Abstract
The incorporation of organocatalysts into protein scaffolds holds the promise of overcoming some of the limitations of this powerful catalytic approach. Previously, we showed that incorporation of the non‐canonical amino acid para‐aminophenylalanine into the non‐enzymatic protein scaffold LmrR forms a proficient and enantioselective artificial enzyme (LmrR_pAF) for the Friedel‐Crafts alkylation of indoles with enals. The unnatural aniline side‐chain is directly involved in catalysis, operating via a well‐known organocatalytic iminium‐based mechanism. In this study, we show that LmrR_pAF can enantioselectively form tertiary carbon centres not only during C−C bond formation, but also by enantioselective protonation, delivering a proton to one face of a prochiral enamine intermediate. The importance of various side‐chains in the pocket of LmrR is distinct from the Friedel‐Crafts reaction without enantioselective protonation, and two particularly important residues were probed by exhaustive mutagenesis.
Keywords: Artificial Enzymes, Iminium Catalysis, Enantioselective Protonation, Non-canonical Amino Acids, Friedel-Crafts Alkylations
BioTrans2021: We created an artificial enzyme consisting of a non‐enzymatic protein (LmrR) containing an unnatural catalytic residue with an aniline side chain (LmrR_pAF). Building on our previous work showing how LmrR_pAF can catalyse a Friedel‐Crafts alkylation of indoles, here we show that when α‐substituted acroleins are applied as substrates the protein scaffold enables enantioselective protonation with good selectivity.
Introduction
The quest to broaden the catalytic repertoire of enzymes is born out of a societal need for greener methods of chemical manufacture.[ 1 , 2 ] Enzymes’ mild operating conditions and high efficiencies are of great appeal, yet the chemistries that they can catalyse are predominantly limited to those that are important for organismal fitness, and not necessarily those which are useful for humankind.[ 3 , 4 ] Strategies to this end include enhancing promiscuous activities of natural enzymes through directed evolution, computational design of ‘de novo’ enzymes from scratch, as well as the construction of hybrid catalysts known as artificial enzymes.[ 1 , 2 , 5 , 6 , 7 , 8 ] This last approach involves the situation of catalytic chemical moieties not exploited by natural enzymes into biomolecular scaffolds such as proteins. Of the many methods to combine these two components, the use of non‐canonical amino acids (ncAAs) whose side‐chains have inherent catalytic properties has recently emerged as an elegant and effective strategy which can reduce the handling steps required for artificial enzyme preparation.[ 9 , 10 , 11 , 12 ] In this method, amber‐stop‐codon‐suppression is used to site‐selectively incorporate the ncAA during protein biosynthesis in response to the amber (TAG) codon.[ 13 , 14 ] The choice of biomolecular scaffold is paramount to success and in this work, as in our previous studies, we employed the homodimeric Lactococcal multi‐drug resistance regulatory protein (LmrR) which has the unusual feature of a large hydrophobic pocket at its dimer interface.[ 15 , 16 ] This protein has proven the perfect catalytic pocket in which to conduct a variety of chemical transformations with rate acceleration and enantio‐induction provided by this protein environment. [17]
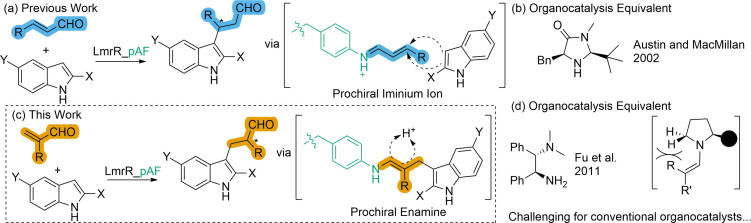
Target transformations for artificial enzymes are typically selected on the basis of their omission amongst Nature's catalytic reactions, as well as their proven synthetic utility. Amino‐catalytic chemistry (often simply referred to as organocatalysis) is a highly powerful set of methodologies, many of which were first demonstrated in the past two decades, and whose remarkable contribution to the field of asymmetric synthesis was acknowledged with the 2021 Nobel Prize in Chemistry. It presents many different transformations worthy of translation into a biocatalytic setting with the use of artificial enzymes, some of which are already demonstrated in aqueous environments.