Abstract
Background
Activated coagulation factor XI (FXIa) contributes to the development and propagation of thrombosis but plays only a minor role in hemostasis; therefore, it is an attractive antithrombotic target.
Objectives
To evaluate the pharmacology of asundexian (BAY 2433334), a small molecule inhibitor targeting FXIa, in vitro and in various rabbit models.
Methods
The effects of asundexian on FXIa activity, selectivity versus other proteases, plasma thrombin generation, and clotting assays were evaluated. Antithrombotic effects were determined in FeCl2‐ and arterio‐venous (AV) shunt models. Asundexian was administered intravenously or orally, before or during thrombus formation, and with or without antiplatelet drugs (aspirin and ticagrelor). Potential effects of asundexian on bleeding were evaluated in ear‐, gum‐, and liver injury models.
Results
Asundexian inhibited human FXIa with high potency and selectivity. It reduced FXIa activity, thrombin generation triggered by contact activation or low concentrations of tissue factor, and prolonged activated partial thromboplastin time in human, rabbit, and various other species, but not in rodents. In the FeCl2‐injury models, asundexian reduced thrombus weight versus control, and in the arterial model when added to aspirin and ticagrelor. In the AV shunt model, asundexian reduced thrombus weight when administered before or during thrombus formation. Asundexian alone or in combination with antiplatelet drugs did not increase bleeding times or blood loss in any of the models studied.
Conclusions
Asundexian is a potent oral FXIa inhibitor with antithrombotic efficacy in arterial and venous thrombosis models in prevention and intervention settings, without increasing bleeding.
Keywords: anticoagulant, asundexian, BAY 2433334, factor XIa inhibitor, thrombosis
Essentials.
Asundexian is a small molecule inhibitor of human activated coagulation factor XI (FXIa).
Asundexian dose‐dependently reduced arterial and venous thrombosis in animal models.
Mono‐ or combination therapy with asundexian did not increase bleeding.
Further evaluation of asundexian in patients with, or at risk of, thrombosis is ongoing.
1. INTRODUCTION
Direct oral anticoagulants (DOACs) have markedly improved the management of thrombotic disorders compared to vitamin K antagonists or heparins. 1 However, bleeding remains a concern and dosing requires a careful balance between efficacy and bleeding risk, particularly in indications with a need for higher therapeutic doses (e.g., under‐treatment conditions) or in which antiplatelets are standard of care and the addition of anticoagulants may be beneficial. Establishing a “sweet spot” in the latter indications remains difficult. So far only dual antiplatelet therapy (DAPT) with rivaroxaban at a very low dose has been successful in the secondary prevention of acute coronary syndrome, coronary artery disease, or peripheral artery disease, albeit with an increased bleeding risk compared to antiplatelets alone. 2 , 3 , 4 , 5 Thus, a need remains for new therapies that offer a reduced risk of bleeding and equivalent or superior antithrombotic efficacy. 1
Factor XI (FXI) is the zymogen of the serine protease activated FXI (FXIa). It is activated by factor XIIa (FXIIa) after contact activation, as part of an amplification loop by thrombin or through autoactivation by FXIa. 6 As such, FXIa or FXI represent interesting therapeutic targets because inhibition of FXIa may block contact pathway–initiated coagulation as well as amplification‐associated clot propagation while leaving the tissue factor pathway intact, a pathway that plays a major role in stopping bleeding after vessel wall injury. Consequently, FXIa inhibitors have the potential to be antithrombotic without compromising hemostasis. 7 This has been confirmed by experimental data with inhibitors of FXIa or with FXI‐depleting strategies, 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 as well as by clinical observations from individuals with congenital FXI deficiency, 16 , 17 and from randomized controlled trials of FXI or FXIa inhibitors. 18 , 19 , 20 , 21
It has long been a challenge to identify potent FXIa inhibitors with a pharmacokinetic profile suitable for oral administration. However, two orally available small‐molecule FXIa inhibitors—milvexian and asundexian—are currently in clinical development. This study investigates the preclinical pharmacology of asundexian (BAY 2433334), which binds to the active center of FXIa.
2. MATERIALS AND METHODS
2.1. In vitro studies
Asundexian, rivaroxaban, dabigatran, and apixaban were synthesized at the laboratories of Bayer AG.
Whole blood of anesthetized mice, rats, and guinea pigs was drawn from the vena cava. Dog blood was taken from the antecubital vein, while rabbit and pig whole blood were drawn from the carotid artery. All animal blood samples were collected from untreated donor animals into citrate‐containing Monovette tubes (Sarstedt), and centrifuged at 2500 ×g for 20 min at 4°C. Citrated plasma samples were stored at −20°C prior to use.
Human blood was obtained by venipuncture from healthy volunteers who had received no medication during the previous 10 days. All blood samples were collected into plastic tubes and diluted 1:10 with 3.8% trisodium citrate. Platelet‐poor plasma was obtained immediately after collection by centrifugation at 2500 ×g for 10 min at 4°C and stored at −20°C. Human plasma was sourced from 10 volunteers and pooled. Human citrated plasma was purchased from Octapharma.
2.1.1. Inhibition of human FXIa and selectivity versus other serine proteases
The potency of asundexian against human FXIa activity in buffer was analyzed in biochemical assays based on the fluorimetric detection of aminomethylcoumarine (AMC) cleaved from a synthetic peptidic substrate by FXIa.
Asundexian was diluted in dimethylsulfoxide (DMSO) to final assay concentrations of 0–50 µM. Human FXIa (Kordia) and the FXIa substrate Boc‐Glu(OBzl)‐Ala‐Arg‐AMC (Bachem I‐1575) were diluted in buffer solutions (pH 7.4) consisting of 50 mM Tris/HCl, 100 mM NaCl, 5 mM CaCl2, and 0.1% bovine serum albumin to final concentrations of 0.15 nM and 5 µM, respectively. Diluted asundexian or DMSO (1 μl) in assay buffer (20 μl), diluted FXIa solution (20 μl), and substrate solution (20 μl) were added to 384‐well microtiter plates (Greiner Bio‐One). Fluorescence (excitation 360 nm, emission 460 nm) was measured using a microtiter plate fluorescence reader (Safire II, Tecan) for 30 min at room temperature. The concentration of asundexian producing 50% inhibition of FXIa activity (IC50) was determined via a nonlinear logistic regression model.
