Abstract
Patients with relapsed warm antibody autoimmune hemolytic anemia (wAIHA) have limited treatment options. Fostamatinib is a potent, orally administered spleen tyrosine kinase inhibitor approved in the United States and Europe for the treatment of adults with chronic immune thrombocytopenia (ITP). This phase 2 study evaluated the response to fostamatinib, administered at 150 mg BID orally with or without food in adults with wAIHA and active hemolysis with hemoglobin (Hgb) <10 g/dL who had failed at least one prior treatment. Hemoglobin levels and safety assessments were performed at visits every 2 weeks. The primary endpoint was Hgb >10 g/dL with an increase of ≥2 g/dL from baseline by week 24 without rescue therapy or red blood cell transfusion. Eleven of 24 (46%) patients achieved the primary endpoint. Increases in median Hgb were detected at week 2 and sustained over time. Median lactate dehydrogenase levels and reticulocyte counts generally declined over time with little change in median haptoglobin levels. The most common adverse events (AEs) were diarrhea (42%), fatigue (42%), hypertension (27%), dizziness (27%), and insomnia (23%). AEs were manageable and consistent with the fostamatinib safety database of over 3900 patients across multiple diseases (rheumatoid arthritis, B‐cell lymphoma, COVID‐19, and ITP). No new safety signals were detected. Fostamatinib may be a promising therapeutic option for wAIHA. A randomized, double‐blind, phase 3 study is nearing completion.
1. INTRODUCTION
Warm autoimmune hemolytic anemia (wAIHA) is an acquired disorder manifested by accelerated red blood cell (RBC) destruction due to the presence of antibodies, usually immunoglobulin G (IgG), that bind to antigens on erythrocytes at physiological temperatures and lead to red cell clearance by spleen and liver. The estimated incidence in adults is 0.8 to 3 per 100 000/year with a prevalence of 17 per 100 000 and a mortality rate of 8%–11%. 1 , 2 , 3 The incidence in the United States is approximately 13 000/year. 4 wAIHA can be either primary or secondary to an underlying disease such as an autoimmune disease (20%), lymphoproliferative disorder (20%), infection, or cancer. 5 Of all the autoimmune hemolytic anemias, 80% are due to wAIHA, with the remaining cases due to cold agglutinin disease (CAD or cold AIHA); up to 30% of patients have mixed disease (warm and cold AIHA). 6
The accelerated clearance of circulating IgG‐coated RBCs by immunoglobulin Fc receptor (FcR) bearing macrophages in the spleen and liver is thought to be the pathogenic mechanism in wAIHA. 7 Immunoglobulin FcRs involved in the recognition of Ig‐coated particles are expressed on all phagocytic cells and play an important role in antibody‐mediated immune responses. 8 They are responsible for such functions as endocytosis, phagocytosis, reactive mediator release, and cell activation/cytotoxicity. 9 Activation of the FcR is associated with a signaling subunit, FcRγ, whose phosphorylation subsequent to receptor binding results in the recruitment and activation of spleen tyrosine kinase (SYK) (Figure S1). 10 , 11 Activated SYK mediates downstream signaling of the activated FcRs in phagocytic cells, resulting in phagocytosis of RBCs. 12 In addition, activation of SYK through the B‐cell receptor (BCR) mediates activation and differentiation of B lymphocytes into antibody secreting plasma cells. 13 , 14 Therefore, inhibition of SYK has potential effects in the treatment of wAIHA through inhibition of phagocytosis and reduction of antibody production.
Fostamatinib disodium hexahydrate is an orally available inhibitor of SYK and consequently inhibits the FcR and BCR signaling pathways. It is indicated for the treatment of adult patients with chronic immune thrombocytopenia (ITP). Fostamatinib is a prodrug that is rapidly converted to R406 in vivo. R406 is a reversible, biologically active, potent inhibitor of immunoglobulin E (IgE)‐ and IgG‐mediated activation of FcR signaling, with the primary target of R406 identified as SYK. 10 Inhibition of SYK was protective against the development of thrombocytopenia and anemia in mouse models of ITP and AIHA, respectively. 10 , 11 Preclinical data have demonstrated that fostamatinib treatment significantly (p < .05) protected against antibody‐induced anemia in mice compared to vehicle. 11 Fostamatinib prevented RBC loss in mice via inhibition of autoantibody‐directed RBC destruction in AIHA, providing a rationale for its clinical investigation.
In previous clinical studies, fostamatinib was evaluated in patients with rheumatoid arthritis (RA), solid tumors, B‐cell malignancies, and ITP. In RA patients, fostamatinib (100–150 mg, twice daily [BID]) was effective in reducing joint tenderness and swelling and in improving functional outcomes. 15 In non‐Hodgkin lymphoma and chronic lymphocytic leukemia patients, fostamatinib (200 mg BID) inhibited a critical B‐cell lymphoma survival pathway, which resulted in tumor cell death and clinical responses. 16 In ITP patients, fostamatinib provided clinical benefit (improved platelet counts, reduced bleeding episodes, and reduced rescue therapy) at doses ranging from 100 to 150 mg orally BID in phase 2 and phase 3 studies. 17 , 18 The approved dosing for ITP is 100 mg BID with an increase to 150 mg BID at 4 weeks or later if needed and tolerated. To maximize the potential for response in patients with wAIHA who have few or no alternative treatment options, the starting oral dose for this study was 150 mg BID. This report describes a phase 2 multicenter, open‐label study that evaluated the response to and safety of fostamatinib treatment in patients with wAIHA who had failed at least one prior treatment regimen.
