Abstract
Despite the release of a growing number of direct‐acting antivirals and evolving policy landscape, many of those diagnosed with hepatitis C virus (HCV) have not received treatment. Those from vulnerable populations are at particular risk of being unable to access treatment, threatening World Health Organization (WHO) HCV elimination goals. The aim of this study was to understand the association between direct‐acting antivirals approvals, HCV‐related policy changes and access to HCV virus treatment in Indiana, and to explore access to treatment by race, birth cohort and insurance type. We performed a retrospective cohort study of adults with HCV from 05/2011‐03/2021, using statewide electronic health data. Nine policy and treatment changes were defined a priori. A Lowess curve evaluated treatment trends over time. Monthly screening and treatment rates were examined. Multivariable logistic regression explored predictors of treatment. The population (N = 10,336) was 13.4% Black, 51.8% was born after 1965 and 44.7% was Medicaid recipients. Inflections in the Lowess curve defined four periods: (1) Interferon + DAA, (2) early direct‐acting antivirals, (3) Medicaid expansion/optimization and (4) Medicaid restrictions (fibrosis/prescriber) removed. The largest increase in monthly treatment rates was during period 4, when Medicaid prescriber and fibrosis restrictions were removed (2.4 persons per month [PPM] in period 1 to 72.3 PPM in period 4, p < 0.001; 78.0% change in slope). Multivariable logistic regression analysis showed being born after 1965 (vs. before 1945; OR 0.69; 95% 0.49–0.98) and having Medicaid (vs. private insurance; OR 0.47; 95% CI 0.42–0.53), but not race was associated with lower odds of being treated. In conclusion, DAAs had limited impact on HCV treatment rates until Medicaid restrictions were removed. Additional policies may be needed to address HCV treatment‐related age and insurance disparities.
Keywords: affordable care act, direct‐acting antiviral, fibrosis, medicaid expansion, young
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- Ab
antibody
- ACA
Affordable Care Act
- COVID‐19
coronavirus disease 2019
- DAA
direct‐acting antivirals
- ECHO
Extension of Community Healthcare Outcomes
- EMR
electronic medical record
- FDA
Food and Drug Administration
- FFS
fee‐for‐service
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IUH
Indiana University Health
- PCR
polymerase chain reaction
- Peg‐IFN
pegylated‐interferon
- PPM
persons per month
- RNA
ribonucleic acid
- US
United States
- USPSTF
United States Preventive Services Task Force
1. INTRODUCTION
There are estimated to be over 70 million people globally and 2.4 million persons in the United States (US) living with hepatitis C virus (HCV). 1 The burden of disease is particularly high amongst vulnerable populations including younger people who inject drugs and racial and ethnic minorities. 1 , 2 Chronic HCV infection is characterized by progressive fibrosis, leading to cirrhosis and the potential development of hepatocellular carcinoma (HCC) and need for liver transplantation. 3
By 2030, the World Health Organization global elimination targets for HCV are 80% of those eligible treated, 90% reduction in incidence of new infections and 65% reduction in liver‐related mortality. 4 Recent medical advances, including the development of the more tolerable and effective direct‐acting antiviral agents (DAAs), make achieving these goals possible. 5 , 6 However, accessing DAAs remain an issue, particularly for vulnerable populations; Black and Hispanic populations, and those insured with Medicaid are less likely to be treated than White and privately insured patients respectively. 7 , 8
Policy changes have been made to reduce barriers to accessing care and receiving HCV treatment. In 2014, the Affordable Care Act (ACA) sought to expand insurance coverage through an optional state‐level expansion of Medicaid. 9 , 10 In February of 2015, Indiana adopted a state‐specific version of Medicaid expansion. 11 Locally, policies to improve access to treatment have been initiated and include Indiana Medicaid's negotiation of supplemental pharmaceutical rebate agreements, adoption of the HCV Extension of Community Healthcare Outcomes (ECHO) programme, and Medicaid's removal of the specialist provider and advanced fibrosis restriction requirements for treatment. 12 , 13 , 14
There is ongoing interest in the impact of policy changes on access to care and treatment. Research on healthcare utilization by race, ethnicity and gender before and after implementation of the ACA showed non‐Hispanic White patients had the greatest improvements, and Black men and women fared the worst with respect to changes in healthcare access. 15 With regard to HCV specifically, we previously showed that following the expansion of Medicaid, fewer Black patients with HCV were waitlisted for liver transplant in states that expanded the programme compared with states that did not. We hypothesized that this trend is related to increased access to treatment in states with expanded programmes leading to lower burdens of decompensated liver disease necessitating waitlisting of Black HCV patients. 16
Advances in HCV treatment and policy changes facilitating access to treatment have the potential to substantially change the chronic liver disease landscape for all patients, including those from vulnerable populations and help us achieve the WHO HCV elimination goals. However, it is unclear which, if any, of these policies have translated into increases in HCV treatment rates. Understanding the impact of policies on treatment trends can inform public health HCV elimination efforts. Therefore, our study aims were to: (1) examine the association between policy changes and DAA approval on HCV treatment trends in Indiana, and (2) identify factors associated with receiving treatment for HCV infection to better understand what, if any, disparities exist in the receipt of HCV treatment in Indiana.
