Abstract
Aims
There are many situations where preclinical models of the human vagina would be valuable for in vitro studies into the pathophysiology of vaginally transmitted diseases, microbicide efficacy, irritability testing, and particularly, for assessing materials to be inserted in the vagina for support of the pelvic floor. The aim of this study is to develop a physiologically relevant, low cost, and ethically suitable model of the vagina using sheep vaginal tissue (SVT) to reduce the need for animal testing in gynecological research.
Methods
Tissue‐engineered (TE) vaginal models were developed by culturing primary vaginal epithelial cells and vaginal fibroblasts, isolated from the native SVTs on decellularized sheep vaginal matrices at an air–liquid interface. Morphological analyses of the models were conducted by performing hematoxylin and eosin staining and further characterization was done by immunohistofluorescence (IHF) of structural proteins and cytokeratins.
Results
Histological analysis of the models revealed a gradual formation of a stratified epithelium on our decellularized matrices and cell metabolic activity remained high for 21 days as measured by the resazurin assay. Our models showed a dose‐dependent response to estradiol‐17β [E2] with an increase in the vaginal epithelium thickness and cellular proliferation under higher E2 concentrations (100–400 pg/ml). The physiological relevance of these results was confirmed by the IHF analysis of Ki67, and cytokeratins 10 and 19 expression.
Conclusion
In this study, we have developed an estradiol‐responsive TE vaginal model that closely mimics the structural and physiological properties of the native SVT.
Keywords: preclinical models, sheep, tissue‐engineered vagina
1. INTRODUCTION
The female vagina is an elastic muscular tube that connects the cervix (lower part of the uterus) to the vulva (female external genitalia). The vagina serves as a canal for evacuation of the contents of the uterus (e.g., menstrual blood, fetus) and has a key role in protecting against bacteria with its local flora, acidic pH, and epithelial barrier. 1 The vaginal epithelium is a nonkeratinised, stratified squamous epithelium that forms a continuous mucosal barrier separating the external microenvironment of the vaginal canal from the underlying lamina propria and prevents the entry of pathogens. 2 Research into advancing the management of vaginal diseases has been limited by the absence of relevant, efficient, and easily accessible in vitro models of the vagina. It is salutary to conclude that newly emerging treatments have all undergone suboptimal preclinical evaluation. For example, the well‐known “vaginal mesh scandal” where the biocompatibility of polymer meshes in the vagina were not tested adequately in preclinical models before inserting into the pelvic floor of women. 3 In practice, experts agree on the need for better preclinical models and testing pathways that can evaluate the efficacy and safety of new therapies applied to the vagina. 4
Current preclinical vaginal models require the use of animals (e.g., mice, rat, or sheep) or human explanted tissues. Animal models have many advantages as data are produced in a complex organ system that can recapitulate whole organism physiology. However, several aspects including species discrepancy, anatomical, and physiological differences of the reproductive tissue and variations in hormone responsiveness limit their applicability. 5 Vaginal explant cultures are often used in preclinical studies but are impractical due to their short life span (approximately a week in culture), limited availability of human explanted tissues, and high variability among donors. 6 Accordingly, in vitro tissue‐engineered (TE) vaginal models can offer potential solutions to these limitations as they are more physiologically relevant and ethically sound.
There are several published approaches to produce three‐dimensional (3D) vaginal models, but none are ideal. Ayehunie et al. 7 first reported the development of a 3D organotypic vaginal‐ectocervical model using human vaginal cells cultured on collagen gel matrix. Later, their model was further developed to produce EpiVaginal™ tissue (VEC‐100) and EpiVaginal™ tissue (VEC‐100‐FT). 8 , 9 While these models showed morphological similarities with the native human vaginal tissue, but the collagen gel substrate fails to accurately model the complexity of the native lamina propria. Other commercially available models of the human vaginal mucosa include human vaginal epithelium (SkinEthic™ HVE) and reconstructed HVE that uses cancer‐derived cell lines to construct the epithelium. The use of immortalized cells limit their physiological relevance 10 and also commercial models can only be used for a few days and are very expensive.
Access to human tissue and primary human vaginal cells is a serious limitation for many researchers; therefore, in this study, we have used sheep vaginal tissue (SVT) as this has been shown to be anatomically close to the human vaginal tissue in terms of similar reproductive anatomy, pathophysiology, hormonal levels, and anatomical development of prolapse. 11 Other advantages include low cost, less ethical concerns than human tissue, and ease of availability as a waste product from the meat industry without the need to sacrifice additional animals for these models.
The HVE physiological and immunological functions are regulated by the two female reproductive hormones: (I) estrogen (estradiol‐17β [E2]) and (II) progesterone (P4). Therefore, the response of any TE model to hormone treatment is an important consideration in model development. Estradiol‐17β [E2] promotes vaginal epithelial cell proliferation, stratification, and cytodifferentiation by binding with the estrogen receptor‐α. 12 Furthermore, it is well documented that estradiol‐17β [E2] administration affects multiple cellular pathways, pathogen defense, inflammatory responses, regulation of oxidative stress, and neovascularization at the molecular level. 13
Molecular understanding of the effects of reproductive hormones on the vaginal epithelium microenvironment and other molecular targets, such as epithelial differentiation proteins (cytokeratins), could enhance our knowledge of the pathophysiology of vaginal diseases, mechanisms of cell growth and differentiation, tumorigenesis, and/or toxicity of drugs that enable researchers to develop improved treatments. Cytokeratins are expressed differently in different epithelial cell types and have been shown to be valuable diagnostic markers for squamous cell carcinomas. 14 For example, in nonkeratinised stratified squamous epithelia, such as the oral mucosa and vaginal tissue, cytokeratin 19 (cyt19) has been reported to be a characteristic marker of the proliferating cells. 15
The aim of this study is to develop a physiologically relevant in vitro animal based TE vaginal model using decellularized‐SVT (de‐SVT). Through exposing this TE vaginal model to estradiol‐17β [E2], we aimed to demonstrate the physiological relevance by investigating its role in maintaining the structure and functionality of the vaginal epithelium in an in vitro environment.