[ 18 , 19 , 20 ] Of the many activation modes demonstrated in amino‐catalysis, the electrophilic activation of enals via the formation of unsaturated iminium ions and their subsequent nucleophilic attack caught our attention due to the diversity of reaction pathways that it allows. [21] Recently we demonstrated that LmrR, with the ncAA para‐aminophenyl alanine (pAF) incorporated at position 15, makes a competent and enantioselective catalyst for the Friedel‐Crafts alkylation of indoles with aliphatic enal substrates, which are activated for nucleophilic attack at the β‐position by iminium ion formation at the catalytic pAF residue (henceforth referred to as FC‐reaction, Figure 1(a)). [22] This transformation, which was first demonstrated with organocatalysis by Austin and MacMillan in 2002 (Figure 1(b)), creates the chiral centre during the C−C bond forming step, and thus stereoselective formation of the iminium ion and controlled approach of indole are important for good enantioselectivity.[ 23 , 24 ] In this study, we turned our attention to the tandem Friedel‐Crafts‐alkylation‐enantioselective‐protonation (henceforth FC‐EP reaction) of indoles with α‐substituted acroleins (Figure 1(c)). Enantio‐induction in this transformation eluded organocatalysis until 2011 when Fu et al. demonstrated good enantioselectivity with a bifunctional amino‐catalyst (Figure 1(d)). [25] The greatest challenge for enantioselectivity with α‐substituted acrolein substrates is that the chiral centre is formed by protonation of the enamine intermediate formed after the C−C bond formation step (Figure 1(c)). The controlled delivery of a proton to one prochiral face of a substrate is notoriously difficult, yet it is a feat achieved by several natural, artificial, and engineered enzymes.[ 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ] Here, we show that LmrR_pAF performs this transformation with good yields and enantioselectivities with a variety of enal and indole substrates. Furthermore, we investigate how pH as well as mutations in the catalytic pocket of LmrR_pAF affect reaction outcomes of both the FC and FC‐EP reactions. The steric demands of α‐substituted aldehydes/enals make them challenging substrates for conventional secondary‐amine‐containing organocatalysts and thus this transformation with LmrR_pAF represents an important step forward for artificial enzymes and demonstrates the broad catalytic potential of the primary‐amine containing catalytic ncAA employed.[ 34 , 35 ]
Figure 1.
(a) our previous work on the Friedel‐Crafts alkylation of indoles with β‐substituted enals using LmrR_pAF as catalyst, which takes place via a prochiral iminium‐ion intermediate. [22] (b) An organocatalyst for this transformation demonstrated by Austin and MacMillan[23]. (c) This work – tandem‐Friedel‐crafts‐alkylation‐enantioselective‐protonation of indoles employing α‐substituted acroleins as substrates via protonation of a prochiral enamine intermediate. (d) Organocatalysts for this transformation require primary‐amine moieties for iminium activation and tertiary‐amine moieties for enantioselective proton delivery[25], steric constraints make α‐substituted acroleins challenging substrates for conventional secondary‐amine‐containing organocatalysts[34,35].
Results and Discussion
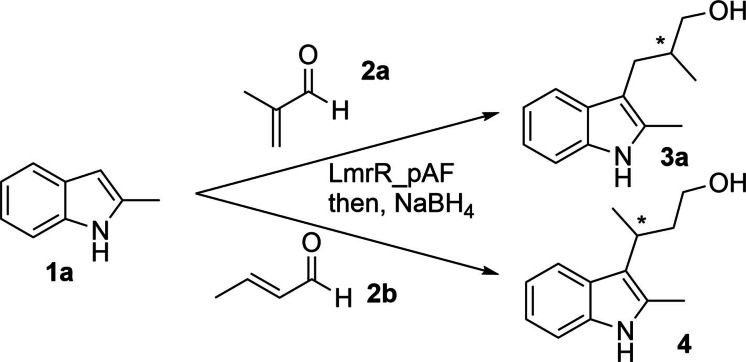
Our initial efforts in this study focussed on conducting a series of control experiments to both establish the activity and selectivity of LmrR_pAF for the FC‐EP reaction between methacrolein 2 a and 2‐methyl‐indole 1 a and to rule out the efficacy of LmrR mutants with canonical amino acids at position 15 in place of pAF (Scheme 1, Table 1). Indeed, 16 hours reaction time with just 2 mol% of LmrR_pAF afforded essentially quantitative yield of product 3 a with an enantiomeric excess of 74 % (as with our previous study, reduction of the aldehyde product to the alcohol 3 a was necessary for normal‐phase HPLC analysis of the reaction mixture). Replacing the pAF residue with either lysine, tyrosine or valine (which is present at this position in the wild‐type sequence) abrogated activity and selectivity for this transformation. Likewise, incorporation of the pAF aniline sidechain into the protein backbone was also essential, since the combination of aniline and LmrR in ratios typically used for supramolecular catalysis with LmrR [36] failed to produce appreciable yields or enantiomeric excess. LmrR_pAF also significantly outperforms aniline itself for this transformation, which affords only 25 % yield of 3 a even at equimolar catalyst loadings.
Scheme 1.
The reaction between 2‐methyl‐indole (1 a) and methacrolein (2 a) produces 3 a after reduction via a tandem enantioselective protonation process (FC‐EP reaction), whilst substitution of methacrolein with crotonaldehyde (2 b) produces 4 after reduction (FC reaction).
Table 1.
