Abstract
Objective
Axonal excitability reflects ion channel function, and it is proposed that this may be a biomarker in painful (vs painless) polyneuropathy. Our objective was to investigate the relationship between axonal excitability parameters and chronic neuropathic pain in deeply phenotyped cohorts with diabetic or chemotherapy‐induced distal symmetrical polyneuropathy.
Methods
Two hundred thirty‐nine participants with diabetic polyneuropathy were recruited from sites in the UK and Denmark, and 39 participants who developed chemotherapy‐induced polyneuropathy were recruited from Denmark. Participants were separated into those with probable or definite neuropathic pain and those without neuropathic pain. Axonal excitability of large myelinated fibers was measured with the threshold tracking technique. The stimulus site was the median nerve, and the recording sites were the index finger (sensory studies) and abductor pollicis brevis muscle (motor studies).
Results
Participants with painless and painful polyneuropathy were well matched across clinical variables. Sensory and motor axonal excitability measures, including recovery cycle, threshold electrotonus, strength–duration time constant, and current–threshold relationship, did not show differences between participants with painful and painless diabetic polyneuropathy, and there were only minor changes for chemotherapy‐induced polyneuropathy.
Interpretation
Axonal excitability did not significantly differ between painful and painless diabetic or chemotherapy‐induced polyneuropathy in a multicenter observational study. Threshold tracking assesses the excitability of myelinated axons; the majority of nociceptors are unmyelinated, and although there is some overlap of the "channelome" between these axonal populations, our results suggest that alternative measures such as microneurography are required to understand the relationship between sensory neuron excitability and neuropathic pain. ANN NEUROL 2022;91:506–520
Neuropathic pain is defined as “pain caused by a lesion or disease affecting the somatosensory system.” 1 The best estimate, based on population based studies, for neuropathic pain prevalence lies between 6.9 and 10% of the general population 2 , 3 ; diabetes and neurotoxic chemotherapy 4 are important causes of neuropathic pain and will become more common as the global population ages, 5 diabetes mellitus incidence increases, 6 and cancer survival after oncologic treatments continues to improve. 7 Current challenges include translating knowledge from preclinical observations in animal models into meaningful clinical outcomes, such as targeted drug therapies, a process that would be facilitated by appropriate pain biomarkers. 8 An important driver for neuropathic pain in peripheral neuropathy is aberrant excitability of sensory neurons, as demonstrated by the efficacy of peripheral lidocaine blocks in reducing established neuropathic pain. 9 Neurophysiological studies of peripheral nerves are therefore attractive as translatable tools that can study the pathophysiology of neuropathic pain in preclinical animal models and patients. 10
Measurement of axonal excitability, using threshold tracking technique, provides in vivo information about ion channel function and axonal resting membrane potential of large sensory and motor fibers. 10 This allows the study of the biophysical properties of human axons and pathophysiological mechanisms of disease. 11 Studies have shown changes in the biophysical properties of nerves in patients with type 1 and 2 diabetes mellitus, and chemotherapy‐induced distal symmetrical polyneuropathy. Multiple axonal excitability measures differ between diabetic patients and healthy nondiabetic study participants, and those with and without diabetic polyneuropathy. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Taken together, these studies provide evidence of altered sodium conductance and channel function, alteration in Na+/K+ pump function, and membrane depolarization.
An increase in axonal excitability, characterized by increased nodal sodium currents, associates with diabetic polyneuropathy‐related neuropathic pain, 21 poorer quality of life, and a more severe diabetic polyneuropathy. 19 Mexiletine suppression of nodal Na+ currents associates with analgesia in patients with diabetic neuropathic pain. 22 Similar to diabetes, chemotherapy‐induced changes in axonal excitability are associated with acute polyneuropathy symptoms. 23 , 24 , 25 , 26 , 27 , 28 Chemotherapy neurotoxicity may mediate its effect through changes of sodium channels. Oxaliplatin causes slowing of sodium channel inactivation that correlates with the intensity of unpleasant or painful sensations. 27 , 29 Despite not measuring the properties of Aδ and C fibers, in vivo human axonal excitability studies suggest an association between an increase in axonal excitability and neuropathic pain or dysesthesias. 21 , 22 , 27 In vivo studies of axonal excitability may therefore provide insights into pathophysiology of neuropathic pain and offer potential as a biomarker.
The aim of this study was to determine whether the axonal excitability profiles of patients can act as a biomarker of chronic neuropathic pain and to determine links between ion channels, resting membrane potential, and neuropathic pain. We set out to investigate whether axonal excitability, assessed using threshold tracking technique, is related to chronic neuropathic pain in deeply phenotyped cohorts with probable or definite diabetic or chemotherapy‐induced polyneuropathy, comparing those with and without neuropathic pain.
Patients and Methods
Study Design and Participants
Three Groups of Study Participants Were Recruited
Study participants with diabetes mellitus were recruited in Oxford, UK, as part of the Pain in Neuropathy Study (PiNS). 30 PiNS is an observational cross‐sectional multicenter study approved by the National Research Ethics Service of the UK (10/H07056/35). The ClinicalTrials.gov Identifier is NCT02672059. Study participants were recruited from diabetes clinics at Oxford University teaching hospitals, Thames Valley primary health practices, and through advertisements in the Thames Valley area.
Study participants with diabetes mellitus were recruited in Aarhus and Odense, Denmark, as part of the International Diabetic Neuropathy Consortium (IDNC). IDNC is a multicenter cross‐sectional study designed to understand polyneuropathy in type 2 diabetes mellitus. Participants were recruited from the Danish Center for Strategic Research in type 2 diabetes cohort (DD2). 31 , 32 Included in the DD2 cohort are more than 9,000 patients with type 2 diabetes. A detailed description of the cohort and the primary clinical results are given elsewhere. A subgroup of the cohort was sent a questionnaire, and invitations were sent to the responders of this questionnaire. Central Denmark Region Committees on Health Research Ethics (1‐10‐72‐130‐16) approved the study. The ClinicalTrials.gov identifier is NCT02947828.
Patients scheduled to receive chemotherapy were recruited from the Department of Oncology, Aarhus University Hospital, Denmark. 7 , 28 This study was approved by the Central Denmark Region Committees on Health Research Ethics (1‐10‐72‐359‐15) and registered in the Danish Data Protection Agency (1‐16‐02‐89‐16). The ClinicalTrials.gov identifier is NCT02654691.
The studies were completed in line with the Declaration of Helsinki. All study participants signed written informed consent. In all the cohorts, data collection for clinical phenotyping, neuropathy grading, and neuropathic pain grading were aligned as part of the DOLORisk consortium. A detailed description of the DOLORisk study protocol is available. 33 The study design is summarized below.
Diabetic Cohort Inclusion/Exclusion Criteria
The inclusion and exclusion criteria were matched between the UK and Danish sites. Patients with type 1 or 2 diabetes mellitus were included in the axonal excitability analysis if they were >18 years of age with a diagnosis of distal symmetrical polyneuropathy (based on a clinical assessment combined with supportive clinical investigations such as abnormal nerve conduction studies, reduced intraepidermal nerve fiber density, or abnormal findings on quantitative sensory testing) or symptoms suggestive of a polyneuropathy. The exclusion criteria were pregnancy, incapacity to give consent or to complete the study questionnaires due to insufficient language command or mental deficiencies, concurrent severe psychological or psychiatric disorders, moderate to severe pain from other causes that may confound assessment or reporting of pain (eg, spinal canal stenosis), central nervous lesions that may complicate somatosensory testing, and patients who are in the opinion of the investigator unsuitable for participation in the study.
