Abstract
Salmonella spp. are reported to have an increased heat tolerance at low water activity (aw; measured by relative vapor pressure [rvp]), achieved either by drying or by incorporating solutes. Much of the published data, however, cover only a narrow treatment range and have been analyzed by assuming first-order death kinetics. In this study, the death of Salmonella enterica serovar Typhimurium DT104 when exposed to 54 combinations of temperature (55 to 80°C) and aw (rvp 0.65 to 0.90, reduced using glucose-fructose) was investigated. The Weibull model (LogS = −btn) was used to describe microbial inactivation, and surface response models were developed to predict death rates for serovar Typhimurium at all points within the design surface. The models were evaluated with data generated by using six different Salmonella strains in place of serovar Typhimurium DT104 strain 30, two different solutes in place of glucose-fructose to reduce aw, or six low-aw foods artificially contaminated with Salmonella in place of the sugar broths. The data demonstrate that, at temperatures of ≥70°C, Salmonella cells at low aw were more heat tolerant than those at a higher aw but below 65°C the reverse was true. The same patterns were generated when sucrose (rvp 0.80 compared with 0.90) or NaCl (0.75 compared with 0.90) was used to reduce aw, but the extent of the protection afforded varied with solute type. The predictions of thermal death rates in the low-aw foods were usually fail-safe, but the few exceptions highlight the importance of validating models with specific foods that may have additional factors affecting survival.
Salmonella enterica is an international food-borne pathogen, which regularly causes large outbreaks of food poisoning. Salmonellosis can be fatal, in addition to the significant morbidity caused, and the cost associated with infection can be very high. A recent study estimated that each case of salmonellosis in the United Kingdom costs approximately £600 ($1,000) on average, through costs to the health sector, direct costs to patients, and lost employment (4, 61). In addition, there are important implications for the food industry through recall of products and lost prestige and income (64).
Most outbreaks of salmonellosis have resulted from the consumption of contaminated meat, eggs, or dairy products, but some large international outbreaks have been associated with foods that have a low water activity (aw; measured by relative vapor pressure [rvp]) (2, 20, 22, 37, 55). The presence of Salmonella cells in low-aw foods raises specific issues for food safety. Outbreak investigations indicate that only a very few Salmonella cells may be required to cause disease when consumed in low-aw foods (22, 53). In addition, it is widely believed that cells suspended at lower aw during thermal inactivation are more heat tolerant than those suspended at a higher aw (6, 14, 19, 21, 38, 58). This has clear implications for food processing when heating is used to ensure the elimination of potential food pathogens including Salmonella.
The Salmonella serotypes responsible for most human infection in the United Kingdom and the United States are Salmonella enterica serovar Enteritidis and S. enterica serovar Typhimurium (1; Public Health Laboratory Service Communicable Disease Surveillance Centre for 1990 to 1999). Outbreaks associated with low-aw foods, however, have been caused by a number of other serovars, such as S. enterica serovar Napoli, S. enterica serovar Agona, and S. enterica serovar Ealing, which were associated with chocolate, a chip-type snack, and infant dried milk, respectively (2, 20, 22, 37, 53, 55). It is interesting that a large proportion of low-aw foods are snack foods, which are a common feature of the modern diet (15, 29). It is possible that increased consumption of these food types could result in more frequent outbreaks of salmonellosis in the future, unless appropriate management steps are taken.
Historically, the heat inactivation of populations of bacteria has been described using first-order kinetics, i.e., D values (18, 36, 59). This makes the assumption that all bacterial cells within a population have the same heat sensitivity, but significant deviations from linearity have been reported elsewhere (5, 9, 26, 33, 40, 60). In such cases, curve fitting will give more accurate descriptions of the data than will D values because shoulders or tailing can be incorporated. The modeling of complete data sets and the ability to predict inactivation kinetics for given combinations of factors provide an invaluable risk assessment tool, for example, in the initial stages of a novel product formulation or in process development.
Despite most data indicating that Salmonella cells are more heat tolerant when in low-aw environments, there are reports describing exceptions to the general rule. The heat tolerance of serovar Typhimurium (65.5 to 75°C with aw reduced using sugar [19]) and S. enterica serovar Anatum (50 to 54°C with aw reduced using glycerol [25]) increased at intermediate aws but decreased at rvp's below 0.75. In addition, an increase in heat tolerance might be observed only when specific carbohydrates are used to reduce aw (47). Clearly, further research is required in order to understand fully the heat tolerance of Salmonella spp. at low aw. Thermal inactivation models are published elsewhere for Salmonella (52 to 59°C and high aw [17] and 55 to 65°C and 0 to 9% NaCl [8]) and for Escherichia coli O157 (52 to 60°C and 8% NaCl [57], 54 to 68°C with 9 or 17% NaCl [11], and 55 to 65°C and 0 to 9% NaCl [8]). There is very little available information, however, on the inactivation of these pathogens at higher temperatures (65 to 80°C) in combination with lower aw (rvp.0.65 to 0.90), particularly when the aw is depressed using solutes other than NaCl. In addition, much of the existing data cover only a narrow range of treatments, were analyzed assuming first-order death kinetics, or were not subsequently evaluated in foods.
In this study, data on the inactivation of serovar Typhimurium definitive type 104 (DT104) at 54 combinations of aw (rvp 0.65 to 0.90, reduced using glucose-fructose) and temperature (55 to 80°C) and six further Salmonella strains at a subset of these conditions were generated. Secondary models were developed for serovar Typhimurium DT104 strain 30 (62) such that inactivation curves could be predicted by interpolation for conditions not tested, and this was evaluated by using intermediate-moisture foods and different solutes to reduce aw. This paper demonstrates that, while low aw is protective for Salmonella at temperatures of >70°C, it promotes more rapid death at lower temperatures.
MATERIALS AND METHODS
Salmonella strains and preparation of cultures.
Salmonella serovar Typhimurium DT104 strain 30 (62) was selected for this study on the basis of its relative tolerance of high temperature and low aw as separate stresses (32, 44). Salmonella serovar Typhimurium DT104 strain 16 (41), serovar Enteritidis PT4 strain E (27), and S. enterica serovar Senftenberg 775W (63) were selected on the basis of their stress tolerance. Serovar Napoli (20), serovar Agona (37), and S. enterica serovar Java (3) were selected to represent a wide range of serovars and relevant low-aw sources. Bacterial strains were recovered from storage at −20°C, and stationary-phase cultures were prepared in tryptone soy broth (TSB; Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) as described previously (45).
Preparation of low-aw (high-sugar) broths.
Low-aw TSB (pH 6.5; Oxoid Ltd.) was prepared as described previously (45) using AnalaR grades of glucose and fructose (in equal proportion) and sucrose or NaCl (BDH, Poole, Dorset, United Kingdom) as the humectants. The aw of the broth was adjusted by adding TSB (rvp 0.99: pH 6.5) to give rvp values between 0.65 and 0.90 and measured at 25°C using an Aqualab CX-3T (Labcell, Basingstoke, Hampshire, United Kingdom) water activity meter with an accuracy of ±0.003. Note that values for rvp and aw are identical in very dilute solutions, but the term aw is defined in terms of ideal equilibrium solutions, and the high-solute broths used in this study are too concentrated to approach ideal behavior.
Increased survival of S. enterica serovar Anatum during heat challenge in TSB compared with that in phosphate buffer has been reported elsewhere (47); therefore, TSB was used in order to produce as fail-safe a model as possible. There was no significant change in pH during heat challenge (data not shown).
Measurement of death rates at high temperature.