Initial results of LmrR_pAF catalysed production of 3 a and control experiments.[a]
|
Catalyst[b] |
Yield[c] [%] |
ee[d] [%] |
|---|---|---|
|
LmrR_pAF (20 μM) |
95±1 |
74±2 |
|
LmrR_V15K (20 μM) |
13±2 |
5±0.5 |
|
LmrR_V15Y (20 μM) |
11±0.7 |
‐6±0.5 |
|
LmrR (20 μM) |
10±0.7 |
6±0.4 |
|
LmrR (20 μM)+aniline (16 μM) |
12±0.2 |
−8±0.1 |
|
Aniline (1 mM)[e] |
25±1 |
N.D. |
[a] Reaction conditions: [1a]=1 mM; [2a]=6 mM; 300 μL volume reaction in phosphate buffer (50 mM, pH 6.5) containing NaCl (150 mM) and DMF (8 vol %). Reactions conducted for 16 hours, followed by reduction to form 3 a by addition of NaBH4 (60 μL, 20 mg mL−1 in 0.5 w/v% NaOH) for analysis. Errors given represent the standard deviation from two experiments with independently produced batches of protein, each conducted in duplicate, to give four total measurements. [b] Concentrations of LmrR dimer. [c] Analytical yields of 3 a determined by chiral normal‐phase HPLC with the use of a calibration curve. [d] Enantiomeric excess determined by chiral normal‐phase HPLC. [e] Error given is standard deviation from an experiment conducted in triplicate. N.D.=not determined.
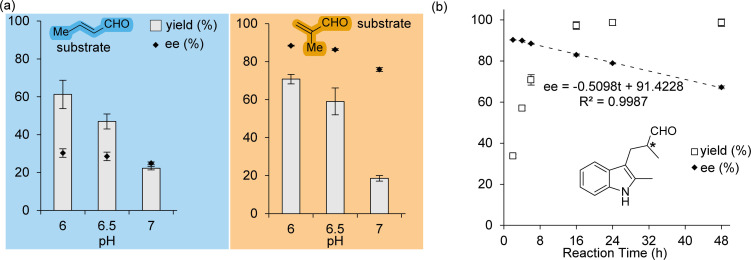
Next, we investigated the effect of pH on the catalytic production of 3 a and 4, to assess whether the abundance of solvent protons is important for catalysis in the enantioselective protonation process required for the production 3 a. We also chose a shorter reaction time and lower enzyme loading for these experiments in order to better observe any effects present. At all pH values measured, the yield of 3 a and 4 is very similar suggesting that positioning the methyl group in either the α‐ or β‐positions of the substrate has little effect on the activity. Both reactions showed a pronounced effect from pH in the region of 6 to 7 (Figure 2(a)). Changing the pH from 6 to 6.5 and to 7 results in a significant loss in product yield, which is likely indicative of the pKa of the iminium ion, whose protonation is crucial for effective catalysis hence the widespread use of acid‐cocatalysts in iminium catalysis. [21]
Figure 2.
(a) Effect of reaction pH on analytical yields and enantiomeric excesses from the formation of 4 (left, blue) and 3 a (right, orange) by LmrR_pAF. Reaction conditions [LmrR_pAF]=10 μM (dimer concentration); [1 a]=1 mM; [2 a]=6 mM or [2 b]=5 mM; 300 μL volume reaction in phosphate buffer (50 mM) containing NaCl (150 mM) and DMF (8 vol %). Reactions conducted for 6 hours at 4 °C, followed by reduction to form 3 a or 4 for analysis by normal‐phase HPLC. (b) LmrR_pAF catalysed production of 3 a monitored over 48 hours, revealing product racemisation. Reaction conditions as in (a), pH=6. In both (a) and (b), errors given represent the standard deviation from two experiments with independently produced batches of protein, each conducted in duplicate.
Much higher enantiomeric excesses are obtained for product 3 a than for 4, which gives the somewhat surprising conclusion that LmrR_pAF can better control the enantioselective delivery of a proton than of the indole substrate. Loss in enantiomeric excess with increasing pH was more pronounced in the case of 3 a than 4, however correcting for the background reaction (which is higher than for 4, Supporting Information Table 2), finds that the enantioselectivity of the catalysed reaction is unaffected. We were interested to find that much higher enantiomeric excesses were obtained for 3 a (up to 88 % at pH 6) than under the first conditions we tested (with longer reaction times and higher catalyst loadings). This led us to suspect that racemisation may be affecting the ultimate enantio‐enrichment in the product, as is well known to occur in water with compounds with stereo‐centres in the α‐ position to a carbonyl functionality. We monitored the production of 3 a over 48 hours and found that whilst full conversion occurs after approximately 12 hours, the enantiomeric excess erodes steadily over the whole period (Figure 2(b)). The enantiomeric excess decreased in a near perfect linear manner, allowing us to determine a rate of decrease of approximately 0.5 % per hour. We previously demonstrated that both the buffer and protein scaffold produced racemisation in another reaction involving enantioselective protonation with LmrR_pAF, and thus a variety of processes are presumably also involved here too. Consequently, shorter reaction times are desirable for obtaining the highest enantiomeric excesses. [31] We explored both lowering the enzyme loading, as well as increasing the substrate concentration obtaining TONs over 200 in some cases. However, in each case there was a concomitant drop in enantiomeric excess (Supporting Information Table 1).