2. METHODS
2.1. Patients
Eligible patients were ≥18 years of age and had a diagnosis of primary or secondary wAIHA. At screening, patient's Hgb level had to be <10.0 g/dL, with a direct antiglobulin test (DAT) positive for IgG, haptoglobin <10 mg/dL, and lactate dehydrogenase >ULN. Patients also had failed at least one prior AIHA treatment regimen. Any other underlying disease was stable/controlled prior to study entry.
Exclusion criteria included cold antibody AIHA, platelet count of <30 000/μL, or secondary AIHA if the underlying disease was not stable or not well‐controlled on current therapy. The study excluded patients with uncontrolled or poorly controlled hypertension, major cardiovascular event within 6 months prior to treatment start, history of deep venous thrombosis, significant infection, acute gastrointestinal symptoms, positive results for HIV, hepatitis B, or hepatitis C virus.
Patients were allowed to continue one concomitant medication for AIHA including corticosteroids, azathioprine, or an erythropoiesis stimulating agent (ESA) if they were on a stable dose for at least 14 days prior to baseline. Patients were to remain on the allowed concomitant AIHA treatment at the same dose during the study. All other AIHA therapeutic agents were discontinued for a minimum required interval prior to starting treatment: 7 days for IVIg, RBC transfusion; 14 days for cyclosporine, mycophenolate mofetil; 6 weeks for rituximab or other anti‐CD20 monoclonal antibody; 8 weeks for alkylating agents; and 30 days or 5 half‐lives (whichever is greater) for investigational agents. Due to the potential for drug–drug interactions with fostamatinib, the following treatments were not permitted or were restricted during the study: CYP3A4 inhibitors and inducers, P‐glycoprotein substrate, and HMG‐CoA reductase inhibitors.
2.2. Study design
The study consisted of two phases: an initial treatment phase and an extension phase. The original treatment period was 12 weeks based on time to response in patients with ITP, which was increased to 24 weeks in a protocol amendment to allow for a potentially longer time to response in patients with AIHA. Some patients completed the study at 12 weeks prior to the protocol amendment. If patients had either met the primary endpoint or shown a beneficial trend in Hgb and tolerated the study drug, they could receive treatment with fostamatinib on their same treatment regimen during the extension phase upon completion of the initial treatment phase. The extension phase continued until the last patient completed the initial treatment phase. The study is now complete, with patients treated for up to 30 months.
Enrolled patients initiated fostamatinib at 150 mg BID. Patients administered the study drug orally once in the morning and once in the evening, with or without food, throughout the treatment period. For patients experiencing adverse events (AEs) requiring dose reduction, the 150 mg BID dose could be adjusted to 100 mg BID, 150 mg QD, or 100 mg QD, or discontinued. For patients receiving QD dosing, fostamatinib was administered in the morning. Hemoglobin levels, hemolysis parameters (LDH, haptoglobin, reticulocyte count), and safety assessments were conducted at each clinic visit. Patients were seen every 2 weeks for the first 12 weeks, every third week till week 24, and every 6 weeks during the extension phase.
2.3. Study endpoints and analysis
These analyses are based on a February 2020 data cut‐off date. The primary endpoint was the proportion of patients who achieved a hemoglobin response by week 24 or, prior to the protocol amendment, by week 12; hemoglobin response was defined as a Hgb level of >10 g/dL and ≥2 g/dL higher than the baseline Hgb, not attributable to RBC transfusion or other rescue medication. A post hoc endpoint was an increase in Hgb level of ≥1.5 g/dL from baseline, not attributable to either RBC transfusion or other rescue medication. Hemoglobin values obtained within 14 days of an RBC transfusion or within 28 days of rescue treatment were attributed to RBC transfusion or rescue treatment. Allowed rescue therapies included RBC transfusion, IVIg (up to 1 g/kg/day for 1–3 days), IV methylprednisolone (up to 1 g/day for 1–3 days), oral dexamethasone (up to 40 mg/day for 1–2 days), or oral prednisone (up to 1 mg/kg/day for 1–3 days).
The safety population included all patients who received ≥1 dose of fostamatinib (n = 26). The efficacy evaluable population included all patients who received ≥75% of intended doses of fostamatinib unless they discontinued due to AEs (n = 24). Two patients who received ≤75% of intended doses and were part of the initial cohort (n = 17) were replaced per protocol.
2.4. Statistical analyses
The sample size for the study was determined from the following specifications: use of Simon's two‐stage study design, achievement endpoint of Hgb response by week 24, one‐sided exact binomial test, unacceptable response rate (p 0) ≤20%, acceptable response rate (p 1) ≥40%, one‐sided significance level α = 0.01, and power = 90%. Based on these specifications, 17 patients were to be enrolled in stage 1. If fewer than four patients achieved a Hgb response by week 24, lack of response would be concluded. Otherwise, the null hypothesis would be rejected, and the primary endpoint was achieved. These critical values were determined based upon values of 0.10 for both alpha (type I error) and beta (type II error). Descriptive statistics were provided for other endpoints and for AEs.
3. RESULTS
3.1. Patient disposition and drug exposure
Twenty‐six patients received ≥1 dose of fostamatinib. Median treatment compliance was 98.8%. Median exposure to fostamatinib was 173 (range: 32–913) days in all patients, 326 (range: 34–764) days in responders (n = 11), and 85 (range: 32–913) days in nonresponders (n = 13). Among the 26 patients treated with fostamatinib, 19 (73%) completed the initial treatment phase and 7 (27%) discontinued treatment (Figure 1). Of the 12 who entered the extension phase, 5 (42%) completed the extension phase and 7 (58%) discontinued.