2. METHODS
2.1. Study population
The study population included patients seen at any Indiana University Health (IUH) practices across the state of Indiana. IUH is the largest network of physicians in Indiana, and it is a statewide integrated healthcare system with 19 hospitals and 178 outpatient practices or testing services across Indiana. 17 , 18 A statewide electronic medical record (EMR) was used to extract demographic, clinical and treatment‐related data. The study was approved by the institutional review board and was compliant with ethical conduct of research.
The primary analysis included patients over 18 years of age diagnosed with HCV from May 2011 through March 2021. Patients were considered to have a diagnosis of HCV if they had a positive HCV ribonucleic acid (RNA) polymerase chain reaction (PCR) quantitative test or if they had a documented HCV genotype.
2.2. Policy changes
With input from experts in the field including a hepatologist/epidemiologist, epidemiologist and scientist from the Indiana Department of Health, direct‐acting antiviral approval by the Food and Drug Administration (FDA) and HCV‐related policy changes that may have impacted HCV treatment trends in the state of Indiana were identified a priori (Figure 1). We chose to focus on Medicaid policy changes because private insurer policy changes for HCV treatment have often followed the Center for Medicare and Medicaid lead. 19 Specifically, nine DAA approval and HCV‐related policy change dates were identified: (1) in May 2011, the first DAAs, telaprevir and boceprevir were approved to be used in combination with pegylated‐interferon (peg‐IFN) for the treatment of HCV 20 ; (2) in November and December 2013, simeprevir and sofosbuvir, respectively, were approved for use in combination with peg‐IFN 20 ; (3) in October 2014, ledipasvir/sofosbuvir became the first oral only DAA regimen released for the treatment 20 ; (4) in February 2015, Indiana adopted a state‐specific version of Medicaid expansion called Healthy Indiana Plan (HIP) 2.0 under the ACA that provided coverage for residents under the poverty line 11 ; (5) in September 2016, Indiana Medicaid moved DAA drug coverage from managed care to a pharmacy fee‐for‐service (FFS) benefit (Medicaid ‘carve out’), which permits Medicaid programmes to negotiate supplemental rebate agreements with pharmaceutical manufacturers 21 ; (6) in August 2017, glecaprevir/pibrentasvir was approved for treatment of genotypes 1–6 HCV and was priced at a substantially lower cost than previously released DAAs 20 ; (7) in 2018, Indiana began the HCV‐ECHO programme, providing gastroenterology consultation to primary care providers interested in treating their patients with HCV 13 ; (8) in July 2019, Indiana Medicaid removed an advanced fibrosis requirement for HCV treatment 14 ; and (9) and finally in October 2020, Indiana Medicaid also removed the requirement for a specialist prescriber. 14 Of note, Indiana Medicaid has never imposed sobriety restrictions on HCV treatment.
FIGURE 1.
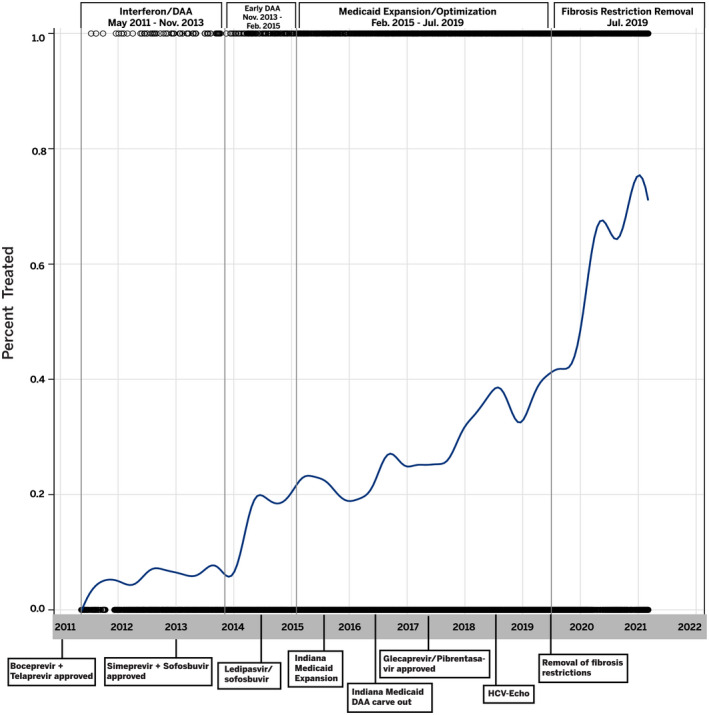
Hepatitis C virus–related policy changes and DAA Approval timeline, study periods and treatment trends. Abbreviations: HCV, Hepatitis C virus; HCV‐ECHO, Extension for Community Healthcare Outcomes; IFN, interferon
2.3. Demographic and clinical variables
To better understand factors associated with initiating HCV treatment, the following exposures of interest were explored: age, race/ethnicity, gender and insurance type. During the course of our study period, the United States Preventive Services Task Force (USPSTF) updated their recommendation for HCV screening from one that only recommended universal screening for people born between 1945 and 1965, to one that recommended universal screening for all adults ages 18–79. 22 To explore specifically how birth cohort screening guidelines may have impacted treatment trends, age was grouped into the following birth cohorts: those born before 1945, those born between 1945 and 1965 and those born after 1965. Race/ethnicity was defined as non‐Hispanic White, non‐Hispanic Black, Hispanic and non‐Hispanic other. Patients were defined as having cirrhosis, chronic kidney disease, hepatocellular carcinoma or history of hepatic encephalopathy or ascites by the presence of ICD‐9 or ICD‐10 codes. Insurance was classified as Medicaid (Medicaid alone or Medicare with Medicaid), Medicare, private and other (self‐pay/uninsured).
