Abstract
Objectives
To evaluate the efficacy and safety of tamsulosin and Hachimijiogan or Ryutanshakanto in patients with lower urinary tract symptoms due to benign prostatic hyperplasia.
Methods
A prospective, randomized, double‐blind method was used to determine the efficacy and safety of the combination or placebo at baseline and 4, 8, and 12 weeks of study. The International Prostate Symptom Score, quality of life index, complete voiding diary, and National Institutes of Health‐Chronic Prostatitis Symptom Index were studied. Uroflowmetery and postvoid residual urine volume were measured and compared. Laboratory tests including prostate‐specific antigen were performed.
Results
In all groups, International Prostate Symptom Score and quality of life showed improvement, but no significant differences were shown among the groups. Prostate volume increased after treatment, and uroflowmetric parameters showed improvements after treatment without significance among the three groups. The total score of the National Institutes of Health‐Chronic Prostatitis Symptom Index showed a significant improvement in all groups, without significant differences among the groups. Only the pain sub‐score of the National Institutes of Health‐Chronic Prostatitis Symptom Index showed a significant decrease in the tamsulosin with Ryutanshakanto group compared to the control group. A total of 11 adverse reactions occurred, but they were mild and not related to the study drugs.
Conclusion
Ryutanshakanto can provide pain relief in patients with chronic prostatitis and chronic pelvic pain syndrome. If more research is conducted, Hachimijiogan and Ryutanshakanto may be applied as add‐on treatments in patients with storage symptoms with alpha‐blocker monotherapy.
Keywords: benign prostatic hyperplasia, Hachimijiogan, Ryutanshakanto, tamsulosin, traditional herbal medicine
Abbreviations & Acronyms
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- BPH
benign prostatic hyperplasia
- BSW
benefit, satisfaction, and willingness to continue
- BUN
blood urea nitrogen
- DBP
diastolic blood pressure
- HJG
Hachimijiogan
- IPSS
International Prostate Symptom Score
- LUTS
lower urinary tract symptoms
- LUTS/BPH
lower urinary tract symptoms due to benign prostatic hyperplasia
- NIH‐CPSI
National Institutes of Health‐Chronic Prostatitis Symptom Index
- PSA
prostate‐specific antigen
- Q‐max
maximum flow rate
- QoL
quality of life
- RST
Ryutanshakanto
- SBP
systolic blood pressure
- TURP
transurethral resection of the prostate
Introduction
BPH is a common diagnosis of progressive enlargement of the prostate in older men and the prevalence increases with an aging population. 1 , 2 BPH is one of the leading causes of LUTS, causing significant morbidity and decreasing the QoL of older men. 3
The first‐line treatment for LUTS/BPH is α‐blockers alone or with 5α‐reductase inhibitors, although they have shown different effects on symptoms and disease progression. 4 , 5 Moreover, adverse effects have been observed in patients receiving first‐line treatment, including dizziness, ejaculatory dysfunction, orthostatic hypotension, headache, dry mouth, and rhinitis. 6
Traditional herbal medicines, such as HJG and RST, were suggested to improve symptoms of patients with LUTS/BPH. In 2003, Yoshimura et al. reported that 41 patients treated with HJG had improved uroflowmetric parameters than patients treated with tamsulosin. 7 Yagi et al. suggested that adding HJG to patients with α‐blockers was effective in improving the QoL and IPSS of LUTS/BPH patients. 8 Furuya et al. reported that RST may have a therapeutic effect on pain and discomfort after TURP. 9 Jin et al. studied the effect of HJG on human‐kit cytochrome P450 and UDP‐glucuronosyltransferase in 2018 and found that the herbal drugs showed a little suppressive effect on the human microsomal drug‐metabolizing enzymes even at high concentrations, suggesting that it does not affect tamsulosin metabolism and lacks any herb–drug interaction. 10
These results indicate that herbal medicines may have a supplementary effect in improving LUTS/BPH and QoL in patients with adverse effects or in whom conventional medical treatment is ineffective. However, there is insufficient evidence, and the results suggest the low level of recommendations. Detailed, large‐scale placebo‐controlled clinical trials to compare the efficacy and effectiveness of combination treatment with herbal medicines have not yet been conducted.