Chemotherapy Cohort Inclusion/Exclusion Criteria
Patients operated for high‐risk colon or breast cancer were included from a prospective questionnaire study that included patients scheduled for adjuvant docetaxel for high‐risk breast cancer or adjuvant oxaliplatin for high‐risk colorectal cancer at Aarhus University Hospital, Denmark from 2011 to 2012. 34 The results of the 5‐year follow‐up data have been published. 7 , 28 This the first study to assess the axonal excitability effects of docetaxel.
Study participants from both groups attended an appointment during which deep phenotyping was completed. This included questionnaires about qualitative aspects and intensity of neuropathic pain, pain distribution, psychological well‐being, and quality of life. Participants underwent a structured neurological examination, quantitative sensory testing, and nerve conduction studies. 30 , 32 , 33 Thereafter, the presence of distal symmetrical polyneuropathy and neuropathic pain was determined.
Definition of Neuropathy and Neuropathic Pain
Published criteria to diagnose distal symmetrical polyneuropathy 35 and chronic neuropathic pain 1 were used and were consistent across all study sites.
Distal Symmetrical Polyneuropathy
Possible distal symmetrical polyneuropathy is defined as the presence of either sensory symptoms (ie, numbness, paresthesias, burning in the toes, feet, or legs) or sensory signs (ie, symmetric decrease of distal sensation or unequivocally decreased or absent ankle reflexes).
Probable distal symmetrical polyneuropathy includes any two or more of the following: sensory symptoms, decreased distal sensation, or unequivocally decreased or absent ankle reflexes.
Definite distal symmetrical polyneuropathy is defined as the presence of sensory symptoms or signs of neuropathy with an abnormality on a confirmatory test, which includes either nerve conduction studies or a validated measure of small fiber neuropathy (ie, abnormal thermal thresholds on quantitative sensory testing or reduced intraepidermal nerve fiber density).
Only participants who satisfied criteria for probable or definite distal symmetrical polyneuropathy underwent neuropathic pain grading.
Neuropathic Pain
The grading of the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain was used to grade neuropathic pain. 1 Possible neuropathic pain fulfils criteria 1 and 2. Probable neuropathic pain fulfils criteria 1, 2, and 3. Definite neuropathic pain fulfils all 4 criteria:
Pain with a distinct neuroanatomically plausible distribution—pain symmetrically distributed in the extremities.
A history suggestive of a relevant lesion or disease affecting the peripheral or central somatosensory system—diagnosis of diabetes mellitus or history of chemotherapy treatment with a history of neuropathy symptoms including decreased sensation or positive sensory symptoms in the toes, feet, or legs.
Demonstration of distinct neuroanatomically plausible distribution of neuropathic pain—presence of clinical signs of distal symmetrical polyneuropathy.
Demonstration of the relevant lesion or disease by at least one confirmatory test—abnormality on either the nerve conduction tests or intraepidermal nerve fiber density.
Only study participants who satisfied criteria for no neuropathic pain, and probable or definite neuropathic pain, proceeded to axonal excitability assessment and analysis.
Axonal Excitability Testing
Axonal excitability was measured with the threshold tracking technique. 11 , 36 The recording system was in line with current consensus guidelines. 10 The stimulus site was the median nerve at the wrist, and the recording sites were the index finger for sensory, and abductor pollicis brevis muscle for motor excitability studies. Red Dot (3M, Two Harbors, MN; Oxford) and BlueSensor (Ambu, Ballerup, Denmark; Denmark) were the stimulus electrodes. Recording electrodes were the Natus (Pleasanton, CA) disposable wide ring electrode, which encircles the index finger for the sensory studies, and the disposable Natus (Oxford) and Ambu BlueSensor (Denmark) surface electrode, which was placed over the motor point of abductor pollicis brevis muscle. QTracS software (©UCL Institute of Neurology, London, UK, available from Digitimer at www.Digitimer.com) was used to acquire and analyze the axonal excitability measures. Standardized axonal excitability protocols, TROND (Oxford) and DOLORisk (Denmark), were used to assess multiple measures of axonal excitability. 36 We measured the stimulus–response curve, strength–duration properties, threshold electrotonus, current–threshold relationship, and recovery cycle. 10 , 11 , 36 For statistically significant differences in axonal excitability to be considered biologically relevant, a consistent change should be seen in at least two separate measures of axonal excitability.
There were differences between the TROND and DOLORisk protocols. The DOLORisk protocol did not include the current–threshold relationship; −20% hyperpolarizing conditioning stimulus during threshold electrotonus; 0.3‐millisecond duration test stimulus assessing strength–duration properties; and fewer test stimuli during strength–duration properties, threshold electrotonus, and recovery cycle. The −70% hyperpolarizing conditioning stimulus was added to the DOLORisk protocol. With fewer measurements, the DOLORisk protocol is quicker to perform than the TROND protocol. Skin temperature over the median nerve stimulus site was maintained between 32 and 34°C during the recordings.
Statistical Analysis
SPSS Statistics version 27 (IBM, Armonk, NY) was used for statistical analysis. Parametric data are expressed as mean ± standard deviation (SD), and nonparametric data as median ± interquartile range. The clinical and axonal excitability measure data were compared between the painful and painless polyneuropathy groups with unpaired t tests (parametric data) and Mann–Whitney tests (nonparametric data). Analysis of covariance was used to test for axonal excitability measures differences while controlling for any confounding effects of age. The sensory nerve excitability parameters were the dependent variables, neuropathic pain was the fixed factor, and age was the covariate. Statistical significance was set at p < 0.05 and was Bonferroni adjusted for the number of tests. For threshold tracking analysis, the probability value was corrected for the number of excitability measures used as independent variables, including strength–duration properties, threshold electrotonus, recovery cycle, stimulus properties, and current–threshold relationship. For the unpaired t tests, 95% confidence intervals were Bonferroni adjusted for the number of independent comparisons. The formula for Bonferroni‐corrected 95% confidence intervals is 1 – 0.05/(calculated confidence intervals).
Graphs were generated in Prism (GraphPad Software, San Diego, CA). Thresholds tracking figures were generated in QTracP and plotted as mean ± SD.
Results
Study Participants with Diabetes Mellitus
A total of 306 patients with diabetes were recruited; 151 were recruited in Oxford, UK, and 155 were recruited in Aarhus/Odense, Denmark (Fig 1). Only participants who met criteria for probable or definite distal symmetrical polyneuropathy were included in the analysis. Therefore, 239 participants were included in the analysis, with 67 participants excluded because they did not meet criteria for probable or definite distal symmetrical polyneuropathy. Each cohort was analyzed separately due to the large participant numbers in each cohort, and different threshold tracking protocols.
FIGURE 1.