Table 2.
Reaction outcomes of competition experiments with equal concentrations of 2 a and 2 b with 1 a, catalysed by LmrR_pAF(mutants).[a]
|
Catalyst[b] |
Yield 4 [b] |
ee 4 [c] |
Yield 3 a [b] |
ee 3 a [c] |
4 a:3 a |
|---|---|---|---|---|---|
|
[%] |
[%] |
[%] |
[%] |
||
|
LmrR_pAF |
46±1 |
22±0.8 |
55±0.7 |
86±0.5 |
1 : 1.2 |
|
LmrR_pAF_RGN |
38±2 |
50±0.4 |
20±0.7 |
37±0.7 |
2 : 1 |
[a] Reaction conditions: [LmrR_pAF] or [LmrR_pAF_RGN]=10 μM (dimer concentration); [1 a]=1 mM; [2 a]=[2 b]=6 mM; 300 μL volume reaction in phosphate buffer (50 mM, pH 6) containing NaCl (150 mM) and DMF (8 vol %). Reactions conducted for 6 hours at 4 °C, followed by reduction to form 3 a or 4 for analysis by HPLC. [b] Yields determined by HPLC on a normal phase with use of calibration curves. [c] Enantiomeric excesses determined by HPLC on a chiral normal phase. Errors given represent the standard deviation from two experiments with independently produced batches of protein, each conducted in duplicate, to give four total measurements.
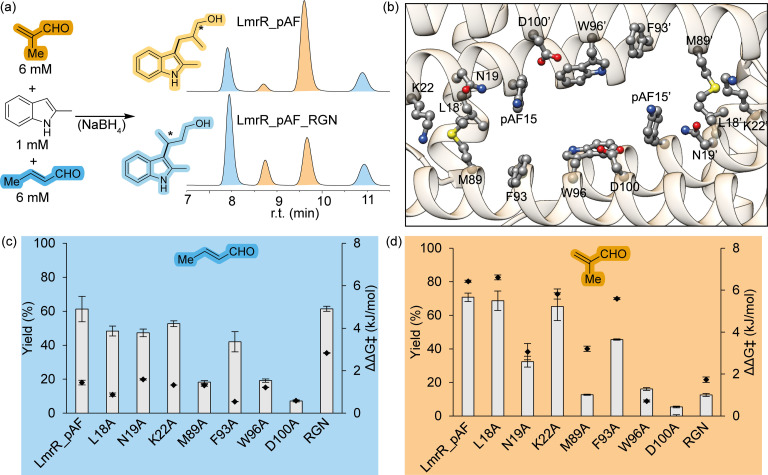
In competition experiments employing equal concentrations of crotonaldehyde (2 b) and methacrolein (2 a) with 2‐methylindole (1 a) LmrR_pAF shows almost no preference for production of either 3 a or 4, albeit with the far higher enantioselectivity for 3 a already noted (Figure 3(a), Table 2). However, when we tested LmrR_pAF_RGN, which was the result of directed evolution for the Friedel‐Crafts reaction with the linear trans‐2‐hexenal as screening substrate, we found that this triple mutant has a twofold preference for production of 4 over 3 a. 4 is produced by LmrR_pAF_RGN with a higher enantiomeric excess than by LmrR_pAF, with an overall lower conversion in the same time frame and large loss in enantiomeric excess for 3 a.
Figure 3.
(a) representative chiral normal‐phases HPLC traces obtained in competition experiments employing both substrates 2 a and 2 b together with indole 1 a to produce mixtures of products 3 a (orange) and 4 (blue) (Table 2). (b) Positions in LmrR_pAF subject to mutagenesis (PDB: 6I8N). Effect of various mutants on reaction outcomes producing product 4 (c) in blue and 3 a (d) in orange. ΔΔGǂ (the difference in the Gibbs’ free energy of activation for the production of the two product enantiomers) was calculated from the enantiomeric ratio (e.r.) according to the equation ΔΔGǂ=RTln(e.r.). In (c) and (d), errors given represent the standard deviation from two experiments with independently produced batches of protein, each conducted in duplicate.