FIGURE 1.
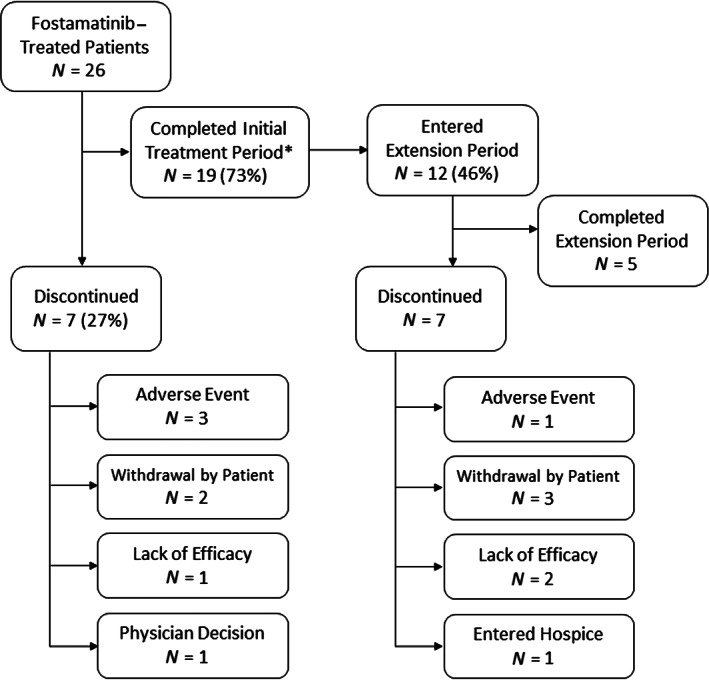
Study flow diagram. *Six patients completed the study at 12 weeks prior to the protocol amendment that extended the initial treatment period to 24 weeks
3.2. Patient demographics
Most patients had primary wAIHA (79%). Baseline characteristics and prior therapies are presented in Table 1. The mean duration of wAIHA in patients was 5.88 (median 1.85 [range: 0.3–25.6]) years at study entry, and most patients had more than one prior treatment. A higher percentage of responders were female, had secondary AIHA, and had fewer prior medications than nonresponders. There was no difference in response based on concomitant corticosteroid use. All patients had screening Hgb counts <10 g/dL, and nearly all (96%) had baseline (day 1) Hgb counts <10 g/dL.
TABLE 1.
Baseline characteristics and prior treatment of all enrolled patients
| Characteristic | Responders (N=11) | Non‐responders (N=13) | All patients (N=24) |
|---|---|---|---|
| Age, median (range) | 56.0 (30‐82) | 65.0 (27‐88) | 61.0 (27‐88) |
| Gender | |||
| Female | 8 (72.7%) | 6 (46.2%) | 14 (58.3%) |
| Male | 3 (27.3%) | 7 (53.8%) | 10 (41.7%) |
| Race | |||
| White | 9 (81.8%) | 10 (76.9%) | 19 (79.2%) |
| Asian | 1 (9.1%) | 1 (7.7%) | 2 (8.3%) |
| Other | 1 (9.1%) | 1 (7.7%) | 2 (8.3%) |
| Black or African American | 0 (0%) | 1 (7.7%) | 1 (4.2%) |
| Disease type | |||
| Primary | 7 (63.6%) | 12 (92.3%) | 19 (79.2%) |
| Secondary | 4 (36.4%) | 1 (7.7%) | 5 (20.8%) |
| Lymphoproliferative disorder | 2 (18.2%) | 1 (7.7%) | 3 (12.5%) |
| Ulcerative colitis | 1 (9.1%) | 0 | 1 (4.2%) |
| Systemic lupus erythematosus | 1 (9.1%) | 0 | 1 (4.2%) |
| AIHA duration (years) | |||
| Mean (SD) | 6.79 (8.973) | 5.12 (5.311) | 5.88 (7.103) |
| Median (range) | 1.06 (0.3, 25.6) | 2.04 (0.5, 17.9) | 1.85 (0.3, 25.6) |
| DAT positive for IgG | 10 (90.9%) | 13 (100%) | 23 (95.8%) a |
| Number of unique prior AIHA treatments | |||
| >1 treatment | 5 (45.5%) | 10 (76.9%) | 15 (62.5%) |
| 1 treatment | 6 (54.5%) | 3 (23.1%) | 9 (37.5%) |
| Prior AIHA treatments | |||
| Corticosteroids | 11 (100%) | 13 (100%) | 24 (100%) |
| Rituximab | 4 (36.4%) | 8 (61.5%) | 12 (50.0%) f |
| ESAs | 0 | 1 (7.7%) | 1 (4.2%) |
| Prior splenectomy | 2 (18.2%) | 3 (23.1%) | 5 (20.8%) |
| Concomitant AIHA treatments | |||
| Corticosteroids (prednisone) | 6 (54.5%) | 9 (69.2%) | 15 (62.5%) g |
| Azathioprine | 1 (9.1%) | 0 | 1 (4.2%) |
| ESAs | 0 | 1 (7.7%) | 1 (4.2%) |
| Hemoglobin (g/dL) median (range) b | 8.80 (6.8‐10.6) | 9.00 (7.0‐9.9) | 9.00 (6.8‐10.6) |
| ≥10 g/dL | 1 (9.1%) | 0 | 1 (4.2%) |
| <10 g/dL | 10 (90.9%) | 13 (100%) | 23 (95.8%) |
| LDH (U/L) median (range) c | 414.0 (233‐781) | 424.0 (225‐815) | 419.0 (225‐815) |
| Reticulocyte (109/L) median (range) d | 279.4 (8‐350) | 310.0 (54‐768) | 301.0 (8‐768) |
| Haptoglobin (g/L) median (range) e | 0.070 (0.07‐2.38) | 0.080 (0.06‐0.30) | 0.070 (0.06‐2.38) |
Abbreviations: IgG, Immunoglobulin G; LDH, lactate dehydrogenase.