The present study is the first to describe the efficacy and safety of combination therapy with tamsulosin, HJG, and RST using prospective, randomized, double‐blind methods.
Methods
Chemical preparations
Two traditional herbal medicines, Hachimijiogan (palmijihwang ‐ hwan in Korean; Baweidihuang‐wan in Chinese) and Ryutanshakanto (Yongdamsagan‐tang in Korean; Longdanxiegan‐tang in Chinese) were prepared. High‐performance liquid chromatography analysis of the two traditional herbal medicines was conducted according to a previously reported analytical protocol 11 , 12 to confirm the marker compounds of two medicines and no marker compounds in any of the placebo samples.
Patients
The present study was conducted within the Chungnam National University Hospital between 14 January 2019 and 31 December 2020. The study was approved by the Institutional Review Board (IRB 2018‐11‐015‐025) and was performed after receiving written informed consent from patients.
Men aged 45 years or above with a history of LUTS secondary to BPH of at least 6 months were eligible. The patients agreed not to use BPH medications during the study, except the study medications. The inclusion criteria were as follows: men aged 45 or above, IPSS over 8 points at baseline, Q‐max 5–15 mL/s with more than 125 mL voided volume at screening, clinical diagnosis of BPH based on medical history and physical examination, willingness and ability to provide written informed consent, and compliance with study procedures.
The exclusion criteria were immediate necessity for surgery during the study period, previous prostatic or urethral surgery or other invasive procedures to treat BPH; history or evidence of prostate cancer and bladder cancer; clinically significant renal insufficiency (serum creatinine >2.0 mg/dL) or hepatic insufficiency (AST or ALT >100 U/L), history of any adverse effects (syncope or orthostatic hypotension) or hypersensitivity after using alpha‐blockers, history of urethral stricture, use of indwelling Foley catheter or clean intermittent catheterization, history of stroke or spinal cord injury that may result in urinary symptoms or changes in flow rate, or ineligible patients based on the judgment of the investigator (Fig. 1).
Fig. 1.
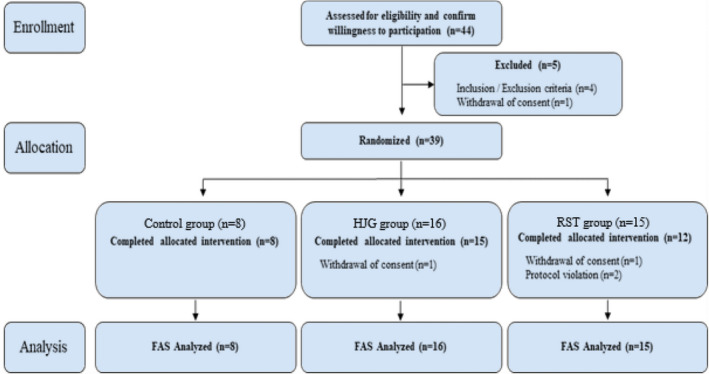
Flow of study participants: from patient enrollment to analysis.
Study design
This study was conducted as a double‐blind clinical study, where both the subjects and investigators (assessors) are blinded. The subjects only know that two different types of drugs are used—the study drug and placebo—but are not informed which is which. Subjects are assigned by block randomization, without additional stratification and are assigned to the control group or experimental groups according to a pre‐generated randomization table, in the order of their subject number. The investigators are not involved in the randomization and remain blinded from group allocation. To maintain blinding, the randomization table and drug assignment are not disclosed until the completion of the trial unless otherwise indicated, such as an emergency.