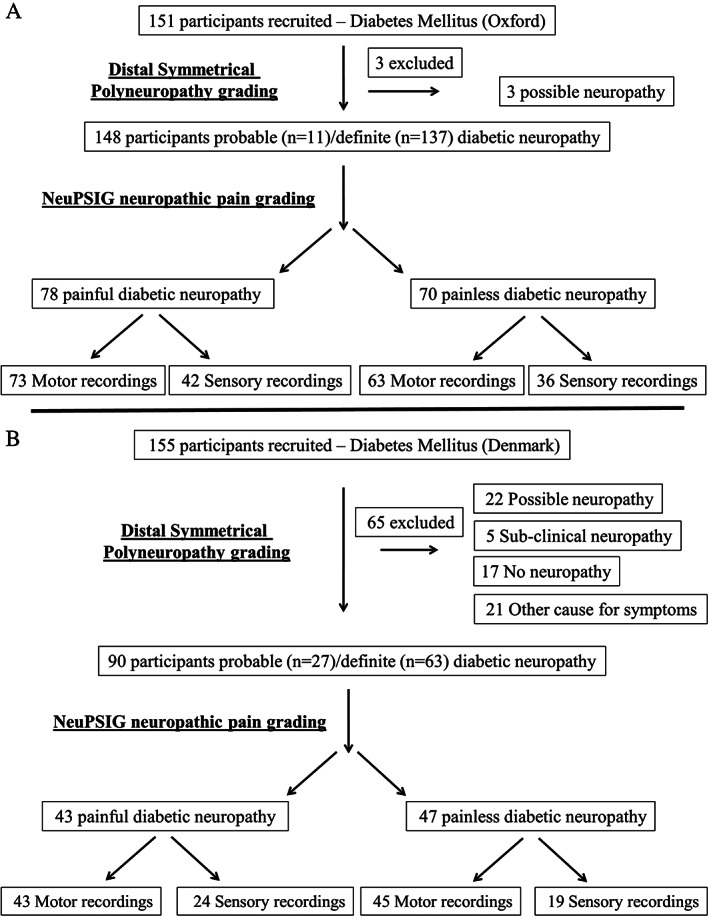
Flow diagram of participants recruited in Oxford and Denmark with diabetes mellitus. (A) In Oxford, 3 participants were excluded because they met criteria for only possible distal symmetrical diabetic polyneuropathy. (B) In Denmark, 65 participants were excluded because they did not meet criteria for probable or definite diabetic distal symmetrical polyneuropathy. NeuPSIG = Neuropathic Pain Special Interest Group.
A higher percentage of participants were included in Oxford than in Denmark (see Fig 1). The difference in inclusion was because of different recruitment pathways. General practice (GP) or hospital records were available for the majority of participants recruited in Oxford, increasing the likelihood of a distal symmetrical polyneuropathy being present at the time of recruitment. Participants were separated into those with painful or painless diabetic polyneuropathy. Sensory nerve axonal excitability recordings were not obtained in a significant percentage of participants (see Fig 1). Low sensory nerve amplitudes and high skin impedance, which generates significant signal interference in patients with diabetic polyneuropathy, contribute to the lower number of successful sensory nerve recordings when compared to motor nerve recordings.
In both the Oxford and Danish cohorts, the gender distribution, body mass index, and hemoglobin A1c did not differ between participants with painless and painful diabetic polyneuropathy. Some of the clinical variables differed between study participants recruited with painful and painless diabetic polyneuropathy (Table 1). In Oxford, Toronto Clinical Scoring System (TCSS) score, as a measure of neuropathy severity, and Douleur Neuropathique 4 (DN4) scores, screening for neuropathic pain, were higher for participants with painful diabetic polyneuropathy. Clinical differences remain for those study participants in whom sensory axonal recordings were obtained. However, the TCSS scores were lower, suggesting that axonal excitability testing success was likelier in those participants with a milder neuropathy. In Denmark, the DN4 scores were higher in those participants with painful diabetic polyneuropathy and the TCSS scores were not different. To test whether age is a confounder for the statically significant associations in Table 1, analysis for covariance was performed. DN4 and TCSS scores were the dependent variables, neuropathic pain was the fixed factor, and age was the covariate. Analysis of covariance shows that DN4 and TCSS were statistically significant (p < 0.01) between participants with painful and painless polyneuropathy.
TABLE 1.
Summary of Clinical Variables of Study Participants with Painful and Painless Diabetic Distal Symmetrical Polyneuropathy, Recruited in Oxford and Aarhus/Odense, Denmark
| Variable | Painless Diabetes Distal Symmetrical Polyneuropathy | Painful Diabetes Distal Symmetrical Polyneuropathy | p | CI of Effect Sizes |
|---|---|---|---|---|
| Oxford | ||||
| Sample size, n | 70 | 78 | ||
| Age, yr | 70.9 ± 9.8 | 67.4 ± 11.9 | 0.07 | −0.02 to 0.63 |
| Gender, males, n (%) | 47 (66.2%) | 57 (73.1%) | 0.38 | NA |
| BMI, kg/m2 | 28.8 ± 6.1 | 29.7 ± 4.7 | 0.37 | −0.48 to 0.20 |
| HbA1c, % | 7.4 ± 1.3 | 7.8 ± 1.5 | 0.17 | −0.57 to 0.10 |
| HbA1c, mmol/mol | 58 ± 15 | 62 ± 16 | ||
| TCSS score, adjusted (range) | 8 (5.0–9.0) | 11 (7.8–13.3) | <0.01 a | −4.0 to −2.0 |
| DN4 (range) | 2 (1–3) | 5 (4–7) | <0.01 a | −4.0 to −3.0 |
| Denmark | ||||
| Sample size, n | 47 | 43 | ||
| Age, yr | 66.0 ± 9 | 64.7 ± 10.5 | 0.53 | −0.28 to 0.55 |
| Gender, males, n (%) | 32 (68.1%) | 23 (53.5%) | 0.20 | NA |
| BMI, kg/m2 | 32.7 ± 6.1 | 33.1 ± 6.8 | 0.80 | −0.47 to 0.36 |
| HbA1c, % | 6.7 ± 0.8 | 7.1 ± 0.8 | 0.05 | −0.84 to 0.01 |
| HbA1c, mmol/mol | 50 ± 9 | 54 ± 9 | ||
| TCSS score, adjusted (range) | 7 (4–10) | 8 (6–11) | 0.07 | 0.0 to 3.0 |
| DN4 (range) | 2 (0–3) | 5 (3–5) | <0.01 a | 2.0 to 3.0 |
Unpaired t tests (parametric data) and Mann–Whitney tests (nonparametric data) comparing axonal excitability measures between those with painful and painless diabetic distal symmetrical polyneuropathy. Effect sizes: 99.0% confidence intervals (Bonferroni corrected); for parametric data, the Cohen d confidence intervals are shown; for nonparametric data, the independent samples Hodges–Lehman median differences are shown. Statistical significance was set at p < 0.05.
Statistically significant.
BMI = body mass index; CI = confidence interval; DN4 = Douleur Neuropathique 4 (a screening tool for neuropathic pain); HbA1c = hemoglobin A1c; NA = not applicable; TCSS = Toronto Clinical Scoring System (a composite clinical score that is a measure for neuropathy severity).
Axonal Excitability Measures
Axonal excitability measures include the recovery cycle, threshold electrotonus, strength–duration time constant, and current–threshold relationship (Fig 2). In the Oxford cohort, no differences in the recovery cycle, threshold electrotonus, strength–duration properties, and current–threshold relationship were found between study participants with and without neuropathic pain (see Fig 2, Table 2). There were no differences in motor nerve excitability measures between study participants with painful and painless diabetic distal symmetrical polyneuropathy. In the Danish cohort, there were no differences in either the sensory or the motor nerve excitability measures between study participants with painful and painless diabetic polyneuropathy. There were no statistically significant differences in both cohorts when controlling for any confounding effects of age (see Table S1).
FIGURE 2.