2.4. HCV treatment
Treatment initiation was defined as having at least one EMR order for an interferon product, ribavirin or a DAA HCV therapy during the study period (Table S1).
2.5. Outcomes
We performed a descriptive analysis to examine monthly treatment rates defined as the number of patients treated per month during the four periods that were identified during our analysis of the nine policy and DAA approval dates described above. The primary outcome was changed in treatment percentage over each period. The secondary outcome was predictor of treatment initiation.
2.6. Data analysis
Categorical variables were described with number and percentage, and the treatment groups were compared with the chi‐square test. The numbers of persons treated per month and the number of HCV‐Ab tests per month were calculated for each period. Only the first treatment date was considered for patients treated more than once. To evaluate the impact of HCV‐related policy and DAA approvals on treatment, multivariable logistic regression was used to estimate the change in treatment percentage between periods, controlling for age, race/ethnicity, gender, cirrhosis status and insurance type. Odds ratios with 95% confidence intervals were calculated. Additionally, the estimated slope of the four periods and the change in the slope between periods were calculated. Multivariable logistic regression was used to evaluate factors associated with receiving treatment while controlling for period. For the multivariable analysis, birth cohort, gender, race/ethnicity and insurance type, and history of cirrhosis, chronic kidney disease, hepatocellular carcinoma, ascites and hepatic encephalopathy were included in the final model due to their plausible association with the outcome. Human immunodeficiency virus (HIV) was not included in final models due to small sample size. A similar subset analysis was performed amongst just those patients with Medicaid. Statistical analyses were performed using SAS Enterprise guide 7.15.
3. RESULTS
A total of 10,336 patients with HCV were seen in the IUH system during the study period. The population was 57.4% male and 13.4% non‐Hispanic Black; 51.8% was born after 1965 and 44.7% of the sample was Medicaid recipients (Table 1). Total, 33.2% (n = 3432) of HCV‐positive patients initiated treatment. Fewer people born after 1965 had been treated compared to those born between 1945 and 1965 (26.4% vs. 40.9%; p < 0.0001). In addition, fewer women received treatment, compared to men (31.7% vs. 34.4%; p = 0.0037) (Table 1).
TABLE 1.