One hundred fifty microliters of a stationary-phase Salmonella culture was inoculated into 15 ml of low-aw TSB, giving an initial cell density of approximately 107 CFU ml−1. To allow Salmonella cells to adapt to the low-aw environment (as would occur in a low-aw food ingredient prior to heat processing), cells were held at 21°C for 1 h prior to heat challenge. On some occasions, control cells were heat challenged immediately, in order to establish the possible effect of the 1-h pretreatment. Cultures were heat challenged as described previously (45) using a submerged heating coil (12). Strain 30 was challenged at 55, 60, 65, 70, 72, 74, 76, 78, and 80°C and rvp's of 0.65, 0.70, 0.75, 0.80, 0.85, and 0.90 (54 combinations), whereas the other Salmonella strains were challenged at 60, 65, and 72°C and rvp's of 0.65, 0.80, and 0.90 (9 combinations). Dilutions were made in maximal recovery diluent (Oxoid), and viable counts were performed using the method of Miles and Misra (46) with plating onto blood agar and incubation for 48 h at 37°C to ensure optimal recovery of injured cells that might have had prolonged lag periods (30).
To investigate the effect of solute used to reduce aw, strain 30 was heat challenged at 55, 60, and 74°C at low aw achieved using NaCl and sucrose. The lowest achievable aw prior to saturation was rvp 0.75 for NaCl and rvp 0.80 for sucrose. These values were compared with inactivation at rvp 0.90 achieved using each solute. There was no 1-h pretreatment prior to heat challenge, since the resulting degree of survival and injury could vary considerably with solute (44).
Evaluation of the models in foods.
The models were evaluated in six low- or intermediate-moisture foods (coconut cake, pecorino cheese, pepperoni sausage, strawberry jam, dried apricots, and peanut butter). These experiments were performed in New Jersey, using foods purchased in a local retail store, whereas data for the broth model were generated in the United Kingdom.
Each food was homogenized by blending. The rvp was measured using an earlier model (CX-2) of the meter described above (Decagon Devices Inc., Pullman, Wash.). The pH was measured three times using a pH meter (Corning Inc., Corning, N.Y.), and a mean value was calculated. No attempt was made to adjust the natural pH of the food. Aliquots (2 g) of the food were added to each of 10 bags which were inoculated directly into the base with 20 μl of a stationary-phase culture of DT104 strain 30, giving an initial cell density of approximately 107 CFU ml−1. The food was mixed, spread thinly along the bottom of the bag, and allowed to stand for 1 h at 21°C. One bag was removed to a water bath at 21°C as a time zero sample, and the remainder were weighted and suspended in a preheated water bath, such that the sample was >15 cm below the water level. The required challenge temperature was achieved in less than 5 s. Bags were removed at predetermined time intervals into the 21°C water bath. Tenfold dilutions of the foods were made by adding 18 ml of diluent to the bags, and further 10-fold dilutions were made in a microtiter plate, as described above. Dilutions were plated onto blood agar and xylose lysine desoxycholate agar, with plate incubation for 48 h at 37 °C. There was minimal background flora observed from any of the foods on blood agar following incubation, and Salmonella bacteria were enumerated from these plates, since selective xylose lysine desoxycholate agar did not adequately recover injured cells.
Statistical analysis, curve fitting, and data modeling.
A minimum of three replicate trials for each combination of temperature, rvp, serovar, and solute was performed. The data were analyzed in Excel. The raw data (CFU milliliter−1) were converted into the log10 of the surviving fraction of bacteria (LogS) at a given time, t.
(i) Curve fitting.
Curves were fitted using the Weibull model, LogS = −btn, where LogS is the log10 of survival ratio at time t, and b and n are the scale and shape parameters, respectively (49). The Weibull model can describe linear inactivations or curves with shoulders or tailing and is mathematically simple. Values for b and n were derived for each inactivation curve in the Solver function of Excel, aiming to minimize the sum of the squares of LogS (observed) minus LogS (predicted). Curves were fitted by instructing Solver to minimize the residual sum of squares by iteratively changing b and n. The convergence criterion was set to 1 × 10−9, and the nonnegative option was selected. Default settings for other options were used. Note that Solver will occasionally fail to converge and an error message will occur to this effect. This can be overcome by substituting some small positive value (e.g., 0.000001) for the first (i.e., zero) time point. The derived values for b and n were used to predict the time required to obtain a 3-log10 or 5-log10 reduction in cell concentration.
When the death curves dropped below the detection threshold of the experiment (20 CFU ml−1), a value of 5 was used for the first censored observation only, with further censored data being excluded. This value was chosen since there was no significant difference between the fitted b and n values when using censored observations of 5 or 20 (data not shown) and because the square root of the threshold value (in this case close to 5) has been used previously (Keith Jewell, personal communication).
To validate the use of Excel Solver for curve fitting, 10 individual data sets were fitted using both Solver and the nonlinear regression module of Statistica (a statistical analysis package; Statsoft, Tulsa, Okla.), and these gave reasonable agreement (P < 0.05).
(ii) Response surface modeling.
To simplify the numerical computation and make the regression coefficients more comparable, the experimental variables rvp and temperature were first normalized as follows:
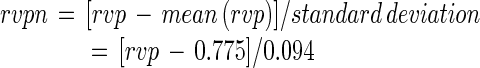 |
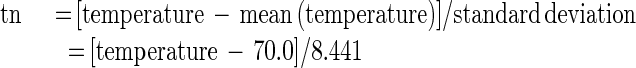 |
where rvpn and tn are normalized rvp and normalized temperature, respectively.
Inspection of frequency plots of b and n (data not shown) showed that the distribution of n was approximately normal but that of b was highly skewed. A high degree of skewness indicates that the variable should be transformed before regression analysis, in order to improve the goodness of fit. A Box-Cox transformation was used, in this case a power transformation such that btrans = b0.3.
Multiple regression analysis was performed using SAS software (SAS Institute, Cary, N.C.) to estimate the response of btrans and n to rvpn and tn. All main effects, interactions, and quadratic terms were included, and a table of regression coefficient estimates and P values was prepared (Table 1). The relative importance of each factor can be judged by the P value, with factors with small P values being most influential and predictive of the response variable.
TABLE 1.
Regression coefficient estimates for btrans (R2 = 0.9672) and n (R2 = 0.5219)
| Variable |
btrans
|
n
|
||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
| Intercept | 1.0423 | <0.0001 | 0.7337 | <0.0001 |
| tn | 0.4519 | <0.0001 | −0.1518 | <0.0001 |
| rvpn | 0.0391 | 0.0018 | −0.0038 | 0.8785 |
| tn × rvpn | 0.1057 | <0.0001 | −0.1294 | <0.0001 |
| tn2 | 0.0691 | <0.0001 | −0.0662 | 0.0261 |
| rvpn2 | −0.0014 | 0.9257 | −0.0054 | 0.8666 |
RESULTS
Inactivation of DT104 strain 30 at high temperature and low aw (achieved using glucose-fructose).