The selectivity of asundexian versus other serine proteases of the hemostatic system was evaluated in buffer in biochemical assays based on the fluorimetric detection of AMC released from specific substrates for respective proteases as described above. The details for each assay are described in Appendix S1 in supporting information. IC50 values were determined via a nonlinear logistic regression model.
2.1.2. Inhibition of FXIa and plasma kallikrein in plasma
The FXIa inhibitory effects of asundexian in plasma following contact activation were investigated using similar methodologies to those described above. Asundexian in 3 µl DMSO was added to 50 µl of pooled human plasma or rabbit plasma to yield final concentrations of 0___50 μM. To 20 μl of these mixtures, 20 μl of a kaolin/cephalin suspension (C.K. Prest, Diagnostica Stago) were added. After incubation for 3 min at 37°C, 20 μl of an aqueous FXIa substrate solution (Bachem I‐1575; final assay concentration 400 μM) with a plasma kallikrein inhibitor (BAY 2292992; final assay concentration 5.6 μM) were added. Fluorescence (excitation 360 nm, emission 460 nm) was measured for 40 min at 37°C, and the slope of fluorescence generation during the first 5 min was calculated. The rate of inhibition was determined relative to the slope of samples without inhibitor; IC50 values were calculated as described above.
To investigate the inhibitory effects on plasma kallikrein after contact activation in plasma, 20 µl of a mixture of human plasma with asundexian in DMSO were added to 20 μl of the kaolin/cephalin suspension. After incubation for 3 min at 37°C, 20 μl of an aqueous plasma kallikrein substrate solution (H‐Pro‐Phe‐Arg‐AMC; Bachem I‐1295; final concentration 50 µM) together with 3.3 µg/ml corn trypsin inhibitor (CTI; Haematologic Technologies) were added and fluorescence was measured for 40 min at 37°C. IC50 values were determined as described above.
2.1.3. Effect of asundexian on thrombin generation in plasma
For this assay, asundexian was diluted in DMSO to final concentrations of 0___30 μM. Samples of pooled human plasma (78 μl) were transferred to 96‐well microtiter plates (Greiner Bio‐One) and mixed with 2 μl/well of the serial dilutions of asundexian or DMSO alone. Samples (20 μl) of a solution containing phospholipids (final concentration 4 μM) and either tissue factor (final concentrations 5 or 0.1 pM, respectively) or 2.5% ellagic acid (Actin® FS, Siemens) were added, and the mixture incubated for 10 min at 37°C. Thrombin generation was initiated by addition of 20 μl of a FluCa buffer solution containing the substrate Z‐Gly‐Gly‐Arg‐AMC and CaCl2. Fluorescence measurements (excitation 390 nm, emission 460 nm) commenced immediately using a Fluoroskan Ascent fluorometer (Thermo Fisher). A plot of thrombin concentration versus time was calculated from the slope of the fluorescence curves using the Thrombin Calibrator and thrombinoscope software (Thrombinoscope BV). Lag time and peak thrombin concentration were calculated from the thrombogram.
2.1.4. Effect of asundexian on clotting times in plasma
The effects of asundexian on clotting times were assessed by measurement of activated partial thromboplastin time (APTT) and prothrombin time (PT) in plasma from various species, including rabbit, dog, pig, guinea pig, mouse, and rat, as well as in human citrated plasma.
For APTT measurement, plasma was incubated with increasing concentrations of asundexian (0–37.5 µM) for 3 min at 37°C, after which 50 μl aliquots were incubated for 3 min with 50 μl of an APTT reagent (C.K. Prest, Diagnostica Stago). Coagulation was initiated by addition of 50 μl of 0.025 M CaCl2, and the clotting time measured by an automated coagulometer (AMAX 200, Trinity Biotech). The concentration of asundexian producing a 1.5‐fold increase in APTT was reported as the EC150.
Prothrombin time was measured in plasma samples incubated with increasing concentrations of asundexian (0–37.5 µM) for 3 min at 37°C. Samples (50 μl) were mixed with 100 μl of thromboplastin (RecombiPlasTin, Instrumentation Laboratory), and the clotting time measured at 37°C using an automated coagulometer.
2.2. In vivo studies
Further methodological details can be found in Appendix S1, method 2 and Figure S5.
2.2.1. Combined arterial thrombosis (FeCl2‐injury) and bleeding time model in rabbits
The antithrombotic effects of asundexian in a prevention setting were assessed in a rabbit arterial thrombosis model simultaneously with determination of effects on ear bleeding time. Two series of experiments were performed. In the first series, various intravenous and oral regimens of asundexian were compared to intravenous rivaroxaban and/or control (vehicle). The second series investigated the combination of intravenous asundexian plus DAPT (aspirin [acetylsalicylic acid] and ticagrelor), versus DAPT alone or apixaban plus DAPT. Details of used regimens in these experiments are shown in Table S1 in supporting information.
Anesthesia was induced and maintained in male New Zealand White rabbits (2.7–3.0 kg) with ketamine and xylazine (intramuscular bolus of 40 mg/kg +5 mg/kg, and 800 mg +80 mg in 12 ml with an infusion rate of 5 ml/hour via an ear vein, respectively). Femoral artery and vein were exposed and cannulated for arterial blood sampling and intravenous drug administration, respectively. Thrombosis was induced 30 min after treatment by exposing the carotid artery to FeCl2 (13% in 100 μl water, Merck KGaA) on a filter paper for 5 min. Thirty minutes after application of FeCl2, the injured artery was excised, and the thrombus removed for weighing.
Shortly after FeCl2 injury and simultaneously to thrombus generation, ear bleeding was initiated by making a standardized 5 mm skin cut using a scalpel blade parallel to the external vein of the ear, avoiding direct injury of a visible vessel. Blood was removed gently by swabbing with a filter paper at 10‐s intervals. Bleeding time was recorded as the time at which bleeding stopped. Equivalent techniques were applied to evaluate bleeding times from the gum mucosa.
