Abstract
Background and Aims
Transmural healing has emerged as a treatment target in Crohn’s disease (CD). We investigated whether transmural healing assessed with intestinal ultrasound (IUS) is associated with improved clinical outcomes in patients with CD in clinical remission.
Methods
Patients with CD in clinical remission at baseline (HBI <4) having IUS between August 2017 and June 2020 with at least 6‐months’ follow‐up were retrospectively studied. Time to medication escalation, corticosteroid use and CD‐related hospitalisation or surgery were compared by the presence or absence of sonographic healing, defined as bowel wall thickness ≤3 mm without hyperemia on color Doppler, inflammatory fat, or disrupted bowel wall stratification. Factors associated with survival were analyzed by Kaplan–Meier analysis using Cox proportional‐hazard model.
Results
Of 202 consecutive patients (50% male), sonographic inflammation was present in 61%. During median follow‐up of 19 (IQR 13–27) months, medication escalation occurred in 52%, corticosteroid use in 23%, hospitalisation in 21%, and CD‐related surgery in 13%. Sonographic healing was significantly associated with a reduced risk of medication escalation (p = 0.0018), corticosteroid use (p = 0.0247), hospitalisation (p = 0.0102), and surgery (p = 0.083). On multivariable analysis, sonographic healing was significantly associated with an increased odds of medication escalation‐free survival (hazard ratio [HR]:1.94; 95% CI 1.23–3.06; p = 0.004) and corticosteroid‐free survival (HR:2.41; 95% CI 1.24–4.67; p = 0.009), but not with hospitalisation or surgery.
Conclusion
In patients with CD in clinical remission, sonographic healing is associated with improved clinical outcomes. Further studies are needed to determine whether sonographic healing should be a treatment target.
Keywords: Crohn’s disease, intestinal ultrasound, prognostic factor, transmural healing
Patients with CD in clinical remission at baseline (HBI <4) having IUS between August 2017 and June 2020 with at least 6‐months’ follow‐up were retrospectively studied. Sonographic healing was significantly associated with a reduced risk of medication escalation (p = 0.0018), corticosteroid use (p = 0.0247), hospitalization (p = 0.0102) and surgery (p = 0.083).

1. BACKGROUND
Crohn’s disease (CD) is a chronic inflammatory condition with relapsing symptoms that correlate poorly with mucosal inflammatory burden. The aim of treatment in CD is to achieve and maintain remission. Historically, clinical remission has been the predominant therapeutic target. 1 However, up to 40% of patients who achieve clinical remission will have persistent endoscopic mucosal inflammation which is associated with inferior clinical outcomes including an increased risk of clinical relapse, medication escalation, hospitalisation, and surgery. 2 , 3 By comparison, some patients with persistent clinical activity have no objective evidence of inflammation. Mucosal healing has therefore emerged as the contemporary clinical target in the treatment of IBD. 2 , 3 , 4
Assessing for mucosal healing requires endoscopic evaluation, which is invasive and costly. Furthermore, it is increasingly being recognised that transmural healing is associated with improved clinical outcomes and reduced long‐term disease complications when compared with mucosal healing, with some proposing that transmural healing should be considered a deeper therapeutic target when treating CD. 5 , 6 Transmural healing can be assessed with magnetic resonance enterography (MRE), computer tomography (CT), and intestinal ultrasound (IUS). MRE is constrained by access and cost and CT is associated with radiation exposure. IUS accurately detects CD activity, extent, and complications and is minimally invasive, easily repeated, and is associated with low cost. 7 Hence, there is growing interest in sonographic healing as a treatment target in CD. 5 , 8 , 9 However, as yet, sonographic healing remains incompletely defined and it is unclear if transmural sonographic healing is associated with improved clinical outcomes.
This study therefore aimed to explore the clinical implications of achieving sonographic over and above clinical remission. The prevalence and predictors of sonographic transmural healing in patients with confirmed CD in clinical remission were determined, and the association between sonographic inflammation and clinical endpoints of medication escalation, corticosteroid use, hospitalisation, and surgery was assessed.
2. METHODS
A retrospective study of patients with CD was performed at The Royal Melbourne Hospital, a tertiary center. Consecutive patients who underwent IUS for IBD between August 2017 and June 2020 were identified. Patients with a confirmed diagnosis of CD were required to have baseline IUS with measurements and recording of findings in at least the sigmoid, descending, transverse, ascending colon, and terminal ileum. Only patients in clinical remission at the time of IUS and with 6 months of hospital follow‐up were included. Clinical remission was defined as Harvey Bradshaw Index ≤4. Patients with disease confined to the rectum, isolated perianal disease, stoma in situ, or suboptimal IUS assessment were excluded.
2.1. Medical record abstraction
IUS reports were retrieved through our electronic radiology record (Karisma RIS, Kestral) for data extraction. Patient data that were collected from the electronic medical record system (EPIC) included age of disease onset, smoking history, CD phenotype according to Montreal classification 10 (B1, inflammatory; B2, stricturing; B3, penetrative), disease location, and medications at the time of IUS (anti‐inflammatory agents and/or steroids, immunomodulators, biologic agents). CRP and fecal calprotectin were recorded if available within 3 months of the baseline IUS.
2.2. Sonographic assessment
IUS was performed using a Canon Aplio i800 (Canon Medical Systems Corporation) by three gastroenterologists with more than 2 years and 1000 scans of sonographic experience. Each patient underwent systematic scanning of the abdomen, with each segment of colon (sigmoid/descending/transverse/ascending) and terminal ileum assessed for maximal bowel wall thickness (BWT) (mm) and the presence of hyperemia on color Doppler, loss of bowel wall stratification, mesenteric inflammatory fat, and complications (stricture, abscess, fistula) was recorded (yes/no). A standardised template for reporting was utilised. The BWT was considered normal if a maximal value ≤3 mm taken from central interface between lumen contents and mucosa measured out to the serosa. Hyperemia/vascularity was defined as positive persistent color Doppler signal within the wall. The presence of inflammatory fat was defined as increased echogenicity and/or wrapping of mesenteric fat surrounding bowel wall on cross‐sectional views of bowel segments. Disrupted bowel wall stratification was categorised as poor definition between the mucosal, submucosal, and muscularis propria layers. Complications included the presence of one or more strictures (fixed luminal narrowing associated with increased BWT with or without pre‐stenotic dilatation), fistula (hypoechoic track at area of abnormal BWT with or without hyperechoic content seen tracking outside the bowel wall, between loops, or between the loop and bladder), abscess/phlegmon (anechoic area). The combined endpoint of sonographic healing was defined as a BWT ≤3 mm without hyperemia on color Doppler, inflammatory fat or disrupted bowel wall stratification. Sonographic inflammation was therefore defined as abnormal BWT and/or hyperemia and/or abnormal bowel wall stratification and/or mesenteric inflammatory fat reported as binary variables (yes/no). No intravenous or oral contrast was utilised and patients were not required to fast.