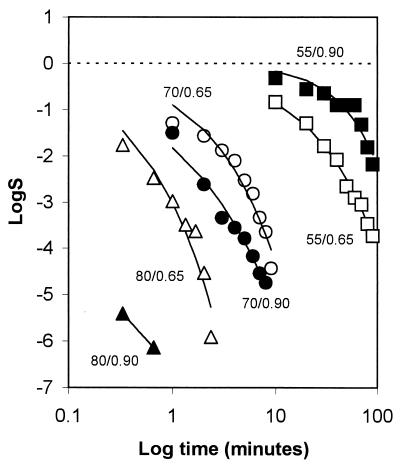
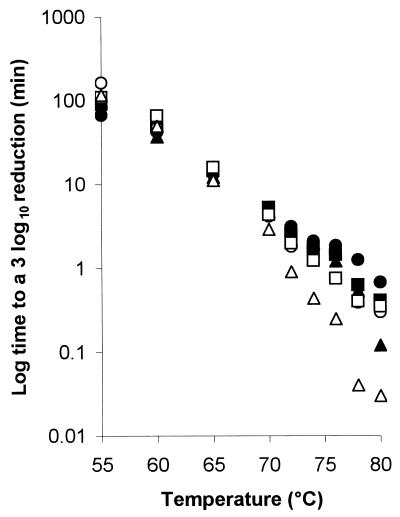
The semilogarithmic inactivation curves were nonlinear but could be described by the Weibull model. The inactivation curve for each set of conditions can be derived by substituting the values for b and n from Tables 2 and 3 into the Weibull equation. Values for n were usually <1, indicating that the curvature was concave upwards, with distinct tailing during inactivation (Table 2). Values for b also showed clear trends (Table 3). For any given aw, b increased from 55 to 80°C. That is, the slope of the curve became steeper, indicating that there was faster death at the higher temperatures (as expected). At 55 and 60°C, b tended to be higher at the lower aw tested, indicating that cells died faster when exposed to the dual stress of temperature and low aw. At temperatures of ≥70°C, however, the b value was greater at rvp 0.90 than at rvp 0.65, and this was very pronounced at high temperature. At 80°C, for example, the b value was 7.11 at rvp 0.90 whereas it was 3.63 at rvp 0.65 (Table 3). This indicates that, at high temperature, low aw protected the cell against thermal death (Fig. 1) with a longer time to obtain a 3-log10 reduction (Fig. 2). The resulting models for b and n were
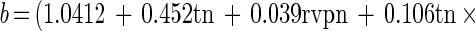 |
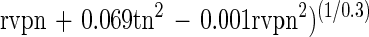 |
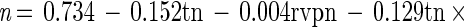 |
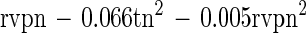 |
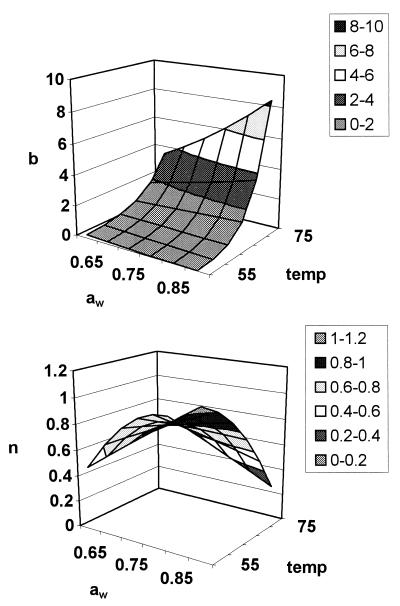
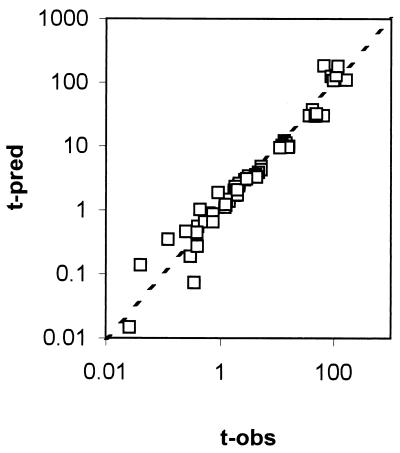
These equations can be used to derive b and n for any given conditions of (normalized) temperature and rvp in the model range. Three-dimensional representations of these models are given in Fig. 3. For both models, normal probability plots of residuals were inspected and no significant deviations from normality were observed (data not shown). The models were used to predict b and n values for all conditions in the experimental design matrix. The predicted values of b and n were substituted into the Weibull equation and used to predict survival curves for all treatments. Observed values were compared with these predicted survival curves and compared favorably, and a plot of observed and predicted time to obtain a 3-log10 reduction in cell concentration showed good agreement (Fig. 4).
TABLE 2.
Mean values of n (representing curvature in the Weibull model) derived from experimental data observations for the inactivation of serovar Typhimurium DT104 at 54 combinations of aw and temperature
| aw (rvp) | Temp (°C)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 55 | 60 | 65 | 70 | 72 | 74 | 76 | 78 | 80 | |
| 0.65 | 0.72 | 0.55 | 0.82 | 0.71 | 0.76 | 0.85 | 0.76 | 0.35 | 0.50 |
| 0.70 | 0.60 | 0.69 | 0.70 | 0.64 | 0.83 | 0.70 | 0.81 | 0.57 | 0.73 |
| 0.75 | 0.61 | 0.56 | 0.78 | 0.67 | 0.65 | 1.06 | 0.80 | 0.45 | 0.24 |
| 0.80 | 0.59 | 0.72 | 0.73 | 0.63 | 0.85 | 0.76 | 0.55 | 0.45 | 0.49 |
| 0.85 | 1.16 | 0.89 | 0.72 | 0.62 | 1.07 | 0.67 | 0.56 | 0.42 | 0.53 |
| 0.90 | 1.26 | 1.30 | 0.79 | 0.50 | 0.63 | 0.35 | 0.34 | 0.27 | 0.24 |
TABLE 3.
Mean values of b (representing rate of inactivation in the Weibull model) derived from experimental data observations for serovar Typhimurium DT104 at 54 combinations of aw and temperature
| aw (rvp) | Temp (°C)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 55 | 60 | 65 | 70 | 72 | 74 | 76 | 78 | 80 | |
| 0.65 | 0.15 | 0.38 | 0.36 | 0.94 | 1.26 | 1.60 | 1.87 | 2.77 | 3.63 |
| 0.70 | 0.20 | 0.21 | 0.47 | 1.03 | 1.31 | 2.03 | 2.23 | 3.89 | 5.78 |
| 0.75 | 0.18 | 0.40 | 0.42 | 1.06 | 1.82 | 1.45 | 2.56 | 3.96 | 4.97 |
| 0.80 | 0.15 | 0.19 | 0.39 | 1.22 | 1.80 | 2.47 | 3.52 | 4.57 | 5.36 |
| 0.85 | 0.01 | 0.07 | 0.41 | 1.20 | 1.44 | 2.61 | 3.51 | 4.41 | 5.22 |
| 0.90 | 0.01 | 0.02 | 0.45 | 1.77 | 3.17 | 4.00 | 4.82 | 7.21 | 7.11 |
FIG. 1.
Graph of the log surviving serovar Typhimurium DT104 at 55°C (squares), 70°C (circles), and 80°C (triangles) and rvp 0.65 (open symbols) or 0.90 (closed symbols) plotted against log time in minutes, demonstrating the effect of the two water activities on the heat tolerance at three temperatures. The dashed line represents the initial CFU of Salmonella milliliter−1.
FIG. 2.
Graph of the log10 time to obtain a 3-log10 reduction in the concentration of serovar Typhimurium DT104 for each aw (rvp 0.65 [closed circle], 0.70 [closed square], 0.75 [closed triangle], 0.80 [open circle], 0.85 [open square], and 0.90 [open triangle]) against the challenge temperature, demonstrating that the protective effect of low water activity is apparent only at temperatures of ≥70°C.
FIG. 3.