2. MATERIALS AND METHODS
2.1. Source of SVT and primary vaginal cells
Intact sheep urogenital tracts were collected from healthy sheep killed at R.B Elliott & Son, Ltd. Tissues were processed within 24 h of surgical removal and treated with Cambridge Antibiotic Solution (CAS‐04‐301) (source BioScience Healthcare UK, Ltd). Sheep vaginas were dissected across the length of vaginal tissue vertically until the vulva at the posterior end.
2.2. Decellularization of SVT
Vaginal tissues measuring 4 cm2 were decellularized using two different methods (Table 1) on a Platform Rocker STR6 at 40 r/min for 5 days. Following decellularization, the tissue samples were washed extensively with sterile phosphate‐buffered saline (PBS) and stored at 4°C until use.
Table 1.
Methods used for the decellularization of SVT
| Steps | Detergent method | Hypertonic + detergent method | Duration of treatment |
|---|---|---|---|
| 1) Primary decellularization solution | 0.25% wt/vol sodium deoxycholate + 0.25% vol/vol Triton X‐100 in PBS supplemented with 1% CAS | 1 M NaCl in PBS supplemented with 1% CAS | Incubated for overnight on a Platform Rocker STR6 at 40 r/min |
| 2) Epithelium removal | Gentle scraping with sterile forceps | Gentle scraping with sterile forceps | – |
| 3) Washing (twice) | Sterile PBS | Sterile PBS | 30 min each |
| 4) Secondary decellularization solution | 0.25% wt/vol sodium deoxycholate + 0.5% vol/vol Triton X‐100 in 100 ml PBS supplemented with 1% CAS | 0.25% wt/vol sodium deoxycholate + 0.5% vol/vol Triton X‐100 in 100 ml PBS supplemented with 1% CAS | Incubated for 4 days on a Platform Rocker STR6 at 40 REV/min |
| 5) Washing (twice) | Sterile PBS | Sterile PBS | 30 min each |
| 6) Storage of decellularized matrices | At 4°C in sterile PBS supplemented with 1% vol/vol P/S | At 4°C in sterile PBS supplemented with 1% vol/vol P/S | Upto 1 month |
Abbreviations: CAS, Cambridge Antibiotic Solution; PBS, phosphate‐buffered saline; P/S, 100 IU/ml penicillin, 100 μg/ml streptomycin; SVT, sheep vaginal tissue.
2.3. Isolation and culture of sheep vaginal epithelial cells and fibroblasts
SVT was used for isolating vaginal epithelial cells and fibroblasts and cultured following the technique of Rheinwatd and Green. 16 Briefly, 0.2 cm2 tissue sections were incubated at 4°C overnight in 0.1% wt/vol Difco trypsin (Sigma‐Aldrich). The underlying lamina propria was separated and epithelial cells were collected by scraping the epithelium and the upper surface of the lamina propria. Freshly isolated epithelial cells were centrifuged (1000 rpm, 5 min), resuspended in fresh Green's media (Table 2), and cultured with a feeder layer of irradiated murine 3T3 fibroblasts at 37°C, 5% CO2.
Table 2.
Composition of Green's medium
| Component and supplier | Volume and stock solution | Final concentration | Storage |
|---|---|---|---|
| Dulbecco's modified Eagle's medium (GIBCO) | 330 ml | 66% vol/vol | 4°C |
| Nutrient mixture F12 (Ham's F12) (GIBCO) | 108 ml | 21.6% vol/vol | 4°C |
| Fetal bovine serum (Advanced Protein Products Ltd.) | 50 ml | 10% vol/vol | −20°C |
| Penicillin/streptomycin | 5 ml of 10 000 IU/ml penicillin and 10 000 μg/ml streptomycin | 100 IU/ml penicillin 100 μg/ml streptomycin | −20°C |
| Amphotericin B | 1.25 ml of 250 μg/ml | 0.625 μg/ml | −20°C |
| Adenine | 2 ml of 6.25 μg/ml | 0.025 μg/ml | −20°C |
| Insulin | 2.5 ml of 1 mg/ml | 5 μg/ml | 4°C |
| 3,3,5‐triiodothyronine/apotransferrin | 0.5 ml of 1.36 μg/ml T3 and 5 mg/ml apotransferrin | 1.36 ng/ml T3 and 5 μg/ml apotransferrin | −20°C |
| Hydrocortisone | 80 μl of 2.5 mg/ml | 4 μg/ml | 4°C |
| Epidermal growth factor | 25 μl of 100 μg/ml | 5 ng/ml | −20°C |
| Cholera toxin | 500 μl of 8.47 μg/ml | 8.47 ng/ml | 4°C |
Note: All components are from Invitrogen unless otherwise indicated.
Vaginal fibroblasts were isolated from the minced lamina propria of the native tissue by collagenase treatment, 0.5% wt/vol collagenase A solution (collagenase type 1, ≥125 CDU/mg solid; Sigma‐Aldrich) overnight at 37°C with 5% CO2. The solution was centrifuged (2000 rpm, 10 min), cells resuspended in fresh Dulbecco's modified Eagle's medium (Table 3), and cultured at 37°C, 5% CO2. Both cell types were grown to 70%–80% confluency, harvested by trypsinization, cryopreserved until use, and used between Passage 2 and 3 in all experiments.
Table 3.