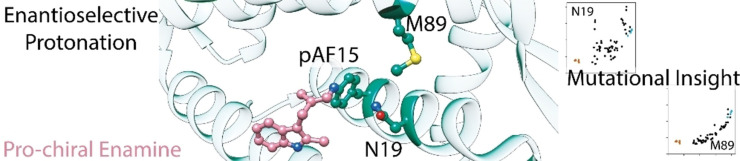
Noting this large divergence in substrate preference and enantioselectivity engendered by the mutations in LmrR_pAF_RGN we hypothesised that the two reaction pathways to produce either 3 a or 4 utilise the pocket of LmrR to promote catalysis in different manners. To test this, we performed alanine‐scanning at 7 positions inside the pocket of LmrR encompassing both polar and apolar residues (Figure 3(b)‐(d)). Since the enantiomeric excesses produced in each reaction are so different, and to present mutational effects on this parameter on a linear scale we show ΔΔGǂ i. e. the level of energy discrimination that a particular mutant provides between the transition states leading to either enantiomer of 3 a or 4. Alanine mutations at positions L18, K22 and F93 produced only minor effects on catalysis outcomes for the production of 3 a and 4. At positions W96 and D100, large detrimental effects were observed for both yield and enantioselectivity of 3 a and 4 (although LmrR_pAF_W96A has similar enantioselectivity for production of 4), in line with previous results highlighting the importance of these residues in the majority of LmrR‐based artificial enzymes.[ 30 , 36 , 37 , 38 ] Substitution of alanine at positions N19 and M89, however, showed distinctly different effects on the outcomes of the FC and FC‐EP reactions. Whilst for the FC‐EP reaction both of these mutants show severely reduced yields and enantioselectivities, the only significant effect on the FC reaction is a reduced yield in the case of LmrR_pAF_M89A.
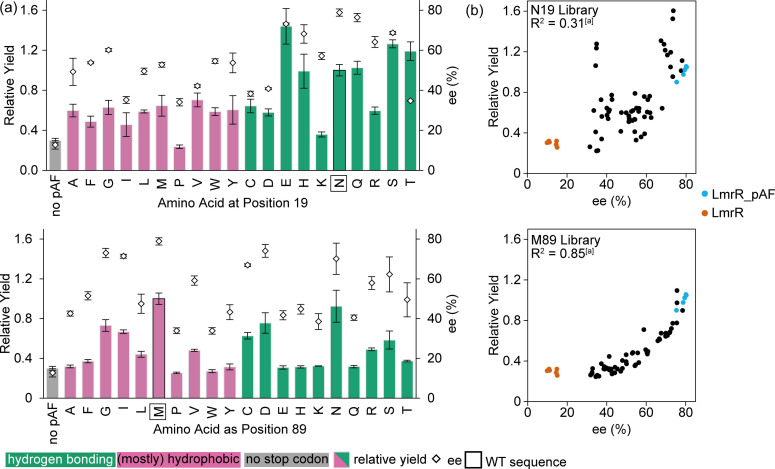
In order to learn more about the roles the side‐chains at N19 and M89 play in the FC‐EP reaction, we prepared every mutant at each of these positions by QuikChange® PCR and expressed them together with LmrR with the wild‐type valine at position 15 instead of pAF, and LmrR_pAF as negative and positive controls, respectively. We then lysed the cells and used the cell‐free extract directly for catalysis, analysing the enantioselectivity and yield of reactions rapidly with super‐critical fluid chromatography (SFC). We also analysed the soluble fraction of the cultures by SDS‐PAGE to qualitatively inspect the relative expression levels of the 38 mutants (see Supporting Information Figure S1). Only the proline mutants showed particularly poor soluble expression, which is unsurprising given that both positions 19 and 89 are situated in α‐helices. In general, mutations at position 19 have significant effects on soluble protein production, whilst the library of mutants at position 89 showed relatively uniform expression.
At position N19, the most functional replacements also contain hydrogen‐bonding side‐chains: glutamic acid, histidine, glutamine and serine (although notably the cysteine and aspartic acid mutants do not perform well) (Figure 4(a), top). The fact that mutants at position 19 show a poor correlation in yield and enantiomeric excess suggests that sidechains in this position can affect the rate‐determining or chiral‐centre‐forming steps via separate mechanisms (Figure 4(b), top). This is well illustrated by LmrR_pAF_N19T, which affords 3 a with increased yield, but much lower enantioselectivity, than LmrR_pAF. Conversely, at position 89, functional replacements for the methionine side‐chain can be found with a variety of sizes and polarities (Figure 4(a), bottom). Unlike the N19 library, this library shows a strong correlation between the yield and enantioselectivity obtained with a given mutant. Any effect that a side‐chain at position 89 has on the yield of 3 a is also reflected proportionally in the enantiomeric excess, implying that sidechains at this position affect both the rate‐determining and enantioselective protonation steps via a single mechanism, (Figure 4(b), bottom). There are many plausible ways in which the residues at positions 19 and 89 may be involved in the catalytic mechanism, however the experiments conducted here cannot determine decipher which role they play.
Figure 4.