Data not available for 1 patient.
Inclusion criteria Hgb of <10 g/dL was at screening (not Baseline/Day 1). One patient with Baseline Hgb >10 g/dL had a Screening Hgb of 9 g/dL (Day ‐15).
Time between last dose of rituximab and study initiation was mean 941 days and median 343 days (range 108‐2842 days).
In patients receiving concomitant prednisone at baseline, the dose was mean 24 mg/day and median 12.5 mg/day (range 0.5‐80 mg/day).
3.3. Response to treatment
Fostamatinib treatment resulted in 11 of 24 patients (46%) achieving the primary endpoint (Hgb >10 g/dL with a change of ≥2 g/dL from baseline) by week 24, with 1 late responder at week 30 (total of 12 responders [50%]). Six (25%) achieved the primary endpoint by week 2. The post hoc endpoint (increase of ≥1.5 g/dL in Hgb from baseline) was met by 15 of 24 patients (63%). Figure 2A,B shows the median Hgb and median change in Hgb in all efficacy‐evaluable patients (n = 24).
FIGURE 2.
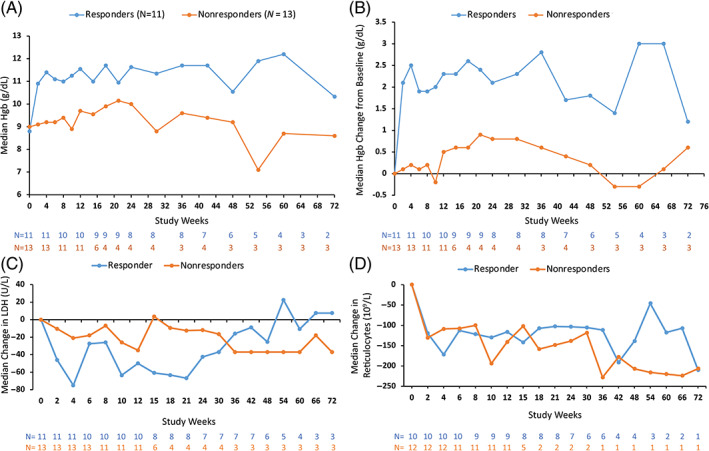
Fostamatinib improved markers of red cell hemolysis. (A) Median Hgb in the efficacy evaluable population (n = 24) categorized by response. (B) Median change in Hgb from baseline in the efficacy evaluable population (n = 24) categorized by response. (C) Median change in LDH from baseline categorized by response. (D) Median change in reticulocytes from baseline categorized by response. Hgb, hemoglobin; LDH, lactate dehydrogenase
The change in Hgb values was most pronounced in patients who achieved the primary endpoint. Median Hgb values exceeded 10 g/dL by week 2 in responders and remained above 10 g/dL for the duration of treatment (Figure 2A). Median Hgb values increased from baseline by >2.0 g/dL for responders and by 0.2 g/dL for nonresponders by week 4 (Figure 2B). Four of 5 (80%) secondary AIHA patients were responders (Hgb >10 g/dL with increase of 2 g/dL), while 7 of 19 (37%) of primary AIHA patients were responders.
Median LDH levels (Figure 2C) and reticulocyte counts (Figure 2D) generally declined over time with little change in median haptoglobin levels (Figure S2).
3.4. Rescue therapy
Certain therapeutic rescue regimens for AIHA were permitted for patients with Hgb <10 g/dL in the investigator's judgment (see Section 2). During the study, 10 patients (38%) required at least one rescue medication or RBC transfusion: 7 (27%) received prednisone (pulse or taper), 1 (4%) received hydrocortisone (pulse), 1 (4%) received immunoglobulin, and 7 (27%) received RBC transfusion. Among the 12 patients identified as responders, 3 (25%) received rescue treatment. Hgb values obtained with 14 days of RBC transfusion or within 28 days of rescue treatment were excluded from evaluation of response to treatment.
3.5. Safety
All 26 patients reported an AE (Table 2). The most common AE was diarrhea, which occurred in 42% of patients and was mild in all but 1 patient. Diarrhea was managed with dose modification (reduction or interruption), over‐the‐counter medication, or changes in dietary regimen. Fatigue was also common, and 4 of 8 cases were considered possibly/probably related to treatment. Hypertension was mild or moderate and managed with changes in the dose or type of concurrent antihypertensive medication, the addition of an antihypertensive medication, and if needed, study drug dose modification (reduction or interruption). Dizziness and insomnia AEs were mild and considered in the majority of patients to be unlikely related to study drug. There were no discernible differences in AEs between responders and nonresponders.
TABLE 2.