Composition of supplemented Dulbecco's modified Eagle's medium (DMEM)
| Component and supplier | Volume and stock solution | Final concentration | Storage |
|---|---|---|---|
| DMEM (GIBCO) | 445 ml | 89% vol/vol | 4°C |
| Fetal bovine serum (Advanced Protein Products Ltd.) | 50 ml | 10% vol/vol | −20°C |
| Penicillin/streptomycin (Invitrogen) | 5 ml of 10 000 IU/ml penicillin and 10 000 μg/ml streptomycin | 100 IU/ml penicillin | −20°C |
2.4. TE in vitro vaginal models
Models were constructed using de‐epithelialised, de‐SVT as scaffolds. Decellularized tissues (generated according to protocols in Table 1) were aseptically cut (1.5 × 1.5 cm2), placed in a six‐well cell culture plate with stainless steel rings on top, and seeded with a coculture of vaginal fibroblasts (3 × 105 cells/model) and epithelial cells (6 × 105 cells/model). The models were cultured in Green's media for 3 days under submerged conditions and then for an additional 3 weeks at an air–liquid interface (ALI) on stainless steel grids at 37°C, 5% CO2.
2.5. Assessment of cell viability
The metabolic activity of cells cultured on de‐SVT scaffolds and human decellularized dermis (collected under NHS research ethics approval YH/15/0177) was measured by resazurin assay as previously described. 17 Absorbance at λ570 nm was measured in a colorimetric plate reader (Bio‐TEK; NorthStar Scientific, Ltd.) after 24 h, 7 and 14 days in culture at ALI. Cell‐free decellularized tissues were included as controls. At Day 14, samples were fixed with 3.7% formaldehyde for subsequent histological analysis.
2.6. Histology
2.6.1. Hematoxylin and eosin staining
All tissue sections were processed in Leica TP 1020 Tissue Processor. Samples embedded in paraffin (using HistoCore Arcadia; Leica Biosystems) were cut (5 μm thick) using a microtome (HistoCore AUTOCUT; Leica Biosystems) and mounted on Polysine™ adhesion microscopic slides (Epredia). Hematoxylin and eosin staining was performed according to the protocol described by Suzuki et al. 18 Slides were observed and imaged with a light microscope (Motic).
2.6.2. Picosirius red staining
Collagen analysis of decellularized tissues was performed by picosirius red staining on 5.0‐μm‐thick tissue sections as described previously. 19 Slides were deparaffinized and rehydrated in a series of ethanol dilutions and then stained with Weigert's hematoxylin for 8 min at room temperature (RT) followed by washing. Picosirius red stain (0.1% Direct Red 80 [Sigma‐Aldrich]) was added to sections, incubated for 1 h at RT, and washed with two changes of acidified water. Slides were dehydrated and mounted to be imaged with a light microscope (Motic).
2.7. Examination of the effect of estradiol‐17β [E2] on the TE vaginal models
Estradiol‐17β (Sigma‐Aldrich) stock solution was prepared by solubilising estradiol in absolute ethanol (conc. 2 × 10−3 mg/ml), then a second stock solution (conc. 2000 pg/ml) was prepared in sterile PBS using serial dilutions. The working solutions were prepared in Green's media at five concentrations (25, 50, 100, 200, and 400 pg/ml). These concentrations were based on clinical definitions of borderline (11–44 pg/ml), normal menstruating female follicular phase (21–251 pg/ml), and “supraphysiological” (258–498 pg/ml) estradiol‐17β serum levels, as recommended by the Endocrine Society guidelines. 20
2.8. Immunohistofluorescence/immunofluorescence
Ki67, and cytokeratin 10 (cyt10) and cyt19 expressions in TE vaginal models (SVT) and estradiol‐17β induced TE models was measured. Slides were deparaffinized and rehydrated using a graded series of ethanol (100% to 95% to 70%) and antigen retrieval was performed by adding 100 μl of trypsin/CaCl2 solution onto each tissue section in a humidified chamber for 20 min at 37°C. Slides were washed with PBS at RT and sections permeabilised by adding 0.5% Tween20 (20 min at RT). Followed by 30 min incubation with a serum‐free protein blocking buffer (ab64226; Abcam). Tissue sections were incubated with the primary antibody Anti‐Ki67 antibody (ab15580) (1:100 dilution; Abcam) or anti‐cyt10 antibody [EP16071HCY]‐(ab76318) (1:100 dilution; Abcam) or cyt19 antibody [NBP1‐78278SS] (1:100 dilution; Novus Biologicals) overnight in a humidified chamber. Slides were then incubated with the secondary antibody; donkey anti‐rabbit IgG H&L (λex 652 nm, λem 668 nm) (Alexa Fluor® 647) preadsorbed (ab150063) (1:200 dilution; Abcam) for 1 h at RT and counterstained with 4′,6‐diamidino‐2‐phenylindole (λex 359 nm, λem 457 nm) (ab228549) (1:800 dilution; Abcam), mounted with DPX, and imaged with an epifluorescence microscope (Olympus IX73).
2.8.1. Imaging
Samples were imaged using an Olympus IX73 inverted microscope (Life Science Solutions, GB) and a RETIGA 6000 (Imaging®) interfaced to a Dell computer using Micro‐Manager V 1.4.23 20210215 software and analyzed using ImageJ software (National Institute of Health).
2.9. Statistics
Statistical analysis was performed using GraphPad Prism V9.1.0 (216). A one‐way analysis of variance was performed using Welch test to analyze the difference between the means (represented as mean ± SD) of groups. Three or more groups were compared using Dunnett's post hoc test and a p < 0.05 was considered statistically significant for differences between means of each group. All experiments were run in triplicate (N = 3) with three samples for each parameter (n = 9).
3. RESULTS
de‐SVT was obtained after 5 days treatment using two different decellularization treatments: 1) detergent method and 2) hypertonic + detergent method (Figure 1A). The de‐SVT samples were delicate on handling and the epithelium came off easily following both decellularization protocols. Isolated primary vaginal epithelial cells and fibroblasts depicted typical morphological characteristics when observed under light microscope (Figure 1B).
Figure 1.
