Abstract
Beekeepers report significant honey bee deaths during and after almond bloom. These losses pose a major problem for the California almond industry because of its dependence on honey bees as pollinators. The present study aimed to determine if combinations of pesticides applied during almond bloom during daylight hours were a possible explanation for these losses. In this study we aimed to mimic the spray application route of exposure to pesticides using a Potter Spray Tower to treat adult honey bees with commonly encountered pesticides and pesticide combinations at multiples of the maximum recommended field application rates. Tested insecticides included Altacor® and Intrepid®, and tested fungicides included Tilt®, Pristine®, Luna Sensation®, and Vangard®. Synergistic toxicity was observed when the fungicide Tilt (active ingredient propiconazole) was applied with the insecticide Altacor (chlorantraniliprole), though neither caused significant mortality when applied independently. The study also looked at the effect of adding a spray adjuvant, Dyne‐Amic®, to pesticide mixtures. Dyne‐Amic was toxic to honey bees at concentrations above the maximum recommended field application rate, and toxicity was increased when combined with the fungicide Pristine (pyraclostrobin and boscalid). Addition of Dyne‐Amic also increased toxicity of the Tilt and Altacor combination. These results suggest that application of Altacor and Tilt in combination with an adjuvant at the recommended field application rates could cause mortality in adult honey bees. These findings highlight a potential explanation for honey bee losses around almond bloom, emphasize that the safety of spray adjuvants to bees should not be assumed, and provide support for recommendations to protect bees from pesticides through application at night when bees are not foraging. Environ Toxicol Chem 2022;41:1042–1053. © 2022 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Keywords: Pesticides, Mixture toxicology, Cytochrome P450, Pesticide regulation, Invertebrate toxicology, Synergistic, Potter tower, Apis mellifera
Pesticide and pesticide adjuvant treatments are applied on almonds as mixtures. Common treatments were converted from acre‐sized field application rates to Petri dish–sized rates and applied as a spray on honey bees using a Potter Spray Tower. Results from acute toxicity assays were related back to field exposure in California almonds.
INTRODUCTION
Managed honey bees (Apis mellifera) play an essential role in the agricultural industry as pollinators for many different crops (Genersch, 2010; Morse & Calderone, 2000). Honey bees are of particular importance for almond production in California (Morse & Calderone, 2000). Wild pollinator populations are essential for the biodiversity of local ecosystems and provide pollination services to crops; however, the pollination required for some crops cannot be met by native pollinator populations, and therefore managed honey bees are required for pollination of large‐scale crops such as almonds (Bushmann & Drummond, 2020; Kremen et al., 2002). Honey bees managed by commercial beekeepers are transported all over the country to provide pollination services, with >75% of the total US colonies recruited for almond pollination (Goodrich et al., 2019). Honey bee deaths have been reported by commercial beekeepers during and after almond bloom (Flottum, 2014). Because of the economic importance of honey bees as pollinators, determining the cause of these losses and providing potential solutions is one focus of honey bee research (Pettis & Delaplane, 2010; vanEngelsdorp & Meixner, 2010). Many different factors such as viruses, parasites, and agrochemicals could affect honey bee health and contribute to these deaths (Genersch, 2010). The present study aims to add to our understanding of the potential causes for honey bee deaths around almond pollination and will focus on one commonly cited factor, agrochemicals.
Agrochemicals are often cited by beekeepers as a potential cause of honey bee deaths (Flottum, 2014; Genersch, 2010). Almond growers apply a range of agrochemicals to control pests with the intention of improving crop health and yields (Durant, 2020). These chemicals are often applied as a “tank mix,” a mixture containing multiple products (Flottum, 2014). Mixtures may include pesticides such as fungicides and insecticides and often include one or more pesticide adjuvants, which are added to improve spray performance (Castro et al., 2014; Mullin et al., 2016). Honey bees can be exposed to these agrochemicals or mixtures when foraging on almonds; however, the ways in which honey bees are exposed can affect their toxicity (Chambó, 2016; Poquet et al., 2015; Ranz, 2020; Villa et al., 2000). The present study will focus on the direct contact route of exposure and assess the toxicity resulting from this exposure pathway. Direct contact exposure occurs when the exterior of a bee comes into contact with a pesticide (Chambó, 2016; Ranz, 2020). In the field, contact exposure could happen when a honey bee comes into contact with agrochemical residues on a plant surface or if the honey bee is directly sprayed during application (Ranz, 2020). Honey bees can also be exposed through other pathways such as drift into a colony, runoff into water, or oral exposure through consumption of contaminated pollen or nectar (Ranz, 2020). While it is important to consider effects of agrochemicals on both individual honey bees and whole colonies when considering different routes of exposure, the present study uses a spray tower to assess the contact route of exposure in individual bees. However, it is important to note that bees exposed directly to sprays likely take up some of the pesticide through the spiracles and may receive oral exposure through grooming (Zhu et al., 2015). It is likely that multiple exposure pathways are interacting at the field level, so using a spray tower allows for these multiple exposure routes to be included during toxicity evaluation (Chambó, 2016; Ranz, 2020; Zhu et al., 2015).
Another aspect determining the hazard that agrochemicals present to honey bees is the duration of exposure. In acute toxicity assays, organisms are exposed to a toxicant for a short period of time to determine the dose that will cause mortality or effects that will result in death (Ochoa, 2002), whereas in chronic toxicity assays, the organism is exposed to a toxicant throughout its natural lifetime (Laws, 2003). Sublethal effects are often studied in chronic toxicity assays because the stressors are applied at low levels that are not lethal to the organism for long periods of time (Beiras, 2018; Laws, 2003). To identify all hazards and complete a comprehensive assessment of the factors causing honey bee losses, research should encompass all the ways in which bees, as individuals and as a colony, can be exposed to agrochemicals. The present study focuses on identifying acute lethal effects in individual bees exposed through direct spray application. To more accurately identify potential hazards of agrochemical mixtures to honey bees, additional research on chronic sublethal effects should be performed at the individual and colony levels.
When pesticides undergo risk assessment for registration, they are only assessed as independent active ingredients (US Environmental Protection Agency [USEPA], 2015). However, previous work has demonstrated that insecticide–fungicide mixtures can be more toxic to bees than their constituents, and the effect of pesticide mixtures may be contributing to losses reported by beekeepers (Biddinger et al., 2013; Johnson et al., 2013; Pilling et al., 1995; Thompson & Wilkins, 2003; Vandame & Belzunces, 1998; Wade et al., 2019; W. Zhu et al., 2014). It is interesting to note that many of these studies suggest that fungicides with cytochrome P450 inhibition as their mode of action can cause synergistic toxicity in honey bees when combined with certain insecticides (Johnson et al., 2013; Pilling et al., 1995; Vandame & Belzunces, 1998; Wade et al., 2019; W. Zhu et al., 2014). Demethylation inhibitor (DMI) fungicides, such as Tilt®, are hypothesized to inhibit cytochrome P450 enzymes in bees (Pilling et al., 1995). Because piperonyl butoxide (PBO) is an established cytochrome P450 inhibitor in insects, it was used to test this hypothesized mechanism (Hodgson & Levi, 1999; Willoughby et al., 2007).
Another area of nontarget toxicity research that needs exploration is the effects of pesticide formulations versus active ingredients (Mullin et al., 2015). The product that honey bees are exposed to in real‐world applications is the formulated product, whereas active ingredients are typically used for regulatory testing in laboratory studies. In addition to the active ingredient, formulated products include “inert ingredients,” the identities of which are held as trade secrets. Most toxicological studies on pesticides focus on the effects of the pesticide active ingredient, but it is possible that the inert ingredients in the pesticide formulations alter the profile of bee toxicity.
The primary goal of the present study was to simulate daylight application of formulated pesticides and adjuvants that honey bee foragers may encounter during almond bloom at field‐level exposure rates. To simulate exposure in the laboratory, we first identified which pesticides are most widely used in flowering almonds. Pesticide usage data from the California Pesticide Information Portal (CalPIP; https://calpip.cdpr.ca.gov/), a database cataloging all agricultural pesticide applications occurring in California, were analyzed to determine treatments that were applied during bloom (Figure 1). The CalPIP database also revealed that pesticides were often applied to the same parcel of land on the same day, presumably as a tank mix.