2.3. Assessment of clinical outcomes
Clinical outcomes were calculated and defined as time from IUS to event. Escalation of medication was defined as need for change in medication maintenance including change of biologic agent or escalation of dose, addition or change of immunomodulator in combination therapy for CD, or escalation from immunomodulator to biologic agent due TO disease activity. Corticosteroid use was defined as the requirement for an increase in dosage or new course of oral corticosteroids for active CD symptoms. Hospitalisation was recorded if overnight admission was required for disease activity or refractory disease. Surgery was recorded separately if deemed to be related to active disease or refractory symptoms. Outpatient endoscopy was not included in admission
2.4. Statistical analysis
Continuous variables were summarised using medians and inter‐quartile ranges (IQR). Categorical variables were expressed as a percentage and number of the cohort. Kaplan–Meier analysis were performed to compare medication escalation‐free survival, corticosteroid‐free survival, hospitalisation‐free survival, and surgery‐free survival in those with and without sonographic healing. p values for Kaplan–Meier analysis were performed using log rank test. The Mann–Whitney U test and Kruskal–Wallis were used to compare continuous variables, and Pearson chi‐square test was used to compare categorical variables. Univariate and multivariable Cox proportional‐hazard regression analysis was performed to identify predictors of medication escalation, corticosteroid use, surgery and hospitalisation. IUS variables which were included in the univariate analysis included thickened BWT, bowel wall hyperemia, mesenteric inflammatory fat, and disease complications. Due to collinearity with sonographic inflammation, BWT, hyperemia, inflammatory fat, and bowel wall stratification were removed from the multivariable analysis and a combined outcome of sonographic inflammation was included. All variables with p < 0.05 on univariate analysis were retained and integrated into the multivariable models. The multivariable analysis was performed using the cox regression. A value of p ≤ 0.05 was considered significant. All data analyses were performed using Stata 16.0. The study was approved by the local institutional ethics review board (QA2020208).
3. RESULTS
3.1. Patients
1043 patients had documented IBD and a paired IUS; following exclusion, 202 patients were included in the study (Figure 1).
FIGURE 1.
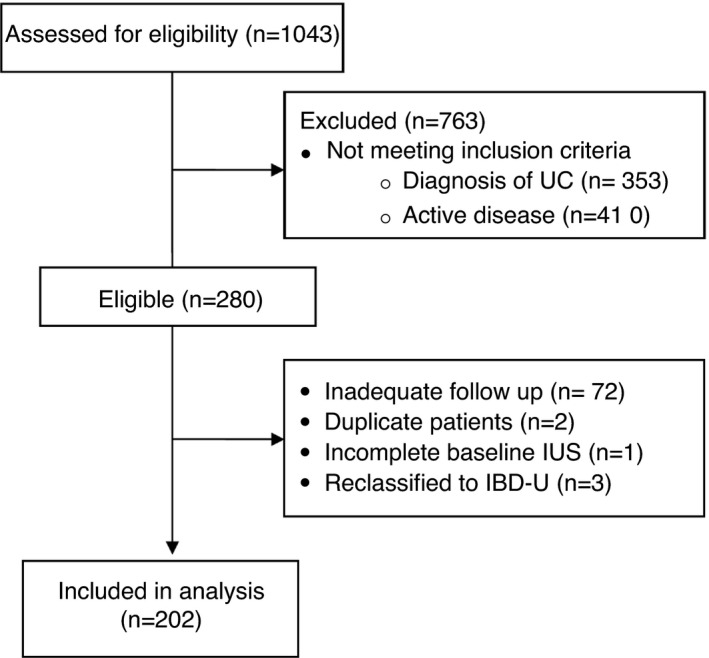
Patient flow chart
Patients’ demographics and clinical characteristics are shown in Table 1. Fifty percent were male with a median age at diagnosis of CD of 24 (IQR 18–34) years and median duration of disease at time of IUS was 9 (4–16) years. One hundred and forty (66%) patients were on a biologic at baseline IUS. The majority of patients had ileal involvement (ileal disease in 32% and ileo‐colonic in 46%). The median follow‐up was 19 (13–27) months.
TABLE 1.