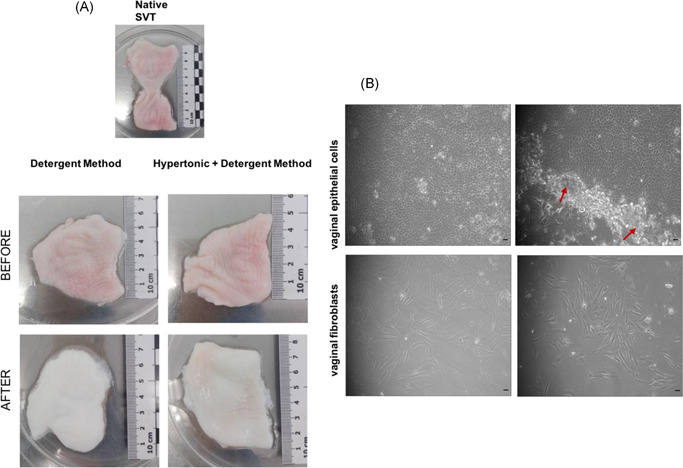
(A) Gross appearance of the sheep vaginal tissue (SVT) before and after decellularization in two different solutions showing visible differences in appearance as the decellularized tissue is whiter in color and delicate upon handling with forceps compared to the native SVT. (B) Light microscopy images of primary sheep vaginal epithelial cells and fibroblasts isolated from sheep vagina under ×10 magnification. Epithelial cells showing cobblestone‐like appearance when cultured on irradiated mouse 3T3s (shown with red arrow heads surrounding the colonies). Vaginal fibroblasts showing flat, elongated morphology (scale bar = 100 μm)
Histological analysis of the de‐SVT showed complete epithelium removal following both treatments (Figure 2A); however, the detergent method was more effective in complete removal of cellular components in the lamina propria. No purple stained nuclei could be seen in the detergent method samples compared to the hypertonic + detergent method where nuclei of vaginal fibroblasts were still present after 5 days of treatment. Picosirius red staining showed that the collagen was largely preserved by both decellularization methods. de‐SVT from the detergent method supported higher cellular metabolic activity of cultured primary sheep vaginal fibroblasts and epithelial cells compared to both de‐epithelialised dermis and de‐SVT from the hypertonic + detergent method (SVT‐B) (Figure 2B). Thus, all subsequent experiments were performed on de‐SVT processed with only the detergent method.
Figure 2.
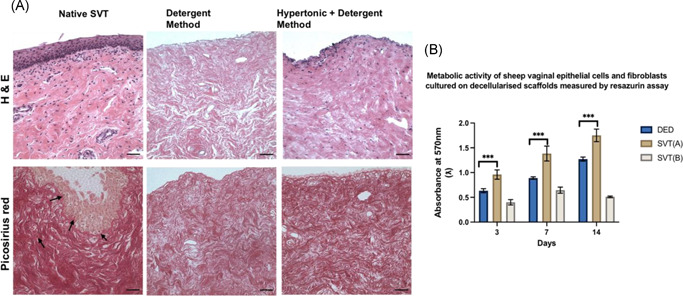
(A) Hematoxylin and eosin (H&E) stained sections of sheep vaginal tissue (SVT) samples after 5 days treatment in two different decellularization solutions. Native SVT showing intact vaginal stratified squamous epithelial lining, invaginations, and underlying lamina propria. Purple stained nuclei of the basal cells forming the demarcation between the epithelial tissue above and the connective tissue below. Decellularized‐SVT (de‐SVT) by the detergent method showed removal of epithelial lining and absence of cellular components (no noticeable purple‐stained nuclei) compared to the other decellularization technique where the cellular components were not completely removed from the connective tissue layer as purple‐stained nuclei were still visible. Picosirius red staining of the native SVT showing deep red stained collagen fibers in the extracellular matrix of the native SVT and cellular components could be seen stained light brown‐colored nuclei (pointed with black arrow heads) in both epithelial layer and lamina propria. de‐SVT (by both methods) showed absence of cellular components (visibly no brown stained nuclei) with collagen (red‐colored fibers) in the matrix remained largely conserved. Scale bar = 100 μm. (B) Metabolic activity of primary sheep vaginal fibroblasts and epithelial cells cultured on both de‐SVT estimated by resazurin assay over 14 days (n = 9 ± SD for each group at each time point, ***p < 0.05). DED, de‐epithelialised dermis; SVT(A), decellularization by detergent method; SVT(B), decellularization by hypertonic + detergent method
Our recellularized models showed histological similarities to the native SVT (Figure 3A), and there was a gradual thickening of a stratified epithelium over three weeks at an ALI culture. Between Days 7 and 14, the vaginal epithelium became more stratified (7–9 layers). After 21 days, the upper layers of the TE models began to keratinize with epithelial cells appearing more flattened. The most superficial 2–3 layers of the epithelium started to flake off from the surface of the models. At Day 27, the TE vaginal models' epithelia detached from the underlying lamina propria as the integrity of the basement membrane was compromised under prolonged ALI culture. The metabolic activity of the vaginal epithelial cells and fibroblasts cultured on de‐SVT showed increasing viability up to 3 weeks in ALI culture but eventually it declined beyond 3 weeks (Figure 3B). Hence, models were not cultured beyond three weeks in subsequent experiments.
Figure 3.
