. J Pediatr. 2014;164(3):463–467 e461. [DOI] [PubMed] [Google Scholar]
- 33. Colloca L, Barsky AJ. Placebo and nocebo effects. New Engl J Med. 2020;382(6):554–61. [DOI] [PubMed] [Google Scholar]
- 34. Carroccio A, Giambalvo O, Blasca F, Iacobucci R, D’Alcamo A, Mansueto P. Self‐reported non‐celiac wheat sensitivity in high school students: demographic and clinical characteristics. Nutrients. 2017;9(7):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoppe C, Gøbel R, Kristensen M, Lind MV, Matthiessen J, Christensen T, et al. Intake and sources of gluten in 20‐ to 75‐year‐old Danish adults: a national dietary survey. Eur J Nutr. 2017;56(1):107–17. [DOI] [PubMed] [Google Scholar]
- 36. Khan A, Suarez MG, Murray JA. Nonceliac gluten and wheat sensitivity. Clin Gastroenterol Hepatol. 2020;18(9):1913–1922 e1911. [DOI] [PubMed] [Google Scholar]
- 37. Karhus LL, Thuesen BH, Skaaby T, Rumessen JJ, Linneberg A. The distribution of HLA DQ2 and DQ8 haplotypes and their association with health indicators in a general Danish population. United European Gastroenterol J. 2018;6(6):866–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arslain K, Gustafson CR, Baishya P, Rose DJ. Determinants of gluten‐free diet adoption among individuals without celiac disease or non‐celiac gluten sensitivity. Appetite. 2021;156:104958. [DOI] [PubMed] [Google Scholar]
- 39. Hansen LBS, Roager HM, Sondertoft NB, Gobel RJ, Kristensen M, Valles‐Colomer M, et al. A low‐gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nat Commun. 2018;9(1):4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pinto‐Sanchez MI, Nardelli A, Borojevic R, De Palma G, Calo NC, McCarville J, et al. Gluten‐free diet reduces symptoms, particularly diarrhea, in patients with irritable bowel syndrome and antigliadin IgG. Clin Gastroenterol Hepatol. 2021;19(11):2343–52.e8. [DOI] [PubMed] [Google Scholar]
- 41. Aziz I, Trott N, Briggs R, North JR, Hadjivassiliou M, Sanders DS. Efficacy of a gluten‐free diet in subjects with irritable bowel syndrome‐diarrhea unaware of their HLA‐DQ2/8 genotype. Clin Gastroenterol H. 2016;14(5):696–U699. [DOI] [PubMed] [Google Scholar]
- 42. Shepherd SJ, Gibson PR. Nutritional inadequacies of the gluten‐free diet in both recently‐diagnosed and long‐term patients with coeliac disease. J Hum Nutr Diet. 2013;26(4):349–58. [DOI] [PubMed] [Google Scholar]
- 43. Jamieson JA, Neufeld A. Food sources of energy and nutrients among Canadian adults following a gluten‐free diet. PeerJ. 2020;8:e9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten‐free diet. Am J Gastroenterol. 2006;101(10):2356–9. [DOI] [PubMed] [Google Scholar]
- 45. Valvano M, Longo S, Stefanelli G, Frieri G, Viscido A, Latella G. Celiac disease, gluten‐free diet, and metabolic and liver disorders. Nutrients. 2020;12(4):940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cadenhead JW, Wolf RL, Lebwohl B, Lee AR, Zybert P, Reilly NR, et al. Diminished quality of life among adolescents with coeliac disease using maladaptive eating behaviours to manage a gluten‐free diet: a cross‐sectional, mixed‐methods study. J Hum Nutr Diet. 2019;32(3):311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caminero A, McCarville JL, Zevallos VF, Pigrau M, Yu XB, Jury J, et al. Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology. 2019;156(8):2266–80. [DOI] [PubMed] [Google Scholar]
- 48. Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol 2021;116(1572–0241 [Electronic]):17–44. [DOI] [PubMed] [Google Scholar]
- 49. Tinggaard J, Aksglaede L, Sorensen K, Mouritsen A, Wohlfahrt‐Veje C, Hagen CP, et al. The 2014 Danish references from birth to 20 years for height, weight and body mass index. Acta Paediatr. 2014;103(2):214–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