Three-dimensional representation of the b-aw-temperature relationship (top) and the n-aw-temperature relationship (bottom), demonstrating the effect of aw on the survival of high temperature by serovar Typhimurium DT104.
FIG. 4.
Plot of observed (from experimental data) and predicted (using the models generated using the experimental data) time to obtain a 3-log10 reduction in cell concentration.
Incubation at low aw (achieved using glucose-fructose) for 1 h at 21°C had no effect on the thermal death of serovar Typhimurium DT104 strain 30 (data not shown), and therefore data for the effect of solute type can be compared directly.
Heat tolerance of different Salmonella strains at low aw (achieved using glucose-fructose).
The inactivation curve for each set of conditions can be derived by substituting the values for b and n from Tables 4 and 5 into the Weibull equation. With the other Salmonella strains tested, the time to obtain a 3-log10 reduction at 60°C was always lower at rvp 0.65 than at rvp 0.90 but at 72°C the opposite was observed. No clear trends in heat tolerance at 65°C were observed, presumably because this is the approximate temperature at which the reversal in effect of low aw occurs. For example, the slowest death was at rvp 0.65 for serovar Typhimurium strain 16; rvp 0.80 for serovar Typhimurium strain 30, serovar Agona, serovar Java, and serovar Senftenberg 775W; and rvp 0.90 for serovar Enteritidis strain E.
TABLE 4.
Values for n in the equation LogS = −b × tn, when used to describe inactivation curves for Salmonella serovars exposed to high temperature and low awa
| Serovar and/or strain | Value for n at heat challenge temp and water activity (rvp)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 60°C
|
65°C
|
72°C
|
|||||||
| 0.65 | 0.80 | 0.90 | 0.65 | 0.80 | 0.90 | 0.65 | 0.80 | 0.90 | |
| Typhimurium DT104 strain 30 | 0.55 | 0.72 | 1.30 | 0.82 | 0.73 | 0.79 | 0.76 | 0.85 | 0.63 |
| Typhimurium DT104 strain 16 | 0.91 | 0.68 | 1.42 | 1.01 | 0.95 | 0.88 | 0.49 | 0.72 | 0.42 |
| Enteritidis PT4 strain E | 0.37 | 0.56 | 0.82 | 0.48 | 0.83 | 1.12 | 0.62 | 0.59 | 0.45 |
| Napoli | 0.85 | 0.87 | 1.44 | 1.19 | 0.76 | 1.05 | 1.07 | 1.01 | 0.44 |
| Agona | 0.68 | 0.46 | 0.59 | 0.60 | 0.72 | 0.51 | 0.90 | 0.64 | 0.33 |
| Java | 0.60 | 0.67 | 0.56 | 0.51 | 0.66 | 0.52 | 0.90 | 0.68 | 0.45 |
| Senftenberg 775W | 0.47 | 0.63 | 0.54 | 0.43 | 0.68 | 0.65 | 0.53 | 0.74 | 0.46 |
If n = 1, then the inactivation is a straight line (when plotted as LogS versus t); when n < 1, then tailing is observed; when n > 1, the curve has a shoulder.
TABLE 5.
Values for b in the equation LogS = −b × tn, when used to describe inactivation curves for Salmonella serovars exposed to high temperature and low aw
| Serovar and/or strain | Value for b at heat challenge temp and water activity (rvp)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 60°C
|
65°C
|
72°C
|
|||||||
| 0.65 | 0.80 | 0.90 | 0.65 | 0.80 | 0.90 | 0.65 | 0.80 | 0.90 | |
| Typhimurium DT104 strain 30 | 0.38 | 0.19 | 0.02 | 0.36 | 0.39 | 0.45 | 1.26 | 1.80 | 3.17 |
| Typhimurium DT104 strain 16 | 0.09 | 0.23 | 0.01 | 0.19 | 0.23 | 0.29 | 0.17 | 0.10 | 0.54 |
| Enteritidis PT4 strain E | 0.99 | 0.41 | 0.13 | 1.24 | 0.33 | 0.14 | 0.16 | 0.21 | 0.52 |
| Napoli | 0.15 | 0.10 | 0.01 | 0.15 | 0.55 | 0.22 | 0.01 | 0.02 | 0.44 |
| Agona | 0.32 | 0.70 | 0.40 | 0.95 | 0.58 | 1.18 | 0.03 | 0.20 | 0.95 |
| Java | 0.42 | 0.23 | 0.45 | 1.20 | 0.55 | 1.04 | 0.02 | 0.14 | 0.47 |
| Senftenberg 775W | 0.68 | 0.32 | 0.52 | 1.46 | 0.54 | 0.78 | 0.27 | 0.11 | 0.52 |
Overall, serovar Senftenberg 775W, serovar Java, and serovar Agona were the least heat-tolerant isolates for the aw range tested. Of the Salmonella strains from outbreaks, only serovar Napoli showed heat tolerance similar to that of serovar Typhimurium and serovar Enteritidis. Salmonella serovar Enteritidis was always relatively heat sensitive at rvp 0.65 but was one of the most heat-tolerant strains at rvp 0.90. For each inactivation with n < 1, an increase in time to obtain a 3-log10 reduction was usually associated with an increase in n value, such that it was closer to 1 (Table 8). In other words, increased heat tolerance of Salmonella was usually associated with more linear death kinetics. For all treatments, higher n values were usually associated with the more heat-tolerant Salmonella serovars (serovar Typhimurium, serovar Enteritidis, and serovar Napoli).
TABLE 8.
Effect of reduced water activity on the heat tolerance of Salmonella strains, measured as the time in minutes to obtain a 3-log10 reduction (standard error)
| Serovar and/or strain | Value at heat challenge temp (°C) and water activity (rvp)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 60°C
|
65°C
|
72°C
|
|||||||
| 0.65 | 0.80 | 0.90 | 0.65 | 0.80 | 0.90 | 0.65 | 0.80 | 0.90 | |
| Typhimurium DT104 strain 30 | 45 (6) | 48 (6) | 49 (3) | 13 (1) | 16 (2) | 11 (1) | 3.5 (0.6) | 1.9 (0.3) | 0.93 (0.1) |
| Typhimurium DT104 strain 16 | 47 (6) | 43 (5) | 49 (1) | 15 (1) | 13 (2) | 13 (1) | 4.0 (0.5) | 1.8 (0.1) | 1.0 (0.1) |
| Enteritidis PT4 strain E | 20 (5) | 33 (4) | 44 (0.8) | 6.4 (0.4) | 14 (2) | 15 (1) | 1.9 (0.2) | 1.5 (0.3) | 0.78 (0.0) |
| Napoli | 35 (1) | 50 (4) | 50 (1) | 12 (0.7) | 9.2 (0.5) | 12 (0.1) | 3.9 (0.2) | 2.3 (0.0) | 1.4 (0.1) |
| Agona | 28 (1) | 23 (7) | 32 (5) | 7.0 (0.4) | 9.7 (0.3) | 6.1 (0.7) | 2.5 (0.1) | 1.2 (0.2) | 0.52 (0.0) |
| Java | 27 (1) | 47 (2) | 28 (0.8) | 5.6 (0.2) | 13 (0.4) | 7.7 (0.3) | 3.5 (0.3) | 1.6 (0.2) | 1.0 (0.3) |
| Senftenberg 775W | 24 (1) | 35 (2) | 27 (0.9) | 5.4 (0.4) | 12 (0.3) | 8.2 (0.3) | 1.5 (0.3) | 1.3 (0.0) | 0.77 (0.0) |
Effect of solute type on heat tolerance of serovar Typhimurium DT104 strain 30.