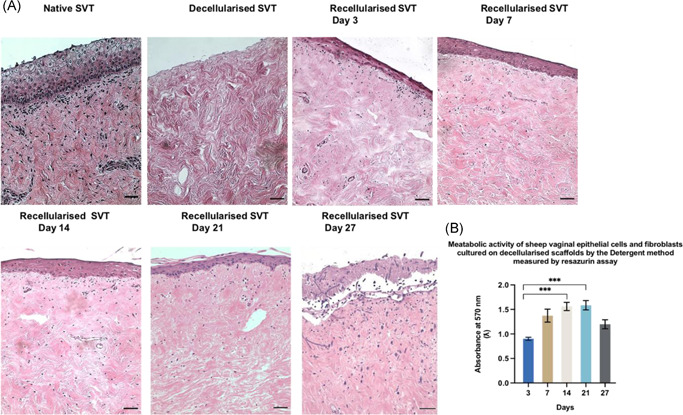
Hematoxylin and eosin stained sections of the TE sheep vaginal models. (From top left to bottom right) de‐SVT from detergent method showed absence of epithelial layer and cellular components. (A) Recellularized tissues showed a gradual formation of a stratified epithelial layer from Days 3–7 (3–5 layers) that became denser and more stratified between Days 7 and 14 (7–9 layers). At Day 21 of the ALI culture, superficial layers of the epithelia were seen more keratinized, and at Day 27, the epithelia were observed detached from the underlying matrix (scale bar = 100 μm). (B) Metabolic activity of primary sheep vaginal fibroblasts and epithelial cells cultured on the de‐SVT by the detergent method estimated by resazurin assay over 27 days at ALI culture conditions (n = 9 ± SD for each group at each time point, ***p < 0.05). ALI, air–liquid interface; de‐SVT, decellularized sheep vaginal tissue; TE, tissue engineered
Figure 4A demonstrates the effect of different concentrations of estradiol 17‐β [E2] on the TE vaginal model epithelium. Our results showed that increasing E2 concentrations (from 50 to 400 pg/ml) in the culture medium led to an increase in the epithelium thickness and stratification (Figure 4A). A higher concentration of E2 (from 100 to 400 pg/ml) also had a significant effect on cellular metabolic activity on the models compared to the control (without E2) (Figure 4B).
Figure 4.
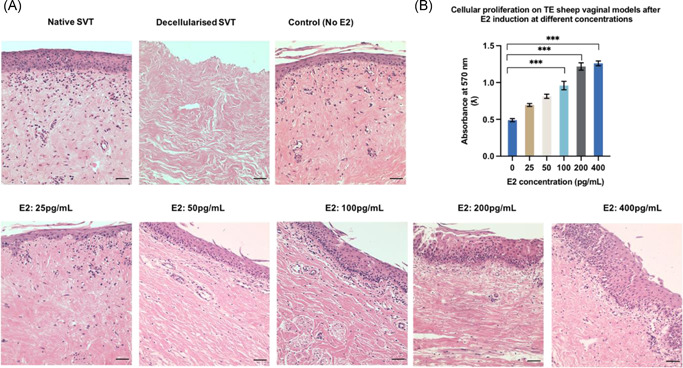
(A) Hematoxylin and eosin stained sections of sheep TE vaginal models after estradiol induction on models cultured at air–liquid interface (ALI) upto 3 weeks. TE models treated with higher concentrations of estradiol‐17β [E2] (100–400 pg/ml) showed more stratified and cornified epithelium (10–19 layers) compared to the control (without E2) (5–7 layers). Scale bar = 100 μm. (B) Metabolic activity of primary vaginal epithelial cells and fibroblasts seeded on decellularized‐SVT estimated by resazurin assay after 3 weeks at ALI under E2 induction (n = 9 ± SD for each group, ***p < 0.05). SVT, sheep vaginal tissue; TE, tissue engineered
Immunohistofluorescence detection of Ki67 showed that there were more proliferative cells in models cultured with increasing dose of E2 (Figure 5). Higher concentrations of E2 (50–400 pg/ml) increased the number of Ki67 positive cells in both the epithelium and the lamina propria of the TE vaginal models compared to the control (without E2) as shown in Figure 5.
Figure 5.
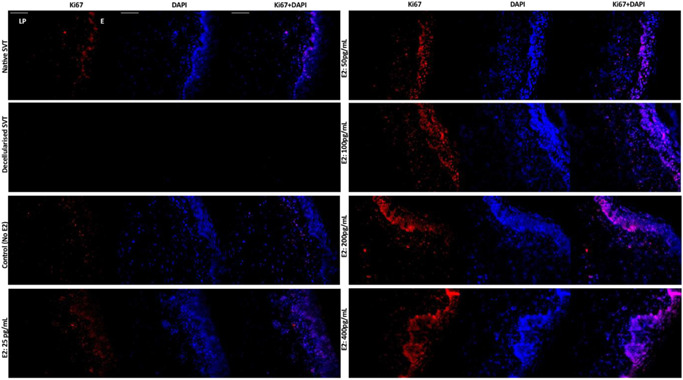
Immunohistofluorescence detection of Ki67, a marker for proliferative cells in the TE sheep vaginal models. The basal cells of the vaginal epithelium are stained intensely for Ki67 (red channel) in the native sheep vaginal tissue (top left), whereas no cellular expression for either Ki67 or DAPI could be seen in the decellularized sheep vaginal tissue (SVT) (negative control). In the reconstructed TE vaginal models, under estradiol‐17β [E2] induction, a dose‐dependent response of cells positive for Ki67 could be seen with an increase in E2 concentration. An increase in E2 concentration (from 50 to 400 pg/ml) showed an increase in the intensity and number of positive cells for Ki67 in both the vaginal epithelium (E) and the underlying lamina propria (LP). All tissue sections were counterstained with DAPI (blue channel). Scale bar = 100 μm (applies to all). DAPI, 4′,6‐diamidino‐2‐phenylindole; TE, tissue engineered
Staining for cyt10 was observed in the superficial and suprabasal epithelium of the native SVT and TE models (Figure 6). Models cultured in all E2 concentrations (25–400 pg/ml) showed more cyt10 positive cells compared to the untreated models. cyt19 was not expressed in the native SVT or TE models treated with no or low levels of E2 (0–50 pg/ml) (Figure 7). The expression of cyt19 in the suprabasal and superficial epithelium of TE models cultured in high concentrations of E2 (100–400 pg/ml) indicated an abnormal pattern of keratin production.
Figure 6.