Demographic and clinical characteristics of patients with hepatitis C virus by treatment status
| Variable |
Total (n = 10,336) |
Not treated (n = 6904) |
Treated (n = 3432) |
p‐Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age group | <0.0001 | |||
| Born 1945–1965 | 4759 (46.0) | 2813 (59.1) | 1946 (40.9) | |
| Born after 1965 | 5353 (51.8) | 3940 (73.6) | 1413 (26.4) | |
| Born before 1945 | 224 (2.2) | 151 (67.4) | 73 (32.6) | |
| Race/ethnicity | <0.0001 | |||
| Hispanic | 184 (1.8) | 117 (63.5) | 67 (35.7) | |
| Non‐Hispanic Black | 1388 (13.4) | 862 (62.1) | 526 (37.8) | |
| Non‐Hispanic other | 487 (4.7) | 354 (72.7) | 133 (27.3) | |
| Non‐Hispanic White | 8277 (80.1) | 5571 (67.3) | 2706 (32.7) | |
| Gender | 0.0037 | |||
| Women | 4405 (42.6) | 3011 (68.4) | 1394 (31.7) | |
| Men | 5931 (57.4) | 3893 (65.6) | 2038 (34.4) | |
| Healthcare Characteristics | <0.0001 | |||
| History of cirrhosis | ||||
| No | 7613 (73.7) | 5486 (72.1) | 2127 (27.9) | |
| Yes | 2723 (26.4) | 1418 (52.1) | 1305 (47.9) | |
| History of ascites | ||||
| No | 9706 (94.0) | 6472(66.7) | 3234 (33.3) | |
| Yes | 628 (6.1) | 431(68.6) | 197 (31.4) | |
| History of hepatic encephalopathy | ||||
| No | 10105 (97.8) | 6734(66.6) | 3371 (33.4) | |
| Yes | 229 (2.2) | 169(73.8) | 60 (26.2) | |
| History of chronic kidney disease | ||||
| No | 9760 (94.4) | 6544(67.1) | 3216 (33.0) | |
| Yes | 574 (5.6) | 359 (62.5) | 215 (37.5) | |
| History of hepatocellular carcinoma | ||||
| No | 10207 (98.8) | 6841 (67.0) | 3366 (33.0) | |
| Yes | 127 (1.2) | 62 (48.8) | 65 (51.2) | |
| History of human immunodeficiency virus | ||||
| No | 10065 (97.4) | 6776 (98.2) | 3289 (95.8) | |
| Yes | 211(2.04) | 128 (1.85) | 83 (2.42) | |
| Insurance status | <0.0001 | |||
| Medicaid | 4621(44.7) | 3252 (70.4) | 1369 (29.6) | |
| Other | 1786 (17.3) | 1424 (79.7) | 362 (20.3) | |
| Private | 2107 (20.4) | 1135 (53.9) | 972 (46.1) | |
| Medicare | 1822 (17.6) | 1093 (60.0) | 729 (40.0) | |
3.1. Defining time periods
After dates for the nine policy changes and DAA approvals were identified, a Lowess curve was created to evaluate HCV treatment trends over the study period. Inflections in the curve identified distinct periods, collapsing the nine policy changes and DAA approvals into four periods: (1) Interferon + DAA, (2) early DAA, (3) Medicaid expansion/optimization and (4) Medicaid restrictions removed (Figure 1).
3.2. HCV treatment per period
The average time from positive test to treatment initiation was 33 days. After beginning treatment, 83.0% of patients had at least one negative RNA captured in the IU health system. Treatment rates increased over the 4 periods with a monthly treatment rate of 2.4 PPM (persons per month) in period 1, 9.3 PPM in period 2, 32.8 PPM in period 3 and 72.3 PPM in period 4. The increase in PPM was not significantly different for Period 2 compared with Period 1. However, the increase was significantly different for both Period 3 and Period 4 compared with Period 1 (p‐values < 0.0001). In multivariable regression analyses, the % change in slope for Period 2 was 7.47%, Period 3 was −52.41% and the largest increase was seen for Period 4 (Medicaid restrictions removed) with an increase of 79.07% compared with Period 1 (Figure 1). In multivariable logistic regression analyses (Table 2), factors associated with decreased odds of HCV treatment included being born after 1965, compared to those born before 1945 (aOR 0.70; 95% CI 0.49–0.99). In addition, insurance type was associated with treatment, with every other insurance type having lower odds of treatment compared with private insurance (aORs = 0.36–0.63). Having a history of cirrhosis was associated with increased odds of being treated (aOR 2.76; 95% CI 2.45–3.11), while having ascites (aOR 0.56; 95% CI 0.46–0.69) and encephalopathy (aOR 0.66; 95% CI 0.47–0.92) were associated with decreased odds of treatment. Race/ethnicity and gender were not significant in the multivariable regression analysis.
TABLE 2.