Figure 1.
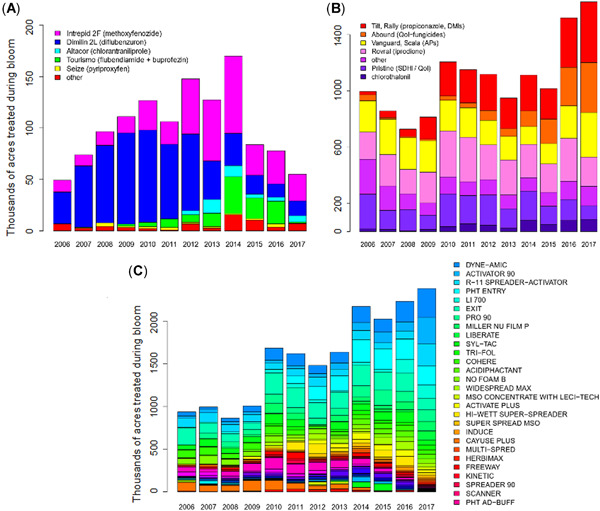
Usage data of insecticides (A), fungicides (B), and pesticide adjuvants (C) applied during almond bloom (February 15–March 15) in California. The height of each box represents acres receiving that treatment. The legends in each chart indicate the color corresponding to each treatment. Data were summarized from the California Pesticide Information Portal. AP = anilino‐pryimidines; DMI = demethylation inhibitor; MSO = methylated seed oil; SDHI = succinate dehydrogenase inhibitor; QoI = quinone outside inhibitors.
The insecticides that were applied to the largest area of almonds during bloom (February 15–March 15) in 2017 were Intrepid 2F®, Dimilin 2L®, and Altacor® (Figure 1). Methoxyfenozide (Intrepid) is a diacylhydrazine‐based ecdysone receptor agonist insecticide and causes premature molting in insects (Carlson et al., 2001; Sparks & Nauen, 2015). Previous studies showed that methoxyfenozide has low intrinsic toxicity to bees (Mommaerts et al., 2006; Y. C. Zhu et al., 2015); however, applications of methoxyfenozide at field‐relevant concentrations decreased worker survival over a 10‐day period (Fisher et al., 2018). Diflubenzuron (Dimilin 2L) has also shown low toxicity to adult bees (Barker & Taber, 1977; Emmett & Archer, 1980; Tasei, 2001), but adverse effects in larval and developing queens have been observed (Chon et al., 2017; Johnson & Percel, 2013; Thompson et al., 2005). Diflubenzuron is an insect growth regulator and affects insect molting by inhibition of chitin synthesis (Matsumura, 2010; Sparks & Nauen, 2015). Chlorantraniliprole (Altacor) affects the ryanodine receptors in insects to cause paralysis (Isaacs et al., 2012; Qi & Casida, 2013; Sparks & Nauen, 2015). Chlorantraniliprole shows low intrinsic toxicity to honey bees (Dinter et al., 2010; Y. C. Zhu et al., 2015).
The fungicides that were applied to the largest area of blooming almonds in 2017 were Tilt®, Vangard®, and Pristine® (Figure 1). Propiconazole (Tilt) is classified as a DMI fungicide which acts to inhibit synthesis of ergosterol, an important compound for fungal growth (Kwok & Loeffler, 1993). Cyprodinil (Vangard) is classified as an anilino‐pyrimidine fungicide which acts by inhibiting methionine biosynthesis (Mosbach et al., 2017). Pristine is classified as a quinone “outside” inhibitor and a succinate dehydrogenase inhibitor because it has two active ingredients with different modes of action. The two active ingredients in the fungicide Pristine, pyraclostrobin and boscalid, target cytochrome b and complex II, respectively, to inhibit mitochondrial respiration (Avenot & Michailides, 2010; Karadimos et al., 2005).
Additional components in tank mixtures are the pesticide adjuvants, which are broadly defined as any compound that can improve pesticide performance (Winand, 2021). Some adjuvants may be used to improve the mixing of pesticides in the tank while others enhance the spreading and deposition of the pesticide on the plant (Castro et al., 2014; Winand, 2021). Even though pesticide adjuvants can alter the performance of a pesticide in a number of different ways, these products are exempt from the same regulatory testing that is required for pesticide active ingredients because the principal functioning agents constituting adjuvants are classified as “inert” under the Federal Insecticide Fungicide and Rodenticide Act (USEPA, 2013a, 2013b). Likely because there is relatively little regulation and a wide variety of ways in which pesticide adjuvants can potentially improve pesticide performance, a much greater range of adjuvant products are applied than pesticides (Figure 1). Tank mixtures may even include multiple adjuvants intended for different purposes. Because pesticide adjuvants are intended to improve the performance of pesticides, it is possible that the toxicity of pesticides to bees could also be increased with their addition.
There has been relatively little research focused on the toxicity of pesticide adjuvants to bees, despite the wide use of these products. Studies on the effects of pesticide adjuvants to honey bees have focused largely on the organosilicone surfactants (OSS). These studies showed that OSS can cause learning impairment and acute toxicity in adult honey bees and chronic toxicity in honey bee larvae through ingestion (Ciarlo et al., 2012; Fine et al., 2017; Mullin et al., 2015). Another study showed that surfactants can cause acute toxicity to adult bees when applied as a spray (Goodwin & McBrydie, 2000). One limitation of studies with adjuvants is the lack of knowledge on their chemical makeup because of their classification as inerts. Chen and Mullin (2014) attempted to use liquid chromatography paired with mass spectrometry to identify the components of certain nonylphenol ethoxylate and octylphenol ethoxylate surfactants. More studies like these are needed to better understand adjuvant compositions and aid in a complete understanding of the hazards adjuvants pose to honey bees. The present study tested Dyne‐Amic®, one of the most popular pesticide adjuvants applied to California almonds. Dyne‐Amic is classified as an OSS and methylated seed oil. Dyne‐Amic was chosen because of its widespread use in almonds during bloom and because of the documented negative effects of OSS on honey bees.
The goal of the present study was to identify common almond tank mixture treatments that are potentially hazardous to honey bees when applied during daylight hours by mimicking spray application of pesticides in a laboratory setting. Formulated products were applied at multiples of the maximum recommended field application rate. All results are presented relative to the maximum concentration recommended on the product label so that findings can be directly related to managing honey bee risk in field applications. The present study reports principal findings of acute toxic effects from independent and tank mixture formulations, including a common spray adjuvant, on adult honey bees.
MATERIALS AND METHODS
Test chemicals
The following pesticide formulations were used in the present study; Tilt (41.8% propiconazole; Syngenta Crop Protection), Pristine Fungicide (12.8% pyraclostrobin and 25.2% boscalid; BASF), Luna Sensation® (21.4% fluopyram and 21.4% trifloxystrobin; Bayer Crop Science), Vangard WG (75.0% cyprodinil; Syngenta Crop Protection), Altacor Insect Control (35.0% chlorantraniliprole; FMC), Intrepid 2F Insecticide (22.6% methoxyfenozide; Dow AgroSciences), Mustang Maxx® (9.15% zeta‐cypermethrin; FMC), the pesticide adjuvant formulation Dyne‐Amic (99.0% principal functioning agents, methyl esters of C16–C18 fatty acids, polyalkyleneoxide modified polydimethylsiloxane, alkylphenol ethoxylate; Helena Chemical). Technical‐grade PBO (purity 90%) was acquired from Sigma‐Aldrich. Solutions were prepared using deionized water and stored at 4 °C.
Honey bees
Bees used in the present study were collected and handled following the guidelines of the Organisation for Economic Co‐operation and Development (1998). Late‐stage sealed worker broods were collected from May to September 2019 from eight different colonies at the Ohio State University Wooster Campus, managed according to standard beekeeping practices. No antibiotics were used to control bacterial diseases in the 3 years prior, and only formic acid and oxalic acid were used for Varroa mite control, as required. Frames were placed in a plastic nuc box (Bee Brief; Nod Apiary Products) and stored in a dark and humid (60%–80% relative humidity) incubator set at 32 °C (model HH030‐AA; Darwin Chambers). Newly emerged bees were brushed from frames into wooden bulk bee cages (11 × 14 × 22 cm) every 24 h. All bees in a bulk cage came from the same source colony and ranged in number from 200 to 1000, depending on the emergence rate of bees from the frame. The number of bees in each cage was estimated by weight (100 mg/bee). Bulk cages were stored in the incubator for 72 h, to allow the cuticle to harden, before bees were taken from the cage for acute toxicity testing in smaller groups. Bees in bulk cages were provisioned with fresh 1:1 (w/w) sugar water solution and did not have access to pollen.