Use of sucrose and NaCl to reduce the aw of the challenge broth revealed that the temperature-dependent effects of low aw were still observed when using these solutes in place of glucose-fructose but that the extent of the effects varied with solute type (Table 6).
TABLE 6.
Effect of inactivation of serovar Typhimurium DT104 strain 30 at low aw (achieved using sucrose and NaCl), measured as the time in minutes to achieve a 3-log10 reduction in cell concentration (standard error)
| Humectant | Temp (°C) | Value at rvp:
|
Effect of low aw | P | ||
|---|---|---|---|---|---|---|
| 0.75 | 0.80 | 0.90 | ||||
| NaCl | 55 | 23.5 (2.9) | 35.5 (2.0) | Detrimental | 0.03 | |
| 60 | 11.1 (3.2) | 13.9 (2.2) | Detrimental | 0.05 | ||
| 68 | 0.74 (0.05)a | 0.76 (0.08)a | No effect | 0.79 | ||
| 70 | 0.40 (0.02)a | 0.32 (0.05)a | Protective | 0.20 | ||
| 72 | 0.38 (0.05)a | 0.22 (0.00)a | Protective | 0.47 | ||
| Sucrose | 55 | 198 (20) | 682 (170) | Detrimental | 0.04 | |
| 60 | 56.4 (16) | 56.3 (4.0) | No effect | 1.00 | ||
| 74 | 1.91 (0.04) | 0.97 (0.06) | Protective | 0.003 | ||
| Glucose-fructose | 55 | 92.3 (21) | 198 (67) | 144 (28) | Detrimental | |
| 60 | 38.9 (7.1) | 48.0 (5.4) | 52.4 (5.3) | Detrimental | ||
| 74 | 2.01 (0.17) | 1.43 (0.37) | 0.71 (0.03) | Protective | ||
Time to a 5-log decrease given due to rapid death.
At 55 and 60°C, the presence of NaCl in the challenge broth with an aw close to saturation (rvp 0.75) was detrimental compared with the effect at a higher aw (rvp 0.90; P = 0.03 and 0.05, respectively). At 68°C, there was no difference in death rate at the two aws tested (P = 0.79). At 70 and 72°C, the lower aw gave marginal protection (P = 0.20 and 0.47, respectively), and at 74°C, cell death was too rapid for accurate measurements to be taken. At 55°C, a broth containing sucrose (rvp 0.80) was detrimental to heat tolerance compared with the effect of a higher aw (rvp 0.90; P = 0.04); at 60°C, there was no difference in effect; and at 74°C, significant protection was observed (P = 0.003). Sucrose was more protective at all temperatures than was glucose-fructose, which in turn was more protective than NaCl (Table 6). The difference in observed death rates at 55°C and rvp 0.90 between use of sucrose and use of NaCl to reduce aw was nearly 20-fold.
Evaluation of the thermal inactivation models using food products.
With pecorino cheese, pepperoni sausage, strawberry jam, and dried apricots, death occurred at the rate predicted by the models or higher at each temperature (Table 7), indicating that the models gave conservative (fail-safe) predictions for these foods. The discrepancies between the observed and predicted times to a 3-log10 reduction are probably the result of variations in pH, fat content, and other factors between the foods and the sugar broths.
TABLE 7.
Survival of serovar Typhimurium DT104 in sugar solutions and in low aw foods at 55 to 74°C, expressed as the time to obtain a 3-log10 decrease in cell concentration
| Food | Challenge temp (°C) | Death rate predicted in sugar solution
|
Death rate observed in foods
|
||||
|---|---|---|---|---|---|---|---|
| aw | pH | Time to obtain a 3-log decrease (min) | aw | pH | Time to obtain a 3-log decrease (min) | ||
| Pecorino cheese | 55 | 0.87 | 6.5 | 143 | 0.87 | 5.7 | 10 |
| 65 | 9.5 | 0.7 | |||||
| 74 | 1.1 | 0.8 | |||||
| Coconut cake | 55 | 0.86 | 6.5 | 67a | 0.86 | 6.2 | 111a |
| 65 | 9.5 | 18 | |||||
| 74 | 1.2 | 0.7 | |||||
| Pepperoni sausage | 55 | 0.84 | 6.5 | 123 | 0.84 | 5.2 | 13 |
| 65 | 9.6 | 1 | |||||
| 74 | 1.3 | 0.7 | |||||
| Strawberry jam | 55 | 0.82 | 6.5 | 114 | 0.82 | 3.0 | 15 |
| 65 | 9.7 | 3 | |||||
| 74 | 1.4 | 0.3 | |||||
| Dried apricots | 55 | 0.64 | 6.5 | 206 | 0.64 | 4.0 | 44 |
| 65 | 12 | 6 | |||||
| 74 | 2.5 | 1 | |||||
| Peanut butter | 55 | 0.50b | 6.5 | 13,679a | 0.50b | 6.1 | 98a |
| 65 | 5.6a | 24a | |||||
| 74 | 1.7a | 6a | |||||
Where the fall in bacterial concentration was less than 3 log10, the time to decrease the population by 1.5 log10 is given.
Prediction for a food with an aw well outside the range of the model.
In coconut cake and peanut butter, however, Salmonella sometimes survived for longer than predicted (Table 7). The predicted and observed times to achieve a 3-log10 reduction were <2-fold different at each temperature in coconut cake but >100-fold different at 55°C in peanut butter. The peanut butter had an rvp that was significantly below the intended range of the model, and these data confirm that extrapolating a model far beyond its intended range should be avoided.
The aw and pH of the six low- and intermediate-moisture foods are given in Table 7. In strawberry jam, a 1- to 2-log10 decrease in cell concentration was observed during the 1-h pretreatment at 21°C (P = 0.00001), presumably due to the very low pH, but in all other foods there was no significant change in cell concentration during pretreatment.
DISCUSSION
Much of the thermal inactivation data generated in this study showed significant tailing, particularly at the higher temperatures tested, and this is consistent with published data for other bacteria under similar conditions (60). Since linear descriptions of cellular death would not accurately describe the data, curves were fitted to the inactivation data using the Weibull model (49). Other investigators have used the logistic (13) and Gompertz (10, 43) models and other models (52).
A polynomial function derived by multiple regression analysis was used for the secondary inactivation models, in common with previous researchers (13, 34), whereas others have used Arrhenius-Eyring (51) and linear Arrhenius-Davey (16) models. By modeling a response surface, reliable predictions of thermal inactivation under conditions that have not been tested but are within the range of the experimental matrix can be generated by interpolation. The secondary models were produced with data generated using serovar Typhimurium DT104 strain 30, and to confirm the main observations of the models, six further strains of Salmonella were analyzed at a subset of conditions. These were serovar Typhimurium DT104 strain 16, serovar Enteritidis (reported elsewhere to be relatively tolerant to low aw [44]), serovar Senftenberg 775W (reported to be more heat tolerant than other Salmonella strains at high aw [23, 40, 48]), and three outbreak strains (serovars Napoli, Agona, and Java).
Existing inactivation data for Salmonella and related organisms at high temperature and low aw were compared to the data from this study. Gibson (19) presented D values for heat tolerance (55 to 70°C) of serovar Typhimurium and serovar Senftenberg at rvp 0.71 to 0.99 (reduced using sucrose, adding glucose for rvp's of <0.85). Gibson's work gave similar results for five comparable combinations of temperature and rvp, but at rvp 0.90 and 60°C our data indicated approximately fourfold-higher heat tolerance, using glucose-fructose in place of sucrose. Sumner et al. (58) looked at sucrose solutions (rvp 0.83 to 0.98) with 3-h osmotic equilibration prior to heat challenge at 66 to 77°C. Results were comparable at 74°C and 0.90, but at rvp <0.90 or a temperature of <74°C our data indicated a lower level of heat tolerance, using glucose-fructose in place of sucrose.