Baseline clinical characteristics
| Baseline characteristics (n = 202) | Patients with sonographic healing (n = 79) | Patients without sonographic healing (n = 123) | |
|---|---|---|---|
| Median (IQR) or percentage (n) | p value | ||
| Age at diagnosis (years) | 21 IQR (17–32) | 25 IQR(19–37) | 0.27 |
| Gender (male) | 48 (38) | 51 (63) | 0.67 |
| Duration of disease (years) | 8 IQR (3–13) | 9 IQR (4–19) | 0.76 |
| Previous bowel resection | 29 (23) | 37 (46) | 0.23 |
| Current smoking | 20 (16) | 37 (45) | 0.014* |
| Disease phenotype | |||
| B1 (inflammatory) | 63.3 (50) | 29.3 (36) | <0.00* |
| B2 (stricturing) | 17.7 (14) | 52.0 (64) | <0.00* |
| B3 (penetrating) | 19.0 (15) | 18.7 (23) | 0.959 |
| Medication at time of IUS | |||
| Oral prednisolone/budesonide | 6 (7.6) | 12.2 (15) (2 missing) | 0.30 |
| 5‐aminosalicilic acid | 10.1 (8) | 14.7 (19) (2 missing) | 0.28 |
| 6‐mercaptopurine | 8.9 (7) | 6.5 (8) | 0.13 |
| Azathioprine | 35.4 (28) | 39 (48) | |
| Methotrexate | 5.6 (4) | 13 (16) | 0.07 |
| Anti‐tumor necrosis factor | |||
| Infliximab | 39.2 (31) | 25.2 (31) | 0.04* |
| High dose Infliximab | 5.1 (4) | 7.3 (9) | 0.52 |
| Adalimumab | 10.1 (8) | 22.0 (27) | 0.03 |
| High dose adalimumab | 2.2 (2) | 2.4 (3) | 0.97 |
| Ustekinumab | 6.7 (6) | 6.5 (8) | 0.77 |
| Vedolizumab | 0 | 4.1 (5) | 0.14 |
| High dose Vedolizumab | 1.3 (1) | 1.6 (2) | 0.84 |
| Other | 1.3 (1) | 0 | 0.21 |
At IUS, 79 (39%) patients demonstrated sonographic healing. Only a single patient had positive Doppler with normal BWT. Notably the presence of mesenteric inflammatory fat or loss of bowel wall stratification was seen in approximately 30% of all patients with sonographic inflammation, and was not observed in any of those patients who had BWT ≤3 mm. 11
3.2. Medication escalation‐free survival
In 103 patients (51%), medication was escalated 196 (89–402) days after the IUS (Appendix Table A1).
APPENDIX TABLE A1.
Description of medication escalation
| Medication escalation | Number (%) |
|---|---|
| Total n = 103 | |
| New corticosteroids | 47 (46) |
| New immunomodulator/methotrexate | 17 (17) |
| New biologic | 17 (17) |
| Increased dose immunomodulator/methotrexate | 16 (16) |
| Increased dose of biologic | 29 (28) |
| Change in biologic due to disease activity | 20 (19) |
| Addition of allopurinol to optimise thiopurine | 3 (3) |
Patients in clinical remission with evidence of sonographic inflammation at baseline were more likely to require medication escalation compared to those with sonographic healing, (Figure 2A) with a median times to escalation of 1.1 versus 3.0 years, respectively. On univariate analysis, evidence of sonographic inflammation (HR 1.95; 95% CI 1.27–2.98; p = 0.002) and penetrative (Montreal B3) versus inflammatory (Montreal B1) disease phenotype (HR 1.80; 95% CI 1.07–3.01; p = 0.026) was significantly associated with need for medication escalation (Table 2). On multivariable analysis, only sonographic inflammation remained significant with a HR of 1.94 95% CI 1.23–3.06; p = 0.004 (Table 2).
FIGURE 2.
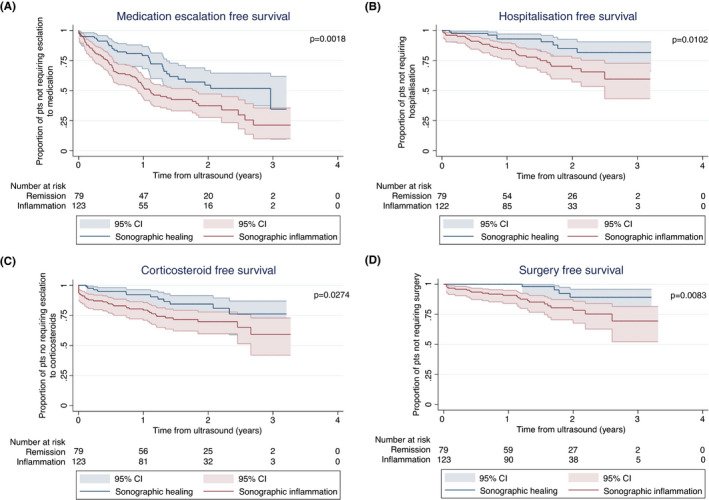
Kaplan–Meier analysis of the effect of sonographic healing versus sonographic inflammation on (A) medication escalation‐free survival, (B) corticosteroid‐free survival, (C) hospitalisation‐free survival, (D) surgery‐free survival
TABLE 2.
Univariate and multivariable predictors of clinical outcome measures in patients with Crohn’s disease
| Variable | Medication escalation n = 103 HR (95% CI), p value | Corticosteroid use n = 47 HR (95% CI), p value | Hospitalisation n = 42 HR (95% CI), p value | Surgery n = 26 HR (95% CI), p value | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | |
| Gender | 1.15 (0.78–1.70), p = 0.46 | 1.25 (0.71–2.23), p = 0.44 | 0.86 (0.47–1.58), p = 0.63 | 0.58 (0.26–1.27), p = 0.17 | ||||
| Penetrative versus inflammatory (B2 v sB1) | 1.26 (0.81–1.98), p = 0.30 | 1.34 (0.716–2.52), p = 0.36 | 2.14 (1.00–4.57), p = 0.050* | 1.63 (0.73–3.67), p = 0.24 | 3.72 (1.05–13.22) p = 0.042* | 2.16 (0.59–7.98), p = 0.25 | ||
| Stricturing versus inflammatory (B3 vs. B1) | 1.80 (1.07–3.01), p = 0.026* | 1.34 (0.78–2.32), p = 0.28 | 0.789 (0.327–1.91), p = 0.60 | 2.67 (1.15–6.19), p = 0.022* | 1.93 (0.80–4.63), p = 0.14 | 7.25 (2.02–26.02), <p = 0.002* | 4.95 (1.36–18.04), p = 0.015* | |
| Smoking | 1.0 (0.65–1.53), p = 0.10 | 1.47 (0.81–2.67) p = 0.21 | 1.22 (0.63–2.35), p = 0.56 | 2.32 (1.02–5.31), p = 0.045* | 2.05 (0.92–4.55) p = 0.08 | |||
| Age at diagnosis of CD (y) | 0.99 (0.97–1.00), p = 0.06 | 1.01 (0.99–1.03), p = 0.23 | 0.99 (0.96–1.01), p = 0.22 | 0.99 (0.95–1.02) p = 0.35 | ||||
| Disease duration (years from diagnosis to IUS) | 1.01 (0.99–1.03), p = 0.26 | 1.02 (0.99–1.05), p = 0.17 | 1.03 (1.0–1.06), p = 0.075 | 1.01 (0.97–1.05), p = 0.74 | ||||
| Baseline Immunomodulator | 1.04 (0.70–1.54), p = 0.84 | 0.41 (0.23–0.74), p = 0.003* | 0.37 (0.20–0.67), p = 0.001* | 1.17 (0.63–2.19), p = 0.62 | 4.13 (1.42–11.99, p = 0.009* | 3.74 (1.28–10.97), p = 0.016* | ||
| Baseline biologic | 1.35 (0.87–2.09), p = 0.18 | 0.59 (0.33–1.06), p = 0.08 | 2.79 (1.18–6.63), p = 0.020* | 2.54(1.06‐6.08), p = 0.036* | 3.57 (1.07–11.90), p = 0.038* | 2.41 (0.71–8.18), p = 0.16 | ||
| Sonographic inflammation | 1.95 (1.27–2.98), p = 0.002* | 1.94 (1.23–3.06), p = 0.004* | 2.06 (1.07–3.96), p = 0.031* | 2.41 (1.24–4.67), p = 0.009* | 2.55 (1.22–5.33), p = 0.013* | 2.16 (0.98–4.74), p = 0.06 | 3.80 (1.31–11.02), p = 0.014* | 2.55 (0.84–7.69), p = 0.10 |
Significant p < 0.05.