Preparation for acute toxicity tests
Spray treatments were applied at multiples of the maximum label field application rate scaled to the area of the circular spray plate for the Potter Spray Tower (9‐cm diameter). The pesticides tested in the present study are referred to only by their formulated product name. Table 1 lists the active ingredients and application information that can be found on the label.
Table 1.
Label information on pesticides testeda
| Product name | a.i. | Registrant | Revision date | % a.i. | Rate on almonds (lb a.i./acre) |
|---|---|---|---|---|---|
| Altacor | Chlorantraniliprole | FMC | 7/2/18 | 35.0 | 0.099 |
| Intrepid | Methoxyfenozide | Corteva Agriscience | 7/5/17 | 22.6 | 0.380 |
| Tilt | Propiconazole | Syngenta Crop Protection | 9/6/19 | 41.8 | 0.220 |
| Pristine | Pyraclostrobin | BASF | 10/30/18 | 12.8 | 0.116 |
| Pristine | Boscalid | BASF | 10/30/18 | 25.2 | 0.228 |
| Vangard | Cyprodinil | Syngenta Crop Protection | 3/26/20 | 75.0 | 0.469 |
| Luna Sensation | Fluopyram | Bayer CropScience | 6/12/19 | 21.4 | 0.102 |
| Luna Sensation | Trifloxystrobin | Bayer CropScience | 6/12/19 | 21.4 | 0.102 |
aLabel information, including the trade name of the formulated product, active ingredient and percentage, registrant, and the revision date of the label, are listed in this table. Each pesticide label was determined to have no language in the environmental hazards statement prohibiting application around honey bees. The maximum recommended application rate on almonds is represented as pounds of active ingredient per acre.
a.i. = active ingredient.
Equations 1 and 2 display the calculations used to determine how much of each liquid or solid formulated product was added to 1 ml of deionized water used for the spray solution. The circular spray plate of the Potter Tower had a diameter of 9 cm and an area of 0.00636 m2 as represented in Equations 1 and 2. These equations were adjusted to create solutions of varying concentrations relative to the maximum recommended application rate for almonds listed on the pesticide label.
| (1) |
| (2) |
A concentrated stock solution (100× maximum field application rate) of each independent pesticide was prepared by mixing the desired amount of formulated product in deionized water in a 20‐ml glass scintillation vial. Aliquots from each concentrated stock in the combination treatment were mixed and diluted to the desired multiple of the field application rate (1×, 3×, 5×, 10×, 30×). The single or combined agrochemicals were then applied to bees in the form of a spray.
Technical‐grade PBO, a model inhibitor of cytochrome P450 monooxygenase enzymes, was applied in combination with Altacor. A concentrated stock solution was prepared by mixing PBO with water. There is no recommended field application rate for technical‐grade PBO, so the concentration recommended for Tilt was used. Mixtures with PBO were created in the same way as other pesticide mixtures.
The concentration of the adjuvant Dyne‐Amic was held constant over all treatments in which it was included and was added at the maximum recommended field application rate, which was 2% of the final solution. Each treatment was created as a 10‐ml solution, so 200 µl of Dyne‐Amic was added to each experimental solution during dilutions.
Acute toxicity tests with the Potter Spray Tower
Bulk cages of 3‐day‐old adult worker honey bees were anesthetized with 2 min of exposure to CO2. Groups of 20 anesthetized bees, all originating from the same colony, were then separated into plastic‐coated paper cups (490 ml, UNIQ 8 oz Cups; Frozen Dessert Supplies) and covered with #20 cotton cheesecloth secured with a rubber band. Each cup of 20 bees was sprayed with a single concentration for one treatment. A treatment may contain multiple agrochemicals combined. A range of concentrations for each treatment were tested, and each treatment contained at least three replicates at all concentrations. All cups of bees in a replicate contained bees from the same colony; however, different replicates contained bees from separate colonies. Bees from at least three different colonies were tested for each treatment. For spray treatment, each group of bees was again anesthetized with 15–20 s of CO2, transferred to a 9‐cm glass Petri dish with filter paper, and placed onto the spray plate of the Potter Spray Tower. The Potter Spray Tower was set to spray at 68.9 kPa (10 psi) for all treatments. Bees were then sprayed with 1 ml of the designated treatment, transferred back into the labeled cup, and fed with a double‐punctured 1.5‐ml microcentrifuge tube filled with fresh sugar syrup (1:1 w/w). Distilled water was sprayed as the negative control, and Mustang Maxx, an insecticide that is highly toxic to honey bees, was applied at the field application rate as the positive control. The Potter Tower was cleaned with water and acetone between each treatment. Bees were returned to the incubator, and the number of dead bees was recorded after 48 h.
In the present study, treatment concentrations are represented as multiples of the label rate as opposed to standard International System units to facilitate the presentation of concentrations for tank‐mix combinations and to better relate results to field application scenarios. For example, a treatment concentration of 1× represents the concentration that a honey bee forager might experience in the field during almond pollination. Therefore, 1× represents one multiple of the maximum label rate, and 10× represents 10 times the label rate.
Statistical analysis
Raw data from the toxicity test were analyzed in R Studio (Ver 1.2.5001). First, the drc package (Ritz et al., 2015) was used to create two‐parameter log‐logistic models for each treatment, and the dose–response relationship for each model was evaluated. Second, relevant statistics including the median lethal concentration (LC50) estimates and their 95% confidence intervals (CIs) were extracted from the dose–response curves. Third, the ecotox package (Hlina et al., 2021) was used to compare LC50 values for treatments with a significant dose–response relationship using the LC50 ratio test (Wheeler et al., 2006).
A nonlinear regression analysis was performed on raw data from toxicity tests using the drc package (Ritz et al., 2015). The generalized log‐logistic model used for dose–response analysis within the drc package is represented in Equation 3. In interpreting Equation 3, it is important to note that a negative slope estimate corresponds with an increasing dose–response relationship (Ritz et al., 2015). The parameters in the model are the slope parameter (b), the lower limit of response (c), the upper limit of response (d), and the LC50 estimate (e). A two‐parameter log‐logistic model, in which the c and d parameters were fixed at 0 and 1, respectively, was created using the drm function. The fit for each model was evaluated using the modelFit function, which used a goodness of fit χ 2. Models with the highest χ 2 values from the goodness of fit test were adjusted by removing the lowest dosage concentrations and then checked visually. All fits were acceptable after adjustments were made. Each model was then evaluated with the noEffect function, which used a likelihood ratio test to determine if the slope (b) for each dose–response curve was significantly different from 0. The noEffect function was used to determine which treatments showed a significant dose–response relationship (p < 0.05). The LC20 estimate, or the treatment concentration at which 20% mortality of adult honey bees was observed, was defined as the cutoff for significant mortality. The LC20 and LC50 estimates and their 95% CIs were determined for each model exhibiting a significant dose–response relationship using the ED function. Treatments with an LC20 estimate above the highest concentration tested were not considered to show a significant does–response relationship and were removed from further analysis.
| (3) |
A generalized linear model was created for each treatment that showed a significant dose–response relationship (noEffect p < 0.05) with the glm function. The generalized linear models were then analyzed using the ecotox package (Hlina et al., 2021). The ratio_test function, which uses the LC50 ratio test developed by Wheeler et al. (2006), was used to compare LC50 estimates of relevant treatments. Comparisons with the LC50 ratio test were used to compare the relative toxicity of two treatments and determine if LC50 estimates for two treatments were significantly different. In the present study, the LC50 ratio test was used to determine if less complex mixtures (Treatment 1) showed significantly different mortality than higher‐order mixtures (Treatment 2). Ratios of LC50s (LC50 Treatment 1/LC50 Treatment 2) were determined to estimate the fold change in toxicity for all comparisons. A ratio >1 represented an increase in toxicity from Treatment 1 to Treatment 2 in the comparison, and a ratio <1 represented a decrease in toxicity. Treatments were determined to have significantly different LC50 estimates if the p value for the LC50 ratio test was <0.05.