All Salmonella strains tested demonstrated that low aw (rvp 0.65 compared with 0.90) was detrimental to survival at 55 or 60°C, whereas at ≥70°C the lower aw was always protective. The most heat-tolerant serovars over the range of conditions tested were serovar Typhimurium DT104, serovar Enteritidis PT4, and serovar Napoli. Strains isolated from outbreaks associated with low-aw foods did not appear to be more heat tolerant at low aw than did other strains. This indicates that Salmonella strains from outbreaks associated with low-aw foods may not have particular characteristics promoting their survival during heat processing and subsequent storage in low-aw foods but that their characteristics may instead relate to the contamination source. The temperature-dependent effect of low aw on heat tolerance was independent of the solute type used to reduce aw, although the extent of protection afforded did vary. Sucrose was generally more protective than was glucose-fructose, which in turn resulted in significantly lower thermal death rates than with NaCl at all temperatures tested.
Most published reports indicate that the heat tolerance of Salmonella increases as the aw decreases (14, 21, 38, 58), but a small number of reports indicate that heat tolerance of Salmonella may increase or decrease at low aw (6, 19, 25). We propose that the temperature-dependent effects of low aw on the heat tolerance of Salmonella reflect different targets for death at low temperatures than at high temperatures. The high-temperature target(s) appears to be protected by low aw, perhaps through improved stability of proteins, reduced mobility of water, or the direct effects of solutes, whereas the lower-temperature target(s) is clearly not protected by low aw. For example, the high-temperature targets could be the ribosomes, since it is reported that their heat stability is increased at low aw (56). Air-dried cells, as well as those suspended at low aw, exhibit increased tolerance (38); therefore, general dehydration probably gives rise to the observed increased heat tolerance at the higher temperatures tested. Gibson stated that proteins and other macromolecules are more stable in the dry state (19). Corry studied the turbidity of serovar Typhimurium as a measure of the degree of plasmolysis, and this correlated with the degree of protection afforded by the high concentration of solutes during heating at 65°C (14).
Published data indicate that serovar Senftenberg strain 775W is significantly more heat tolerant than are most other Salmonella strains at optimal aw (rvp 0.995) but not at low aw (6, 19, 21). Our data confirm that serovar Senftenberg strain 775W was relatively heat sensitive over the rvp range 0.65 to 0.90. A disparity between the behavior of strain 775W and that of serovar Typhimurium was reported previously, with the heat tolerance of 775W being virtually unaffected by reducing the aw (rvp 0.94 to 0.997 [24]). These data indicate that there is an unusual interaction between heat tolerance and aw in this strain. They also show clearly that serovar Senftenberg strain 775W is not an appropriate strain to estimate the efficacy of thermal processes for low-aw foods.
Validation of the models was performed with six retail foods, selected to represent a wide range of low- and intermediate-moisture food types. In addition, some of the foods had particular properties that could increase the heat tolerance of Salmonella. In other words, the foods were selected to challenge the ability of the model to produce fail-safe predictions of thermal inactivation. Pepperoni sausage was used since it has been demonstrated that Salmonella strains attached to muscle tissue may be more resistant to heat than strains that are not (28). Coconut cake was chosen since desiccated coconut has previously been associated with outbreaks of Salmonella (3, 7). Peanut butter has a high fat content, which has been reported elsewhere to protect microorganisms against high temperature (33, 42, 54), although other reports are less conclusive (35, 39).
Salmonella died more quickly in pecorino cheese, pepperoni sausage, strawberry jam, and dried apricot than would be predicted by the broth models. This indicates that predictions are fail-safe, as is required in order to design safe processes. The more rapid death observed is likely to be due partly to the additional stress of low pH in some of the foods, compared to the model predictions based on data generated at pH 6.5. Unfortunately there is currently no model available to generate predictions for relevant combinations of high temperature, low aw, and low pH. Predicted inactivations for coconut cake (55 and 65°C) and peanut butter (65 and 74°C), however, were more rapid than those actually observed in the foods. The predicted and observed times to achieve a 3-log10 reduction differed by approximately twofold in coconut cake. With peanut butter (rvp 0.50), however, death was far slower than predicted by the model, and this highlights the dangers associated with extrapolating a predictive model beyond its intended range. The peanut butter had a relatively neutral pH and a very high fat content (53% [wt/wt]), and these factors may account for the differences seen.
It is clearly important to evaluate laboratory-based models with real foods, since the individual properties of foods will have a great effect on the survival of microorganisms within foods. Other researchers have demonstrated increased heat tolerance of microorganisms in foods compared with that predicted in broth models (31, 50). In addition, the food validation studies reported here indicate that pH is an important factor influencing the survival of Salmonella at low aw when exposed to heat, and this requires further investigation.
In conclusion, the greater heat sensitivity at low aw (rvp 0.65 compared with 0.90) at the lower inactivation temperatures (55 and 60°C) could have implications for food process design, development of new food formulations, and risk assessment. It is clearly important that thermal processes for low-aw foods are designed using thermal inactivation data generated in low-aw systems. Therefore, it is hoped that these data will make a positive contribution to food safety for manufacturers of low-aw foods whose process involves a heat treatment step.
ACKNOWLEDGMENTS
We gratefully acknowledge funding from Nabisco Inc. and the Public Health Laboratory Service.
We also thank Martin Cole for his involvement in initiating the project and Micha Peleg, Louise Slade, and Cindy Stewart for useful discussions.