3.3. Corticosteroid‐free survival
New corticosteroids were prescribed in 47 (23%) patients during follow‐up after 190 (35–435) days. At baseline, 10 patients (5%) continued to wean corticosteroids and were not included in the definition of new corticosteroid use. Of the 49 patients requiring new corticosteroids, 20 (10%) had new prednisolone or IV hydrocortisone and 27 (13%) had budesonide. The presence of sonographic inflammation at baseline IUS was associated with need for corticosteroid use (Figure 2B). On univariate and multivariable analysis baseline immunomodulator use (HR 0.41; 95% CI 0.23–0.74; p = 0.003) and sonographic inflammation (HR 2.06 95% CI 1.07–3.96; p = 0.031) were significantly associated with corticosteroid use (Table 2).
3.4. Hospitalisation‐free survival
There were 42 (20%) patients hospitalised over the study period 309 (136–551) days from the index IUS. The presence of sonographic inflammation was significantly associated with reduced hospitalisation‐free survival, p = 0.0102 (Figure 2C). On univariate analysis, Montreal phenotype, baseline biologic use, and sonographic inflammation were associated with reduced hospitalisation‐free survival (Table 2). On multivariable analysis, only baseline biologic use remained significantly associated with hospital‐free survival with HR of 2.49; 95% CI 1.04–5.96; p = 0.04. There was a trend for patients with sonographic inflammation to have higher likelihood of requiring hospitalisation (HR 2.16; 95% CI 0.98–4.74; p = 0.06).
3.5. Surgery‐free survival
Twenty‐six (13%) patients underwent CD‐related surgery after 425 (146–606) days; nine patients underwent ileo‐cecal resection, seven examinations under anesthesia, six colectomies, two isolated small bowel surgeries, and two patients had laparotomy and adhesiolysis. The presence of sonographic inflammation was associated with reduced surgery‐free survival, p = 0.0083 (Figure 1D). On univariate analysis the risk of surgery was increased with the presence of B2 (penetrating) or B3 (stricturing) phenotype compared to B1 (inflammatory) phenotype (HR 3.72; 95% CI 1.05–13.22; p = 0.042, and HR 7.25; 95% CI 2.02–26.02; p = 0.002, respectively). Smoking (HR 2.32; 95% CI 1.02–5.31; p = 0.045), use of immunomodulator (HR 4.13; 95% CI 1.42–11.99; p = 0.009), and biologics at baseline (HR 3.57; 95% CI 1.07–11.90; p = 0.038) were associated with increased risk of surgery. On multivariable analysis, stricturing phenotype (HR 4.95; 95% CI 1.36–18.04; p = 0.015) and baseline immunomodulator use (HR 3.74; 95% CI 1.28–10.97; p = 0.016) remained significantly associated with surgery. Sonographic inflammation was not associated with risk of surgery.
3.6. Sub‐analysis of sonographic parameters
When exploring the relationship between individual sonographic parameters and clinical outcome measured using univariate analysis, no single sonographic parameter predicted all four outcome measures (Table 3). BWT was associated with an increased risk of medication escalation (HR 1.87; 95%CI 1.22–2.85; p = 0.004), hospitalisation (HR 2.24; 95%CI 1.1–4.56; p = 0.027), and surgery (HR 2.92; 95% CI 1.1–7.74; p = 0.031). Loss of wall stratification was associated with medication escalation (HR 2.81; 95% CI 1.81–4.36; p ≤ 0.001) (Figure 3A), hospitalisation (HR 2.09; 95% CI 1.07–4.08; p = 0.032) (Figure 3C), and surgery (HR 4.92; 95% CI 2.27–10.67; p < 0.0001) (Figure 3D). Hyperemia was significantly associated with need for corticosteroids (HR 2.36; 95% CI 1.35–4.13; p = 0.027). Mesenteric fat hypertrophy was associated with increased risk of medication escalation (HR 2.08; 95% CI 1.33–3.24; p = 0.001) and surgery (HR 3.69; 95% CI 1.70–8.04; p = 0.001), but showed no significant relationship with corticosteroid use or risk of hospitalisation. The presence or absence of complications such as stricture or abscess was not significantly associated with any outcome (Table 3).
TABLE 3.