RESULTS AND DISCUSSION
Spray toxicity assays for independent and combined insecticide and fungicide treatments
In the present study, we defined any treatment that produced a significant dose–response relationship as a potentially toxic treatment to honey bees and then evaluated the hazard to honey bees from this treatment relative to the field application rate. Figure 2 shows the dose–response model for Altacor. In this model, no substantial mortality was observed at any applied concentrations, so a proper dose–response curve could not be created for the insecticide Altacor. This is supported by the noEffect test because Altacor did not show a significant dose–response relationship (p > 0.05). Of all single‐pesticide treatments, the fungicide Vangard was the only treatment that showed a significant dose–response relationship (Table 2, top bracket). However, the LC20 estimate was above the maximum concentration tested. Therefore, we can conclude that none of the independent insecticides and fungicides tested caused significant mortality in adult honey bees.
Figure 2.
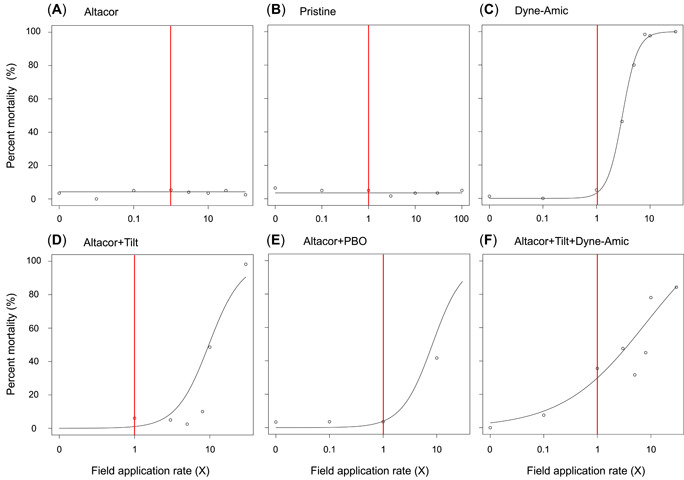
Dose–response curves for the insecticide Altacor (A), the fungicide Pristine (B), the adjuvant Dyne‐Amic (C), Altacor + Tilt (D), Altacor + piperonyl butoxide (E), and Altacor + Tilt + Dyne‐Amic (F). The treatment concentrations are reported as the relative field application rate (×), with 1× representing the maximum recommended field application rate found on the formulated product label (indicated with vertical red line). Each point represents the average response (percent mortality) at the treatment concentration. PBO = piperonyl butoxide.
Table 2.
Results from Stage 1 analysis of the dose–response relationship for all treatments applieda
| Treatment | n | Dose levels | χ 2 | p |
|---|---|---|---|---|
| Independent | ||||
| Mustang Maxx | 640 | 4 | 146 | <0.001* |
| Altacor | 1320 | 6 | −0.931 | 1.0 |
| Intrepid | 660 | 6 | −656 | 1.0 |
| Tilt | 1240 | 6 | −272 | 1.0 |
| Pristine | 580 | 6 | −476 | 1.0 |
| Luna Sensation | 300 | 5 | −190 | 1.0 |
| Vangard | 400 | 5 | 4.32 | 0.038* |
| Dyne‐Amic | 580 | 8 | 513 | <0.001* |
| Insecticide + fungicide | ||||
| Altacor + Tilt | 1580 | 8 | 354 | <0.001* |
| Altacor + Pristine | 760 | 6 | −0.0841 | 1.0 |
| Altacor + Luna Sensation | 300 | 5 | −332 | 1.0 |
| Altacor + Vangard | 300 | 5 | −342 | 1.0 |
| Intrepid + Tilt | 380 | 5 | 8.98 | 0.00272* |
| Intrepid + Pristine | 340 | 5 | −91.9 | 1.0 |
| Intrepid + Luna Sensation | 300 | 5 | −41.3 | 1.0 |
| Intrepid + Vangard | 300 | 5 | −416 | 1.0 |
| Altacor + PBO | 300 | 5 | <0.001* | |
| + Dyne‐Amic | ||||
| Altacor + Dyne‐Amic | 400 | 5 | −149 | 1.0 |
| Intrepid + Dyne‐Amic | 200 | 5 | 1.01 | 0.31 |
| Tilt + Dyne‐Amic | 440 | 5 | −529 | 1.0 |
| Pristine + Dyne‐Amic | 280 | 5 | 19.8 | <0.001* |
| Luna Sensation + Dyne‐Amic | 280 | 5 | 20.0 | 0.00880* |
| Vangard + Dyne‐Amic | 500 | 5 | 36.4 | <0.001* |
| Altacor + Tilt + Dyne‐Amic | 920 | 8 | 379 | <0.001* |
| Altacor + Pristine + Dyne‐Amic | 600 | 5 | 198 | <0.001* |
| Altacor + Luna Sensation + Dyne‐Amic | 300 | 5 | 102 | <0.001* |
| Altacor + Vangard + Dyne‐Amic | 500 | 5 | −80.9 | 1.0 |
| Intrepid + Tilt + Dyne‐Amic | 300 | 5 | −14.9 | 1.0 |
| Intrepid + Pristine + Dyne‐Amic | 280 | 5 | −105 | 1.0 |
| Intrepid + Luna Sensation + Dyne‐Amic | 300 | 5 | −5.37 | 1.0 |
| Intrepid + Vangard + Dyne‐Amic | 300 | 5 | 3.83 | 0.050 |
aTreatments are organized in brackets by level of complexity. Independent treatments are in the top bracket, insecticide + fungicide combinations are in the middle bracket, and the bottom bracket includes all pesticide treatments from the top and middle brackets with the addition of 2% Dyne‐Amic. The total number of bees tested for each treatment is indicated by n. Degrees of freedom was 1 for all tests. Dose levels in this table represent the numbers of different doses tested for each treatment. The value and p value for each likelihood ratio test are presented, and treatments with p < 0.05 (indicated with an asterisk) represent a significant dose–response relationship.
*p < 0.05.
PBO = piperonyl butoxide.
For the combination treatments with insecticides and fungicides, the combinations of Intrepid + Tilt and Altacor + Tilt showed significant dose–response relationships (Table 2, middle bracket). However, the LC20 estimate for Intrepid + Tilt was above the maximum concentration tested. None of the other insecticide and fungicide combination treatments produced a dose–response curve, which suggests that all other insecticide + fungicide treatments tested were not acutely toxic to honey bees at the applied concentrations.
Figure 2 shows the dose–response model created for Altacor + Tilt. In this model, substantial mortality was observed at the 10× and 30× concentrations, so a dose–response curve could be fit. Therefore, no substantial mortality was observed at the label rate for the combination of Altacor + Tilt. The LC50 for Altacor + Tilt was determined to be 9.70×. While the LC50 for Altacor + Tilt is higher than the recommended field application rate this is not outside the realm of possibility for mixing errors, and concentrations closer to the field application rate could cause significant mortality that is <50%.
The results from the independent and combined treatment assays suggest that Altacor + Tilt was the only insecticide–fungicide combination eliciting a significant dose–response relationship despite the observation that neither Altacor nor Tilt, when applied alone, demonstrated significant toxicity to bees at the concentrations tested.