Factors associated with hepatitis C Virus treatment for the total population in multivariable regression
| Variable |
aOR (95% CI) (N = 10,336) |
|
|---|---|---|
| Demographic characteristics | ||
| Age group | ||
| Born 1945–1965 | 1.26 (0.90–1.76) | |
| Born after 1965 | 0.70 (0.49–0.99) | |
| Born before 1945 | Ref. | |
| Race/ethnicity | ||
| Hispanic | 1.08 (0.77–1.54) | |
| Non‐Hispanic Black | 1.00 (0.87–1.15) | |
| Non‐Hispanic Other | 0.97 (0.77–1.23) | |
| Non‐Hispanic White | Ref. | |
| Gender | ||
| Female | 1.02 (0.93–1.13) | |
| Male | Ref. | |
| Healthcare characteristics | ||
| History of cirrhosis | ||
| No | Ref. | |
| Yes | 2.76 (2.45–3.11) | |
| History of ascites | ||
| No | Ref. | |
| Yes | 0.56 (0.46–0.69) | |
| History of hepatic encephalopathy | ||
| No | Ref. | |
| Yes | 0.66 (0.48–0.92) | |
| History of chronic kidney disease | ||
| No | Ref. | |
| Yes | 0.94 (0.76–1.15) | |
| History of hepatocellular carcinoma | ||
| No | Ref. | |
| Yes | 1.02 (0.68–1.53) | |
| Insurance status | ||
| Medicaid | 0.47 (0.42–0.53) | |
| Other | 0.36 (0.31–0.43) | |
| Private | Ref. | |
| Medicare | 0.63 (0.55–0.73) | |
| Period | Est. Slope | % Change |
| Period | ||
| Period 1 (DAA + IFN) | 0.0016 | Ref. |
| Period 2 (Early DAA) | 0.0017 | 7.47% |
| Period 3 (Medicaid expansion and optimization) | 0.0008 | −52.41% |
| Period 4 (Medicaid restrictions removed) | 0.0029 | 79.07% |
3.3. HCV treatment per time period in medicaid population
Treatment rates for in the subset with Medicaid insurance were compared across the 4 periods. As with the full cohort, treatment rates increased over the 4 periods with a monthly treatment rate of 0.70 PPM in period 1, 2.9 PPM in period 2, 11.7 PPM in period 3 and 33.6 PPM in period 4 (Figure 2). The increase in PPM was not significantly different for Period 2 compared with Period 1. However, the increase was significantly different for both Period 3 and Period 4 compared with Period 1 (p's < 0.0001). While HCV treatment rates did increase over the four periods, the change in the slope did not always increase, compared with Period 1. Specifically, the slope of Periods 2 and 3 decreased, compared with Period 1 (−5.00% and −26.67% respectively). However, as with the total population, the largest increase was seen for Period 4 with an increase of 170.00% compared with Period 1 (p < 0.0001). In the multivariable logistic regression analysis, non‐Hispanic, other race/ethnicity had significantly higher odds of initiating treatment compared with non‐Hispanic White patients (aOR 1.86; 95% CI 1.31–2.66) (Table 3).
FIGURE 2.
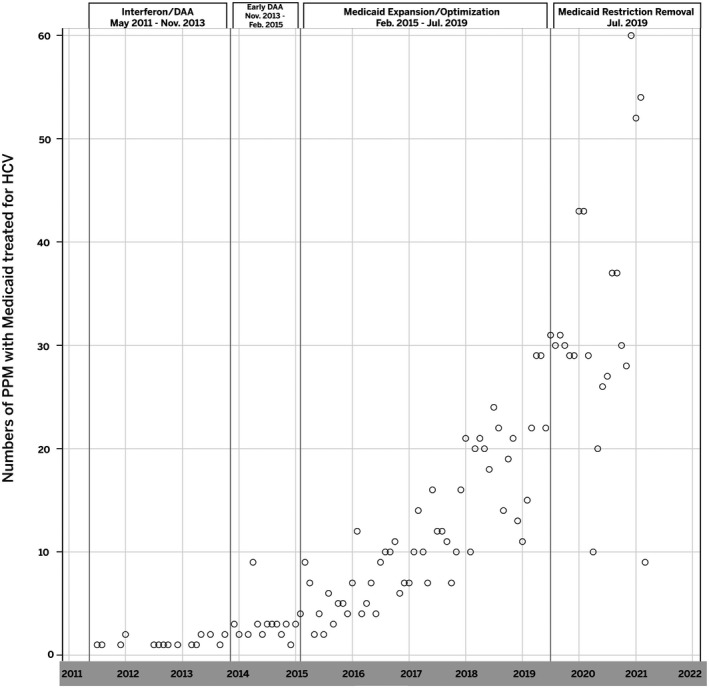
Hepatitis C Virus Patient Treatment Per Month with Medicaid Insurance. Abbreviations: HCV, Hepatitis C virus; PPM, persons per month
TABLE 3.