At the start of the thrombosis experiment, blood was drawn and collected in 2.9 ml citrated tubes (Sarstedt). Plasma was obtained by centrifugation at 2500 ×g for 10 min at 4°C and was stored at −20°C. Rabbit plasma concentrations of asundexian were determined by liquid chromatography–tandem mass spectrometry using an internal standard. The applied sample preparation procedure involved protein precipitation followed by high‐performance liquid chromatography (HPLC) separation and tandem mass spectrometric detection. The HPLC system was coupled in‐line to a tandem mass spectrometer (Sciex API 5500) via a Turbo Spray interface. Plasma concentrations were logarithmically transformed and plotted against thrombus weight to determine the half‐maximal effective concentration (EC50).
Rate of FXIa inhibition by asundexian was assessed fluorimetrically and the APTT was measured mechanically as described above.
2.2.2. Combined venous thrombosis (FeCl2‐injury) and bleeding time model in rabbit
Anesthesia was induced and maintained in male New Zealand White rabbits (2.7–3.0 kg) using the same regimen described above. A femoral artery and vein were exposed and cannulated for arterial blood sampling and intravenous drug administration, respectively. Thrombosis was induced 30 min after intravenous treatment with asundexian by exposing the jugular vein to FeCl2 (13% in 100 μl water, Merck KGaA) on a filter paper for 5 min. After an additional 30 min, the injured part of the vein was excised, and the thrombus removed for weighing. The effect of drug treatment on ear bleeding was assessed as described above.
2.2.3. Liver injury in a rabbit model
The effect of asundexian was evaluated in a rabbit liver‐injury model compared with vehicle and dabigatran.
Anesthesia was induced and maintained in male New Zealand White rabbits (2.5–3.3 kg) as described above. The rabbits were maintained at 37°C throughout the experiment. Forty‐five minutes before the liver‐injury procedure was initiated, rabbits received intravenous injections of 6 or 12 mg/kg asundexian, 0.3 or 1 mg/kg dabigatran, or vehicle (10% ethanol/40% polyethylene glycol/50% sodium chloride [0.9%]). The abdominal cavity was opened directly beneath the costal arch, and the liver was exposed. A triangular wedge (1 cm length and 1 cm width) was removed from the outer edge of the liver, and non‐adhesive absorbent swabs (Medtronic Inc.) were placed in the wound in 30‐s intervals for 30 min. The pre‐weighed swabs were collected and the magnitude of blood loss was determined.
Intravenous blood samples were obtained at the start of the bleeding procedure. APTT and PT were measured as described above.
2.2.4. Arterio‐venous shunt model in preventive and intervention settings
The in vivo antithrombotic effects of asundexian in an arterio‐venous (AV) shunt model were assessed using male New Zealand White rabbits (2.7–3.0 kg). Anesthesia was induced and maintained as described previously.
A femoral artery and vein were used for blood sampling and intravenous drug administration, respectively. Pieces of Braun venous catheter (1.4/2.1 mm inner/outer diameter), filled with physiological saline, were tightly implanted into the left jugular vein and right carotid artery and connected with a 4 cm polyethylene 240 tube containing a 6 cm loop of nylon wire (Mitchell fishing line, 0.14 mm diameter). Thrombosis experiments were started by opening the shunt.
In the prevention protocol, asundexian was administered as an intravenous bolus (0.3, 1, or 3 mg/kg) 10 min prior to opening the shunt, while asundexian was administered as intravenous bolus (1 or 5 mg/kg) 10 min after start of the experiment in the intervention protocol. After shunt opening for 15 min in the prevention setting and 30 min in the intervention protocol, the catheter was excised and the nylon wire, including the attached thrombus, was removed for weighing.
After blood collection in 2.9 ml citrated tubes and plasma preparation, plasma concentrations of asundexian, rates of inhibition of FXIa, and APTT prolongation were determined as described above.
2.3. Statistical methods
Descriptive statistics were prepared for all variables after confirmation of normality (in vitro: mean ±standard error of the mean [SEM] or standard deviation [SD]; in vivo: median with 25th and 75th percentiles). Differences in thrombus weights and in bleeding times between asundexian and comparators were analyzed by Kruskall–Wallis analysis followed by Dunn’s multiple comparison tests.
Graphs were prepared and all statistical analyses were performed using GraphPad Prism software and adjusted alpha errors of P < .05 were considered statistically significant.
2.4. Ethical approval
Collection of human blood by venipuncture was approved by the Ethics Committee of the North Rhine Medical Association (Ärztekammer Nordrhein). All animal experiments were approved by the State Office for Environment, Nature and Consumer Protection North Rhine‐Westphalia (Landesamt fuer Umwelt, Natur und Verbraucherschutz NRW), Recklinghausen, Germany.
3. RESULTS
3.1. In vitro properties of asundexian
3.1.1. Inhibition of human FXIa and selectivity versus a hemostasis protease panel
In vitro fluorometric assays showed that asundexian produced a concentration‐dependent, reversible reduction in human FXIa activity, with a mean (± SD) IC50 value of 1.0 (± 0.17) nM in buffer (Figure 1A; Figure S4 in supporting information), as IC50 values were determined at substrate concentrations much lower than the substrate, Ki ≈ IC50. Asundexian did not inhibit other proteases, including thrombin, FXa, FIXa, FXIIa, FVIIa, urokinase, tissue plasminogen activator, plasmin, activated protein C, trypsin, and chymotrypsin at concentrations more than 1000‐fold higher, except for plasma kallikrein, which had a mean (± SD) IC50 of 6.7 (± 1.5) nM (Table S2 in supporting information).
FIGURE 1.