Univariate sonographic parameter predictors of complication outcome measures in patients with Crohn’s disease
| Sonographic parameters | Medication escalation | Corticosteroid use | Hospitalisation | Surgery |
|---|---|---|---|---|
| HR (95% CI), p value | HR (95% CI), p value | HR (95% CI), p value | HR (95% CI), p value | |
| Abnormal BWT (BWT >3 mm) | 1.87 (1.22–2.85), p = 0.004* | 1.85 (0.98–3.51), p = 0.059 | 2.24 (1.1–4.56), p = 0.027* | 2.92 (1.1–7.74), p = 0.031* |
| Hyperemia | 2.19 (1.48–3.24), p = 0.0001* | 2.36 (1.35–4.13), p = 0.027* | 1.34 (0.72–2.50), p = 0.36 | 1.28 (0.581–2.82), p = 0.54 |
| Mesenteric inflammatory fat | 2.08 (1.33–3.24), p = 0.001* | 1.46 (0.74–2.87), p = 0.27 | 1.72 (0.86–3.42), p = 0.12 | 3.69 (1.70–8.04), p = 0.001* |
| Complications | 1.41 (0.95–2.12), p = 0.09 | 1.68 (0.94–3.00), p = 0.08 | 1.36 (0.73–2.53), p = 0.34 | 1.54 (0.71–3.37), p = 0.27 |
| Bowel wall stratification | 2.81 (1.81–4.36), p ≤ 0.001* | 1.44 (0.71–2.89), p = 0.31 | 2.09 (1.07–4.08), p = 0.032* | 4.92 (2.27–10.67), p < 0.0001* |
p < 0.05.
FIGURE 3.
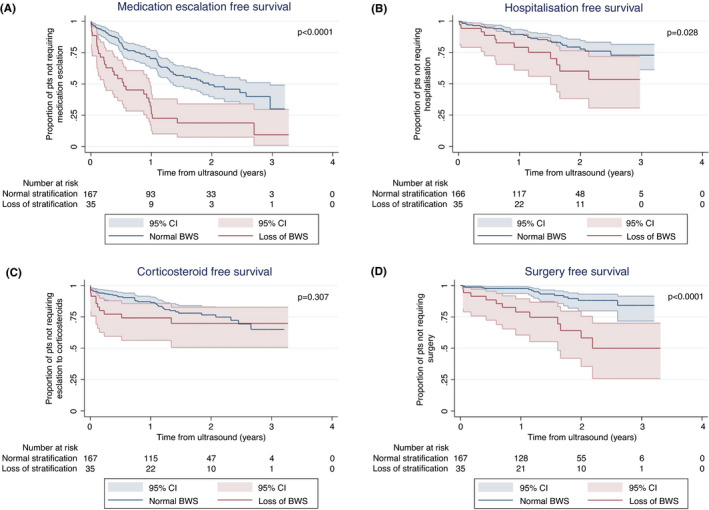
Kaplan–Meier analysis of the effect of loss of bowel wall stratification (BWS) on (A) medication escalation‐free survival, (B) corticosteroid‐free survival, (C) hospitalisation‐free survival, (D) surgery‐free survival
3.7. Sonographic inflammation and risk of clinical complications more than 90 days from IUS
In order to limit the possible impact of clinician decision making solely in response to IUS findings on clinical outcomes, patients who experience poor outcome (medication escalation, corticosteroid use, hospitalisation, or surgery) within 90 days of IUS were excluded from the following analysis. In our study, 27 patients had medication escalation, 24 patients had corticosteroids, 15 patients were hospitalised and 5 underwent surgery within 90 days of their IUS. When these patients were excluded from their sub‐group analysis, the presence of sonographic inflammation remained significantly associated with risk of escalation in medication after 90 days with a HR 1.73 (CI 0 1.1–2.8), p = 0.02. Furthermore, sonographic inflammation remained significantly associated with risk of hospitalisation, HR 2.7 (1.2–6.2), p = 0.01 and with surgery after 90 days, HR 2.97 (CI 1.0–8.8), p = 0.03. Sonographic inflammation was not significantly associated with corticosteroid use after 90 days, HR 1.52 (0.69–3.4), p = 0.28.
3.8. CRP and fecal calprotectin
Baseline CRP was available for 73% of patients. The median CRP was 2.2 (0.9–5.25) mg/L. CRP was classified as normal (≤5 mg/L) in 74%. An abnormal CRP at baseline did not significantly predict any clinical outcomes (Appendix Table A2). A single patient had CRP recorded 1 month following treatment escalation which is not felt to impact the results. Fecal calprotectin was available for 37% patients, of these results 42% were returned within 2 weeks of IUS. Calprotectin was elevated >150 μg/g in 36% and >50 μg/g in 72%. Median calprotectin was 71.4 (34–139) in those with healing (n = 33) versus 126.5 (53–388) in patients with sonographic inflammation (n = 42), p = 0.079. Elevation in fecal calprotectin >50 μg/g was associated with medication escalation (HR 2.29; 95% CI 1.03–5.06; p = 0.03) and corticosteroid use (HR 10.7; 95% CI 0.14–80.48; p = 0.001) but not risk of hospitalisation (HR 0.55; 95% CI 0.17–1.59; p = 0.26) or surgery (HR 3.05; 95% CI 0.34–26.90; p = 0.26). Higher thresholds for elevation in fecal calprotectin >150 or >250 μg/g was not associated with clinical complication outcomes (Appendix Table A2). The presence of sonographic remission in combination with CRP and calprotectin remission did not significantly predict medication escalation, steroid use, hospitalisation, or surgery (data not shows) in the 68 patients with available data.
APPENDIX TABLE A2.
Serum and stool biomarkers and clinical complications
| Medication escalation | Corticosteroid use | Hospitalisation | Surgery | |
|---|---|---|---|---|
| HR (95% CI), p value | HR (95% CI), p value | HR (95% CI), p value | HR (95% CI), p value | |
| Elevated CRP (>5 mg/L) | 1.36 (0.83–2.24), p = 0.23 | 1.00 (0.46–2.13), p = 0.10 | 1.32 (0.61–2.87), p = 0.48 | 1.49 (0.57–3.9), p = 0.41 |
| Elevated fecal calprotectin (>250 μg/g) | 1.48 (0.76–2.86), p = 0.26 | 2.22 (0.93–5.29), p = 0.071 | 1.3 (0.39–4.31), p = 0.67 | 3.91 (0.77–19.80), p = 0.11 |
| Elevated fecal calprotectin (>150 μg/g) | 1.55 (0.081–2.95), p = 0.19 | 2.11 (0.89–4.98), p = 0.09 | 1.05 (0.31–3.51), p = 0.93 | 3.37 (0.66–17.16), p = 0.15 |
| Elevated Fecal Calprotectin (>50 μg/L) | 2.29 (1.03–5.06), p = 0.03* | 10.7 (0.142–80.48), p = 0.001* | 0.55 (0.17–1.59), p = 0.26 | 3.05 (0.34–26.90), p = 0.26 |
p < 0.05.