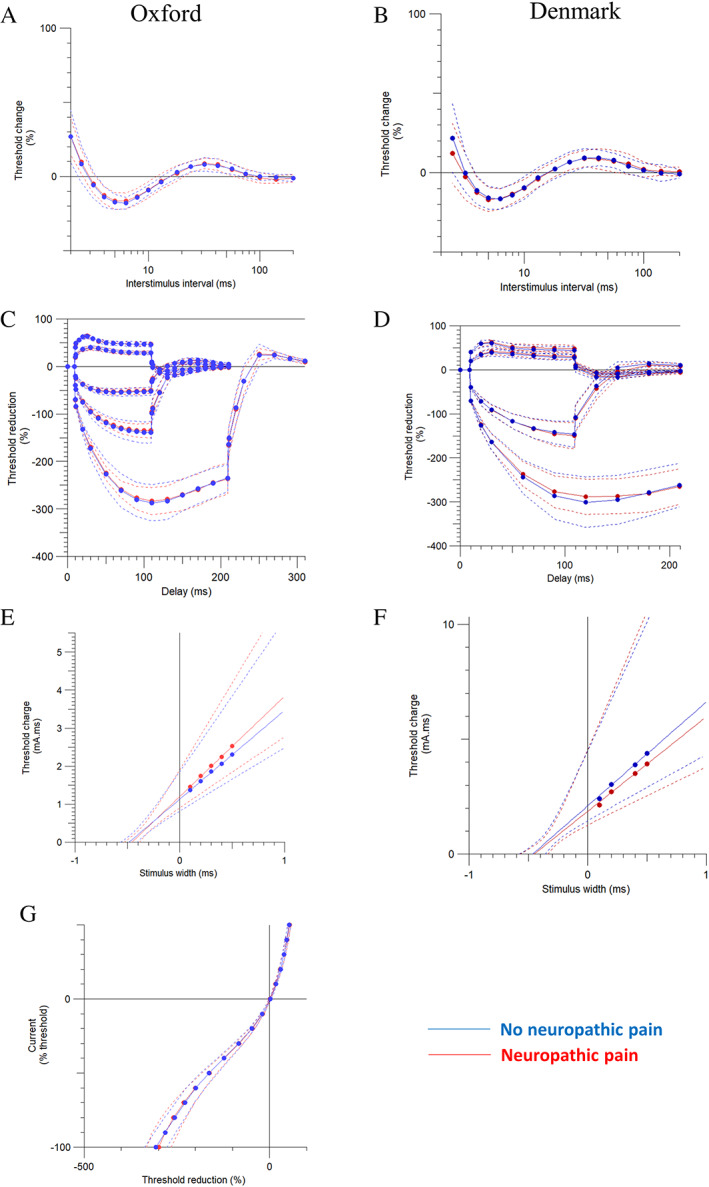
Sensory nerve excitability measures, which include (A, B) recovery cycle, (C, D) threshold electrotonus, (E, F) strength–duration properties, and (G) current‐threshold relationship between study participants with painful and painless diabetic distal symmetrical polyneuropathy recruited in Oxford and Aarhus/Odense, Denmark. No significant differences were found. Data are shown as mean ± standard deviation. [Color figure can be viewed at www.annalsofneurology.org]
TABLE 2.
Summary of Key Variables Derived from Sensory Axonal Excitability Studies of Study Participants with Painful and Painless Diabetic Distal Symmetrical Polyneuropathy, Recruited in Oxford and Aarhus/Odense, Denmark
| Summary table of key diabetic DSP sensory nerve excitability parameters | Painless polyneuropathy | Painful polyneuropathy | Unpaired t‐ test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | p Value | CI Effect Sizes | CI of the Difference | |
| Oxford | |||||||||
| Strength‐duration properties | |||||||||
| Strength‐duration time constant (ms) | 36 | 0.5 | 0.1 | 42 | 0.5 | 0.1 | 0.20 | −0.16 to 0.74 | −0.01 to 0.06 |
| Threshold electrotonus | |||||||||
| TEd peak (%) | 36 | 61.0 | 3.7 | 42 | 60.9 | 3.3 | 0.95 | −0.43 to 0.46 | −1.52 to 1.63 |
| TEd 90–100 ms (%) | 36 | 46.6 | 5.2 | 42 | 46.8 | 4.0 | 0.89 | −0.48 to 0.41 | −2.21 to 1.92 |
| TEh 90–100 ms (%) | 36 | −138.9 | 22.6 | 42 | −135.2 | 14.7 | 0.40 | −0.65 to 0.25 | −12.27 to 4.72 |
| S2 accommodation | 36 | 14.3 | 3.7 | 42 | 14.1 | 2.6 | 0.79 | −0.38 to 0.51 | −1.24 to 1.63 |
| Recovery Cycle | |||||||||
| RRP (ms) | 25 | 3.0 | 0.5 | 33 | 2.9 | 0.3 | 0.76 | −0.42 to 0.61 | −5.85 to 2.40 |
| Superexcitability (%) | 29 | −17.1 | 4.3 | 38 | −15.9 | 4.8 | 0.27 | −0.76 to 0.22 | −3.49 to 1.01 |
| Subexcitability (%) | 29 | 8.3 | 3.8 | 38 | 8.3 | 3.4 | 0.97 | −0.47 to 0.49 | −1.72 to 1.79 |
| Current‐Threshold relationship | |||||||||
| Resting I/V slope | 34 | 0.55 | 0.08 | 42 | 0.59 | 0.12 | 0.10 | −0.82 to 0.10 | −0.08 to 0.01 |
| Minimum I/V slope | 34 | 0.25 | 0.03 | 42 | 0.24 | 0.04 | 0.43 | −0.27 to 0.64 | −0.01 to 0.02 |
| Denmark | |||||||||
| Strength‐duration properties | |||||||||
| Strength‐duration time constant (ms) | 19 | 0.5 | 0.1 | 24 | 0.5 | 0.1 | 0.94 | −0.62 to 0.58 | −0.07 to 0.06 |
| Threshold electrotonus | |||||||||
| TEd peak (%) | 19 | 58.9 | 7.1 | 24 | 60.8 | 5.7 | 0.33 | −0.31 to 0.91 | −2.02 to 5.84 |
| TEd 90–100 ms (%) | 19 | 44.4 | 8.6 | 24 | 48.5 | 7.4 | 0.10 | −0.10 to 1.13 | −0.81 to 9.03 |
| TEh 90–100 ms (%) | 19 | −145.4 | 29.0 | 24 | −148.8 | 29.2 | 0.71 | −0.72 to 0.50 | −21.44 to 14.64 |
| S2 accommodation | 19 | 14.5 | 4.3 | 24 | 12.3 | 4.6 | 0.12 | −1.10 to 0.12 | −4.96 to 0.57 |
| Recovery Cycle | |||||||||
| RRP (ms) | 18 | 3.2 | 1.0 | 18 | 3.3 | 1.1 | 0.86 | −0.59 to 0.71 | −0.40 to 0.48 |
| Superexcitability (%) | 19 | −16.1 | 6.1 | 24 | −16.5 | 6.6 | 0.81 | −0.68 to 0.53 | −4.42 to 3.46 |
| Subexcitability (%) | 19 | 10.4 | 3.9 | 24 | 10.6 | 5.4 | 0.89 | −0.56 to 0.65 | −2.75 to 3.16 |
Unpaired t‐tests comparing unadjusted means of axonal excitability measures between those with painful and painless distal symmetrical polyneuropathy. No statistically significant differences were found. After Bonferroni correction, the corrected statistical significance thresholds are p = 0.0125 (Oxford, 4 comparisons) and p = 0.0167 (Denmark, 3 comparisons). p values for unpaired t‐tests are unadjusted and should be compared to the Bonferroni corrected statistical significance thresholds. 99.5% (Oxford) and 99.4 % (Denmark) confidence intervals (Bonferroni corrected) for Cohen’s d effect sizes and differences are shown.
CI = confidence interval.
When participants recruited in Oxford were stratified according to the presence of paresthesias (“tingling”/“pins and needles”) independent of neuropathic pain, no differences were found in axonal excitability measures. Neuropathic pain and paresthesias are significantly associated (Fisher exact test, p < 0.01). For all the study participants with paresthesias, 80.2% experience neuropathic pain, and of those with no paresthesias, 14.3% experience neuropathic pain. Dynamic brush‐evoked allodynia was only elicited from 3 participants (2.4%) with painful diabetic polyneuropathy. Dynamic brush‐evoked allodynia was not elicited in those with painless diabetic polyneuropathy. However, 46.6% and 50.7% of participants reported pain provoked or increased by brushing or pressure over the painful area. When participants recruited in Oxford were stratified according to reported “allodynia” independent of neuropathic pain, no differences were found in axonal excitability measures.