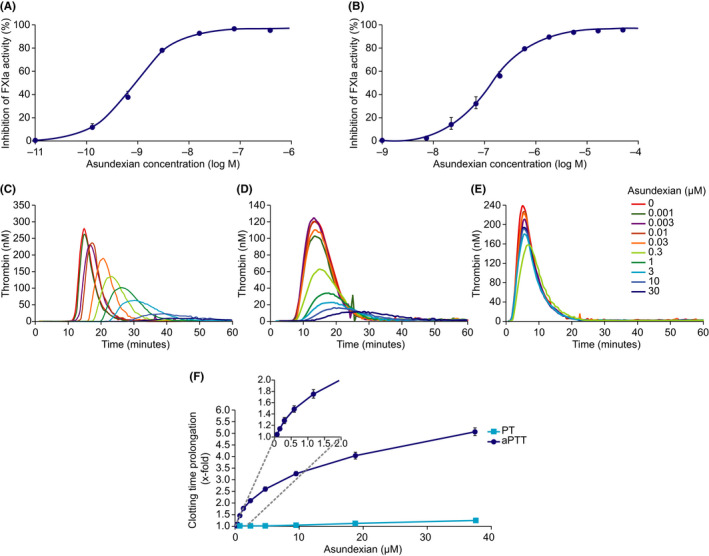
Effect of asundexian on human FXIa activity in buffer (A; n = 6) and human plasma (B; n = 4). Results are presented as mean ±SEM. Representative thrombograms showing the effect of asundexian on thrombin generation initiated by the FXII activating agent ellagic acid (Actin® FS) 2.5% (C), or 0.1 pM or 5 pM tissue factor (D and E). Effect of asundexian on clotting time prolongation in human plasma, with the inset expanding the curve for APTT over the asundexian 0–2 μM range (F). Results are presented as mean ±SEM (n = 2___9). APTT, activated partial thromboplastin time; FXIa, activated coagulation factor XI; PT, prothrombin time; SEM, standard error of the mean
3.1.2. Inhibition of FXIa or plasma kallikrein in plasma
When triggering FXI activation via the contact activation pathway, asundexian reduced FXIa activity in human plasma with a mean (± SD) IC50 of 0.14 (± 0.04) µM (Figure 1B). In rabbit plasma, asundexian was 3.8‐fold less potent (IC50 0.54 [± 0.11] µM). Under similar conditions, but with an adequate substrate, human plasma kallikrein was inhibited with a mean (± SD) IC50 of 1.23 (± 0.07) µM.
3.1.3. Impact on thrombin generation in plasma
Following initiation of the coagulation system by either a low concentration (0.1 pM) of tissue factor or a FXII activating agent (Actin® FS), asundexian markedly inhibited thrombin formation, with an IC50 for peak height of 0.3 μM (representative examples in Figure 1C,D). While asundexian resulted in a concentration‐dependent effect on the lag time following contact activation by Actin® FS, this effect was small when triggered using a low tissue factor (TF) concentration.
By contrast, asundexian had no effect on thrombin generation in the presence of a high concentration (5 pM) of tissue factor (representative example in Figure 1E).
3.1.4. Effect of asundexian on clotting times in plasma
Asundexian produced a concentration‐dependent prolongation of APTT in human plasma, with a mean (± SD) EC150 of 0.61 (± 0.10) μM (Figure 1F). By contrast, asundexian had little to no effect on PT. Experiments using plasma from other species showed that asundexian prolonged APTT to a similar extent in pig plasma (EC150 0.51 μM), and to a lesser extent in rabbit (EC150 1.50 μM), dog (EC150 1.59 μM), and guinea‐pig plasma (EC150 2.13 μM), but had virtually no effect in mouse or rat plasma (EC150 >12.5 μM; Table 1).
TABLE 1.
Prolongation of APTT by asundexian in plasma (mean, n = 2–9)
| Human | Rabbit | Dog | Pig | Guinea pig | Mouse | Rat | |
|---|---|---|---|---|---|---|---|
| APTT prolongation, EC150, μM | 0.61 | 1.50 | 1.59 | 0.51 | 2.13 | >12.5 | >12.5 |
Abbreviations: APTT, activated partial thromboplastin time; EC150, effective concentration to achieve 50% prolongation.
3.2. In vivo profile of asundexian
3.2.1. Evaluation in an arterial FeCl2‐injury rabbit model after intravenous or oral administration and in combination with DAPT
Prophylactic intravenous administration of asundexian produced a dose‐dependent reduction in thrombus weight compared to control in the FeCl2‐injury model (Figure 2A) with no increase in bleeding time (Figure 2B). Thrombus weight decreased from a mean (± SEM) of 18.7 (± 1.1) mg in the control group to 14.0 (± 1.8) mg (25% reduction) in animals receiving asundexian 0.6 mg/kg, and 2.2 (± 0.6) mg (88% reduction) in animals receiving 20 mg/kg (Figure 2A). The EC50 of asundexian was 380 μg/L. Following oral administration of asundexian 10 mg/kg and 30 mg/kg, thrombus weight was significantly reduced by 30% (P < .05) and 91% (P < .001) compared to the control group, respectively, with no prolongation of ear bleeding times at either dose (Figure S1 in supporting information).
FIGURE 2.
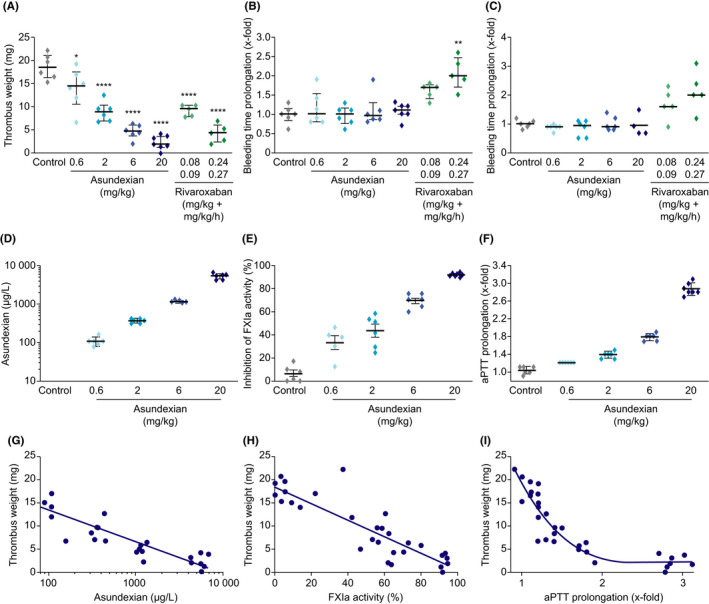
Effects of asundexian versus rivaroxaban on thrombus weight following FeCl2‐induced damage to the carotid artery (A), ear bleeding time (B), and gum bleeding time (C) in rabbits. Plasma concentrations of asundexian (D), inhibition of FXIa activity (E), and APTT prolongation (F) according to asundexian dose, and correlation of plasma concentrations (G), inhibition of FXIa (H), and APTT prolongation (I) with thrombus weight. Asundexian was administered as an intravenous bolus, whereas rivaroxaban was administered as an intravenous bolus followed by continuous infusion. Results are presented as individual values and median with 25th and 75th percentiles (Parts A–F). * P < .05, *** P < .001. APTT, activated partial thromboplastin time; FXIa, activated coagulation factor XI
Intravenous administration of rivaroxaban had a similar dose‐dependent effect on thrombus weight as intravenous asundexian, with mean reductions of 51% and 77% for the 0.08 mg/kg +0.09 mg/kg/hour and 0.24 mg/kg +0.27 mg/kg/hour doses, respectively (Figure 2A). In contrast to asundexian, rivaroxaban prolonged ear bleeding time compared to controls (significant for the higher dose; Figure 2B). Similar results were obtained for gum bleeding (Figure 2C).