Factors Associated with HCV Treatment amongst Medicaid Recipients
| Variable |
aOR (95% CI) (n = 4621) |
|
|---|---|---|
| Demographic characteristics | ||
| Age group | ||
| Born 1945–1965 | 2.92 (0.56–15.20) | |
| Born after 1965 | 1.44 (0.28–7.51) | |
| Born before 1945 | Ref. | |
| Race/ethnicity | ||
| Hispanic | 0.73 (0.41–1.28) | |
| Non‐Hispanic Black | 1.02 (0.80–1.30) | |
| Non‐Hispanic Other | 1.86 (1.31–2.66) | |
| Non‐Hispanic White | Ref. | |
| Gender | ||
| Female | 1.04 (0.90–1.21) | |
| Male | Ref. | |
| Healthcare characteristics | ||
| History of cirrhosis | ||
| No | Ref. | |
| Yes | 2.80 (2.30–3.40) | |
| History of ascites | ||
| No | Ref. | |
| Yes | 0.57 (0.41–0.78) | |
| History of hepatic encephalopathy | ||
| No | Ref. | |
| Yes | 0.69 (0.42–1.13) | |
| History of chronic kidney disease | ||
| No | Ref. | |
| Yes | 0.53 (0.35–0.80) | |
| History of hepatocellular carcinoma | ||
| No | Ref. | |
| Yes | 1.05 (0.51–2.16) | |
| Period | Est. Slope | % Change |
| Period | ||
| Period 1 (DAA + IFN) | 0.0012 | Ref. |
| Period 2 (Early DAA) | 0.0011 | −5.00% |
| Period 3 (Medicaid expansion and optimization) | 0.0009 | −26.67% |
| Period 4 (Medicaid restrictions removed) | 0.0032 | 170.00% |
4. DISCUSSION
Recent advances in the treatment of HCV have produced DAA therapies that are highly effective with minimal side effects. 20 HCV and its downstream sequelae of end‐stage liver disease and HCC could be eliminated if screening and treatment were available to all those infected with the virus. Unfortunately, many barriers to treatment remain with access to DAA being particularly challenging for vulnerable populations. 8 HCV‐related policy changes have been made in silos around the country with an effort to increase access to health care and DAAs. 23 However, it was unclear whether these policy changes translated into increased treatment rates. Furthermore, it was unknown whether these changes would help improve treatment access for vulnerable populations. In this study, we identified four periods that were associated with HCV treatment and screening rates for patients being treated across the state of Indiana. We found the largest increase in treatment rates was seen when the requirements for advanced fibrosis and specialist prescriber were lifted, with relatively less of an increase seen during the DAA‐alone approval period. DAAs had limited impact on HCV treatment until Medicaid restrictions were removed. However, despite the increasing treatment rates, disparities remain for those who are younger and those who are privately insured.
There was a steady increase in the numbers of patients treated per month in each period, with 72.3 patients treated per month in period 4 (Medicaid restriction removed), compared with 2.4 patients per month in period 1. The American Association for the Study of Liver Diseases (AASLD) recommends treatment of all patients with chronic HCV, with prioritization of those with more advanced liver disease when resources are limited. 24 This led many Medicaid programmes to only cover the cost of treatment for those with F3 or greater fibrosis. 25 However, this has been met with legal challenges in some states, including Indiana, which resulted in the state removing liver damage restrictions as a prerequisite for treatment. 14 Little published data exist on the association between the removal of fibrosis restrictions and HCV treatment. One study used Markov modelling to demonstrate that removing restrictions to HCV access, including disease severity restrictions, resulted in significantly lower healthcare expenditures due to expanded access and improving health. 26 In a study examining the HCV cascade of care in San Francisco, 42% of patients had received treatment in 2018 compared with 18% in 2015. 27 Both California and Indiana have been given a grade of A+ by hepatitis C: State of Medicaid Access project for their lack of fibrosis, sobriety or provider restrictions suggesting that states with less restrictions are seeing higher treatment rates over time. 25 Data have suggested that treatment by nonspecialist providers with compact didactic training was as safe and effective as that provided by specialist. 28
While treatment rates have increased for patients in our health system over the study period, some populations remained undertreated compared to their counterparts. Patients with Medicaid, Medicare and other types of insurance were less likely to be treated than patients with private insurance. In a National Health and Nutrition Examination Survey dataset that predated the ACA, patients with HCV were more likely to be uninsured or covered by Medicaid. 7 The ongoing disparity in access to treatment amongst Medicaid recipients, even in states that have expanded Medicaid and removed treatment restrictions, highlights the need for additional innovative strategies to help vulnerable populations access treatment.
Our study did not find racial disparities in HCV treatment. However, in a Kaiser cohort in Northern California studied between 2014 and 2016, DAA initiation was 30% lower in non‐Hispanic Blacks compared with White patients. 29 Given our large sample size, we did have 80% power to detect an odds ratio of 1.19; thus, we would have expected to detect a disparity as large as 30% if it were present in our cohort. The lack of racial disparity seen in our cohort may reflect improving access over time, given our study population explored treatments initiated as recently as 2021.
Finally, we did find that patients born after 1965 were less likely to be treated than older patients in our cohort. In a small cohort of 60 young adults (younger than 30 years old) who used injection drugs in San Francisco, 30 patients accepted referral for HCV care but only five initiated and completed HCV treatment. 30 Youth‐tailored services that help overcome stigma related to care may be needed to help improve treatment uptake. Furthermore, previously, anyone not born between 1945 and 1965 had to disclose a behavioural risk factor for HCV infection (e.g. injection drug use) in order to be screened. 31 The recent Centers for Disease Control and USPSTF‐directed changes in screening recommendations removing age restrictions should also help to alleviate the stigma of screening and improve treatment across the continuum.