4. DISCUSSION
We have demonstrated that, in patients with CD in clinical remission, achieving sonographic healing is associated with reduced clinical complications including medication escalation, corticosteroid use, hospitalization, and surgery. When examining clinical complications occurring greater than 90 days following IUS, sonographic inflammation remains associated with an increased with of clinical complications including medication escalation, hospitalisation, and surgery. To our knowledge, this is the first exploration of sonographic healing associated with risk of clinical complications in CD despite clinical remission.
Objective disease assessment is now acknowledged as the cornerstone to preventing progressive disease and disability. 12 The utility of cross‐sectional imaging to establish transmural healing as an objective target above that of mucosal healing is also emerging. 13 Previously this has been most well established with MRI. Indeed, the benefits of achieving a combination of MRI and endoscopic healing or solely MRI healing are established with lower rates of surgery, bowel damage progression, hospitalisation, CD‐related drug discontinuation, or escalation in therapy than endoscopic mucosal healing alone. 5 MRI transmural healing offers superior outcomes over endoscopic mucosal healing in several studies with >70% decrease in major adverse outcomes compared to mucosal healing alone. 5 , 14 Similar evidence is emerging for sonographic healing. 15 In a prospective study among patients who had completed 2 years of anti‐TNF treatment, sonographic healing at enrolment was associated with significantly increased rates of sustained steroid‐free clinical remission, reduced hospitalisation, and reduced need for surgery at 12 months. Ninety‐six percent of patients who achieved sonographic healing sustained steroid‐free clinical remission, 9% were hospitalised, and 0% underwent surgery compared to 75%, 28%, and 10%, respectively, in patients with only mucosal healing and 41%, 67%, and 36%, respectively, in patients who had not achieved either type of healing (p = 0.01). 8 IUS is therefore promising with a number of advantages given its sensitivity at detecting mucosal inflammation, immediacy through point of care results, and its ability to be repeated at short duration with greater patient acceptance, low cost, and lack of preparation needed. 16 , 17 Our study additionally highlights its utility as a method of stratifying risk of flare in patients who have achieved clinical remission. 18 We demonstrated that patients achieving transmural healing have a longer median time to medication escalation (3.0 vs. 1.1 years), and a reduced need for corticosteroid use, hospitalisation, and surgery related to CD, demonstrating the utility of stratifying patients by sonographic healing status. The lag between the detection of sonographic inflammation and a disease flare has been replicated with histological inflammation predicting clinical flare, 2 however, IUS offers obvious advantages over endoscopy. IUS is a noninvasive tool to identify patients failing to achieve sonographic healing that may require more intense proactive monitoring, and a lower threshold for medical escalation should be considered. Future studies are now required to prospectively explore the role of sonographic transmural healing as a treatment target and how it compares to MRI transmural healing, endoscopic healing, and even histological healing. The concept of incremental levels of healing offering superior outcomes is attractive and it is likely that targets will include the use of composite objective endpoints incorporating transmural healing.
In this study, sonographic healing was defined as the presence of BWT ≤3 mm without hyperemia or inflammatory fat and with preserved wall stratification. In fact, since inflammatory fat and loss of wall stratification was only seen in those with increased BWT, sonographic healing can be more simply defined as BWT ≤3 mm without hyperemia (Appendix Table A3). This is supported by both a recent prospective study 19 and consensus statements by international experts in IBD where BWT remains the cornerstone sonographic parameter, with severity and acuity of inflammation qualified by additional findings of hyperemia, wall stratification, and inflammatory fat. 20 While hyperemia is less commonly seen outside of the setting of mural thickening (in our study only on a single occasion) it is an important parameter of active inflammation and should be assessed in each segment regardless of wall thickness. Studies have shown that the presence of hyperemia correlates with acute histological inflammation, 21 and in our study was the only factor to predict corticosteroid‐use, echoing findings showing histological activity but not mucosal inflammation predicted corticosteroid use. 2
APPENDIX TABLE A3.
Rates of abnormal bowel wall stratification and mesenteric fat hypertrophy in patients divided into sonographic remission and sonographic inflammation
| Sonographic remission (BWT ≤3 mm and normal CDI), number (%) | Sonographic inflammation (BWT >3 mm and/or hyperemia on CDI), number (%) | Total number | |
|---|---|---|---|
| Normal BWS | 79 (39) | 88 (44) | 167 (83) |
| Loss of BWS | 0 | 35 (17) | 35 (17) |
| MFH absent | 79 (39) | 87 (43) | 166 (82) |
| MFH present | 0 | 36 (18) | 36 (18) |
If BWT and hyperemia represent sonographic healing, how then do addition sonographic findings inform our assessment? In our study disrupted bowel wall stratification was noted in 30% of those with sonographic inflammation. This may be indicative of more severe disease with wall stratification most strongly associated with clinical complications including medication escalation, hospitalisation, and surgery. Inflammatory fat also occurred in approximately 30% of patients with sonographic inflammation and was associated with risk of medication escalation, and surgery and although felt to reflect active inflammation that is dynamic in responsive to therapy it remains more vulnerable to inter‐observer variability. 20 , 22 Sonographic complications occurred in 64 of 202 patients and were predominantly strictures (78%) with 27/50 occurring at anastomosis. These complications were not independently associated with poor outcomes, likely reflecting persistence of stricturing disease even when inflammation is controlled. The CONSTRICT definition of stricture was not used, therefore luminal narrowing in the absence of pre‐stenotic dilatation may reflect a milder severity of stricturing leading to lower rates of surgery. This is supported by other studies that have demonstrated sustained clinical remission occurring following treatment with control of inflammation around the stricture despite the majority of patients having persistent evidence of strictures on imaging. 23 Allocca et al. 19 noted a significant relationship between active disease at IUS and negative clinical outcome at 12 months, utilising the same two IUS parameters identified in this study, BWT and hyperemia, which were developed into a bowel ultrasound score. A bowel ultrasound score >3.52 reflected active endoscopic inflammation and was significantly associated with increased risk for treatment escalation and surgery, but not the risk of hospitalisation or corticosteroids use, compared to an inactive bowel ultrasound score. Although the score requires validation, it highlights the central role that BWT and hyperemia play in IUS disease assessment, further confirming the utility of IUS as a noninvasive assessment tool.