Ex vivo measurements revealed a dose‐dependent increase in asundexian plasma concentrations, FXIa inhibition, and APTT prolongation following intravenous administration of asundexian (Figure 2D–F). At the EC50 of 380 μg/L, APTT was prolonged by a factor of approximately 1.4. There was a strong negative correlation between thrombus weight and APTT prolongation (R 2 = .76; Figure 2I), consistent with the strong negative correlation seen between thrombus weight and asundexian plasma level and FXIa activity inhibition, respectively (Figure 2G,H). Following oral administration, asundexian 10 mg/kg and 30 mg/kg prolonged ex vivo APTT by 1.15‐fold and 1.45‐fold, respectively, and reduced FXIa activity by a mean (± SD) of 17% (± 22%) and 44% (± 29%), respectively, compared to the control group (data not shown).
Coadministration of intravenous asundexian with aspirin and ticagrelor produced additional dose‐dependent reductions in thrombus weight: an additional 32% with an asundexian dose of 2 mg/kg, 70% with a 6 mg/kg dose, and 98% with a 12 mg/kg dose (Figure 3A). However, it did not further increase bleeding time beyond that observed following coadministration of aspirin and ticagrelor alone (Figure 3B). Coadministration of intravenous apixaban 1 and 3 mg/kg with aspirin and ticagrelor was also associated with a significant additional reduction of 46% and 83% in thrombus weight, respectively (Figure 3A). However, apixaban significantly increased bleeding time compared to aspirin and ticagrelor alone by 2.8‐fold and 6.6‐fold, respectively (Figure 3B).
FIGURE 3.
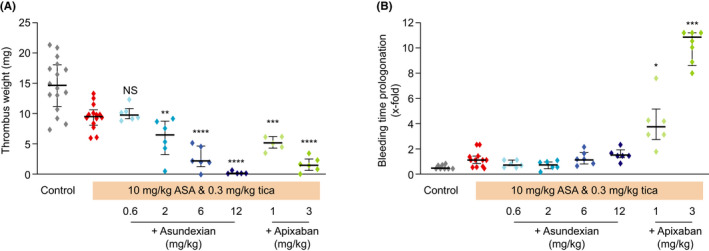
Effects of asundexian in combination with aspirin/ticagrelor versus apixaban in combination with aspirin/ticagrelor on thrombus weight following FeCl2‐induced damage to the carotid artery (A) and ear bleeding time (B) in rabbits. Results are presented as individual values and medians (± interquartile range). Adjusted P‐values versus control: *P < .05, **P < .01, ***P < .001, ****P < .0001. ASA, acetylsalicylic acid (aspirin); Tica, ticagrelor
3.2.2. Evaluation of asundexian in a combined FeCl2‐injury venous thrombosis and ear bleeding rabbit model
Intravenous asundexian produced a dose‐dependent reduction in thrombus weight (Figure 4A) in the venous FeCl2‐injury model, from a mean (± SEM) of 6.1 (± 1.6) mg in the control group to 4.5 (± 1.2) mg (25% reduction), 1.6 (± 0.5) mg (74% reduction), or 0.6 (± 0.6) mg (90% reduction) in animals receiving asundexian 0.6, 2, or 6 mg/kg, respectively. No ear bleeding time prolongation was observed in any of the dose groups (Figure 4B).
FIGURE 4.
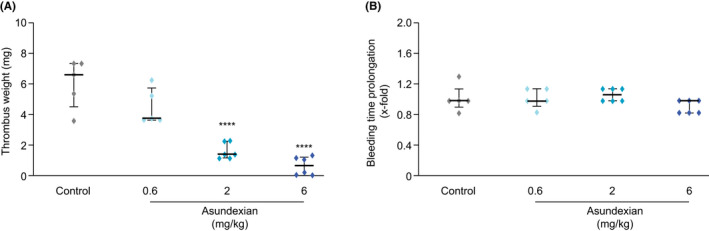
Effects of asundexian on thrombus weight (A) and ear bleeding time prolongation (B) following FeCl2‐induced damage to the jugular vein in rabbits. Asundexian was administered as an intravenous bolus. Results are presented as individual values and medians (± interquartile range). **** P < .001
3.2.3. Impact of asundexian on bleeding in the rabbit liver‐injury model
In this model, neither 6 nor 12 mg/kg asundexian led to any significant effect on bleeding time or blood loss compared to controls (Figure 5). By contrast, dabigatran 0.3 mg/kg increased bleeding time approximately 5‐fold compared to the mean value of 5.7 min (340 s) in controls, and with a dose of 1 mg/kg dabigatran bleeding continued beyond the maximum observation period of 30 min (Figure 5).
FIGURE 5.
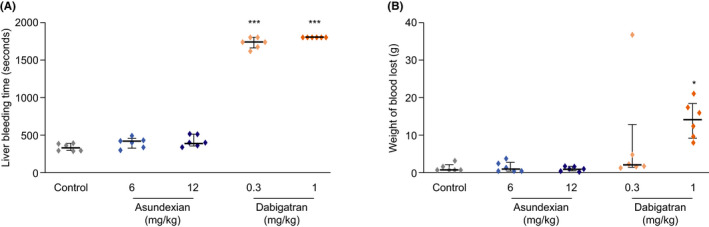
Effect of intravenously administered asundexian 6 mg/kg and 12 mg/kg on bleeding time (A) and blood loss (B) in the rabbit liver‐injury model. Results are presented as medians with interquartile ranges (n = 6). * P < .05, ** P < .001, *** P < .0001 versus controls
Both doses of asundexian significantly prolonged APTT (low dose: 1.7‐fold increase; high dose: 2.3‐fold increase), but had no effect on PT, whereas the higher dose, but not the lower dose, of dabigatran significantly prolonged both APTT (low dose: 1.4‐fold increase; high dose: 2.2‐fold increase) and PT (low dose: 1.1‐fold increase; high dose: 1.7‐fold increase).