REFERENCES
- 1.Angulo F J, Swerdlow D L. Salmonella Enteritidis infections in the United States. J Am Vet Med Assoc. 1998;213:1729–1731. [PubMed] [Google Scholar]
- 2.Anonymous. An outbreak of Salmonella agona due to contaminated snacks. Commun Dis Rep Wkly. 1995;5:29–32. [PubMed] [Google Scholar]
- 3.Anonymous. Salmonella java phage type Dundee—rise in cases. Commun Dis Rep Wkly. 1999;9:77. [PubMed] [Google Scholar]
- 4.Anonymous. Infectious intestinal diseases (IID) study summary report. London, United Kingdom: Her Majesty's Stationery Office; 2000. [Google Scholar]
- 5.Archer J, Jervis E T, Bird J, Gaze J E. Heat resistance of Salmonella weltevreden in low-moisture environments. J Food Prot. 1998;61:969–973. doi: 10.4315/0362-028x-61.8.969. [DOI] [PubMed] [Google Scholar]
- 6.Baird-Parker A C, Boothroyd M, Jones E. The effect of water activity on the heat resistance of heat sensitive and heat resistant strains of salmonellae. J Appl Bacteriol. 1970;33:515–522. doi: 10.1111/j.1365-2672.1970.tb02228.x. [DOI] [PubMed] [Google Scholar]
- 7.Berginski R, Pareth G, Brunn W. Microbiological investigations of desiccated coconut. Dtsch Lebensm-Rundsch. 1998;94:211–214. [Google Scholar]
- 8.Blackburn C D, Curtis L M, Humpheson L, Billon C, McClure P J. Development of thermal inactivation models for Salmonella enteritidis and Escherichia coli O157:H7 with temperature, pH and NaCl as controlling factors. Int J Food Microbiol. 1997;38:31–44. doi: 10.1016/s0168-1605(97)00085-8. [DOI] [PubMed] [Google Scholar]
- 9.Breand S, Fardel G, Flandrois J P, Rosso L, Tomassone R. Model of the influence of time and mild temperature on Listeria monocytogenes nonlinear survival curves. Int J Food Microbiol. 1998;40:185–195. doi: 10.1016/s0168-1605(98)00032-4. [DOI] [PubMed] [Google Scholar]
- 10.Chhabra A T, Carter W H, Linton R H, Cousin M A. A predictive model to determine the effects of pH, milkfat, and temperature on thermal inactivation of Listeria monocytogenes. J Food Prot. 1999;62:1143–1149. doi: 10.4315/0362-028x-62.10.1143. [DOI] [PubMed] [Google Scholar]
- 11.Clavero M R S, Beuchat L R, Doyle M P. Thermal inactivation of Escherichia coli O157:H7 isolated from ground beef and bovine feces, and suitability of media for enumeration. J Food Prot. 1998;61:285–289. doi: 10.4315/0362-028x-61.3.285. [DOI] [PubMed] [Google Scholar]
- 12.Cole M B, Jones M W. A submerged-coil heating apparatus for investigating thermal inactivation of micro-organisms. Lett Appl Microbiol. 1990;11:233–235. [Google Scholar]
- 13.Cole M B, Davies K W, Munro G, Holyoak C D, Kilsby D C. A vitalistic model to describe the thermal inactivation of Listeria monocytogenes. J Ind Microbiol. 1993;12:232–239. [Google Scholar]
- 14.Corry J E L. The effect of sugars and polyols on the heat resistance of salmonellae. J Appl Bacteriol. 1974;37:31–43. doi: 10.1111/j.1365-2672.1974.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 15.Cross A T, Babicz D, Cushman L F. Snacking patterns among 1,800 adults and children. J Am Diet Assoc. 1994;94:1398–1403. doi: 10.1016/0002-8223(94)92542-9. [DOI] [PubMed] [Google Scholar]
- 16.Davey K R, Daughtry B J. Validation of a model for predicting the combined effect of three environmental factors on both exponential and lag phases of bacterial growth: temperature, salt concentration and pH. Food Res Int. 1995;28:233–237. [Google Scholar]
- 17.Ellison A, Anderson W, Cole M B, Stewart G S A B. Modelling the thermal inactivation of Salmonella typhimurium using bioluminescence data. Int J Food Microbiol. 1994;23:467–477. doi: 10.1016/0168-1605(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 18.Esty J R, Meyer K F. The heat resistance of spores of Clostridium botulinum and allied anaerobes. J Infect Dis. 1922;31:650–663. [Google Scholar]
- 19.Gibson B. The effect of high sugar concentration on the heat resistance of vegetative microorganisms. J Appl Bacteriol. 1973;36:365–376. doi: 10.1111/j.1365-2672.1973.tb04118.x. [DOI] [PubMed] [Google Scholar]
- 20.Gill O N, Sockett P N, Bartlett C L R, Vaile M S B. Outbreak of Salmonella napoli infection caused by contaminated chocolate bars. Lancet. 1983;i:574–577. doi: 10.1016/s0140-6736(83)92822-2. [DOI] [PubMed] [Google Scholar]
- 21.Goepfert J M, Iskander I K, Amundson C H. Relation of the heat resistance of salmonellae to the water activity of the environment. Appl Microbiol. 1970;19:429–433. doi: 10.1128/am.19.3.429-433.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood M H, Hooper W L. Chocolate bars contaminated with Salmonella napoli: an infectivity study. BMJ. 1983;286:1394. doi: 10.1136/bmj.286.6375.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hockin J C, D'Aoust J Y, Bowering D, Jessop J H, Khanna B, Lior H, Milling M E. An international outbreak of Salmonella nima from imported chocolate. J Food Prot. 1989;52:51–54. doi: 10.4315/0362-028X-52.1.51. [DOI] [PubMed] [Google Scholar]
- 24.Horner K J, Anagnostopoulos G D. Effect of water activity on heat survival of Staphylococcus aureus, Salmonella typhimurium and Salm. senftenberg. J Appl Bacteriol. 1975;38:9–17. doi: 10.1111/j.1365-2672.1975.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh F, Acott K, Elizondo H, Labuza T P. The effect of water activity on the heat resistance of vegetative cells in the intermediate moisture range. Lebensm-Wiss Technol. 1975;8:78–81. [Google Scholar]
- 26.Humpheson L, Adams M R, Anderson W A, Cole M B. Biphasic thermal inactivation kinetics in Salmonella enteritidis PT4. Appl Environ Microbiol. 1998;64:459–464. doi: 10.1128/aem.64.2.459-464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey T J, Williams A, McAlpine K, Jørgensen F, O'Byrne C. Pathogenicity in isolates of Salmonella enterica serotype Enteritidis PT4 which differ in RpoS expression: effects of growth phase and low temperature. Epidemiol Infect. 1998;121:295–301. doi: 10.1017/s0950268898001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey T J, Wilde S J, Rowbury R J. Heat tolerance of Salmonella typhimurium DT104 isolates attached to muscle tissue. Lett Appl Microbiol. 1997;25:265–268. doi: 10.1046/j.1472-765x.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson B, Hackett A F. Eating habits of 11-14 year old schoolchildren living in less affluent areas of Liverpool, UK. J Hum Nutr Diet. 1997;10:135–144. [Google Scholar]
- 30.Jordan K N, Hall S, McClure P J. Osmotic stress on dilution of acid-injured Escherichia coli O157:H7. Lett Appl Microbiol. 1999;28:389–393. doi: 10.1046/j.1365-2672.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 31.Jørgensen F, Stephens P J, Knochel S. The effect of osmotic shock and subsequent adaptation on the thermotolerance and cell morphology of Listeria monocytogenes. J Appl Bacteriol. 1995;79:274–281. [Google Scholar]
- 32.Jørgensen F, Leach S, Wilde S J, Davies A, Stewart G S A B, Humphrey T J. Invasiveness in chickens, stress resistance and RpoS status of wild-type Salmonella enterica subsp. enterica serovar Typhimurium definitive type 104 and serovar Enteritidis phage type 4 isolates. Microbiology. 2000;146:3227–3235. doi: 10.1099/00221287-146-12-3227. [DOI] [PubMed] [Google Scholar]
- 33.Juneja V K, Eblen B S. Heat inactivation of Salmonella typhimurium DT104 in beef as affected by fat content. Lett Appl Microbiol. 2000;30:461–467. doi: 10.1046/j.1472-765x.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- 34.Juneja V K, Marmer B S, Phillips J G, Miller A J. Influence of the intrinsic properties of food on thermal inactivation of spores of nonproteolytic Clostridium botulinum: development of a predictive model. J Food Safety. 1995;15:349–364. [Google Scholar]