In the cohort recruited from Aarhus, 53.5% and 65.1% of participants reported pain provoked or increased by brushing or pressure over the painful area. As with the Oxford cohort, when participants recruited in Aarhus were stratified according to reported “allodynia” independent of neuropathic pain, no differences were found in axonal excitability measures.
Study Participants Who Received Chemotherapy
A total of 63 patients were recruited in Aarhus, Denmark 5 years after receiving chemotherapy (Fig 3). Only participants who met the criteria for probable or definite polyneuropathy were included in the analysis. Therefore, 39 participants were included in the analysis, with 24 participants excluded because they did not have a polyneuropathy or met criteria for possible polyneuropathy. A further 3 were excluded because they did not undergo axonal excitability testing. Thereafter, participants were separated into those with painful or painless chemotherapy‐induced polyneuropathy. There were no differences in clinical variables between study participants with painful and painless chemotherapy‐induced polyneuropathy (Table 3).
FIGURE 3.
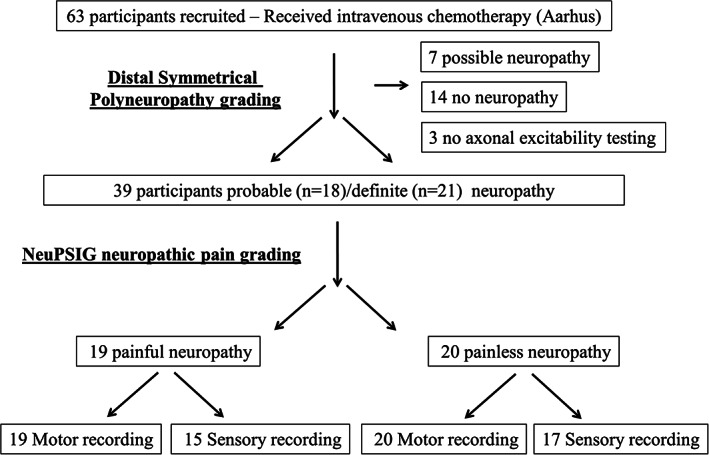
Flow diagram of participants recruited in Aarhus, Denmark who received chemotherapy. Participants were excluded if they did not meet criteria for probable or definite distal symmetrical polyneuropathy. NeuPSIG = Neuropathic Pain Special Interest Group.
TABLE 3.
Summary of Clinical Variables of Study Participants, Recruited in Aarhus, with Chemotherapy Distal Symmetrical Polyneuropathy
| Variable | Painful Chemotherapy‐Induced Neuropathy | Painless Chemotherapy‐Induced Neuropathy | p | CI of Effect Sizes |
|---|---|---|---|---|
| Age, yr | 65.2 ± 10.8 | 65.7 ± 8.3 | 0.89 | −0.67 to 0.58 |
| Gender, males, n (%) | 11 (57.9%) | 10 (50%) | 0.70 | NA |
| BMI, kg/m2 | 27.5 ± 4.4 | 25.3 ± 3.5 | 0.10 | −0.10 to 1.18 |
| TCSS score, adjusted (range) | 6 (4–9) | 6 (5–7) | 0.88 | −2.0 to 1.0 |
| DN4 (range) | 4 (3–6) | ND | NA | NA |
Unpaired t tests (parametric data) and Mann–Whitney tests (nonparametric data) comparing axonal excitability measures between those with painful and painless chemotherapy‐induced distal symmetrical polyneuropathy. Effect sizes: 98.3% confidence intervals (Bonferroni corrected); for parametric data, the Cohen d CIs are shown, and for nonparametric data, the independent samples Hodges–Lehman median differences are shown. Statistical significance was set at p < 0.05. No statistically significant differences were found.
BMI = body mass index; CI = confidence interval; DN4 = Douleur Neuropathique 4; NA = not applicable; ND = not determined; TCSS = Toronto Clinical Scoring System.
Axonal Excitability Measures
Sensory axonal excitability measures include the recovery cycle, threshold electrotonus, and strength–duration time constant. The measures were similar across groups except for lower S2 accommodation and TEd40 (accommodation) measurements in those with neuropathic pain (Fig 4, Table 4).
FIGURE 4.
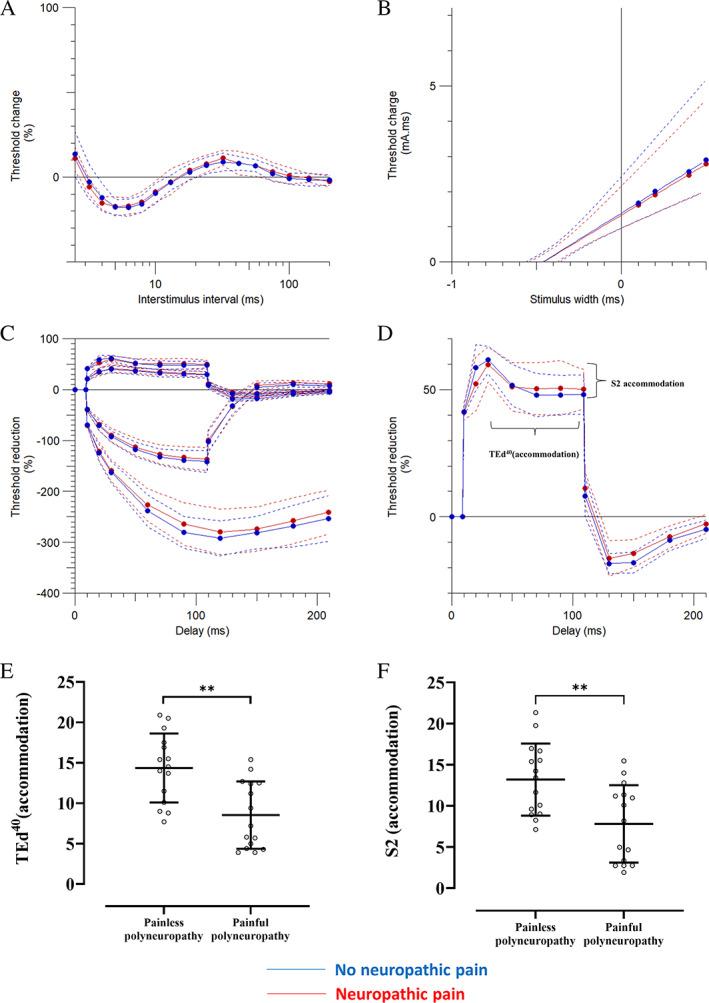
Sensory nerve excitability measures between study participants with painful and painless chemotherapy‐induced distal symmetrical polyneuropathy, recruited in Aarhus. These include (A) recovery cycle, (B) strength–duration time constant, (C) threshold electrotonus, and (D) threshold changes during 100‐millisecond 40% depolarizing current. There were significant differences between the groups in (E) S2 accommodation and (F) TEd40 (accommodation). Data are shown as mean ± standard deviation. **p < 0.01. TEd40 (accommodation) = maximum drop from TEd40 (peak) during 100‐millisecond depolarization. [Color figure can be viewed at www.annalsofneurology.org]
TABLE 4.