Most common (>10% of patients) adverse events (AEs)
| Preferred term | All patients N = 26 n (%) | Maximum severity grade | ||
|---|---|---|---|---|
| Mild (grade 1) n (%) | Moderate (grade 2) n (%) | Severe (grade 3) n (%) | ||
| Patients with at least 1 AE | 26 (100%) | 2 (8%) | 12 (46%) | 12 (46%) |
| Diarrhea | 11 (42%) | 10 (38%) | 1 (4%) | 0 |
| Fatigue | 11 (42%) | 6 (23%) | 4 (15%) | 1 (4%) |
| Hypertension | 7 (27%) | 3 (12%) | 4 (15%) | 0 |
| Dizziness | 7 (27%) | 6 (23%) | 1 (4%) | 0 |
| Insomnia | 6 (23%) | 6 (23%) | 0 | 0 |
| Nausea | 5 (19%) | 5 (19%) | 0 | 0 |
| Upper respiratory tract infection | 6 (23%) | 6 (23%) | 0 | 0 |
| Anemia | 4 (15%) | 0 | 2 (8%) | 2 (8%) |
| Jaundice | 4 (15%) | 2 (8%) | 1 (4%) | 1 (4%) |
| Neutrophil count decreased | 4 (15%) | 2 (8%) | 1 (4%) | 1 (4%) |
| Pyrexia | 4 (15%) | 3 (12%) | 0 | 1 (4%) |
| Cough | 4 (15%) | 4 (15%) | 0 | 0 |
| Headache | 4 (15%) | 3 (12%) | 1 (4%) | 0 |
| Pain in extremity | 4 (15%) | 4 (15%) | 0 | 0 |
| Abdominal pain | 3 (12%) | 2 (8%) | 1 (4%) | 0 |
| Alanine aminotransferase increased | 3 (12%) | 1 (4%) | 2 (8%) | 0 |
| Alopecia | 3 (12%) | 2 (8%) | 1 (4%) | 0 |
| Aspartate aminotransferase increased | 3 (12%) | 2 (8%) | 1 (4%) | 0 |
| Atrial fibrillation | 3 (12%) | 1 (4%) | 2 (8%) | 0 |
| Blood bilirubin increased | 3 (12%) | 0 | 3 (12%) | 0 |
| Constipation | 3 (12%) | 2 (8%) | 1 (4%) | 0 |
| Dyspnea | 3 (12%) | 2 (8%) | 1 (4%) | 0 |
| Arthralgia | 3 (12%) | 0 | 2 (8%) | 1 (4%) |
| Hypokalemia | 3 (12%) | 1 (4%) | 1 (4%) | 1 (4%) |
| Muscle spasms | 3 (12%) | 2 (8%) | 1 (4%) | 0 |
| Oropharyngeal pain | 3 (12%) | 3 (12%) | 0 | 0 |
| Rash | 3 (12%) | 2 (8%) | 1 (4%) | 0 |
| Urinary tract infection | 3 (12%) | 1 (4%) | 2 (8%) | 0 |
| Weight increased | 3 (12%) | 3 (12%) | 0 | 0 |
Note: Patients are counted for the most severe event in a given row. Less common events (those occurring in <10% of patients) were not listed but contributed to the total number (top row).
Abbreviation: AE, adverse event.
Three patients (12%) in the initial treatment phase required a dose reduction (to 100 mg BID) due to liver toxicity, neutropenia, and diarrhea. Twelve patients (46%) had treatment interrupted or withdrawn due to AEs, and 4 (15%) of these were treatment related.
Seven patients (27%) reported at least one serious adverse event (SAE). These SAEs were anemia in 2 patients and, in 1 patient each, acute myocardial infarction, fall, hemoglobin decrease, inappropriate antidiuretic hormone secretion, infection, pneumonia, rhabdomyolysis, sepsis, skin necrosis, systemic inflammatory response syndrome, and urinary tract infection; all were considered by the investigators as not related to the study drug.
Two patients had events leading to death in this study. A 50‐year‐old female, receiving concomitant azathioprine, died 58 days after the last dose of fostamatinib due to events of skin necrosis and infection. An 83‐year‐old male, whose medical history was significant for chronic lymphocytic leukemia (in remission at study entry), died due to polymicrobial pneumonia 48 days after the last dose of fostamatinib. Both SAEs were considered as not related to study drug and related to underlying comorbidities with long‐standing prednisone.