CRP and fecal calprotectin have been embraced as noninvasive biomarkers, 24 , 25 used to monitor Crohn’s disease. This study demonstrated that both sonographic remission and strict threshold stool biomarker results (calprotectin <50 μg/g), but not CRP are predictive of complications. IUS presents an attractive adjunct to assess disease activity in patients with CD, particularly in those with clinical remission, given that up to 15% of patients may not mount CRP elevation, CRP has lower sensitivity in isolated ileal inflammation and fecal calprotectin is often not available. 26 Furthermore, IUS is preferred by patients as a monitoring tool 27 and allows for immediate action on results. We acknowledge limitations in our retrospective design impacting the timing and incomplete availability of results.
In our study, 40% achieved sonographic healing, leaving the majority of patients in clinical remission with objective evidence of sonographic inflammation. The clinical risks and benefits of escalating therapy to aim to achieve sonographic transmural healing above that of biochemical or endoscopic remission is still yet to be fully quantified. It is also unclear if targeting sonographic transmural healing is feasible or provides benefit above that of endoscopic healing. Thus, currently one must still be guarded in translating these associations into clinical practice. However, simple measures to optimise treatment strategies should be used if transmural inflammation is seen and, in patients who achieve sonographic transmural healing, their improved prognosis can be acknowledged and less stringent clinical surveillance or follow‐up may be considered.
Limitations in this study include the single‐center retrospective design, the relatively small sample size with a heterogeneous patient cohort with varied disease duration and treatment experience. Routine care continued for patients and may be a source of bias for clinicians who were not blinded to IUS results. Second, mandated six monthly applications for government funded biologic therapy may have impacted the follow‐up times. The study is further limited by lack of universal paired endoscopic assessment, calprotectin, or CRP data, to confirm endoscopic remission and biochemical remission. To address the issue of bias due to unblinded IUS results, analysis of outcomes excluding complications occurring within 90 days of IUS was undertaken. This analysis confirmed the utility of IUS in predicting outcomes and demonstrated that sonographic inflammation does predict worse long‐tern outcome occurring after 90 days. Prospective studies are required to validate our findings. Ultrasound is operator dependent; however, these criticisms are also applicable to MRI, endoscopy, and histology where inter‐rater variability is often found to have similar or lower (histology) accuracy in determining disease activity. 1 , 28 International training standards and consensus guidelines ensure clinicians performing IUS have appropriate training and experience to minimise variability in quality. Ultrasound has been shown to have excellent inter‐observer agreement for BWT and substantial agreement on hyperemia, acknowledging that central reading of IUS would strengthen prospective research going forward. 29 , 30 The selection of patients from a hospital setting who report clinical remission may introduce bias, missing patients who have been in deep remission and less likely to be engaged in hospital care referred for disease reassessment with IUS.
In conclusion, we have demonstrated that patients with CD in clinical remission with sonographic inflammation have poorer clinical outcomes than those who achieve sonographic healing. We propose that IUS be incorporated at routine clinical review to allow for real‐time objective assessment and prognostication of the patient. Future prospective trials are essential to examine the role of sonographic transmural healing as a treatment target.
COMPETING INTEREST/DISCLOSURES
BC has served as a consultant, advisory board member, or received grants from AbbVie, Ferring, Janssen, Pfizer, Fresenius Kabi, Falk Pharama, Gilead, Celgene, and Takeda.
PG has served as a consultant or advisory board member for Anatara, Atmo Biosciences, Falk Pharma, Immunic Therapeutics, Novozymes, Novoviah, Comvita, and Takeda. He has received research grants for investigator‐driven studies from Atmo Biosciences. He holds shares in Atmo Biosciences.
RV has served as a consultant for Janssen.
There are no author financial disclosures or relevant conflict of interest for DT, AP, NM. RG, AB, ZA, and AA.
AUTHOR CONTRIBUTIONS
Rose Vaughan: Conceptualization (equal); data curation (equal); formal analysis (lead); investigation (equal); methodology (equal); project administration (lead); software (equal); visualization (supporting); writing – original draft (lead); writing – review and editing (lead). Douglas Peter Tjandra: Data curation (supporting); writing – review and editing (supporting). Ashwin S Patwardhan: Data curation (supporting). Nicholas Mingos: Data curation (supporting). Robert Gibson: Supervision (supporting); writing – review and editing (supporting). Alex Boussioutas: Methodology (supporting); supervision (equal); writing – review and editing (supporting). Zaid S Ardalan: Visualization (supporting); writing – review and editing (supporting). Aysha Al‐Ani: Writing – review and editing (supporting). Peter Gibson: Supervision (supporting); visualization (supporting); writing – review and editing (supporting). Britt Christensen: Formal analysis (supporting); methodology (supporting); resources (supporting); supervision (lead); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting).