Our study explores the association of HCV‐related policy changes and DAA approval with treatment and screening trends in a large, current cohort of patients seen in multiple healthcare settings across the state of Indiana. Our study limitations include that we only captured data from patients treated within the IU Health system. While IU Health is the largest healthcare system in the state, it is possible that people were diagnosed at IU health and treated in other healthcare systems. While this would impact any report of the proportion of patients treated, it should not impact the ability to explore trends and factors associated with being treated in the IU health system. It should also be noted that our study population differed slightly from the broader population in Indiana. Specifically, our population had a lower proportion reporting Hispanic ethnicity (1.8% vs. 7.3% statewide); therefore, disparities in this population may not have been identified. 32 In addition, all patients do not get post‐treatment RNA levels drawn within the IU health system, so we could not reliably report HCV eradication rates. In Period 4, it should be noted that there were five months between the removal of the prescriber restriction and the end of the study period, so it is likely the majority of the changes reflect removing the fibrosis restriction. Lastly, the fourth time period occurred during the coronavirus disease 2019 (COVID‐19) pandemic and may have impacted treatment trends differentially in vulnerable groups. Further work will be needed to explore the impact of COVID‐19 pandemic on chronic liver disease care.
Here, we demonstrate that treatment rates for HCV have continued not only to improve over the last decade, likely the result of the release of efficacious therapies, but also as a result of HCV‐related policy changes. Despite AASLD guidelines and clear guidance from Center from Medicare and Medicaid that current restrictions violate federal law, as of May 2021, four states continued to impose fibrosis restrictions,18 states had prescriber restrictions, 13 required a period of sobriety or abstinences and 18 required drug or alcohol screening or counselling. 33 If we are to eliminate HCV, federal and state regulators will need to change policies to be consistent with established treatment guidelines. Further, we hope these data provide evidence that indeed removal of these barriers is associated with increased HCV treatment uptake. Despite being only one of eight states with an A+ rating for Medicaid HCV treatment access, disparities remain. 25 If viral hepatitis elimination targets are to be more than just aspirational, innovative outreach programmes that bridge the divide between practice to target the young and those who may be underinsured, including integrating treatment with other services, telehealth programmes and outreach services are needed. 23
Supporting information
Table S1
ACKNOWLEDGMENT
The authors thank Julianne Nanzer for her assistance in design of the figures for this manuscript.
Nephew LD, Wang Y, Mohamed K, et al. Removal of medicaid restrictions were associated with increased hepatitis C virus treatment rates, but disparities persist. J Viral Hepat. 2022;29:366–374. doi: 10.1111/jvh.13661
Funding information
This work was supported by a grant from the National Institute of Health an Indiana Clinical and Translational Science Institute KL2TR002530 (B. Tucker Edmonds, PI) and UL1TR002529 (S. Moe and S. Wiehe, co‐PIs) award
REFERENCES
- 1. Patel AA, Bui A, Prohl E, et al. Innovations in hepatitis C screening and treatment. Hepatol Commun. 2021;5(3):371‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall EW, Rosenberg ES, Sullivan PS. Estimates of state‐level chronic hepatitis C virus infection, stratified by race and sex, United States, 2010. BMC Infect Dis. 2018;18(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheung MCM, Walker AJ, Hudson BE, et al. Outcomes after successful direct‐acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65(4):741‐747. [DOI] [PubMed] [Google Scholar]
- 4. Dore GJ, Bajis S. Hepatitis C virus elimination: laying the foundation for achieving 2030 targets. Nat Rev Gastroenterol Hepatol. 2021;18(2):91‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coppola N, Pisaturo M, Zampino R, Macera M, Sagnelli C, Sagnelli E. Hepatitis C virus markers in infection by hepatitis C virus: In the era of directly acting antivirals. World J Gastroenterol. 2015;21(38):10749‐10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121(17):2874‐2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bush H, Paik J, Golabi P, de Avila L, Escheik C, Younossi ZM. Impact of hepatitis C virus and insurance coverage on mortality. Am J Manag Care. 2019;25(2):61‐67. [PubMed] [Google Scholar]
- 8. Kim NJ, Locke CJ, Park H, Magee C, Bacchetti P, Khalili M. Race and hepatitis C care continuum in an underserved birth cohort. J Gen Intern Med. 2019;34(10):2005‐2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sommers BD, Blendon RJ, Orav EJ, Epstein AM. Changes in utilization and health among low‐income adults after medicaid expansion or expanded private insurance. JAMA Intern Med. 2016;176(10):1501‐1509. [DOI] [PubMed] [Google Scholar]