(a) Catalysis results for the FC‐EP reaction producing 3 a using cell‐free extract libraries with every mutant at the N19 (top) and M89 (bottom) positions. Results are an average of a triplicate experiment, and error bars shown reflect the standard deviation of those experiments, except for the results for LmrR (no pAF) which is six repeated experiments (data from both libraries combined) and LmrR_pAF which is five repeated experiments (data from both libraries combined, one sample was calculated to be an outlier by the interquartile method). Analytical yields and enantiomeric excesses were determined by SFC with the use of an internal standard, and are given relative to the mean of the LmrR_pAF samples. (b) Results for each library: N19 shows a weak correlation between yield and ee for the pAF containing samples, whilst M89 shows a strong correlation. LmrR (no pAF) samples shown in orange and LmrR_pAF samples shown in blue. [a] Value obtained by performing a linear fit of the pAF containing members of the library, i. e., LmrR without pAF was not included in the fit.
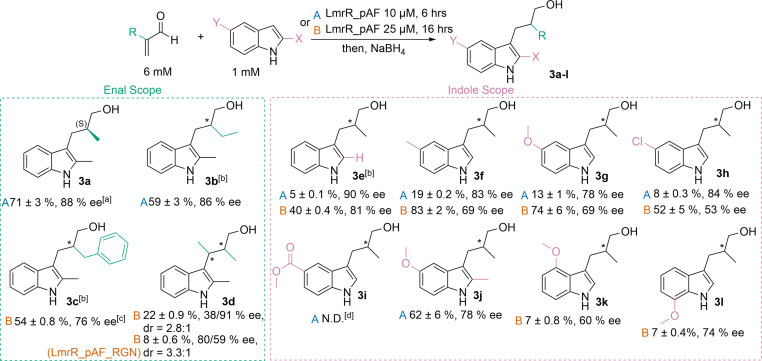
Finally, we assessed the scope of the FC‐EP reaction by LmrR_pAF with regards to both enal α‐substituents as well as indole substituents in the 2‐ and 5‐positions (Figure 5). Methacrolein could be substituted with 2‐ethyl‐acrolein to produce 3 b whilst maintaining good yields and enantioselectivity. Even the bulky 2‐benzyl‐acrolein could be employed successfully as substrate, requiring slightly increased enzyme loadings and reactions times to give good yields and enantioselectivities. The doubly substituted tiglic aldehyde could also be employed to afford product 3 d, however higher catalyst loadings and longer reaction times were required to afford modest yields and enantio‐ and diastereoselectivities. When LmrR_pAF_RGN was used to catalyse the conversion of this substrate, a similar diastereomeric ratio but different enantiomeric excesses were obtained, which may reflect the propensity of this mutant for enantioselective C−C bond formation, rather than enantioselective protonation. Similarly to our previous work, the 2‐methyl substituent has a significant effect on the yields obtained, and thus products 3 e–h required increased catalyst loadings and reaction times to accumulate good yields. [22] Erosion of enantiomeric excess was also observed for these products, with shorter reaction times giving higher selectivities but lower yields, and longer reaction times improving yields at the expense of selectivity. Electron donating substituents on the indole ring proved beneficial for activity (products 3 f and 3 g); the electron withdrawing 5‐chloro substituent in product 3 h did not negatively affect the yield obtained, but did give more rapid erosion of enantiomeric excess. The highly electron‐withdrawing methyl‐ester substituent in 3 i, however, reduced activity to the point that no product was detected. These substituent effects are consistent with the nucleophilic role of indole in the reaction pathway. Product 3 j, with both 2‐methyl and 5‐methoxy substituents, was obtained with good yields and enantiomeric excess whilst still using a low catalyst loading and short reaction time. We also tested indole substrates with methoxy substituents at the 4‐ and 7‐positions and could obtain the corresponding products 3 k and 3 l with modest to good enantiomeric excess, albeit with low yields.
Figure 5.
Substrate scope of FC‐EP reaction catalysed by LmrR_pAF. Analytical yields were determined by HPLC or SFC with the use of a calibration curve. [a] The absolute configuration was assigned by comparison of the order of elution of the enantiomers on chiral HPLC with that reported in the literature [25] where the stereocentre was assigned by analogy to another product. [b] Small unidentified impurities also observed in the chromatogram.[c] nal concentration of 1.5 mM was used due to low solubility of this substrate under reaction conditions. [b] No product was detected by HPLC. Errors given represent the standard deviation from two experiments with independently produced batches of protein, each conducted in duplicate, to give four total measurements. The errors are quoted to one decimal where they are below 1 %. Errors on ee measurements are approximately 1 % or lower in most cases. N.D.=not determined.