4. DISCUSSION
This open‐label, multicenter study evaluated the administration of fostamatinib, an SYK inhibitor, in the treatment of patients with wAIHA who had failed at least one prior AIHA treatment regimen. Fostamatinib increased Hgb levels in previously treated patients with wAIHA, with clinically meaningful Hgb responses observed in nearly half of patients. The Hgb response was rapid and durable. Responders most often were female, had secondary AIHA, and had fewer prior AIHA medications than nonresponders. However, the study was not powered to correlate response with patient characteristics. Markers of hemolysis also improved, with decreases in reticulocytes and LDH in both responders and those who did not meet the strict definition of response (Figure 2). The observed minimal improvement in haptoglobin was not unexpected, as haptoglobin is known to remain decreased even in the presence of normalized Hgb levels (Figure S2). 20
Adult wAIHA is a potentially life‐threatening autoimmune disease, and medical management can be challenging, with no currently approved treatments for wAIHA. 6 Corticosteroids, usually prednisone, are standard first‐line therapy, with a response achieved in 75%–80% of patients, 6 although 50% require long‐term maintenance dosing (often accompanied by several adverse events), and 20%–30% require second‐line therapies. 21 A 2017 guidance recommended rituximab as second‐line therapy, 22 acknowledging the increasing shift away from splenectomy as the traditionally preferred second‐line therapy due to its long‐term risk of severe infection and thrombotic complications. 6 , 12 , 21 , 22 , 23 Rituximab, administered as an intravenous infusion, may be associated with infusion‐related side effects, neutropenia, and a modest increase in infection rates. 21 Rituximab may impede the response to vaccination, which is of current concern since the start of the COVID‐19 pandemic. An international consensus statement published in 2020 provided a harmonized review of the diagnosis and treatment of AIHA, also addressing additional third‐line therapies including immunosuppressant therapies and hematopoietic stem cell transplantation. 6
Comparing fostamatinib responses to other AIHA treatments is difficult due to heterogeneity of study designs, patient populations, and efficacy definitions. The primary endpoint in this study was the achievement of a Hgb response by week 24 defined as a Hgb level of >10 g/dL and ≥2 g/dL higher than the baseline Hgb, not attributable to RBC transfusion or other rescue medication. Two corticosteroid studies evaluating newly diagnosed patients treated with prednisone used a similar endpoint, and responses were observed in 20 of 46 (43%) and 20 of 53 (38%) cases. 3 , 24 Although these rates are similar to the response rate (46%) observed in the current study, the fostamatinib patients were more difficult to treat due to heavy pretreatment and a longer duration of disease. The need for second‐line therapy after steroid treatment is expected, due to either lack of response or emergence of adverse effects, and indeed, in the newly diagnosed population in Roumier et al., half of all responders required second‐line treatment at a mean of 10 ± 8 months after steroids. Furthermore, many of the patients reported AEs, including 20% who developed de novo diabetes and 10% who experienced osteoporosis with fractures. 3
Other studies evaluating patients with either wAIHA or cold AIHA also used similar response criteria. One observed a response only in 6 of 26 (16%) after 4 cycles of rituximab. 25 Two smaller studies reported response (Hgb ≥2 g/dL) in 4 of 11 (36%) and 5 of 11 (45%) patients. 26 , 27 Two more recent studies evaluated rituximab response in newly diagnosed wAIHA. In one randomized, double‐blind study of 32 patients with newly diagnosed wAIHA, 75% of those receiving rituximab (1000 mg administered 2 weeks apart) plus corticosteroids had a response (Hgb >10 g/dL and 2 g/dL increased over baseline) compared with 31% in those receiving only corticosteroids (p = .032). 28 In another randomized study of 64 patients with newly diagnosed wAIHA, 75% of those receiving rituximab (375 mg/m2 weekly for 4 weeks) plus corticosteroids and only 36% of those receiving just corticosteroids attained at least a stable, acceptable hemoglobin level without any need of treatment except <10 mg/day prednisone. 29
In this study, fostamatinib demonstrated a safety and tolerability profile consistent with the existing fostamatinib safety database of 3881 patients and 724 healthy subjects across disease programs studied (rheumatoid arthritis [n = 3437], ITP [n = 163], oncology [n = 204], IgA nephropathy [n = 51], and AIHA [n = 26]). 11 , 15 , 17 , 18 , 30 , 31 , 32 , 33 No new safety signals were detected. The most commonly reported AEs included diarrhea, hypertension, fatigue, dizziness, and insomnia. Gastrointestinal effects were the most commonly reported AEs in fostamatinib studies in patients with other diseases. Hypertension is attributed to the off‐target effect of fostamatinib on VEGF receptor and is a known effect of SYK inhibition. 34 Fatigue is very common in patients with anemia, and without a control group, it is difficult to assess how much of the fatigue is related to disease. Most AEs were mild or moderate and medically managed as needed. All reported SAEs were considered by investigators to be not related to study drug and most likely related to underlying comorbidities or corticosteroid use.
Limitations of this study are the small sample size, lack of a control or comparator arm, lack of DAT results after treatment, and a relatively short duration of follow‐up. Potential comparisons to other studies treating patients with wAIHA should be interpreted with caution due to differences in study design, definitions of response, and patient characteristics, particularly the type and number of prior treatments.
With no approved therapies for wAIHA, many patients with successive relapses or who are exposed to long‐term use of corticosteroids highlight an unmet need in wAIHA. The Hgb response and safety results from this study suggest that the SYK inhibitor fostamatinib demonstrates potential in the treatment of patients with a history of wAIHA who have relapsed following one or more prior treatments. A phase 3 study (NCT03764618) to further investigate the results observed in this study is nearing completion.
Patient consent: All patients provided written informed consent in accordance with the Declaration of Helsinki.
Clinical trial registration: This study was registered at ClinicalTrials.gov (#NCT02612558).
CONFLICT OF INTEREST
David J. Kuter has received research funding from Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Immunovant, Kezar, Principia, Protalex, Rigel, and Takeda (Bioverativ) UCB; and has served in a consulting role for Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Caremark, CRICO, Daiichi Sankyo, Dova, Genzyme, Immunovant, Incyte, Kyowa‐Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Platelet Disorder Support Association, Principia, Protalex, Protalix, Rigel, Sanofi, Genzyme, Shionogi, Shire, Takeda (Bioverativ), UCB, Up‐To‐Date, and Zafgen. Kerry A. Rogers has received research funding from Genentech, AbbVie, Novartis, and Janssen; has served in a consulting role for Acerta Pharma, AbbVie, Genentech, Pharmacyclics, AstraZeneca, and Innate Pharma; and has received travel funding from AstraZeneca. Michael A. Boxer has served on speaker's bureaus for Sanofi and Pharmacosmos. Michael Choi has received research funding from Pharmacyclics, Rigel, Sunesis, TG Therapeutics, and Velosbio. Donald Arnold has received research funding from Novartis and BMS; and has served in a consulting role for Rigel, Novartis, Amgen, UCB, and Principia. Catherine M. Broome has served in a consulting role for Alexion, Argenx, Sanofi, and Appelis. Robert Numerof and Sandra Tong are employees of Rigel Pharmaceuticals. The remaining authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
David J. Kuter and Sandra Tong contributed to the study design; David J. Kuter, Kerry A. Rogers, Michael A. Boxer, Michael Choi, Richy Agajanian, Donald Arnold, Catherine M. Broome, Joshua J. Field, Irina Murakhovskaya, Robert Numerof, and Sandra Tong contributed to acquisition, analysis, and interpretation of data; David J. Kuter and Sandra Tong made substantial contributions to the drafting of the manuscript; Kerry A. Rogers, Michael A. Boxer, Michael Choi, Richy Agajanian, Donald Arnold, Catherine M. Broome, Joshua J. Field, Irina Murakhovskaya, and Robert Numerof made critical revisions to the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Supporting information
Appendix S1: Supplementary information.