- 10. French MT, Homer J, Gumus G, Hickling L. Key provisions of the patient protection and affordable care act (ACA): a systematic review and presentation of early research findings. Health Serv Res. 2016;51(5):1735‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaiser Family Foundation: Status of State Mediciad Expansion Decisons: Interactive Map. https://www.kff.org/medicaid/issue‐brief/status‐of‐state‐medicaid‐expansion‐decisions‐interactive‐map/ Published 2021 Accessed May 31, 2021, 2021
- 12. Gilfford Kathleen WA, Linda W, Rachel D, Marina T, Rachel G. How State Mediciad Programs are Managing Prescription Drug Costs; Results form a State Mediciad Survey for State Fiscal Years 2019 and 2020. https://files.kff.org/attachment/How‐State‐Medicaid‐Programs‐are‐Managing‐Prescription‐Drug‐Costs.pdf Published 2020 Accessed May 31, 2021
- 13. Richard M. Faribanks School of Public Health: Project ECHO. https://fsph.iupui.edu/research‐centers/centers/public‐health‐practice/echo‐project.html Accessed May 31, 2021
- 14. Aclu lawsuit agreement ends unlawful hepatitis c treatment restrictions. https://www.aclu‐in.org/en/press‐releases/aclu‐lawsuit‐agreement‐ends‐unlawful‐hepatitis‐c‐treatment‐restrictions Published 2019 Accessed
- 15. Manuel JI. Racial/ethnic and gender disparities in health care use and access. Health Serv Res. 2018;53(3):1407‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nephew LD, Mosesso K, Desai A, et al. Association of state medicaid expansion with racial/ethnic disparities in liver transplant wait‐listing in the United States. JAMA Netw Open. 2020;3(10):e2019869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rawl SM, Dickinson S, Lee JL, et al. Racial and socioeconomic disparities in cancer‐related knowledge, beliefs, and behaviors in Indiana. Cancer Epidemiol Biomarkers Prev. 2019;28(3):462‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Indiana Unveristy Health ; About Our System. https://iuhealth.org/about‐our‐system Published 2021 Accessed August, 2021
- 19. Medicaid, Private Insurers Begin To Lift Curbs on Pricye Hepatitis C Drugs. Kaister Health Network. https://khn.org/news/medicaid‐private‐insurers‐begin‐to‐lift‐curbs‐on‐pricey‐hepatitis‐c‐drugs/ Published 2016 Accessed June 13, 2021, 2021
- 20. Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct acting anti‐hepatitis C virus drugs: clinical pharmacology and future direction. J Transl Int Med. 2017;5(1):8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaiser Family Foundation : How State Medicaid Programs are Managing Prescription Drug Costs: Results from a State Medicaid Pharmacy Survey for State Fiscal Years 2019 and 2020. https://www.kff.org/report‐section/how‐state‐medicaid‐programs‐are‐managing‐prescription‐drug‐costs‐pharmacy‐benefit‐administration/ Published 2020 Accessed June 2, 2021, 2021
- 22. Force UPST. Screening for hepatitis C virus infection in adolescents and adults: US preventive services task force recommendation statement. JAMA. 2020;323(10):970‐975. [DOI] [PubMed] [Google Scholar]
- 23. Feld JJ, Ward JW. Key elements on the pathway to HCV elimination: Lessons learned from the AASLD HCV special interest group 2020. Hepatol Commun. 2021;5:911‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panel AIHG . Hepatitis C guidance: AASLD‐IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932‐954. [DOI] [PubMed] [Google Scholar]
- 25. Hepatitis C: State of Mediciad Access . https://stateofhepc.org/ Published 2019 Accessed June 2, 2021
- 26. Chou JW, Silverstein AR, Goldman DP. Short‐term budget affordability of hepatitis C treatments for state Medicaid programs. BMC Health Serv Res. 2019;19(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mirzazadeh A, Chen Y‐H, Lin J, et al. Progress toward closing gaps in the hepatitis C virus cascade of care for people who inject drugs in San Francisco. PLoS One. 2021;16(4):e0249585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kattakuzhy S, Gross C, Emmanuel B, et al. Expansion of treatment for hepatitis C virus infection by task shifting to community‐based nonspecialist providers: a nonrandomized clinical trial. Ann Intern Med. 2017;167(5):311‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcus JL, Hurley LB, Chamberland S, et al. Disparities in initiation of direct‐acting antiviral agents for hepatitis C virus infection in an insured population. Public Health Rep (Washington, DC: 1974). 2018;133(4):452‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris MD, Mirzazadeh A, Evans JL, et al. Treatment cascade for hepatitis C virus in young adult people who inject drugs in San Francisco: Low number treated. Drug Alcohol Depend. 2019;198:133‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. U.S. Preventive Services Task Force . Screening for hepatitis C virus infection in adolescents and adults: US preventive services task force recommendation statement. JAMA. 2020;323(10):970‐975. [DOI] [PubMed] [Google Scholar]
- 32. United States Census Bureau . QuickFacts: Indiana Published 2019. Accessed June 29 2020.
- 33. HCV State‐of‐Medicaid Access National Progress Report . https://www.statnews.com/wp‐content/uploads/2021/05/HCV_State‐of‐Medicaid‐Access_May‐2021‐Progress‐Report‐1.pdf Published 2021 Accessed September 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