ACKNOWLEDGEMENTS
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Vaughan RP, Tjandra D, Patwardhan A, Mingos N, Gibson R, Boussioutas A, Toward transmural healing: Sonographic healing is associated with improved long‐term outcomes in patients with Crohn’s disease. Aliment Pharmacol Ther. 2022;56:84–94. 10.1111/apt.16892
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Jensen MD, Ormstrup T, Vagn‐Hansen C, Østergaard L, Rafaelsen SR. Interobserver and intermodality agreement for detection of small bowel Crohn’s disease with MR enterography and CT enterography. Inflamm Bowel Dis. 2011;17(5):1081‐1088. [DOI] [PubMed] [Google Scholar]
- 2. Christensen B, Erlich J, Gibson PR, Turner JR, Hart J, Rubin DT. Histologic healing is more strongly associated with clinical outcomes in ileal Crohn’s disease than endoscopic healing. Clin Gastroenterol Hepatol. 2020;18(11):2518‐2525.e2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peyrin‐Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat‐to‐target. Am J Gastroenterol. 2015;110(9):1324‐1338. [DOI] [PubMed] [Google Scholar]
- 4. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long‐term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15(9):1295‐1301. [DOI] [PubMed] [Google Scholar]
- 5. Lafeuille P, Hordonneau C, Vignette J, et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn’s disease. Aliment Pharmacol Ther. 2021;53(5):577‐586. [DOI] [PubMed] [Google Scholar]
- 6. Turner D, Ricciuto A, Lewis A, et al. STRIDE‐II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570‐1583. [DOI] [PubMed] [Google Scholar]
- 7. Schuler A, Reuss J, Delorme S, Hagendorff A, Giesel F. Costs of clinical ultrasound examinations—an economical cost calculation and analysis. Ultraschall Med. 2010;31(4):379‐386. [DOI] [PubMed] [Google Scholar]
- 8. Castiglione F, Imperatore N, Testa A, et al. One‐year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019;49(8):1026‐1039. [DOI] [PubMed] [Google Scholar]
- 9. Serban ED. Treat‐to‐target in Crohn’s disease: will transmural healing become a therapeutic endpoint? World J Clin Cases. 2018;6(12):501‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kucharzik T, Wilkens R, Maconi G, et al. DOP10 intestinal ultrasound response and transmural healing after ustekinumab induction in Crohn’s disease: week 16 interim analysis of the STARDUST trial substudy. J Crohn’s Colitis. 2020;14(Supplement_1):S046‐S048. [Google Scholar]
- 12. Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis. 2017;23(6):894‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilkens R, Novak KL, Maaser C, Panaccione R, Kucharzik T. Relevance of monitoring transmural disease activity in patients with Crohn’s disease: current status and future perspectives. Ther Adv Gastroenterol. 2021;14:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernandes SR, Rodrigues RV, Bernardo S, et al. Transmural healing is associated with improved long‐term outcomes of patients with Crohn’s disease. Inflamm Bowel Dis. 2017;23(8):1403‐1409. [DOI] [PubMed] [Google Scholar]
- 15. Zorzi F, Ghosh S, Chiaramonte C, et al. Response assessed by ultrasonography as target of biological treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2019;18:2030‐2037. [DOI] [PubMed] [Google Scholar]
- 16. Kucharzik T, Maaser C. Intestinal ultrasound and management of small bowel Crohn’s disease. Ther Adv Gastroenterol. 2018;11:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maaser C, Petersen F, Helwig U, et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut. 2020;69(9):1629‐1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allocca M, Furfaro F, Fiorino G, Peyrin‐Biroulet L, Danese S. Point‐of‐care ultrasound in inflammatory bowel disease. J Crohn’s Colitis. 2021;15:143‐151. [DOI] [PubMed] [Google Scholar]
- 19. Allocca M, Craviotto V, Bonovas S, et al. Predictive value of bowel ultrasound in Crohn’s disease: a 12‐month prospective study. Clin Gastroenterol Hepatol. 2021;20(4):e723‐e740. [DOI] [PubMed] [Google Scholar]
- 20. Goodsall TM, Jairath V, Feagan BG, et al. Standardisation of intestinal ultrasound scoring in clinical trials for luminal Crohn’s disease. Aliment Pharmacol Ther. 2021;53(8):873‐886. [DOI] [PubMed] [Google Scholar]
- 21. Drews BH, Barth TF, Hanle MM, et al. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn’s disease. Eur Radiol. 2009;19(6):1379‐1386. [DOI] [PubMed] [Google Scholar]
- 22. Kucharzik T, Kannengiesser K, Petersen F. The use of ultrasound in inflammatory bowel disease. Ann Gastroenterol. 2017;30(2):135‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schulberg J, Wright E, Holt B, et al. P300 Crohn’s disease strictures respond to drug treatment and treat‐to‐target intense combination therapy is more effective than standard anti‐TNF therapy. The STRIDENT randomised controlled trial. J Crohn’s Colitis. 2021;15(Supplement_1):S328‐S330. [Google Scholar]
- 24. Click B, Vargas EJ, Anderson AM, et al. Silent Crohn’s disease: asymptomatic patients with elevated C‐reactive protein are at risk for subsequent hospitalization. Inflamm Bowel Dis. 2015;21(10):2254‐2261. [DOI] [PubMed] [Google Scholar]
- 25. Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54(3):364‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Consigny Y, Modigliani R, Colombel JF, Dupas JL, Lémann M, Mary JY. A simple biological score for predicting low risk of short‐term relapse in Crohn’s disease. Inflamm Bowel Dis. 2006;12(7):551‐557. [DOI] [PubMed] [Google Scholar]
- 27. Goodsall TM, Noy R, Nguyen TM, Costello SP, Jairath V, Bryant RV. Systematic review: patient perceptions of monitoring tools in inflammatory bowel disease. J Can Assoc Gastroenterol. 2021;4(2):e31‐e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tielbeek JA, Makanyanga JC, Bipat S, et al. Grading Crohn disease activity with MRI: interobserver variability of MRI features, MRI scoring of severity, and correlation with Crohn disease endoscopic index of severity. AJR Am J Roentgenol. 2013;201(6):1220‐1228. [DOI] [PubMed] [Google Scholar]
- 29. Bhatnagar G, Rodriguez‐Justo M, Higginson A, et al. Inflammation and fibrosis in Crohn’s disease: location‐matched histological correlation of small bowel ultrasound features. Abdom Radiol. 2020;46:144‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fraquelli M, Sarno A, Girelli C, et al. Reproducibility of bowel ultrasonography in the evaluation of Crohn’s disease. Dig Liver Dis. 2008;40(11):860‐866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