Conclusion
In this work we have shown how a challenging enantioselective protonation process can be achieved in a protein scaffold by using an organocatalytic mechanism mediated by a non‐canonical amino acid side‐chain. This reactivity responds to mutations in a markedly different manner than the Friedel‐Crafts reaction which does not involve enantioselective protonation. This suggests that the LmrR scaffold acts as a ‘blank canvas’ where the amino‐acid sidechains in the pocket can be utilised in different manners to promote the different catalytic reactions which can be conducted there. In particular, the N19 and M89 positions play crucial roles in the activity shown herein, and the patterns in reactivity and selectivity of mutants at these positions suggest that side‐chains here play roles in both the C−C bonding forming, and enantioselective protonation steps. The FC‐EP reaction is another promiscuous activity of LmrR_pAF building on the hydrazone formation, Friedel‐Crafts and synergistically catalysed processes that we have already demonstrated.[ 11 , 22 , 31 , 39 ] We anticipate that LmrR_pAF will find application in yet further useful and challenging reactions operating via organocatalytic processes, realizing the benefits of biocatalytic processes for a broad array of transformations.[ 1 , 2 ]
Experimental Section
General Procedure for Catalysis with Cell‐Free Extracts and Purified Protein
Reactions were conducted in 300 μL total volume in a 2 mL microcentrifuge tube. Stock solutions of protein in buffer (50 mM NaCl, 150 mM NaH2PO4, pH as specified) to give the specified final concentration and the same buffer was added to make up 276 μL volume. For screening of N19 and M89 mutant libraries, 276 μL of cell‐free lysate was used instead. Stock solutions of indole (25 mM in DMF, 12 μL added, final concentration 1 mM) and enal (150 mM or 750 mM when using cell‐free lystate, 12 μL added to give final concentrations of 6 mM or 18 mM with cell free lysate) substrates were added. The microcentrifuge tubes were then mixed by continuous inversion in a cold room at 4 °C for the specified reaction time. After the reaction time had elapsed, NaBH4 solution (60 μL, 20 mg/mL in 0.5 w/v% NaOH) and 3‐(3‐hydroxypropyl)indole internal standard solution (12 μL, 5 mM in DMF) were added. The micro‐centrifuge tubes were mixed by continuous inversion for a further 30 minutes. For HPLC analysis (Products 3 a, 3 b and 3 d–i): the reaction products and internal standard were then extracted by vortex mixing with EtOAc (1 mL) and the organic extract was dried over Na2SO4, filtered and evaporated to dryness. The residue thus obtained was redissolved by vortex mixing with HPLC grade solvent (heptane:isopropanol 4 : 1, 90 μL) and analysed by HPLC on a normal phase to determine yield and enantioselectivity with a 20 μL injection volume (injection volumes were reduced proportionately when indole concentrations above 1 mM were used). For SFC analysis (cell free extract libraries with product 3 a, purified protein with products 3 c and 3 l) 400 μL n‐butanol was added to the reactions and vortexed for one minute. The layers were separated with the aid of centrifugation (14,500 rpm, 5 minutes) and 150 μL of the organic layer was taken for SFC analysis, using a 10 μL injection volume. For product 3 k, the extraction and sample preparation procedure described for HPLC analysis was used, but the samples were analysed by SFC.
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
Our thanks to Dr. H. van Beek of GECCO Biotech B.V. and Dr. F.S. Aalbers for advice on library preparation and screening, to Dr. Z. Zhou and C. da Settimo Passetti for preparing 2‐benzyl‐acrolein, [40] and to J.L. Sneep for assistance with SFC analysis. This work was supported by the Netherlands Ministry of Education, Culture and Science (Gravitation program no. 024.001.035) and the European Research Council (ERC advanced grant 885396).
R. B. Leveson-Gower, R. M. de Boer, G. Roelfes, ChemCatChem 2022, 14, e202101875.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1. Chen K., Arnold F. H., Nat. Catal. 2020, 3, 203–213. [Google Scholar]
- 2. Leveson-Gower R. B., Mayer C., Roelfes G., Nat. Rev. Chem. 2019, 3, 687–705. [Google Scholar]
- 3. Sheldon R. A., Woodley J. M., Chem. Rev. 2018, 118, 801–838. [DOI] [PubMed] [Google Scholar]
- 4. Sheldon R. A., Brady D., Bode M. L., Chem. Sci. 2020, 11, 2587–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiss G., Çelebi-Ölçüm N., Moretti R., Baker D., Houk K. N., Angew. Chem. Int. Ed. 2013, 52, 5700–5725; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 5810–5836. [Google Scholar]