3.2.4. Impact of asundexian on already initiated thrombus formations in a rabbit AV shunt model
Preventative intravenous administration of asundexian produced a dose‐dependent reduction in thrombus weight (Figure 6A), without increasing bleeding (Figure S2 in supporting information). Thrombus weight was decreased from a mean (± SEM) of 14.8 (± 2) mg in the control group to 12.7 (± 2.2) mg (14% reduction), 6.1 (± 0.9) mg (59% reduction; P < .01), and 4.3 (± 0.5) mg (71% reduction; P < .001) with the intravenous doses of 0.3, 1, and 3 mg/kg, respectively (Figure 6A). Bleeding times following ear incision were not prolonged by asundexian at any dose or time point after administration (Figure S2).
FIGURE 6.
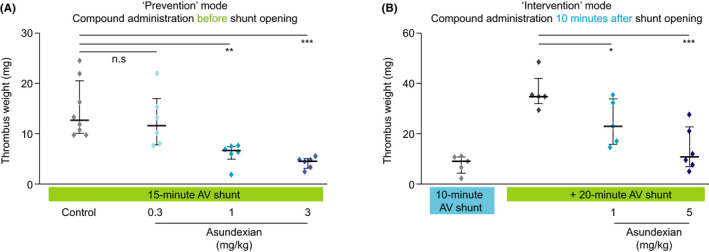
Effects of asundexian in the AV‐shunt model in rabbits on thrombus weight in the prevention protocol (A) and intervention protocol (B). Asundexian was administered as an intravenous bolus 10 min before or 10 min after the shunt was opened, respectively. Results are presented as individual values (diamonds) and mean values (bars) (± interquartile range). * P < .05, ** P < .01, *** P < .001. AV, arterio‐venous
When given during ongoing thrombus formation (interventional protocol), intravenous asundexian significantly reduced thrombus weight from a mean (± SEM) of 36.7 (± 3.2) mg in controls to 24.6 (± 4.1) mg (33% reduction; P < .05), and 14.0 (± 3.5) mg (62% reduction; P < .001) in the 1 mg/kg and 5 mg/kg groups, respectively (Figure 6B).
In the AV shunt model, preventative and interventional asundexian treatment were associated with a dose‐dependent prolongation of APTT (Table S3A,B in supporting information) and reduced FXIa activity (Figure S3 in supporting information).
4. DISCUSSION
The studies reported here show that asundexian is a potent small molecule inhibitor of FXIa activity and coagulation in buffer and plasma of various species and demonstrated antithrombotic effects in rabbit models after intravenous or oral administration without affecting bleeding time or blood loss.
Asundexian produced a direct, potent, and reversible inhibition of FXIa activity in buffer and human plasma with the 94% plasma protein binding of asundexian in human plasma accounting for the difference in potency observed in these two assays. X‐ray cocrystal structures show the binding mode of asundexian in the active site of the activated form FXIa (manuscript in preparation).
Asundexian also demonstrated high selectivity versus other proteases of the coagulation and fibrinolytic system and proteases of the gastrointestinal tract (inhibited ≥1000‐fold less potently). The “close relative” plasma kallikrein was inhibited by asundexian 10‐fold less potently in human plasma. It is therefore possible that at very high doses, where FXIa is fully inhibited, a concomitant plasma kallikrein inhibitory effect may contribute to the biological effect of asundexian.
The results from the thrombin generation assays may reflect different processes for FXI activation: When the coagulation system was activated with the standard concentration of 5 pM TF in human plasma, the high TF concentration resulted in strong thrombin generation via the extrinsic pathway without FXI(a) involvement and consequently no impact of asundexian. When coagulation was triggered via contact activation, asundexian delayed and reduced thrombin generation. Reduced thrombin generation was predominantly observed when low amounts of TF were used as trigger, which can be explained by an initial activation of a small amount of thrombin via the extrinsic pathway followed by initiation of the thrombin amplification loop, which includes FXI activation by thrombin and can thereby be reduced by asundexian.
These findings are in line with the clotting assay results showing a concentration‐dependent prolongation of APTT without affecting PT. Overall, the in vitro results correspond well to the proposed mode of action: fibrin generation initiated by high tissue factor concentrations, found for example in the subendothelial “tissue factor envelope,” is thought to be critical for hemostasis after vessel wall injury and may not be affected by asundexian. In contrast to vitamin K antagonists or DOACs, which block the common pathway of the coagulation system irrespective of the trigger origin, FXIa inhibitors act as selective coagulation modulators via the two specific processes mentioned earlier: reducing coagulation after contact activation by negatively charged macromolecules or surfaces and by attenuating the effect of the amplification loop on clot progression, while leaving the TF pathway as a cornerstone of hemostasis intact. This is consistent with findings in in vitro studies of other inhibitors of FXI and FXIa. 7 , 11
Of note, asundexian prolonged the APTT in the plasma of guinea pigs, rabbits, dogs, pigs, and monkeys, but had little effect in rodent plasma, potentially due to amino acids preventing appropriate binding in the active site. Exchanging two amino acids in the active center of murine FXIa with the equivalent ones from the human enzyme increased the potency of asundexian in such humanized FXI transgenic mouse experiments. 22 However, the in vivo thrombosis studies were carried out in rabbits instead of transgenic mice to allow comparative studies with other anticoagulant approaches.
In arterial or venous thrombosis models in rabbits (FeCl2‐injury or AV shunt), intravenous or oral asundexian administration alone or on top of dual platelet inhibition reduced the thrombus weight versus control in a dose‐dependent manner, without affecting the bleeding times in simultaneously conducted ear or gum bleeding models compared to control. This decoupling of antithrombotic efficacy and bleeding time prolongation is uncommon, as thrombus weight reductions with higher doses of the clinically established DOACs are accompanied by longer bleeding times, as demonstrated herein for the different members of this class used as references in the described studies. Balancing efficacy with bleeding risk appears to be particularly challenging when current anticoagulants are given in combination with DAPT in arterial thrombosis indications. It is therefore encouraging that under these conditions, no further increase of bleeding risk was observed when asundexian was combined with antiplatelets in these animal models.