Summary of Key Variables Derived from Sensory Axonal Excitability Studies of Study Participants with Painful and Painless Chemotherapy‐Induced Polyneuropathy
| Chemotherapy DSP Sensory Nerve Excitability Parameters, Aarhus | Painless Polyneuropathy | Painful Polyneuropathy | p | CI of Effect Sizes | CI of Difference | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||
| Strength–duration properties | |||||||||
| Strength–duration/time constant, ms | 17 | 0.5 | 0.1 | 15 | 0.5 | 0.1 | 0.88 | −0.64 to 0.75 | −0.06 to 0.07 |
| Threshold electrotonus | |||||||||
| TEd peak, % | 16 | 60.0 | 6.0 | 15 | 58.0 | 8.4 | 0.46 | −0.44 to 0.98 | −3.37 to 7.27 |
| TEd 90–100 ms, % | 17 | 50.0 | 12.3 | 15 | 50.2 | 8.4 | 0.94 | −0.72 to 0.67 | −7.99 to 7.45 |
| TEh 90‐100 ms, % | 17 | −140.7 | 21.0 | 15 | −135.8 | 22.3 | 0.52 | −0.92 to 0.47 | −20.58 to 10.70 |
| S2 accommodation | 15 | 13.2 | 4.4 | 15 | 7.8 | 4.7 | <0.01 a | 0.18 to 1.67 | 0.97 to 8.29 |
| Recovery cycle | |||||||||
| RRP, ms | 12 | 3.3 | 0.5 | 12 | 3.0 | 0.2 | 0.06 | −0.04 to 1.62 | −0.02 to 0.65 |
| Superexcitability, % | 17 | −17.4 | 7.2 | 15 | −17.7 | 4.3 | 0.89 | −0.64 to 0.75 | −4.03 to 4.64 |
| Subexcitability, % | 11 | 9.3 | 3.2 | 14 | 11.4 | 6.9 | 0.37 | −1.16 to 0.43 | −6.74 to 2.61 |
Unpaired t tests comparing axonal excitability measures between those with painful and painless chemotherapy‐induced distal symmetrical polyneuropathy. TEd40 and S2 accommodation were significantly higher in those participants with a painless polyneuropathy. After Bonferroni correction, the corrected statistical significance threshold is p = 0.0167. The p values in the table are unadjusted and should be compared to the Bonferroni‐corrected statistical significance threshold. Shown are 99.4% confidence intervals (Bonferroni corrected) for Cohen d effect sizes and differences. TEd and TEh ‐ depolarising and hyperpolarising currents as conditioning stimului during threshold electrotonus.
Statistically significant.
CI = confidence interval; DSP = distal symmetrical polyneuropathy; SD = standard deviation.
There were no differences in motor nerve excitability measures between study participants with painful and painless chemotherapy‐induced polyneuropathy. These measures included the recovery cycle, threshold electrotonus, and strength–duration time constant. When participants were stratified according to the presence of paresthesia (“tingling”/“pins and needles”) independent of neuropathic pain, there were no differences in sensory or motor nerve excitability measures. Dynamic brush‐evoked allodynia was elicited from only 2 participants (10.5%) with painful chemotherapy‐induced polyneuropathy. Dynamic brush‐evoked allodynia was not elicited in those with painless chemotherapy‐induced polyneuropathy. However, 42.1% and 63.2% of participants reported pain provoked or increased by brushing or pressure over the painful area. When participants recruited in Aarhus were stratified according to reported “allodynia” independent of neuropathic pain, no differences were found in axonal excitability measures.
Discussion
Measurements of axonal excitability between study participants with painless and painful distal symmetrical polyneuropathy did not identify consistent differences. Furthermore, neither paresthesias nor reported allodynia was independently associated with changes in axonal excitability. Therefore, in our study, axonal excitability, resting membrane potential, and ion channel function of large myelinated fibers were not related to chronic neuropathic pain or paresthesias in deeply phenotyped cohorts with probable or definite diabetic or chemotherapy‐induced distal symmetrical polyneuropathy.
The assessment of nerve excitability using threshold tracking does not directly determine the excitability of nociceptive fibers. The majority of nociceptors are unmyelinated and do not contribute to the compound sensory nerve action potential that is assessed. However, measuring axonal excitability in large fibers may still detect changes relevant to neuropathic pain. For example, it can detect a functional change in an ion channel that is shared by myelinated and unmyelinated axons. Furthermore, preclinical studies suggest a contribution of myelinated fibers to touch‐evoked allodynia, a pathophysiological phenomenon seen in a subset of patients with neuropathic pain. 37 , 38 A number of nerve excitability studies suggest a link between an increase in myelinated fiber axonal excitability and neuropathic pain or dysesthesias. 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 In particular, persistent Na+ currents were reported to increase in patients with neuropathic pain. There are multiple isoforms of voltage‐gated sodium channels with distinct gating properties, and although some of these (eg, NaV 1.8 and 1.9) show relatively selective expression in unmyelinated nociceptive afferents, others are expressed in both myelinated and unmyelinated afferents (eg, NaV1.7). 39 Voltage‐gated sodium channels are linked to the development of neuropathic pain. Gene variants in voltage‐gated sodium channels can lead to inherited pain syndromes, such as inherited erythromelalgia, and increase the risk for the development of neuropathic pain in acquired disorders, 39 such as diabetes 40 or small fiber neuropathy. 41 After nerve injury, changes of sodium channel expression and gating properties contribute to peripheral neuron hyperexcitability and neuropathic pain. For example, NaV 1.3 expression is significantly upregulated after nerve injury. 42 There are links between voltage‐gated sodium channels expressed at high levels in myelinated fibers and neuropathic pain. For example, NaV1.6 has been linked to pain‐related behavior in a model of chemotherapy‐induced neuropathy in rodents, 43 and gain‐of‐function NaV1.6 variants are associated with trigeminal neuralgia. 44 However, it is unknown to what extent threshold tracking changes can detect changes within NaV1.7 and 1.3 when action potential generation and conduction within myelinated fibers are largely driven by other sodium channels (NaV1.6 and 1.2).
As there is a sodium channel homology between large and small sensory fibers and voltage‐gated sodium channel expression and function changes after nerve injury (in myelinated and unmyelinated fibers), it is plausible that axonal excitability measurements of large fibers may detect ion channel changes that indicate an increase in risk for the development of neuropathic pain or reflect changes within small fibers after nerve injury. This was our rationale for investigating the relationship between our investigation of nerve excitability and neuropathic pain in a large well‐phenotyped cohort.
There were no statistically significant differences in axonal excitability when comparing painful to painless diabetic distal symmetrical polyneuropathy. In chemotherapy‐induced polyneuropathy, the only statistically significant differences identified were in the S2 accommodation and TEd40 (accommodation) measurements recorded from participants with chemotherapy‐induced distal symmetrical polyneuropathy. The S2 accommodation and TEd40 (accommodation) measurements were lower in those participants with neuropathic pain. Slow K+ channels of the KV7 family are responsible for the decline in depolarizing electrotonus after peak threshold change, which is termed the S2 phase of threshold electrotonus. 45 , 46 , 47 , 48 Slow K+ channels are expressed at the node of Ranvier and are responsible for outward rectification, limitation of ectopic firing, and reduced excitability after a train of impulses. 10 , 45 No changes were observed in the subexcitability phase of the recovery cycle, which is another measure of slow K+ channel function. 10
Although a statistical difference was found, the changes are not considered biologically relevant. For a finding to be biologically relevant, it should be seen in at least two separate measures of axonal excitability. S2 phase of threshold electrotonus changes were not replicated in the subexcitability phase of the recovery cycle; both are measures of slow K+ channels. Furthermore, for the S2 phase changes to be meaningfully related to neuropathic pain, we would expect to see the same findings in the other two cohorts of neuropathic pain. The S2 phase of threshold electrotonus changes was not replicated in the painful diabetic polyneuropathy cohorts. Another possible explanation for the statistical differences found was the small sample size of the chemotherapy‐induced polyneuropathy cohort relative to the larger diabetic cohorts.