Spray toxicity assays with the synergist PBO
We hypothesize that the toxicity observed in the Altacor + Tilt combination is the result of a synergistic interaction resulting from the pesticides interacting biochemically. The mode of action for propiconazole, the active ingredient in Tilt, is as a DMI fungicide that works as an inhibitor of cytochrome P450 monooxygenase enzymes, which are important enzymes for detoxification in many organisms (Berenbaum & Johnson, 2015; Burden et al., 1989; Johnson et al., 2013). Chlorantraniliprole, the active ingredient in Altacor, acts on ryanodine receptors to cause paralysis in insects; but this effect is not observed in honey bees, as seen in the present study and others (Qi & Casida, 2013; Sparks & Nauen, 2015). There are two hypotheses regarding potential mechanisms of tolerance to chlorantraniliprole in honey bees. First, tolerance to chlorantraniliprole may be due to low sensitivity or low binding affinity of bee ryanodine receptors to this molecule, as demonstrated in differential binding affinities in ryanodine receptor splice variants in another insect, the rice stem borer Chilo suppressalis (Peng et al., 2017). Second, chlorantraniliprole tolerance may be the result of rapid detoxification through the activity of cytochrome P450 monooxygenase enzymes (P450) or other enzyme families (Hu et al., 2014). Detoxification mediated by P450s has been proposed as a mechanism of tolerance for a range of insecticides, and it has been widely observed that tolerance is reduced in the presence of DMI fungicides (Johnson et al., 2013; Pilling et al., 1995; Vandame & Belzunces, 1998; Wade et al., 2019). Further evidence in support of the role of P450s in chlorantraniprole detoxification is increased transcription of P450 genes in honey bees following exposure to chlorantraniliprole (Christen & Fent, 2017), though these changes in gene expression may not result in elevated P450 enzyme activity (Williams, 2020).
To further test whether P450‐mediated deteoxification plays a role in chlorantraniliprole tolerance, bees were treated with PBO, an established synergist that acts as a universal inhibitor of P450 enzymes (Hodgson & Levi, 1999; Willoughby et al., 2007). Synergists such as PBO act to interfere with detoxification mechanisms in an organism to increase toxicity of pesticides (Capinera, 2008). To test whether P450‐mediated detoxification contributes to the relative safety of chlorantraniliprole in bees, we combined PBO with the insecticide Altacor.
The combination of the insecticide Altacor and the synergist PBO (Altacor + PBO) also reduced adult honey bee survival (Figure 2). The LC50 for Altacor + PBO was estimated to be 8.32×, while Altacor alone did not cause significant mortality. However, the risk to bees posed by the combination of Altacor + PBO is likely minimal because PBO is not known to be combined with this insecticide in practice.
The findings in the present study support the hypothesis that P450 enzymes in honey bees degrade chlorantraniliprole, thus contributing to its low toxicity. In the presence of a P450 inhibitor, such as PBO or propiconazole, chlorantraniliprole is no longer metabolized and can cause mortality in honey bees. Additional biochemical studies on both the metabolism of chlorantraniliprole and inhibition by DMI fungicides are needed to support this proposed mechanism of synergistic toxicity for Altacor + Tilt.
Spray toxicity assays with the pesticide adjuvant Dyne‐Amic
The adjuvant Dyne‐Amic, when applied independently, significantly increased mortality of adult honey bees (Figure 2). The 1× concentration of Dyne‐Amic did not result in significant mortality; however, nearly 50% mortality was observed at the 3× concentration of Dyne‐Amic. This suggests that changes in tank mixture concentration can significantly affect honey bee survival. The LC50 for Dyne‐Amic was estimated to be 3.05× the maximum recommended field application rate.
For the independent pesticide treatments that included 2% Dyne‐Amic, the treatments that showed significant dose–response relationships were Vangard + Dyne‐Amic, Luna Sensation + Dyne‐Amic, and Pristine + Dyne‐Amic (Table 2, bottom bracket). The LC20 estimate for Luna Sensation + Dyne‐Amic was above the maximum concentrated tested. The LC50 for Vangard + Dyne‐Amic was 9320× the maximum recommended field application rate and was determined to be not significantly toxic to honey bees. Therefore, Vangard + Dyne‐Amic was not considered significantly toxic to honey bees (Table 3, bottom bracket).
Table 3.
Median lethal concentrations and 95% confidence intervals (in parentheses) for adult honey bees exposed to common pesticide treatments applied to almonds during blooma
| LC50 | ||||
|---|---|---|---|---|
| Treatment | Slope ± SE | Field application rate (×) | Insecticide a.i. (mg/L) | Fungicide a.i. (mg/L) |
| Independent | ||||
| Mustang Maxx | −1.54 ± 0.17 | 0.51 (0.411–0.617) | 9.1 (7.3–11.0) | – |
| Dyne‐Amic | −3.06 ± 0.32 | 3.05 (2.71–3.40) | – | – |
| Insecticide + fungicide | ||||
| Altacor + Tilt | −2.01 ± 0.13 | 9.70 (8.77–10.64) | 679.0 (614.0–744.6) | 1556.7 (1407.8–1707.1) |
| Altacor + PBO | −1.52 ± 0.22 | 8.32 (6.02–10.62) | 582.4 (421.4–743.4) | 2949.9 (2134.4–3765.3) |
| Pristine + Dyne‐Amic | −1.62 ± 0.30 | 30.65 (21.30–40.01) | – | 2534.4 (1760.9–3308.5) |
| 4989.6 (3466.8–6513.6) | ||||
| + Dyne‐Amic | ||||
| Vangard + Dyne‐Amic | −0.43 ± 0.23 | 1.28E+04 (−4.26E+04 to 6.82E+04) | – | 4.29E+06 (0.00–2.28E+07) |
| Altacor + Tilt + Dyne‐Amic | −0.68 ± 0.06 | 3.41 (2.60–4.22) | 238.7 (182.0–295.3) | 547.3 (417.3–676.9) |
| Altacor + Pristine + Dyne‐Amic | −1.10 ± 0.10 | 5.81 (4.66–6.95) | 406.7 (326.4–486.6) | 480.4 (385.6–574.8) |
| 945.8 (759.1–1131.7) | ||||
| Altacor + Luna Sensation + Dyne‐Amic | −1.13 ± 0.17 | 17.78 (11.94–23.62) | 1244.6 (835.5–1653.2) | 1579.5 (1060.4–2098.1) |
| 1579.5 (1060.4–2098.1) | ||||
aTreatments are organized in brackets by level of complexity. Independent pesticide treatments are in the top bracket, insecticide + fungicide combinations are in the middle bracket, and the bottom bracket includes all previous treatments with the addition of 2% Dyne‐Amic (1×). Slope estimates are reported relative to the field application rate. Median lethal concentrations are represented as multiples of the maximum field application rate and concentration of pesticide active ingredient in milligrams per liter.
LC50 = median lethal concentration; a.i. = active ingredient; PBO = piperonyl butoxide.
The LC50 for Pristine + Dyne‐Amic was determined to be 30.65× the field recommended application rate, which is well outside the range that could be applied in the field, even accounting for mixing errors, though this combination should still be used with caution. Pristine alone did not demonstrate a significant dose–response relationship, and Dyne‐Amic, when applied at the 1× concentration, resulted in little bee mortality. Therefore, significant mortality was not expected in the combined treatment of Pristine and Dyne‐Amic. This unexpected response in the combined treatment could be the result of a synergistic interaction.
For tertiary combination treatments with Dyne‐Amic, the treatments Altacor + Luna Sensation + Dyne‐Amic, Altacor + Pristine + Dyne‐Amic, and Altacor + Tilt+ Dyne‐Amic demonstrated significant dose–response relationships (Table 2, bottom bracket). The LC50 values for Altacor + Luna Sensation + Dyne‐Amic, Altacor + Pristine + Dyne‐Amic, and Altacor + Tilt + Dyne‐Amic were 17.18×, 5.81×, and 3.41× the field application rate, respectively (Table 3, bottom bracket).
A ratio test was performed comparing the addition of Altacor to Pristine + Dyne‐Amic, and the LC50 estimates were found to be significantly different (Figure 3). The treatment Altacor + Pristine + Dyne‐Amic was 5.28 times more toxic than Pristine + Dyne‐Amic, which suggests that the addition of Altacor to Pristine + Dyne‐Amic significantly increased toxicity.
Figure 3.
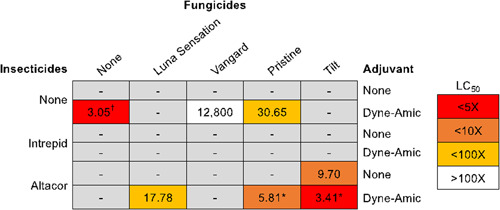
Summary for all independent and combination treatments. Absence of a significant dose–response relationship is represented by a “–” and median lethal concentration (LC50) estimates are presented for treatments with significant dose–response relationships. †Treatments where Dyne‐Amic was not fixed at 1×. The toxicities of the LC50 estimates relative to the recommended field application rate are indicated in color code. Comparisons of LC50 ratios were performed between treatments within the same column. *Significant difference in LC50 estimates (p < 0.05).