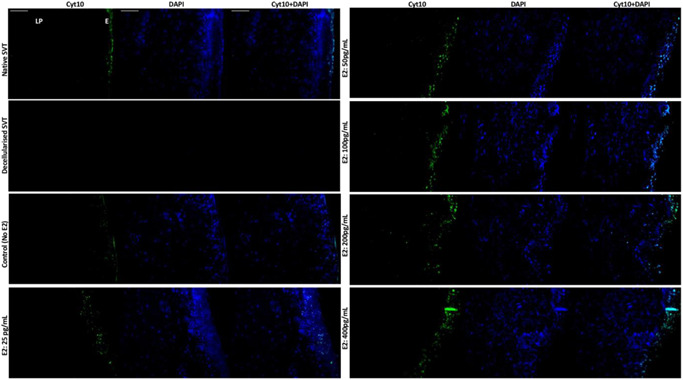
Immunohistofluorescence detection of cytokeratin 10 (cyt10), a marker of stratification in the TE sheep vaginal models. The suprabasal cells and the superficial layers of the vaginal epithelium are stained intensely for cyt10 (green channel) in the native sheep vaginal tissue (top left), whereas no cellular expression for either cyt10 or DAPI could be seen in the decellularized sheep vaginal tissue (SVT) (negative control). In the reconstructed TE vaginal models, under estradiol‐17β [E2] induction, a dose‐dependent response of cells positive for cyt10 could be seen. Higher E2 concentration (from 50 to 400 pg/ml) showed an increase in the intensity and number of positive cells for the expression of cyt10 in the parabasal layers of the vaginal epithelium (E) while none of the vaginal fibroblasts in the lamina propria (LP) were positive for the cyt10 expression. All tissue sections were counterstained with DAPI (blue channel). Scale bar = 100 μm (applies to all). DAPI, 4′,6‐diamidino‐2‐phenylindole; TE, tissue engineered
Figure 7.
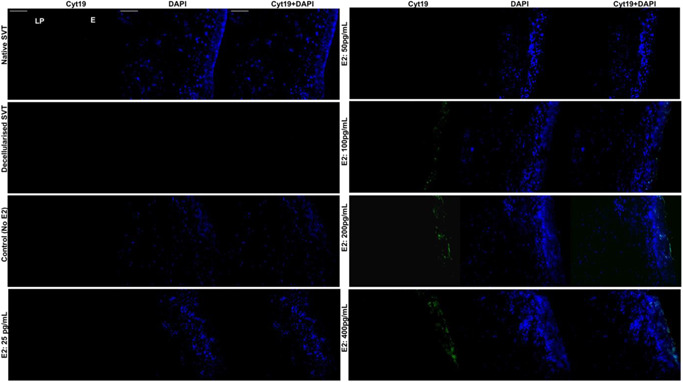
Immunohistofluorescence detection of cytokeratin 19 (cyt19) in the TE sheep vaginal models. The native SVT did not show any cellular expression for cyt19 (either in the epithelium [E] or the lamina propria [LP]); however, the cultured cells were exhibiting DAPI (blue channel) expression (top left). In the decellularized SVT, both cyt19 and DAPI staining was absent (negative control). In the reconstructed TE sheep vaginal models, without estradiol‐17β [E2] and under low E2 concentrations (25–50 pg/ml), there was no cyt19 expression observed from the cultured cells. However, an increase in the E2 concentration (from 100 to 400 pg/ml) induced the epithelial cells present in the transient suprabasal and superficial layers of the TE models' epithelia (E) to express cyt19 in a dose‐dependent manner (green channel). All tissue sections were counterstained with DAPI (blue channel). Scale bar = 100 μm (applies to all). DAPI, 4′,6‐diamidino‐2‐phenylindole; SVT, sheep vaginal tissue; TE, tissue engineered
4. DISCUSSION
In the field of women's reproductive health research, there is an urgent need to access affordable and physiologically relevant preclinical models of vaginal tissue which can be used as an alternative to animal testing, in line with the 3Rs principles. In this study we have developed a TE vaginal model which is structurally similar to the native vaginal tissues of sheep and human. 21 Our models comprised of de‐SVT cultured with primary sheep vaginal epithelial cells and fibroblasts making them accessible, ethically sound and affordable. We have demonstrated that our model was estradiol‐17β [E2] responsive and that it has several advantages over existing preclinical models used in vaginal tissue research.
Existing commercially available in vitro TE vaginal models such as EpiVaginal™ or the HVE models are used in the research community for toxicity and irritability studies of vaginal formulations and understanding the transmission of sexually transmitted infections. 9 These tools are extremely valuable, however, they have several limitations which we aimed to overcome in this study. These models can be prohibitively expensive, have limited culture times, do not include physiologically relevant lamina propria components, and either rely on immortalized cell lines or regular access to human tissue samples. In contrast, our model presented here is developed using cultured expanded primary sheep vaginal cells on decellularized vaginal tissues that showed gradual formation of a stratified epithelium in culture and the resulting vaginal constructs resembled the native SVT histologically.
Managing the ethical, legal, and logistical challenges associated with accessing human tissues can be a barrier to many researchers. Furthermore, small sample size and variability among patients is a serious limitation. Hence, we developed a vaginal model utilizing waste animal tissue from the meat industry, making it widely accessible and affordable. SVT and human vaginal tissue have comparable reproductive anatomy, equivalent epithelial morphology, and structural similarities. 22 For this reason, sheep are commonly used in in vivo studies on vaginal repair as they have similar pelvic tissue components with that of humans. 23 The cultured expanded sheep vaginal cells could be obtained in large volumes and genetically matched models could be created, improving the reproducibility and volume of experiments which could be performed.
Decellularized scaffolds retain the complex biomolecular and physical cues of the native tissue extracellular matrix, that have previously been shown to redirect cell growth and viability by providing a natural microenvironment. 24 Our vaginal matrices were successfully decellularized in 5 days using low concentrations of detergent mix and physical rocking. This technique is much faster compared to the previous reports by Zhang et al., 25 where 10 days was needed for successful decellularization of porcine vaginal tissue. Current vaginal models rely on collagen gels (VEC‐100‐FT and VLC‐100‐FT by MatTek) or polycarbonate membrane filters (SkinEthic™ HVE from EpiSkin) which are not as relevant as the decellularized tissues presented here.