In addition to ear bleeding, gum bleeding times were also measured to simulate mucosal bleedings, which have been observed to be more sensitive to reduced FXI concentrations in humans. 1 However, no increase in gum bleeding times was observed in our models. In the liver injury model, conducted as a representative model for potential trauma bleedings, asundexian had no significant effect on bleeding time or blood loss compared to controls, whereas dabigatran (0.3 mg/kg) as reference increased bleeding time approximately 5‐fold.
In these studies, close correlations were observed between the reductions of thrombus weight versus control and increasing plasma concentrations of asundexian, increasing FXIa inhibition and increasing APTT prolongation. Considered collectively with the human in vitro data described here, these observations support further investigation of asundexian in clinical trials.
Experiments with orally administered asundexian indicate the possible utility of this agent, for example for long‐term thromboprophylaxis. In addition to evaluation in a prophylactic setting, asundexian was also tested in the rabbit AVbshunt model under treatment conditions: when given 10 min after shunt opening, asundexian minimized further clot growth. Therefore, asundexian could have potential in interventional settings like venous thromboembolism treatment.
While the described general pharmacological profile of asundexian is consistent with those of other agents targeting FXIa, including milvexian, 23 osocimab, 7 , 18 , 24 abelacimab, 25 and the antisense oligonucleotide IONIS FXI‐Rx, which blocks the hepatic synthesis of FXI, 19 important differences exist in their route of administration, onset of action, and duration of effect. Asundexian and milvexian can be administered orally, whereas osocimab, abelacimab, and IONIS FXI‐Rx need to be administered subcutaneously or intravenously. The onset of action of asundexian, milvexian, 26 osocimab, 24 and abelacimab 27 is rapid, whereas it takes longer time periods for IONIS FXI‐Rx to lower FXI concentrations to therapeutic levels with a correspondingly long duration of the anticoagulant effect. 19 Osocimab (mean elimination half‐life, 30–44 days 24 ) and abelacimab (elimination half‐life, 25–30 days 27 ) have been shown to have a long duration of effect, whereas milvexian has a half‐life that may be suitable for once‐ or twice‐daily dosing, 26 and phase I data suggest asundexian is a suitable candidate for once‐daily oral anticoagulation 28 , 29 ; this is being further evaluated in phase II.
It is important to note that anticoagulants currently in clinical use were initially proven to be effective in animal models, which also identified their concomitant bleeding risk at high exposures, indicating that mechanisms of thrombus initiation and propagation in animal models are similar to those in humans. 30 However, the limitations of this study are inherent to preclinical studies in that no animal model can fully replicate human pathology. For example, in humans, thrombosis usually develops in diseased vessels, whereas in the animal models used in this study, thrombosis was induced in healthy vessels. While clinically available anticoagulants were used as comparators in our rabbit models, it was not considered ethically feasible to include all DOACs in every model, as these studies have already been published. 31 , 32 , 33 , 34 , 35
In summary, these preclinical findings show that asundexian, an oral, direct, potent, and selective FXIa inhibitor, represents a novel type of selective coagulation modulator by reducing thrombus formation in arterial or venous thrombosis without prolonging bleeding times. Asundexian may therefore have potential as an antithrombotic agent with a minimal bleeding risk. Further, due to the apparent lack of an effect of asundexian on bleeding shown in these studies, higher dosing in humans to further increase efficacy may be possible. The lack of bleeding time prolongation when asundexian is added to DAPT may also support a role for coagulation modulation in the prevention of severe arterial thrombotic events like ischemic stroke or myocardial infarction.
Further investigations are warranted to establish the clinical relevance of these preclinical findings. The efficacy and safety of asundexian are currently being investigated in a number of phase II studies, namely PACIFIC‐AF (NCT04218266) in patients with atrial fibrillation, PACIFIC‐STROKE (NCT04304508) in patients with non‐cardioembolic stroke, and PACIFIC‐AMI (NCT04304534) in patients with acute myocardial infarction.
CONFLICTS OF INTEREST
Stefan Heitmeier, Adrian Tersteegen, Julia Glunz, Jan Stampfuss, Christoph Gerdes, Susanne Roehrig, and Julia Dietze‐Torres are all employees of Bayer AG. At the time this work was conducted, Mayken Visser and Volker Laux were both employees of Bayer AG but have subsequently left. Christoph Gerdes, Julia Glunz, Jan Stampfuss, Susanne Roehrig, and Adrian Tersteegen also own shares in Bayer AG.
AUTHOR CONTRIBUTIONS
All authors contributed to the study concept and design, analysis and/or interpretation of data, and writing or critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript for publication.
Supporting information
App S1
ACKNOWLEDGMENTS
These studies were funded by Bayer AG, Berlin, Germany. Editorial support was provided by Gemma Rogers, PhD, of Oxford PharmaGenesis, Oxford, UK, with funding from Bayer AG.