A ratio test comparing the addition of Dyne‐Amic to Altacor + Tilt showed a significant difference between treatments (Figure 3). The combination Altacor + Tilt + Dyne‐Amic was determined to be 2.91 times more toxic than Altacor + Tilt. These results suggest that the addition of Dyne‐Amic significantly increased the toxicity of the Altacor + Tilt combination.
It is also notable that for the combinations Altacor + Pristine + Dyne‐Amic and Altacor + Tilt + Dyne‐Amic, some bee mortality was observed at 1× the actual field application rate (Figure 3). Altacor + Pristine + Dyne‐Amic showed >10% mortality at the 1× concentration, while Altacor + Tilt + Dyne‐Amic showed 30% mortality. These findings suggest that if adult honey bee foragers encounter the combination Altacor + Pristine + Dyne‐Amic or Altacor + Tilt + Dyne‐Amic at the maximum recommended field application rate, substantial mortality may occur.
In the present study, we focused on the most popular adjuvant used in California almonds during bloom, Dyne‐Amic. The principal functioning agents listed on the Dyne‐Amic include modified vegetable oil and a surfactant blend. Therefore, Dyne‐Amic could be classified as both an oil and a surfactant. Both of these categories generally improve pesticide performance by decreasing surface tension and increasing deposition and penetration of the pesticide (Winand, 2021). We hypothesize that Dyne‐Amic increases the uptake of pesticide active ingredients across the honey bee cuticle, resulting in increased toxicity. During toxicity assays bees that were treated with higher concentrations of Dyne‐Amic appeared visibly “greasy,” possibly as a result of the spreading and penetrating properties of this adjuvant. Additional assays focused on the biochemical and physical interactions between Dyne‐Amic and honey bees would elucidate the mechanism by which Dyne‐Amic can cause toxicity.
It is important to note that young adult bees were used for bioassays in the present study because older forager bees, the cohort most likely to experience direct pesticide exposure from field applications, experience unacceptably high control mortality when maintained under bioassay conditions. Age can be important because a bee's age has been shown to affect its pesticide sensitivity, but whether younger bees are more sensitive than older bees is pesticide‐dependent (Poquet et al., 2016). Young bees fed pollen demonstrate reduced sensitivity to many pesticides (Wahl & Ulm, 1983). To increase the sensitivity of young bees to pesticides and adjuvants and to maximize the conservativeness of the present study, bees were not provided access to pollen. Additional studies are needed to evaluate the sensitivity of bees of different ages, castes, and life stages to field‐relevant combinations of adjuvants and pesticides.
CONCLUSION
These findings suggest that some tank mixtures of insecticides, fungicides, and adjuvants commonly applied to almonds in California during bloom can cause mortality in adult honey bees. These results support the hypothesis that tank mixtures can be more toxic than their constituents and suggest that increasing the complexity of tank mixtures may also increase toxicity to bees. Significant mortality was observed from tank mixture treatments within the range of concentrations that could plausibly be applied to almonds, which suggests that tank mixtures may be a possible explanation for the observed honey bee losses around almond bloom. Additional research is needed to determine if other adjuvant products also increase mixture toxicity or if the effects with Dyne‐Amic are unique. It is also important to note that these tests only studied acute lethal effects from spray application; additional research is needed to determine the chronic and sublethal effects of the treatments applied in the present study as well as effects from other exposure pathways. Finally, the present study highlights treatments that are potentially hazardous to adult honey bees but does not provide a complete risk assessment. The treatments used in the present study are applied at field‐relevant concentrations, which are a simulation of spray exposure; but it is likely that honey bees are exposed to these treatments via multiple exposure pathways in field applications. Therefore, for a more complete understanding of the risks from tank mixtures to honey bees, a formal risk assessment should be completed. The present study provides support for best management practices for almonds advising pesticide applicators to avoid including insecticides or adjuvants in bloom‐time sprays and that applications be made during the night or when bees are not foraging.
Author Contributions Statement
Emily K. Walker: conceptualization, investigation, data curation, formal analysis, methodology, visualization, writing – original draft, and writing – review & editing. Guy N. Brock: formal analysis, methodology, and writing – review & editing. Ryan S. Arvidson: conceptualization, methodology, and writing – review & editing. Reed M. Johnson: conceptualization, formal analysis, visualization, funding acquisition, resources, methodology, project administration, and writing – review & editing.
This article has earned an Open Data and an Open Materials badge for making publicly available the digitally shareable data necessary to reproduce the reported results. The data are available at https://doi.org/10.6084/m9.figshare.14511999.v1. The materials (R Code for statistical analyses) are available at https://doi.org/10.6084/m9.figshare.14512107.v1. Learn more about the Open Practices badges from the Center for Open Science: https://osf.io/tvyxz/wiki.
Acknowledgment
We gratefully acknowledge the efforts and support from J. Lyons, B. Leyda, M. Chapman, S. Suresh, S. Murray, and B. Gross in collecting and maintaining honey bees and in conducting trials. We also acknowledge C. Welty and J. Reed for their help in acquiring formulated pesticides. We thank B. Leslie, R. Lanno, and L. Phelan for their helpful comments and contributions to writing and experimental design. Funding was provided by the Almond Board of California (POLL17) and the State and Federal Appropriations to the Ohio Agricultural Research and Development Center (OHO01277; OHO01355‐MRF).
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (johnson.5005@osu.edu).
REFERENCES
- Avenot, H. F. , & Michailides, T. J. (2010). Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Protection, 29(7), 643–651. 10.1016/j.cropro.2010.02.019 [DOI] [Google Scholar]
- Barker, R. J. , & Taber, S., III. (1977). Effects of diflubenzuron fed to caged honey bees 12. Environmental Entomology, 6(1), 167–168. 10.1093/ee/6.1.167 [DOI] [Google Scholar]
- Beiras, R. (2018). Chapter 14—Sublethal toxicity at the level of organism. In Beiras R. (Ed.), Marine pollution (pp. 233–245) Elsevier. Retrieved August 30, 2021, from: https://www.sciencedirect.com/science/article/pii/B9780128137369000143 [Google Scholar]
- Berenbaum, M. R. , & Johnson, R. M. (2015). Xenobiotic detoxification pathways in honey bees. Current Opinion in Insect Science, 10, 51–58. 10.1016/j.cois.2015.03.005 [DOI] [PubMed] [Google Scholar]
- Biddinger, D. J. , Robertson, J. L. , Mullin, C. , Frazier, J. , Ashcraft, S. A. , Rajotte, E. G. , Joshi, N. K. , & Vaughn, M. (2013). Comparative toxicities and synergism of apple orchard pesticides to Apis mellifera (L.) and Osmia cornifrons (Radoszkowski). PLoS One, 8(9), Article e72587. 10.1371/journal.pone.0072587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden, R. S. , Cooke, D. T. , & Carter, G. A. (1989). Inhibitors of sterol biosynthesis and growth in plants and fungi. Phytochemistry, 28(7), 1791–1804. 10.1016/S0031-9422(00)97862-2 [DOI] [Google Scholar]