The current study is the largest to test axonal excitability differences between painless and painful polyneuropathy. 19 , 21 A combined total of 277 participants underwent excitability testing, compared to the next largest group of 81 participants. 21 In this 2009 study, greater expression of nodal persistent Na+ currents, estimated by latent addition and strength–duration time constant, was present in those participants with neuropathic pain. A similar correlation was found between positive neuropathy symptoms, assessed by the neuropathy‐specific quality of life questionnaire NeuroQoL, and greater nodal persistent Na+ conductance in 37 participants with type 2 diabetes mellitus. 19 Mexiletine suppression, in 17 study participants with polyneuropathy, of nodal Na+ currents was associated with analgesia in patients with neuropathic pain. 22 Changes in Na+ currents were not replicated in our study. We used the strength–duration time constant to study persistent Na+ currents at the node of Ranvier rather than latent addition. The strength–duration time constant is affected by the passive membrane properties. An advantage of latent addition is that it evaluates nodal Na + currents and passive membrane properties separately, 11 , 49 and is thus more sensitive to nodal Na + changes. It is possible that in our studies, strength–duration property measurements were not sensitive enough to detect changes in nodal Na+ channel conductance. Axonal excitability changes associated with diabetic polyneuropathy include reduction in strength–duration time constant, a decrease in superexcitability and subexcitability, and a “fanning in” appearance of threshold electrotonus. In our comparison of painful to painless diabetic polyneuropathy, we did not find differences in these parameters. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 30
Another important difference from previous studies is the stratification of participants into painless and painful polyneuropathy groups. 1 , 35 In the current study, a rigorous approach to distal symmetrical polyneuropathy diagnosis 35 and neuropathic pain grading 1 was used. All participants were assessed in the same manner following standardized published guidelines. In doing so, we were able to compare the axonal excitability data across different cohorts. The revised neuropathic pain grading from 2016 is a significant improvement on previous approaches. 1 It offers a methodical and hierarchical process of diagnosis that can be applied in clinical and research settings. In previous studies, different nonstandardized approaches were used to diagnose polyneuropathy and neuropathic pain, thus limiting comparability.
In our studies, the median sensory and motor nerves were used. A limitation of threshold tracking technique is that reliable measurements from the lower limb sensory and motor nerves innervating the areas of neuropathic pain are not possible. It was not possible to record median nerve sensory axonal measurements in 43.3% of participants with diabetic polyneuropathy; however, motor axonal recordings were recorded from 95% of participants. Recording median sensory response using threshold tracking is technically challenging due to the low sensory nerve amplitudes and high skin impedance, which generates significant signal interference in patients with diabetic polyneuropathy. In the Oxford cohort, the TCSS scores were higher in those participants in whom sensory axonal recordings could not be obtained, consistent with a more severe polyneuropathy. Our studies were therefore biased in that sensory recordings were not obtained from those with more severe diabetic polyneuropathies in both the painless and painful groups. In other studies, the radial sensory and median motor nerves were used. 19 , 21 , 22 The radial sensory nerve is a large sensory nerve. 50 A recordable radial sensory nerve potential is more likely to be obtained than a median sensory nerve potential in severe diabetic polyneuropathy, and this may be a priority for future studies.
Despite the lack of consistent differences in our studies, interrogation of ion channel function and resting membrane potential in myelinated fibers may still provide insights into potential mechanisms of neuropathic pain and paresthesias that are shared across different fiber types. For example, paresthesias arise from changes in large fibers. 51 It is therefore possible that the axonal excitability protocols used are not optimized to detect the relevant hyperexcitability changes that cause paresthesias. Threshold tracking measures changes in large myelinated fibers, whereas microneurography is the only current technique that allows in vivo assessment of thinly myelinated Aδ and unmyelinated C nociceptive fibers. 52 Important future studies will compare the two methods in study participants with painful and painless polyneuropathy, with longitudinal cohorts to correlate neurophysiological changes with neuropathic pain and other symptoms such as paresthesias and allodynia as they evolve over time, and optimization of excitability protocols to detect hyperexcitability changes in large fibers.
Author Contributions
A.C.T., T.S.J., H.B., J.S., N.B.F., H.T., and D.L.H.B. contributed to conception and design of the study. All authors contributed to acquisition and analysis of data. A.C.T., H.T., and D.L.H.B. contributed to drafting a significant portion of the manuscript and figures.
Potential Conflict of Interests
H.B. receives royalties from University College London for sales of his Qtrac software used in this study. The remaining authors have no conflict of interest.
Supporting information
TABLE S1 Summary of Key Variables Derived from Sensory Axonal Excitability Studies of Study Participants with Painful and Painless Diabetic Distal Symmetrical Polyneuropathy, Recruited in Oxford and Aarhus/Odense, Denmark.
Acknowledgments
This project received funding from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement 633491 (DOLORisk). D.L.H.B., A.C.T, N.B.F, and T.S.J are members of the DOLORisk consortium. Research reported in this publication is part of the IDNC research program, which is supported by a Novo Nordisk Foundation Challenge Program grant (NNF14OC0011633). A.C.T is supported by Academy of Medical Sciences Starter Grant SGL022\1086, and is an Honorary Senior Research Fellow and Carnegie‐Wits Diaspora Fellow at the Brain Function Research Group, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. For the purpose of Open Access, the authors have applied a CC BY public copyright license to any author‐accepted manuscript version arising from this submission. We thank the patients who took part in the study; the GPs and staff of the National Institute for Health Research Thames Valley Primary Care Research Network; the staff of the Oxford Centre for Diabetes, Endocrinology, and Metabolism; the Thames Valley Primary Care Research Network for assisting with recruitment; and Dr G. Baskozos, Nuffield Department of Clinical Neurosciences at University of Oxford, for providing assistance with the statistical analysis.