Heitmeier S, Visser M, Tersteegen A, et al. Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa. J Thromb Haemost. 2022;20:1400–1411. doi: 10.1111/jth.15700
Manuscript handled by: Joost Meijers
Final decision: Joost Meijers, 07 March 2022
REFERENCES
- 1. Weitz JI, Fredenburgh JC. 2017 scientific sessions sol sherry distinguished lecture in thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(2):304‐310. [DOI] [PubMed] [Google Scholar]
- 2. Khan SU, Arshad A, Riaz IB, Talluri S, Nasir F, Kaluski E. Meta‐analysis of the safety and efficacy of the oral anticoagulant agents (apixaban, rivaroxaban, dabigatran) in patients with acute coronary syndrome. Am J Cardiol. 2018;121(3):301‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319‐1330. [DOI] [PubMed] [Google Scholar]
- 4. Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994‐2004. [DOI] [PubMed] [Google Scholar]
- 5. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9‐19. [DOI] [PubMed] [Google Scholar]
- 6. Emsley JM, Paul A, Gailani D. Structure and function of factor XI. Blood. 2010;115(13):2569‐2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schaefer M, Buchmueller A, Dittmer F, Straßburger J, Wilmen A. Allosteric inhibition as a new mode of action for BAY 1213790, a neutralizing antibody targeting the activated form of coagulation factor XI. J Mol Biol. 2019;431(24):4817‐4833. [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Cheng Q, Xu L, et al. Effects of factor IX or factor XI deficiency on ferric chloride‐induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3(4):695‐702. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Smith P l, Hsu M‐y, et al. Effects of factor XI deficiency on ferric chloride‐induced vena cava thrombosis in mice. J Thromb Haemost. 2006;4(9):1982‐1988. [DOI] [PubMed] [Google Scholar]
- 10. Cheng Q, Tucker EI, Pine MS, et al. A role for factor XIIa–mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116(19):3981‐3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Younis HS, Crosby J, Huh J‐I, et al. Antisense inhibition of coagulation factor XI prolongs APTT without increased bleeding risk in cynomolgus monkeys. Blood. 2012;119(10):2401‐2408. [DOI] [PubMed] [Google Scholar]
- 12. Tucker EI, Marzec UM, White TC, et al. Prevention of vascular graft occlusion and thrombus‐associated thrombin generation by inhibition of factor XI. Blood. 2009;113(4):936‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamashita A, Nishihira K, Kitazawa T, et al. Factor XI contributes to thrombus propagation on injured neointima of the rabbit iliac artery. J Thromb Haemost. 2006;4(7):1496‐1501. [DOI] [PubMed] [Google Scholar]
- 14. Yau JW, Liao P, Fredenburgh JC, et al. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood. 2014;123(13):2102‐2107. [DOI] [PubMed] [Google Scholar]
- 15. Zhang H, Löwenberg EC, Crosby JR, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116(22):4684‐4692. [DOI] [PubMed] [Google Scholar]
- 16. Preis M, Hirsch J, Kotler A, et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129(9):1210‐1215. [DOI] [PubMed] [Google Scholar]
- 17. Salomon O, Steinberg DM, Koren‐Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111(8):4113‐4117. [DOI] [PubMed] [Google Scholar]
- 18. Weitz JI, Bauersachs R, Becker B, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA. 2020;323(2):130‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verhamme P, Yi BA, Segers A, et al. Abelacimab for prevention of venous thromboembolism. N Engl J Med. 2021;385(7):609‐617. [DOI] [PubMed] [Google Scholar]
- 21. Weitz JI, Strony J, Ageno W, et al. Milvexian for the prevention of venous thromboembolism. N Engl J Med. 2021;385(23):2161‐2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Visser MMK, Wilmen A, Trautwein M, Laux V, Heitmeier S. A way to make rodent FXIa accessible to human FXIa small molecule inhibitors. Res Pract Thromb Haemost. 2019;3(S1):40. [Google Scholar]
- 23. Wong PC, Crain EJ, Bozarth JM, et al. Milvexian, an orally bioavailable, small‐molecule, reversible, direct inhibitor of factor XIa: in vitro studies and in vivo evaluation in experimental thrombosis in rabbits. J Thromb Haemost. 2022;20(2):399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas D, Thelen K, Kraff S, et al. BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: First evaluation of safety, pharmacodynamics, and pharmacokinetics. Res Pract Thromb Haemost. 2019;3(2):242‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koch AW, Schiering N, Melkko S, et al. MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood. 2019;133(13):1507‐1516. [DOI] [PubMed] [Google Scholar]
- 26. Perera V, Wang Z, Luettgen J, et al. First‐in‐human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci. 2022;15(2):330‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yi BA, Freedholm D, Widener N, et al. Pharmacokinetics and pharmacodynamics of abelacimab (MAA868), a novel dual inhibitor of factor XI and factor XIa. J Thromb Haemost. 2022;20(2):307‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas D, Kanefendt F, Schwers S, Unger S, Yassen A, Boxnick S. First evaluation of the safety, pharmacokinetics, and pharmacodynamics of BAY 2433334, a small molecule targeting coagulation factor XIa. J Thromb Haemost. 2021;19(10):2407‐2416. doi: 10.1111/jth.15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kubitza D, Heckmann M, Distler J, Koechel A, Schwers S, Kanefendt F. Pharmacokinetics, pharmacodynamics and safety of BAY 2433334, a novel activated factor XI inhibitor, in healthy volunteers: a randomized phase 1 multiple‐dose study. Br J Clin Pharmacol. 2022. doi: 10.1111/bcp.15230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jagadeeswaran P, Cooley BC, Gross PL, Mackman N. Animal models of thrombosis from Zebrafish to nonhuman primates: use in the elucidation of new pathologic pathways and the development of antithrombotic drugs. Circ Res. 2016;118(9):1363‐1379. [DOI] [PubMed] [Google Scholar]
- 31. Wong PC, Watson CA, Crain EJ. Arterial antithrombotic and bleeding time effects of apixaban, a direct factor Xa inhibitor, in combination with antiplatelet therapy in rabbits. J Thromb Haemost. 2008;6(10):1736‐1741. [DOI] [PubMed] [Google Scholar]
- 32. Wienen W, Stassen J‐m, Priepke H, Ries U j, Hauel N. Antithrombotic and anticoagulant effects of the direct thrombin inhibitor dabigatran, and its oral prodrug, dabigatran etexilate, in a rabbit model of venous thrombosis. J Thromb Haemost. 2007;5(6):1237‐1242. [DOI] [PubMed] [Google Scholar]
- 33. Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59‐7939‐‐an oral, direct factor Xa inhibitor. J Thromb Haemost. 2005;3(3):514‐521. [DOI] [PubMed] [Google Scholar]
- 34. Pinto DJ, Orwat MJ, Koch S, et al. Discovery of 1‐(4‐methoxyphenyl)‐7‐oxo‐6‐(4‐(2‐oxopiperidin‐1‐yl)phenyl)‐4,5,6,7‐tetrahydro‐1H ‐pyrazolo[3,4‐c]pyridine‐3‐carboxamide (apixaban, BMS‐562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem. 2007;50(22):5339‐5356. [DOI] [PubMed] [Google Scholar]
- 35. Biemond B, Perzborn E, Friederich P, Levi M, Buetehorn U, Büller H. Prevention and treatment of experimental thrombosis in rabbits with rivaroxaban (BAY 597939)‐‐an oral, direct factor Xa inhibitor. Thromb Haemost. 2007;97(03):471‐477. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