- Bushmann, S. L. , & Drummond, F. A. (2020). Analysis of pollination services provided by wild and managed bees (Apoidea) in wild blueberry (Vaccinium angustifolium Aiton) production in Maine, USA, with a literature review. Agronomy, 10(9), Article 1413. 10.3390/agronomy10091413 [DOI] [Google Scholar]
- Capinera, J. L. (Ed.). (2008). Synergist. In Encyclopedia of entomology (p. 3671). Springer Netherlands. 10.1007/978-1-4020-6359-6_4517 [DOI] [Google Scholar]
- Carlson, G. R. , Dhadialla, T. S. , Hunter, R. , Jansson, R. K. , Jany, C. S. , Lidert, Z. , & Slawecki, R. A. (2001). The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist. Pest Management Science, 57(2), 115–119. [DOI] [PubMed] [Google Scholar]
- Castro, M. J. L. , Ojeda, C. , & Cirelli, A. F. (2014). Advances in surfactants for agrochemicals. Environmental Chemistry Letters, 12(1), 85–95. 10.1007/s10311-013-0432-4 [DOI] [Google Scholar]
- Chambó, E. (2016). Beekeeping and bee conservation: Advances in research. InTech. 10.5772/61424 [DOI] [Google Scholar]
- Chen, J. , & Mullin, C. A. (2014). Determination of nonylphenol ethoxylate and octylphenol ethoxylate surfactants in beehive samples by high performance liquid chromatography coupled to mass spectrometry. Food Chemistry, 158, 473–479. 10.1016/j.foodchem.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Chon, K. , Lee, H. , Hwang, H. C. , Im, J. , Park, K.‐H. , Paik, M. K. , & Choi, Y.‐S. (2017). The honey bee brood test under semi‐field conditions for the assessment of positive reference chemicals in Korea. Applied Biological Chemistry, 60(5), 569–582. 10.1007/s13765-017-0312-x [DOI] [Google Scholar]
- Christen, V. , & Fent, K. (2017). Exposure of honey bees (Apis mellifera) to different classes of insecticides exhibit distinct molecular effect patterns at concentrations that mimic environmental contamination. Environmental Pollution, 226, 48–59. 10.1016/j.envpol.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Ciarlo, T. J. , Mullin, C. A. , Frazier, J. L. , & Schmehl, D. R. (2012). Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS One, 7(7), Article e40848. 10.1371/journal.pone.0040848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter, A. , Brugger, K. , Frost, N.‐M. & Woodward, M. (2010, October 8–10). Chlorantraniliprole (Rynaxypyr): A novel DuPontTM insecticide with low toxicity and low risk for honey bees (Apis mellifera) and bumble bees (Bombus terrestris) providing excellent tools for uses in integrated pest management [Conference presentation]. Hazards of pesticides to bees. 10th International Symposium of the ICP‐Bee Protection Group, Bucharest, Romania
- Durant, J. L. (2020). Ignorance loops: How non‐knowledge about bee‐toxic agrochemicals is iteratively produced. Social Studies of Science, 50(5), 751–777. 10.1177/0306312720923390 [DOI] [PubMed] [Google Scholar]
- Emmett, B. J. , & Archer, B. M. (1980). The toxicity of diflubenzuron to honey bee (Apis mellifera L.) colonies in apple orchards. Plant Pathology, 29(4), 177–183. 10.1111/j.1365-3059.1980.tb01209.x [DOI] [Google Scholar]
- Fine, J. D. , Cox‐Foster, D. L. , & Mullin, C. A. (2017). An inert pesticide adjuvant synergizes viral pathogenicity and mortality in honey bee larvae. Scientific Reports, 7(1), Article 40499. 10.1038/srep40499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, A, II , Colman, C. , Hoffmann, C. , Fritz, B. , & Rangel, J. (2018). The effects of the insect growth regulators methoxyfenozide and pyriproxyfen and the acaricide bifenazate on honey bee (Hymenoptera: Apidae) forager survival. Journal of Economic Entomology, 111(2), 510–516. 10.1093/jee/tox347 [DOI] [PubMed] [Google Scholar]
- Flottum, K. (2014, April 3). Catch the buzz: Huge bee kill in almonds [News release]. Bee Culture. Retrieved March 21, 2021, from: https://www.beeculture.com/catch-the-buzz-huge-bee-kill-in-almonds/
- Genersch, E. (2010). Honey bee pathology: Current threats to honey bees and beekeeping. Applied Microbiology and Biotechnology, 87(1), 87–97. 10.1007/s00253-010-2573-8 [DOI] [PubMed] [Google Scholar]
- Goodrich, B. K. , Williams, J. C. , & Goodhue, R. E. (2019). The great bee migration: Supply analysis of honey bee colony shipments into California for almond pollination services. American Journal of Agricultural Economics, 101(5), 1353–1372. 10.1093/ajae/aaz046 [DOI] [Google Scholar]
- Goodwin, R. M. , & McBrydie, H. M. (2000). Effect of surfactants on honey bees. New Zealand Plant Protection, 53, 230–234. 10.30843/nzpp.2000.53.3694 [DOI] [Google Scholar]
- Hlina, B. L. , Birceanu, O. , Robinson, C. S. , Dhiyebi, H. , & Wilkie, M. P. (2021). The relationship between thermal physiology and lampricide sensitivity in larval sea lamprey (Petromyzon marinus). Journal of Great Lakes Research, 47, S272–S284. 10.1016/j.jglr.2021.10.002 [DOI] [Google Scholar]
- Hodgson, E. , & Levi, P. E. (1999). 3—Interactions of piperonyl butoxide with cytochrome P450. In D. G. Jones (Ed.), Piperonyl butoxide: The insecticide synergist (pp. 41–53). Academic. Retrieved February 24, 2019, from: http://www.sciencedirect.com/science/article/pii/B978012286975450005X
- Hu, Z. , Lin, Q. , Chen, H. , Li, Z. , Yin, F. , & Feng, X. (2014). Identification of a novel cytochrome P450 gene, CYP321E1 from the diamondback moth, Plutella xylostella (L.) and RNA interference to evaluate its role in chlorantraniliprole resistance. Bulletin of Entomological Research, 104(6), 716–723. 10.1017/S0007485314000510 [DOI] [PubMed] [Google Scholar]
- Isaacs, A. K. , Qi, S. , Sarpong, R. , & Casida, J. E. (2012). Insect ryanodine receptor: Distinct but coupled insecticide binding sites for [N‐C3H3]chlorantraniliprole, flubendiamide, and [3H]ryanodine. Chemical Research in Toxicology, 25(8), 1571–1573. 10.1021/tx300326m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. M. , Dahlgren, L. , Siegfried, B. D. , & Ellis, M. D. (2013). Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS One, 8(1), Article e54092. 10.1371/journal.pone.0054092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. M. , & Percel, E. G. (2013). Effect of a fungicide and spray adjuvant on queen‐rearing success in honey bees (Hymenoptera: Apidae). Journal of Economic Entomology, 106(5), 1952–1957. 10.1603/EC13199 [DOI] [PubMed] [Google Scholar]
- Karadimos, D. A. , Karaoglanidis, G. S. , & Tzavella‐Klonari, K. (2005). Biological activity and physical modes of action of the Qo inhibitor fungicides trifloxystrobin and pyraclostrobin against Cercospora beticola . Crop Protection, 24(1), 23–29. 10.1016/j.cropro.2004.06.004 [DOI] [Google Scholar]
- Kremen, C. , Williams, N. M. , & Thorp, R. W. (2002). Crop pollination from native bees at risk from agricultural intensification. Proceedings of the National Academy of Sciences of the United States of America, 99(26), 16812–16816. 10.1073/pnas.262413599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok, I. M.‐Y. , & Loeffler, R. T. (1993). The biochemical mode of action of some newer azole fungicides. Pesticide Science, 39(1), 1–11. 10.1002/ps.2780390102 [DOI] [Google Scholar]
- Laws, E. A. (2003). Environmental toxicology. In Meyers R. A. (Ed.), Encyclopedia of physical science and technology (3rd ed., pp. 601–625). Academic. Retrieved August 31, 2021, from: https://www.sciencedirect.com/science/article/pii/B0122274105002301 [Google Scholar]
- Matsumura, F. (2010). Studies on the action mechanism of benzoylurea insecticides to inhibit the process of chitin synthesis in insects: A review on the status of research activities in the past, the present and the future prospects. Pesticide Biochemistry and Physiology, 97(2), 133–139. 10.1016/j.pestbp.2009.10.001 [DOI] [Google Scholar]