ACKNOWLEDGMENTS
The authors would like to thank the patients for participating in this study. The authors acknowledge the contributions of all investigators and study site staff to the conduct of this study. Stella Chao, PhD, provided medical writing and editorial assistance to the authors during preparation of this article, which was funded by Rigel Pharmaceuticals.
Kuter DJ, Rogers KA, Boxer MA, et al. Fostamatinib for the treatment of warm antibody autoimmune hemolytic anemia: Phase 2, multicenter, open‐label study. Am J Hematol. 2022;97(6):691‐699. doi: 10.1002/ajh.26508
Prior presentation Data from this article were presented in part at the following meetings: 60th American Society of Hematology Annual Meeting 2018, (December 1–4, 2018; San Diego, CA, USA); 61st American Society of Hematology Annual Meeting 2019, (December 7–10, 2019; Orlando, FL, USA); 62nd American Society of Hematology Annual Meeting 2020, (December 5–8, 2020; Virtual); Thrombosis and Hemostasis Summit of North America (THSNA) 2018, (March 6–8, 2018; San Diego, CA, USA); 24th Congress of the European Hematology Association (EHA) 2019, (June 13–16, 2019; Amsterdam, Netherlands); 23rd Annual International Congress on Hematologic Malignancies (ICHM) 2019, (February 28–March 3, 2019; Miami, FL, USA).
Funding information Rigel Pharmaceuticals, Inc.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request via datasharing@rigel.com.
REFERENCES
- 1. Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29(1):1‐9. doi: 10.1016/j.jaut.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petz LD, Garratty G. Drug‐induced immune hemolytic anemia. Immune haemolytic anemias. Churchill Livingstone; 2004. [Google Scholar]
- 3. Roumier M, Loustau V, Guillaud C, et al. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: new insights based on a single‐center experience with 60 patients. Am J Hematol. 2014;89(9):E150‐E155. doi: 10.1002/ajh.23767 [DOI] [PubMed] [Google Scholar]
- 4. Silberstein P. Autoimmune haemolytic anemia. In: Enna SJ, Bylund DB, eds. xPharm: the comprehensive pharmacology reference. Elsevier; 2007:1‐6. [Google Scholar]
- 5. Dierickx D, Kentos A, Delannoy A. The role of rituximab in adults with warm antibody autoimmune hemolytic anemia. Blood. 2015;125(21):3223‐3229. doi: 10.1182/blood-2015-01-588392 [DOI] [PubMed] [Google Scholar]
- 6. Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020;41:100648. doi: 10.1016/j.blre.2019.100648 [DOI] [PubMed] [Google Scholar]
- 7. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24(3):195‐210. doi: 10.1016/j.tmrv.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 8. Sun P. Structural recognition of immunoglobulins by Fcγ receptors. In: Ackerman ME, Nimmerjahn F, eds. Antibody Fc linking adaptive and innate immunity. Elsevier; 2014:131‐141. [Google Scholar]
- 9. Rosse WF, Hillmen P, Schreiber AD. Immune‐mediated hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2004;2004(1):48‐62. doi: 10.1182/asheducation-2004.1.48 [DOI] [PubMed] [Google Scholar]
- 10. Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex‐mediated inflammation. J Pharmacol Exp Ther. 2006;319(3):998‐1008. doi: 10.1124/jpet.106.109058 [DOI] [PubMed] [Google Scholar]
- 11. Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open‐label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113(14):3154‐3160. doi: 10.1182/blood-2008-07-166439 [DOI] [PubMed] [Google Scholar]
- 12. Barcellini W, Fattizzo B, Zaninoni A. Current and emerging treatment options for autoimmune hemolytic anemia. Expert Rev Clin Immunol. 2018;14(10):857‐872. doi: 10.1080/1744666x.2018.1521722 [DOI] [PubMed] [Google Scholar]
- 13. Davidzohn N, Biram A, Stoler‐Barak L, Grenov A, Dassa B, Shulman Z. SYK degradation restrains plasma cell formation and promotes zonal transitions in germinal centers. J Exp Med. 2020;217(3):e20191043. doi: 10.1084/jem.20191043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roders N, Herr F, Ambroise G, et al. SYK inhibition induces apoptosis in germinal center‐like B cells by modulating the antiapoptotic protein myeloid cell leukemia‐1, affecting B‐cell activation and antibody production. Front Immunol. 2018;9:787. doi: 10.3389/fimmu.2018.00787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor PC, Genovese MC, Greenwood M, et al. OSKIRA‐4: a phase IIb randomised, placebo‐controlled study of the efficacy and safety of fostamatinib monotherapy. Ann Rheum Dis. 2015;74(12):2123‐2129. doi: 10.1136/annrheumdis-2014-205361 [DOI] [PubMed] [Google Scholar]
- 16. Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non‐Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578‐2585. doi: 10.1182/blood-2009-08-236471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo‐controlled trials. Am J Hematol. 2018;93(7):921‐930. doi: 10.1002/ajh.25125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bussel JB, Arnold DM, Boxer MA, et al. Long‐term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94(5):546‐553. doi: 10.1002/ajh.25444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayo Clinic Laboratories . Rochester 2020 Interpretative Handbook; 2020. Accessed December 15, 2020. https://www.mayocliniclabs.com/test‐catalog/pod/MayoTestCatalog‐Rochester–SortedByTestName‐duplex‐interpretive.pdf.