The HVE is composed of 7–14 layers of nonkeratinised squamous epithelial cells which are regulated by the ovarian steroids oestradiol, testosterone and progesterone. 26 Buchanan et al. 12 first explored the role of estradiol 17‐β [E2] in promoting vaginal epithelium stratification, proliferation, and cytodifferentiation in in vivo mouse models. Similar results were reported in the current study which is the first in vitro model to mimic the in vivo physiological response of vaginal epithelium towards E2 induction. In the absence of E2, the vaginal epithelia of our models consisted of 3–5 layers of squamous epithelial cells while models treated with higher E2 concentrations displayed an increase in the epithelium thickness (10–19 layers), enhanced cell proliferation, and an increase in cornified cells in the superficial layers. This data shows that our models closely resemble the native vaginal tissue in terms of oestradiol responsiveness and hence can be a valuable preclinical model in vaginal research where hormone responsive behavior is desirable.
Expression of Ki67 in our models cultured with and without E2 showed a pattern of expression similar to those observed in human vaginal tissue samples. 27 We observed Ki67 expression in cells within the lamina propria and the basal and suprabasal layers of the epithelium. As our models were treated with increasing doses of E2, Ki67 expression increased demonstrating higher levels of epithelial cell proliferation which, in turn, increased epithelium thickness. We also compared the expression of cyt10 and cyt19 in our models with that in the native human vaginal tissue reported in the literature. cyt10 is typically expressed in the suprabasal layers of cornifying stratified epithelia. 28 In our models, cyt10 expression is observed with a similar distribution and intensity as in the previously reported in vivo studies. 12
The expression of cyt19 in our models suggests that a few epithelial cells in the suprabasal transient and superficial layers of the epithelium acquired an abnormal phenotype. Previously, the expression of cyt19 in different epithelial layers in 3D in vitro cervical cancer tissue models 29 and from MCF‐7 cells culture have been reported in literature. 30 The expression of cyt19 in our models suggests that this might be a transitional epithelium. Our models could be used to carry out further studies on the dedifferentiation and abnormal epithelial transformation under “supraphysiological” concentrations of E2 as these models allows us to culture normal epithelial cells under a range of physiological conditions for 3 weeks.
The mechanism of E2‐induced epithelial cell proliferation has clinical implications as this is the key mechanism regulating the controlled cellular differentiation processes in the female genitalia and any alterations in the normal regulatory process may lead to the development of endometrial, vaginal, and/or cervical cancers. 31 To the best of our knowledge, our model is the first in vitro hormone‐responsive model of animal origin that depicts the fundamental role of estradiol‐17β [E2] comparable to other in vivo studies as well as in the native tissue.
5. CONCLUSION
In conclusion, we have developed a full thickness in vitro TE vaginal model based on SVT and primary sheep vaginal epithelial cells and fibroblasts that closely mimics the physiological attributes of the native vaginal tissue. This new model overcomes many of the limitations seen in the existing tools for vaginal tissue research as it incorporates a physiologically relevant lamina propria component, can be cultured for up to 3 weeks, and is more accessible and affordable, expanding its potential use for a range of applications. Our models showed estradiol‐17β [E2] responsiveness in a dose‐dependent manner resulting in changes in the vaginal epithelium thickening, differentiation, and cellular proliferation. These features have confirmed the applicability of our model as a valuable preclinical tool in vaginal tissue research.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
All research is undertaken under the University of Sheffield's research ethics policy (Where patients are involved or experimental animals, then these are governed by patient ethical code of conduct and UK Home Office regulations for the conduct of animal studies using experimental animals). As later explained, neither patients or animals were used in this study. This study did not involve any investigations of patients rather it used animal tissues (sheep) available commercially from local abattoirs.
AUTHOR CONTRIBUTIONS
All authors contributed to the broad design of the study. All experimental work was conducted by PhD student Sarah Shafaat. All authors were involved in preparing the manuscript for submission.
ACKNOWLEDGMENTS
Sarah Shafaat was funded by the University of Sheffield for a Prize Studentship. Dr. Naside Mangir was funded by the Royal Academy of Engineering/Turkish Government. The authors would like to thank the University of Sheffield for a Prize Studentship and the Royal Academy of Engineering/Turkish Government.