- Mommaerts, V. , Sterk, G. , & Smagghe, G. (2006). Bumblebees can be used in combination with juvenile hormone analogues and ecdysone agonists. Ecotoxicology, 15(6), 513–521. 10.1007/s10646-006-0087-z [DOI] [PubMed] [Google Scholar]
- Morse, R. A. , & Calderone, N. W. (2000, March). The value of honey bees as pollinators of U.S. crops in 2000. Bee Culture Magazine, 1–15. [Google Scholar]
- Mosbach, A. , Edel, D. , Farmer, A. D. , Widdison, S. , Barchietto, T. , Dietrich, R. A. , Corran, A. , & Scalliet, G. (2017). Anilinopyrimidine resistance in botrytis cinerea is linked to mitochondrial function. Frontiers in Microbiology, 8. 10.3389/fmicb.2017.02361 [DOI] [PMC free article] [PubMed]
- Mullin, C. A. , Chen, J. , Fine, J. D. , Frazier, M. T. , & Frazier, J. L. (2015). The formulation makes the honey bee poison. Pesticide Biochemistry and Physiology, 120, 27–35. 10.1016/j.pestbp.2014.12.026 [DOI] [PubMed] [Google Scholar]
- Mullin, C. A. , Fine, J. D. , Reynolds, R. D. , & Frazier, M. T. (2016). Toxicological risks of agrochemical spray adjuvants: Organosilicone surfactants may not be safe. Frontiers in Public Health 4, 10.3389/fpubh.2016.00092 [DOI] [PMC free article] [PubMed]
- Ochoa, R. (2002). 14—Pathology issues in the design of toxicology studies. In Haschek W. M., Rousseaux C. G., & Wallig M. A. (Eds.), Handbook of toxicologic pathology (2nd ed., pp. 307–326). Academic. Retrieved August 31, 2021, from: https://doi.org/10.1016/B978-012330215-1/50015-6 [Google Scholar]
- Organisation for Economic Co‐operation and Development . (1998). Test No. 214: Honeybees, acute contact toxicity test. OECD guidelines for the testing of chemicals. 10.1787/9789264070189-en [DOI]
- Peng, Y. C. , Sheng, C. W. , Casida, J. E. , Zhao, C. Q. , & Han, Z. J. (2017). Ryanodine receptor genes of the rice stem borer, Chilo suppressalis: Molecular cloning, alternative splicing and expression profiling. Pesticide Biochemistry and Physiology, 135, 69–77. 10.1016/j.pestbp.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Pettis, J. S. , & Delaplane, K. S. (2010). Coordinated responses to honey bee decline in the USA. Apidologie, 41(3), 256–263. 10.1051/apido/2010013 [DOI]
- Pilling, E. D. , Bromley‐Challenor, K. A. C. , Walker, C. H. , & Jepson, P. C. (1995). Mechanism of synergism between the pyrethroid insecticide λ‐cyhalothrin and the imidazole fungicide prochloraz, in the honeybee (Apis mellifera L.). Pesticide Biochemistry and Physiology, 51(1), 1–11. 10.1006/pest.1995.1001 [DOI] [Google Scholar]
- Poquet, Y. , Kairo, G. , Tchamitchian, S. , Brunet, J.‐L. , & Belzunces, L. P. (2015). Wings as a new route of exposure to pesticides in the honey bee. Environmental Toxicology and Chemistry, 34(9), 1983–1988. 10.1002/etc.3014 [DOI] [PubMed] [Google Scholar]
- Poquet, Y. , Vidau, C. , & Alaux, C. (2016). Modulation of pesticide response in honeybees. Apidologie, 47(3), 412–426. 10.1007/s13592-016-0429-7 [DOI] [Google Scholar]
- Qi, S. , & Casida, J. E. (2013). Species differences in chlorantraniliprole and flubendiamide insecticide binding sites in the ryanodine receptor. Pesticide Biochemistry and Physiology, 107(3), 321–326. 10.1016/j.pestbp.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Ranz, R. E. R. (2020). Modern beekeeping: Bases for sustainable production. InTech. [Google Scholar]
- Ritz, C. , Baty, F. , Streibig, J. C. , & Gerhard, D. (2015). Dose–response analysis using R. PloS One, 10(12). http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0146021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks, T. C. , & Nauen, R. (2015). IRAC: Mode of action classification and insecticide resistance management. Pesticide Biochemistry and Physiology, 121, 122–128. 10.1016/j.pestbp.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Tasei, J.‐N. (2001). Effects of insect growth regulators on honey bees and non‐Apis bees. A review. Apidologie, 32(6), 527–545. 10.1051/apido:2001102 [DOI] [Google Scholar]
- Thompson, H. , & Wilkins, S. (2003). Assessment of the synergy and repellency of pyrethroid/fungicide mixtures. Bull Insectology, 56(1), 131–134. [Google Scholar]
- Thompson, H. M. , Wilkins, S. , Battersby, A. H. , Waite, R. J. , & Wilkinson, D. (2005). The effects of four insect growth‐regulating (IGR) insecticides on honeybee (Apis mellifera L.) colony development, queen rearing and drone sperm production. Ecotoxicology, 14(7), 757–769. 10.1007/s10646-005-0024-6 [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency . (2013a, March 5). Inert ingredients overview and guidance. Retrieved February 22, 2021, from: https://www.epa.gov/pesticide-registration/inert-ingredients-overview-and-guidance
- US Environmental Protection Agency . (2013b, May 13). Pesticide registration manual: Chapter 8—Inert ingredients. Retrieved March 30, 2021, from: https://www.epa.gov/pesticide-registration/pesticide-registration-manual-chapter-8-inert-ingredients
- US Environmental Protection Agency . (2015, August 27). Factsheet on ecological risk assessment for pesticides. Retrieved February 25, 2021, from: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/factsheet-ecological-risk-assessment-pesticides
- Vandame, R. , & Belzunces, L. P. (1998). Joint actions of deltamethrin and azole fungicides on honey bee thermoregulation. Neuroscience Letters, 251(1), 57–60. 10.1016/s0304-3940(98)00494-7 [DOI] [PubMed] [Google Scholar]
- van Engelsdorp, D. , & Meixner, M. D. (2010). A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. Journal of Invertebrate Pathology, 103, S80–S95. 10.1016/j.jip.2009.06.011 [DOI] [PubMed] [Google Scholar]
- Villa, S. , Vighi, M. , Finizio, A. , & Bolchi Serini, G. (2000). Risk assessment for honeybees from pesticide‐exposed pollen. Ecotoxicology, 9(4), 287–297. 10.1023/A:1026522112328 [DOI] [Google Scholar]
- Wade, A. , Lin, C.‐H. , Kurkul, C. , Regan, E. R. , & Johnson, R. M. (2019). Combined toxicity of insecticides and fungicides applied to california almond orchards to honey bee larvae and adults. Insects, 10(1), 10.3390/insects10010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl, O. , & Ulm, K. (1983). Influence of pollen feeding and physiological condition on pesticide sensitivity of the honey bee Apis mellifera carnica . Oecologia, 59(1), 106–128. 10.1007/BF00388082 [DOI] [PubMed] [Google Scholar]
- Wheeler, M. W. , Park, R. M. , & Bailer, A. J. (2006). Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environmental Toxicology and Chemistry, 25(5), 1441–1444. 10.1897/05-320R.1 [DOI] [PubMed] [Google Scholar]
- Williams, J. (2020). Comparative physiology of honey bees (Apis mellifera L.) exposed to chlorantraniliprole [Unpublished doctoral dissertation]. University of Nebraska. https://digitalcommons.unl.edu/dissertations/AAI27838398/
- Willoughby, L. , Batterham, P. , & Daborn, P. J. (2007). Piperonyl butoxide induces the expression of cytochrome P450 and glutathione S‐transferase genes in Drosophila melanogaster . Pest Management Science, 63(8), 803–808. 10.1002/ps.1391 [DOI] [PubMed] [Google Scholar]
- Winand, H. (2021). Spray adjuvants. Penn State Extension. Retrieved February 22, 2021, from: https://extension.psu.edu/spray-adjuvants
- Zhu, W. , Schmehl, D. R. , Mullin, C. A. , & Frazier, J. L. (2014). Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS One, 9(1), Article e77547. 10.1371/journal.pone.0077547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. C. , Adamczyk, J. , Rinderer, T. , Yao, J. , Danka, R. , Luttrell, R. , & Gore, J. (2015). Spray toxicity and risk potential of 42 commonly used formulations of row crop pesticides to adult honey bees (Hymenoptera: Apidae). Journal of Economic Entomology, 108(6), 2640–2647. 10.1093/jee/tov269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (johnson.5005@osu.edu).