- 6. Zeymer C., Hilvert D., Annu. Rev. Biochem. 2018, 87, 131–157. [DOI] [PubMed] [Google Scholar]
- 7. Schwizer F., Okamoto Y., Heinisch T., Gu Y., Pellizzoni M. M., Lebrun V., Reuter R., Köhler V., Lewis J. C., Ward T. R., Chem. Rev. 2018, 118, 142–231. [DOI] [PubMed] [Google Scholar]
- 8. Rosati F., Roelfes G., ChemCatChem 2010, 2, 916–927. [Google Scholar]
- 9. Zhao J., Burke A. J., Green A. P., Curr. Opin. Chem. Biol. 2020, 55, 136–144. [DOI] [PubMed] [Google Scholar]
- 10. Drienovská I., Roelfes G., Nat. Catal. 2020, 3, 193–202. [Google Scholar]
- 11. Drienovská I., Mayer C., Dulson C., Roelfes G., Nat. Chem. 2018, 10, 946–952. [DOI] [PubMed] [Google Scholar]
- 12. Burke A. J., Lovelock S. L., Frese A., Crawshaw R., Ortmayer M., Dunstan M., Levy C., Green A. P., Nature 2019, 570, 219–223. [DOI] [PubMed] [Google Scholar]
- 13. Wang L., Brock A., Herberich B., Schultz P. G., Science 2001, 292, 498–500. [DOI] [PubMed] [Google Scholar]
- 14. Dumas A., Lercher L., Spicer C. D., Davis B. G., Chem. Sci. 2014, 6, 50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madoori P. K., Agustiandari H., Driessen A. J. M., Thunnissen A.-M. W. H., EMBO J. 2009, 28, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeong W. J., Yu J., Song W. J., Chem. Commun. 2020, 56, 9586–9599. [DOI] [PubMed] [Google Scholar]
- 17. Roelfes G., Acc. Chem. Res. 2019, 52, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacMillan D. W. C., Nature 2008, 455, 304–308. [DOI] [PubMed] [Google Scholar]
- 19. Melchiorre P., Marigo M., Carlone A., Bartoli G., Angew. Chem. Int. Ed. 2008, 47, 6138–6171; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 6232–6265. [Google Scholar]
- 20. van der Helm M. P., Klemm B., Eelkema R., Nat. Rev. Chem. 2019, 3, 491–508. [Google Scholar]
- 21. Erkkilä A., Majander I., Pihko P. M., Chem. Rev. 2007, 107, 5416–5470. [DOI] [PubMed] [Google Scholar]
- 22. Leveson-Gower R. B., Zhou Z., Drienovská I., Roelfes G., ACS Catal. 2021, 11, 6763–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin J. F., MacMillan D. W. C., J. Am. Chem. Soc. 2002, 124, 1172–1173. [DOI] [PubMed] [Google Scholar]
- 24. Gordillo R., Carter J., Houk K. N., Adv. Synth. Catal. 2004, 346, 1175–1185. [Google Scholar]
- 25. Fu N., Zhang L., Li J., Luo S., Cheng J. P., Angew. Chem. Int. Ed. 2011, 50, 11451–11455; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 11653–11657. [Google Scholar]
- 26. Mohr J. T., Hong A. Y., Stoltz B. M., Nat. Chem. 2009, 1, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsumoto K., Tsutsumi S., Ihori T., Ohta H., J. Am. Chem. Soc. 1990, 112, 9614–9619. [Google Scholar]
- 28. Matoishi K., Ueda M., Miyamoto K., Ohta H., J. Mol. Catal. B 2004, 27, 161–168. [Google Scholar]
- 29. Miyamoto K., Hirokawa S., Ohta H., J. Mol. Catal. B 2007, 46, 14–19. [Google Scholar]
- 30. Villarino L., Chordia S., Alonso-Cotchico L., Reddem E., Zhou Z., Thunnissen A. M. W. H., Maréchal J.-D., Roelfes G., ACS Catal. 2020, 10, 11783–11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou Z., Roelfes G., ACS Catal. 2021, 11, 9366–9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steck V., Carminati D. M., Johnson N. R., Fasan R., ACS Catal. 2020, 10, 10967–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Z., Calvó-Tusell C., Zhou A. Z., Chen K., Garcia-Borràs M., Arnold F. H., Nat. Chem. 2021, 13, 1166–1172. [DOI] [PubMed] [Google Scholar]
- 34. Melchiorre P., Angew. Chem. Int. Ed. 2012, 51, 9748–9770; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 9886–9909. [Google Scholar]
- 35. Xu L.-W., Luo J., Lu Y., Chem. Commun. 2009, 1807. [DOI] [PubMed] [Google Scholar]
- 36. Bos J., Browne W. R., Driessen A. J. M., Roelfes G., J. Am. Chem. Soc. 2015, 137, 9796–9799. [DOI] [PubMed] [Google Scholar]
- 37. Villarino L., Splan K. E., Reddem E., Alonso-Cotchico L., Lledós A., Gutiérrez de Souza C., Thunnissen A.-M. W. H., Maréchal J.-D., Roelfes G., Angew. Chem. Int. Ed. 2018, 57, 7785–7789; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 7911–7915. [Google Scholar]
- 38. Bos J., Fusetti F., Driessen A. J. M., Roelfes G., Angew. Chem. Int. Ed. 2012, 51, 7472–7475; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 7590–7593. [Google Scholar]
- 39. Zhou Z., Roelfes G., Nat. Catal. 2020, 3, 289–294. [Google Scholar]
- 40. Erkkilä A., Pihko P. M., J. Org. Chem. 2006, 71, 2538–2541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.