References
- 1. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016;157:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155:654–662. [DOI] [PubMed] [Google Scholar]
- 3. Bouhassira D, Lantéri‐Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136:380–387. [DOI] [PubMed] [Google Scholar]
- 4. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gregg EW, Sorlie P, Paulose‐Ram R, et al. Prevalence of lower‐extremity disease in the U.S. adult population ≥40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care 2004;27:1591–1597. [DOI] [PubMed] [Google Scholar]
- 6. Feldman EL, Nave K‐A, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017;93:1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennedsgaard K, Ventzel L, Themistocleous AC, et al. Long‐term symptoms of polyneuropathy in breast and colorectal cancer patients treated with and without adjuvant chemotherapy. Cancer Med 2020;9:5114–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tracey I, Woolf CJ, Andrews NA. Composite pain biomarker signatures for objective assessment and effective treatment. Neuron 2019;101:783–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haroutounian S, Nikolajsen L, Nikolajsen L, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain 2014;155:1272–1279. [DOI] [PubMed] [Google Scholar]
- 10. Kiernan MC, Bostock H, Park SB, et al. Measurement of axonal excitability: consensus guidelines. Clin Neurophysiol 2020;131:308–323. [DOI] [PubMed] [Google Scholar]
- 11. Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 1998;21:137–158. [DOI] [PubMed] [Google Scholar]
- 12. Sung JY, Park SB, Liu YT, et al. Progressive axonal dysfunction precedes development of neuropathy in type 2 diabetes. Diabetes 2012;61:1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Misawa S, Kuwabara S, Kanai K, et al. Nodal persistent Na+ currents in human diabetic nerves estimated by the technique of latent addition. Clin Neurophysiol 2006;117:815–820. [DOI] [PubMed] [Google Scholar]
- 14. Kuwabara S, Ogawara K, Harrori T, et al. The acute effects of glycemic control on axonal excitability in human diabetic nerves. Intern Med 2002;41:360–365. [DOI] [PubMed] [Google Scholar]
- 15. Misawa S, Kuwabara S, Kanai K, et al. Axonal potassium conductance and glycemic control in human diabetic nerves. Clin Neurophysiol 2005;116:1181–1187. [DOI] [PubMed] [Google Scholar]
- 16. Misawa S, Kuwabara S, Ogawara K, et al. Strength‐duration properties and glycemic control in human diabetic motor nerves. Clin Neurophysiol 2005;116:254–258. [DOI] [PubMed] [Google Scholar]
- 17. Krishnan AV, Lin CS, Kiernan MC. Activity‐dependent excitability changes suggest Na+/K+ pump dysfunction in diabetic neuropathy. Brain 2008;131:1209–1216. [DOI] [PubMed] [Google Scholar]
- 18. Bae JS, Kim OK, Kim JM. Altered nerve excitability in subclinical/early diabetic neuropathy: evidence for early neurovascular process in diabetes mellitus? Diabetes Res Clin Pract 2011;91:183–189. [DOI] [PubMed] [Google Scholar]
- 19. Kwai NC, Arnold R, Wickremaarachchi C, et al. Effects of axonal ion channel dysfunction on quality of life in type 2 diabetes. Diabetes Care 2013;36:1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kristensen AD, Gylfadottir S, Itani M, et al. Sensory and motor axonal excitability testing in early diabetic neuropathy. Clin Neurophysiol 2021;132:1407–1415. [DOI] [PubMed] [Google Scholar]
- 21. Misawa S, Sakurai K, Shibuya K, et al. Neuropathic pain is associated with increased nodal persistent Na(+) currents in human diabetic neuropathy. J Peripher Nerv Syst 2009;14:279–284. [DOI] [PubMed] [Google Scholar]
- 22. Isose S, Misawa S, Sakurai K, et al. Mexiletine suppresses nodal persistent sodium currents in sensory axons of patients with neuropathic pain. Clin Neurophysiol 2010;121:719–724. [DOI] [PubMed] [Google Scholar]
- 23. Park SB, Lin CS‐Y, Krishnan AV, et al. Oxaliplatin‐induced neurotoxicity: changes in axonal excitability precede development of neuropathy. Brain 2009;132:2712–2723. [DOI] [PubMed] [Google Scholar]
- 24. Park SB, Lin CS‐Y, Krishnan AV, et al. Early, progressive, and sustained dysfunction of sensory axons underlies paclitaxel‐induced neuropathy. Muscle Nerve 2011;43:367–374. [DOI] [PubMed] [Google Scholar]
- 25. Nasu S, Misawa S, Nakaseko C, et al. Bortezomib‐induced neuropathy: axonal membrane depolarization precedes development of neuropathy. Clin Neurophysiol 2014;125:381–387. [DOI] [PubMed] [Google Scholar]
- 26. Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin and axonal Na+ channel function in vivo. Clin Cancer Res 2006;12:4481–4484. [DOI] [PubMed] [Google Scholar]
- 27. Heide R, Bostock H, Ventzel L, et al. Axonal excitability changes and acute symptoms of oxaliplatin treatment: in vivo evidence for slowed sodium channel inactivation. Clin Neurophysiol 2018;129:694–706. [DOI] [PubMed] [Google Scholar]
- 28. Bennedsgaard K, Ventzel L, Andersen NT, et al. Oxaliplatin‐ and docetaxel‐induced polyneuropathy: clinical and neurophysiological characteristics. J Peripher Nerv Syst 2020;25:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bennedsgaard K, Ventzel L, Grafe P, et al. Cold aggravates abnormal excitability of motor axons in oxaliplatin‐treated patients. Muscle Nerve 2020;61:796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Themistocleous AC, Ramirez JD, Shillo PR, et al. The Pain in Neuropathy Study (PiNS): a cross‐sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 2016;157:1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christensen DH, Nicolaisen SK, Berencsi K, et al. Danish Centre for Strategic Research in type 2 diabetes (DD2) project cohort of newly diagnosed patients with type 2 diabetes: a cohort profile. BMJ Open 2018;8:e017273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gylfadottir SS, Itani M, Krøigård T, et al. Diagnosis and prevalence of diabetic polyneuropathy: a cross‐sectional study of Danish patients with type 2 diabetes. Eur J Neurol 2020;27:2575–2585. [DOI] [PubMed] [Google Scholar]
- 33. Pascal MMV, Themistocleous AC, Baron R, et al. DOLORisk: study protocol for a multi‐centre observational study to understand the risk factors and determinants of neuropathic pain. Wellcome Open Res 2019;3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ventzel L, Jensen AB, Jensen AR, et al. Chemotherapy‐induced pain and neuropathy: a prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain 2016;157:560–568. [DOI] [PubMed] [Google Scholar]
- 35. Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophysiol 2001;112:1575–1585. [DOI] [PubMed] [Google Scholar]
- 37. Dhandapani R, Arokiaraj CM, Taberner FJ, et al. Control of mechanical pain hypersensitivity in mice through ligand‐targeted photoablation of TrkB‐positive sensory neurons. Nat Commun 2018;9:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu Z‐Z, Kim YH, Bang S, et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5‐mediated A‐fiber blockade. Nat Med 2015;21:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bennett DL, Clark AJ, Huang J, et al. The role of voltage‐gated sodium channels in pain signaling. Physiol Rev 2019;99:1079–1151. [DOI] [PubMed] [Google Scholar]
- 40. Blesneac I, Themistocleous AC, Fratter C, et al. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain 2018;159:469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Faber CG, Hoeijmakers JGJ, Ahn H‐S, et al. Gain of function NaV1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012;71:26–39. [DOI] [PubMed] [Google Scholar]
- 42. Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol 1994;72:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen L, Huang J, Benson C, et al. Sodium channel Nav1.6 in sensory neurons contributes to vincristine‐induced allodynia. Brain 2020;143:2421–2436. [DOI] [PubMed] [Google Scholar]
- 44. Tanaka BS, Zhao P, Dib‐Hajj FB, et al. A gain‐of‐function mutation in Nav1.6 in a case of trigeminal neuralgia. Mol Med 2016;22:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baker M, Bostock H, Grafe P, Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol 1987;383:45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tomlinson SE, Bostock H, Grinton B, et al. In vivo loss of slow potassium channel activity in individuals with benign familial neonatal epilepsy in remission. Brain 2012;135:3144–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomlinson S, Burke D, Hanna M, et al. In vivo assessment of HCN channel current (Ih) in human motor axons. Muscle Nerve 2010;41:247–256. [DOI] [PubMed] [Google Scholar]
- 48. Tomlinson SE, Tan SV, Kullmann DM, et al. Nerve excitability studies characterize Kv1.1 fast potassium channel dysfunction in patients with episodic ataxia type 1. Brain 2010;133:3530–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bostock H, Rothwell JC. Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol 1997;498:277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kumbhare D, Robinson L, Buschbacher R. Buschbacher's manual of nerve conduction studies. 3rd ed. New York, NY: Springer Publishing, 2015. [Google Scholar]
- 51. Mogyoros I, Bostock H, Burke D. Mechanisms of paresthesias arising from healthy axons. Muscle Nerve 2000;23:310–320. [DOI] [PubMed] [Google Scholar]
- 52. Ackerley R, Watkins RH. Microneurography as a tool to study the function of individual C‐fiber afferents in humans: responses from nociceptors, thermoreceptors, and mechanoreceptors. J Neurophysiol 2018;120:2834–2846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Summary of Key Variables Derived from Sensory Axonal Excitability Studies of Study Participants with Painful and Painless Diabetic Distal Symmetrical Polyneuropathy, Recruited in Oxford and Aarhus/Odense, Denmark.