- 35.Kadan R S, Marten W H, Micklesen R. Effects of ingredients used in condensed and frozen dairy products on thermal resistance of potentially pathogenic staphylococci. Appl Microbiol. 1963;11:45–49. doi: 10.1128/am.11.1.45-49.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katzin L I, Sandholzer L A, Strong M E. Application of the decimal reduction time principle to a study of the resistance of coliform bacteria to pasteurization. J Bacteriol. 1943;45:265–269. doi: 10.1128/jb.45.3.265-272.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Killalea D, Ward L R, Roberts D, de Louvois J, Sufi F, Stuart J M, Wall P G, Susman M, Schweiger M, Sanderson P J, Fisher I S T, Mead P S, Gill O N, Bartlett C L R, Rowe B. International epidemiological and microbiological study of outbreak of Salmonella agona infection from a ready to eat savoury snack—I. England and Wales and the United States. BMJ. 1996;313:1105–1107. doi: 10.1136/bmj.313.7065.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirby R M, Davies R. Survival of dehydrated cells of Salmonella typhimurium LT2 at high temperatures. J Appl Bacteriol. 1990;68:241–246. doi: 10.1111/j.1365-2672.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 39.Kotrola J S, Conner D E. Heat inactivation of Escherichia coli O157:H7 in turkey meat as affected by sodium chloride, sodium lactate, polyphosphate and fat content. J Food Prot. 1997;60:898–902. doi: 10.4315/0362-028X-60.8.898. [DOI] [PubMed] [Google Scholar]
- 40.Kwast R H, Verrips C T. Heat resistance of Salmonella senftenberg 775W at various sucrose concentrations in distilled water. Eur J Appl Microbiol Biotechnol. 1982;14:193–201. [Google Scholar]
- 41.Leach S A, Williams A, Davies A C, Wilson J, Marsh P D, Humphrey T J. Aerosol route enhances the contamination of intact eggs and muscle of experimentally infected laying hens by Salmonella typhimurium DT104. FEMS Microbiol Lett. 1999;171:203–207. doi: 10.1111/j.1574-6968.1999.tb13433.x. [DOI] [PubMed] [Google Scholar]
- 42.Line J E, Fain A R, Moran A B, Martin L M, Lechowich R V, Carosella J M, Brown W L. Lethality of heat to Escherichia coli O157:H7: D-value and z-value determinations in ground beef. J Food Prot. 1991;54:762–766. doi: 10.4315/0362-028X-54.10.762. [DOI] [PubMed] [Google Scholar]
- 43.Linton R H, Carter W H, Pierson M D, Hackney C R, Eifert J D. Use of a Gompertz equation to predict the effects of temperature, pH and NaCl on the inactivation of Listeria monocytogenes Scott A heated in infant formula. J Food Prot. 1996;59:16–23. doi: 10.4315/0362-028X-59.1.16. [DOI] [PubMed] [Google Scholar]
- 44.Mattick K L, Jørgensen F, Legan J D, Cole M B, Porter J, Lappin-Scott H M, Humphrey T J. The survival and filamentation of Salmonella enterica serovar Enteritidis PT4 and Salmonella enterica serovar Typhimurium DT104 at low water activity. Appl Environ Microbiol. 2000;66:1274–1279. doi: 10.1128/aem.66.4.1274-1279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattick K L, Jørgensen F, Legan J D, Lappin-Scott H M, Humphrey T J. Habituation of Salmonella spp. at reduced water activity and its effect on heat tolerance. Appl Environ Microbiol. 2000;66:4921–4925. doi: 10.1128/aem.66.11.4921-4925.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miles A A, Misra S S. The estimation of bactericidal power of blood. J Hyg. 1938;38:732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moats W A, Dabbah R, Edwards V M. Survival of Salmonella anatum heated in various media. Appl Microbiol. 1971;21:476–481. doi: 10.1128/am.21.3.476-481.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng H, Bayne H G, Garibaldi J A. Heat resistance of Salmonella: the uniqueness of Salmonella senftenberg 775W. Appl Microbiol. 1969;17:78–82. doi: 10.1128/am.17.1.78-82.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peleg M, Cole M B. Reinterpretation of microbial survival curves. Crit Rev Food Sci. 1998;38:353–380. doi: 10.1080/10408699891274246. [DOI] [PubMed] [Google Scholar]
- 50.Quintavilla S, Cattani M, Bolmini L, Mutti P, Barbuti S. Heat inactivation of Escherichia coli O157:H7 in a culture medium and in pork meat treated with a curing agent. Ind Conserve. 1998;73:316–324. [Google Scholar]
- 51.Reichart O. Modelling the destruction of Escherichia coli on the basis of reaction kinetics. Int J Food Microbiol. 1994;23:449–465. doi: 10.1016/0168-1605(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez A C, Smerage G H. System analysis of the dynamics of bacterial spore populations during lethal heat treatment. Trans ASAE. 1996;39:595–603. [Google Scholar]
- 53.Rowe B, Begg N T, Hutchinson D N, Dawkins H C, Gilbert R J, Jacob M, Hales B H, Rae F A, Jepson M. Salmonella ealing infections associated with consumption of infant dried milk. Lancet. 1987;ii:900–903. doi: 10.1016/s0140-6736(87)91384-5. [DOI] [PubMed] [Google Scholar]
- 54.Senhaji A F. The protective effect of fat on the heat resistance of bacteria. J Food Technol. 1977;12:203–216. [Google Scholar]
- 55.Shohat T, Green M S, Merom D, Gill O N, Reisfeld A, Matas A, Blau D, Gal N, Slater P E. International epidemiological and microbiological study of an outbreak of Salmonella agona infection from a ready to eat savoury snack—II. Israel. BMJ. 1996;313:1107–1109. doi: 10.1136/bmj.313.7065.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephens P J, Jones M V. Reduced ribosomal thermal-denaturation in Listeria monocytogenes following osmotic and heat shocks. FEMS Microbiol Lett. 1993;106:177–182. doi: 10.1111/j.1574-6968.1993.tb05955.x. [DOI] [PubMed] [Google Scholar]
- 57.Stringer S C, George S M, Peck M W. Thermal inactivation of Escherichia coli O157:H7. J Appl Microbiol. 2000;88(Suppl. S):79S–89S. doi: 10.1111/j.1365-2672.2000.tb05335.x. [DOI] [PubMed] [Google Scholar]
- 58.Sumner S S, Sandros T M, Harmon M C, Scott V N, Bernard D T. Heat resistance of Salmonella typhimurium and Listeria monocytogenes in sucrose solutions of various water activities. J Food Sci. 1991;56:1741–1743. [Google Scholar]
- 59.Tomlins R I, Ordal J. Thermal injury and inactivation in vegetative bacteria. Soc Appl Bacteriol Symp Ser. 1976;5:153–190. [Google Scholar]
- 60.Verrips C T, Glas R, Kwast R H. Heat resistance of Klebsiella pneumoniae in media with various sucrose concentrations. Eur J Appl Microbiol Biotechnol. 1979;8:299–308. [Google Scholar]
- 61.Wheeler J G, Cowden J M, Sethi D, Wall P G, Rodrigues L C, Tompkins D S, et al. Study of infectious intestinal disease in England: rates in the community, presenting to GPs, and reported to national surveillance. BMJ. 1999;318:1046–1050. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams A, Davies A C, Wilson J, Marsh P D, Leach S, Humphrey T J. Contamination of the contents of intact eggs by Salmonella typhimurium DT104. Vet Rec. 1998;143:562–563. [PubMed] [Google Scholar]
- 63.Winter A R, Stewart G F, McFarlane V H, Solowey M. Pasteurisation of liquid egg products. III. Destruction of Salmonella in liquid whole egg. Am J Public Health. 1946;36:451–460. [PMC free article] [PubMed] [Google Scholar]
- 64.Wong S, Street D, Delgado S I, Klontz K C. Recalls of foods and cosmetics due to microbial contamination reported to the U.S. Food and Drug Administration. J Food Prot. 2000;63:1113–1116. doi: 10.4315/0362-028x-63.8.1113. [DOI] [PubMed] [Google Scholar]