Shafaat S, Mangir N, Chapple C, MacNeil S, Hearnden V. A physiologically relevant, estradiol‐17β [E2]‐responsive in vitro tissue‐engineered model of the vaginal epithelium for vaginal tissue research. Neurourol Urodyn. 2022;41:905‐917. 10.1002/nau.24908
DATA AVAILABILITY STATEMENT
All raw data will be made available on a University of Sheffield shared file repository system
REFERENCES
- 1. Siddique SA. Vaginal anatomy and physiology. Female Pelvic Med Reconstr Surg. 2003;9(6):263‐272. [Google Scholar]
- 2. Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85(1):97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mangir N, Dikici BA, Chapple CR, MacNeil S. Landmarks in vaginal mesh development: polypropylene mesh for treatment of SUI and POP. Nat Rev Urol. 2019;16(11):675‐689. [DOI] [PubMed] [Google Scholar]
- 4. Chapple CR, Cruz F, Deffieux X, et al. Consensus statement of the European Urology Association and the European Urogynaecological Association on the use of implanted materials for treating pelvic organ prolapse and stress urinary incontinence. Eur Urol. 2017;72(3):424‐431. [DOI] [PubMed] [Google Scholar]
- 5. Costin G‐E, Raabe HA, Priston R, Evans E, Curren RD. Vaginal irritation models: the current status of available alternative and in vitro tests. Altern Lab Anim. 2011;39(4):317‐337. [DOI] [PubMed] [Google Scholar]
- 6. Merbah M, Introini A, Fitzgerald W, et al. Cervico‐vaginal tissue ex vivo as a model to study early events in HIV‐1 infection. Am J Reprod Immunol. 2011;65(3):268‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayehunie S, Cannon C, Lamore S, et al. Organotypic human vaginal‐ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine‐care products. Toxicol In Vitro. 2006;20(5):689‐698. [DOI] [PubMed] [Google Scholar]
- 8. Ayehunie S, Cannon C, LaRosa K, Pudney J, Anderson DJ, Klausner M. Development of an in vitro alternative assay method for vaginal irritation. Toxicology. 2011;279(1‐3):130‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kandarova H, Ayehunie S, Cannon C, Larosa K, Hayden P, Klausner M. Use of the organotypic EpiVaginalTM tissue model to screen irritation potential of feminine hygiene ingredients and formulations. Toxicol Lett. 2010;196:S152‐S153. [Google Scholar]
- 10. Amelian A, Wasilewska K, Megias D, Winnicka K. Application of standard cell cultures and 3D in vitro tissue models as an effective tool in drug design and development. Pharmacol Rep. 2017;69(5):861‐870. [DOI] [PubMed] [Google Scholar]
- 11. Couri BM, Lenis AT, Borazjani A, Paraiso MFR, Damaser MS. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev Obstet Gynecol. 2012;7(3):249‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buchanan DL, Kurita T, Taylor JA, Lubahn DB, Cunha GR, Cooke PS. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology. 1998;139(10):4345‐4352. [DOI] [PubMed] [Google Scholar]
- 13. Cotreau MM, Chennathukuzhi VM, Harris HA, et al. A study of 17β‐estradiol‐regulated genes in the vagina of postmenopausal women with vaginal atrophy. Maturitas. 2007;58(4):366‐376. [DOI] [PubMed] [Google Scholar]
- 14. Van Bommel PFJ, Kenemans P, Helmerhorst TJM, Gallee MPW, Ivanyi D. Expression of cytokeratin 10, 13, and involucrin as prognostic factors in low stage squamous cell carcinoma of the uterine cervix. Cancer. 1994;74(8):2314‐2320. [DOI] [PubMed] [Google Scholar]
- 15. Bártek J, Bártková J, Taylor‐Papadimitriou J, et al. Differential expression of keratin 19 in normal human epithelial tissues revealed by monospecific monoclonal antibodies. Histochem J. 1986;18(10):565‐575. [DOI] [PubMed] [Google Scholar]
- 16. Rheinwatd JG, Green H. Seria cultivation of strains of human epidemal keratinocytes: the formation keratinizin colonies from single cell is. Cell. 1975;6(3):331‐343. [DOI] [PubMed] [Google Scholar]
- 17. Czekanska EM. Assessment of cell proliferation with resazurin‐based fluorescent dye. In: Stoddart MJ, ed. Mammalian Cell Viability: Methods and Protocols. Humana Press; 2011:27‐32. 10.1007/978-1-61779-108-6_5 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki Y, Imada T, Yamaguchi I, et al. Effects of prolonged water washing of tissue samples fixed in formalin on histological staining. Biotech Histochem. 2012;87(4):241‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Junqueira LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447‐455. [DOI] [PubMed] [Google Scholar]
- 20. Leinung MC, Feustel PJ, Joseph J. Hormonal treatment of transgender women with oral estradiol. Transgender Heal. 2018;3(1):74‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCracken JM, Calderon GA, Robinson AJ, Sullivan CN, Cosgriff‐Hernandez E, Hakim JCE. Animal models and alternatives in vaginal research: a comparative review. Reprod Sci. 2021;28:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144:S146‐S158. [DOI] [PubMed] [Google Scholar]
- 23. Emmerson S, Mukherjee S, Melendez‐Munoz J, et al. Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials. 2019;225:119495. [DOI] [PubMed] [Google Scholar]
- 24. Hoshiba T, Lu H, Kawazoe N, Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10:1717‐1728. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J‐K, Du R‐X, Zhang L, et al. A new material for tissue engineered vagina reconstruction: acellular porcine vagina matrix. J Biomed Mater Res, Part A. 2017;105(7):1949‐1959. [DOI] [PubMed] [Google Scholar]
- 26. Ildgruben AK, Sjöberg IM, Hammarström M‐LKC. Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet Gynecol. 2003;102(3):571‐582. [DOI] [PubMed] [Google Scholar]
- 27. Baldassarre M, Giannone FA, Foschini MP, et al. Effects of long‐term high dose testosterone administration on vaginal epithelium structure and estrogen receptor‐α and ‐β expression of young women. Int J Impot Res. 2013;25(5):172‐177. [DOI] [PubMed] [Google Scholar]
- 28. Huszar M, Gigi‐Leitner O, Moll R, Franke WW, Geiger B. Monoclonal antibodies to various acidic (type I) cytokeratins of stratified epithelia: selective markers for stratification and squamous cell carcinomas. Differentiation. 1986;31(2):141‐153. [DOI] [PubMed] [Google Scholar]
- 29. Zuk AK, Wen X, Dilworth S, Li D, Ghali L. Modeling and validating three dimensional human normal cervix and cervical cancer tissues in vitro. J Biomed Res. 2017;31(3):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi I, Gudas LJ, Katzenellenbogen BS. Regulation of keratin 19 gene expression by estrogen in human breast cancer cells and identification of the estrogen responsive gene region. Mol Cell Endocrinol. 2000;164(1‐2):225‐237. [DOI] [PubMed] [Google Scholar]
- 31. Chung S‐H, Franceschi S, Lambert PF. Estrogen and ERα: culprits in cervical cancer? Trends Endocrinol Metab. 2010;21(8):504‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data will be made available on a University of Sheffield shared file repository system