- 20. Barcellini W, Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Markers. 2015;2015:635670. doi: 10.1155/2015/635670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zanella A, Barcellini W. Treatment of autoimmune hemolytic anemias. Haematologica. 2014;99(10):1547‐1554. doi: 10.3324/haematol.2014.114561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hill QA, Stamps R, Massey E, Grainger JD, Provan D, Hill A. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017;176(3):395‐411. doi: 10.1111/bjh.14478 [DOI] [PubMed] [Google Scholar]
- 23. DeLoughery TG. Autoimmune hemolytic anemia. Hosp Phys Board Rev Manual. 2013;8(1):2‐11. [Google Scholar]
- 24. Jaime‐Pérez JC, Aguilar‐Calderón P, Salazar‐Cavazos L, Gómez‐De León A, Gómez‐Almaguer D. Treatment of autoimmune hemolytic anemia: real world data from a reference center in Mexico. Blood Res. 2019;54(2):131‐136. doi: 10.5045/br.2019.54.2.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peñalver FJ, Alvarez‐Larrán A, Díez‐Martin JL, et al. Rituximab is an effective and safe therapeutic alternative in adults with refractory and severe autoimmune hemolytic anemia. Ann Hematol. 2010;89(11):1073‐1080. doi: 10.1007/s00277-010-0997-y [DOI] [PubMed] [Google Scholar]
- 26. D'Arena G, Califano C, Annunziata M, et al. Rituximab for warm‐type idiopathic autoimmune hemolytic anemia: a retrospective study of 11 adult patients. Eur J Haematol. 2007;79(1):53‐58. doi: 10.1111/j.1600-0609.2007.00861.x [DOI] [PubMed] [Google Scholar]
- 27. Narat S, Gandla J, Hoffbrand AV, Hughes RG, Mehta AB. Rituximab in the treatment of refractory autoimmune cytopenias in adults. Haematologica. 2005;90(9):1273‐1274. [PubMed] [Google Scholar]
- 28. Michel M, Terriou L, Roudot‐Thoraval F, et al. A randomized and double‐blind controlled trial evaluating the safety and efficacy of rituximab for warm auto‐immune hemolytic anemia in adults (the RAIHA study). Am J Hematol. 2017;92(1):23‐27. doi: 10.1002/ajh.24570 [DOI] [PubMed] [Google Scholar]
- 29. Birgens H, Frederiksen H, Hasselbalch HC, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163(3):393‐399. doi: 10.1111/bjh.12541 [DOI] [PubMed] [Google Scholar]
- 30. Baluom M, Samara E, Grossbard EB, Lau DT. Fostamatinib, a Syk‐kinase inhibitor, does not affect methotrexate pharmacokinetics in patients with rheumatoid arthritis. J Clin Pharmacol. 2011;51(9):1310‐1318. doi: 10.1177/0091270010381496 [DOI] [PubMed] [Google Scholar]
- 31. Maringwa J, Kågedal M, Hamrén UW, Martin P, Cox E, Hamrén B. Pharmacokinetic‐pharmacodynamic modeling of fostamatinib efficacy on ACR20 to support dose selection in patients with rheumatoid arthritis (RA). J Clin Pharmacol. 2015;55(3):328‐335. doi: 10.1002/jcph.406 [DOI] [PubMed] [Google Scholar]
- 32. Weinblatt ME, Genovese MC, Ho M, et al. Effects of fostamatinib, an oral spleen tyrosine kinase inhibitor, in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a phase III, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Arthritis Rheum. 2014;66(12):3255‐3264. doi: 10.1002/art.38851 [DOI] [PubMed] [Google Scholar]
- 33. Weinblatt ME, Kavanaugh A, Genovese MC, et al. Effects of fostamatinib (R788), an oral spleen tyrosine kinase inhibitor, on health‐related quality of life in patients with active rheumatoid arthritis: analyses of patient‐reported outcomes from a randomized, double‐blind, placebo‐controlled trial. J Rheumatol. 2013;40(4):369‐378. doi: 10.3899/jrheum.120923 [DOI] [PubMed] [Google Scholar]
- 34. Skinner M, Philp K, Lengel D, et al. The contribution of VEGF signalling to fostamatinib‐induced blood pressure elevation. Br J Pharmacol. 2014;171(9):2308‐2320. doi: 10.1111/bph.12559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cooper N, Numerof RP, Tong S, Kuter DJ. Fostamatinib for the treatment of warm antibody autoimmune hemolytic anemia (wAIHA): a phase 3, randomized, double‐blind, placebo‐controlled, global study. Blood. 2020;136(Supplement 1):1‐3. doi: 10.1182/blood-2020-140469 32430499 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary information.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request via datasharing@rigel.com.