The sample size was determined based on the investigator’s clinical experience by hypothesizing the difference in the effect sizes between the combined therapy and control groups. All calculations were performed at a 5% significance level and 80% power. The two groups had a minimum hypothesized effect size of 0.4, and subjects were allocated at a 2:2:1 ratio. Furthermore, as this is a pilot clinical trial, 10% of the calculated sample size was determined as the final sample size, with reference to a recent recommendation of minimum sample sizes for pilot studies. Based on the above hypothesis, the sample size was calculated to be 36 for each treatment group and 18 for the control group. Considering a dropout rate of 10%, the target sample size set to 100 patients, with 40 patients for each treatment group and 20 patients for the control group.
Two herbal medicines, HJG and RST which are formulated to have nearly identical size and shape and are packed in the same type of packaging with similar scents, were prepared. Double‐blindness was maintained by adding the herbal medicinal flavor to the placebo. Eligible patients were then randomized to receive fixed doses of tamsulosin 0.2 mg/day plus HJG (Hanpoong Pharm. and Food Co. Ltd., Seoul, Korea) at a dose of 3.0 g as an oral solution three times daily (Group 1); tamsulosin 0.2 mg/day plus RST (Hanpoong Pharm. and Food Co. Ltd.) at a dose of 3.0 g as an oral solution three times daily (Group 2); or tamsulosin 0.2 mg/day plus three times daily placebo (Group 3). HJG, RST, and placebo were to be taken three times daily immediately before meals or 2 h after meals.
Assessments
Demographics included only the initial of name and age of patients. Personal identifiable information was not collected, including the resident registration number, name, and address of patients.
Efficacy of drugs was observed in three different groups to check whether LUTS/BPH had improved. The IPSS (questions 1 to 7) and QoL index (question 8) were checked at baseline and visits 1 to 3 (4, 8, and 12 weeks). The NIH‐CPSI was compared at baseline and visit 3. BSW was checked at baseline and visits 1 to 3. Uroflowmetric values and postvoid residual urine volume were compared at baseline and visit 3. Prostate volume was measured by transrectal ultrasonography at baseline and visit 3. The patients were instructed to complete a voiding diary at the baseline and visit 3. Blood pressure was checked at baseline and at visits 1 to 3. Laboratory tests were conducted to determine PSA, BUN, creatinine, AST and ALT levels; bloody chemistry, complete blood cell count test; and urine microscopy were also conducted. Results of the laboratory tests were compared at the baseline with those at visit 3. All patients completed the physical examination, including height (cm), weight (kg) and BMI (kg/m2) at baseline. At baseline, detailed clinical history was noted, including medical history of present and past diseases, and other treatments. Patients were also asked to use medications for LUTS/BPH that could interfere with symptoms (alpha‐blockers, anticholinergics, sympathomimetic drugs, antihistamines, and herbal preparations), or phosphodiesterase 5 inhibitors (Table S1).
Statistics
All statistical analyses were based on the two‐sided significance level at 5% by the statistical programming language, S. Assuming a missing at random, the missing values were replaced by multiple imputations by Markov Chain Monte Carlo. Both full analysis set and per protocol were analyzed. Patients with prostate volumes larger than 30 g were sub‐grouped and analyzed (Tables S2–S4).
All data are expressed as mean (95% confidence interval). Analysis of covariance was performed to evaluate the efficacy of medications. The analysis of covariance model used the subject groups as fixed effects and baseline values as covariates. We used the paired t‐test for comparisons before and after treatment. Repeated ANOVA was used to verify the serial IPSS changes at every visit.
Results
Patient characteristics
Forty‐four patients were prospectively enrolled. Eight patients in the tamsulosin + placebo group, 16 patients in the tamsulosin + HJG group, and 15 patients in the tamsulosin + RST group were randomly assigned.
There were no significant differences in age, height, weight, BMI, and vital signs among patients of all three groups (Table 1).
Table 1.
Baseline clinical profile of the study subjects
| Demographic | Control group (n = 8) | HJG group (n = 16) | RST group (n = 15) | P‐value† |
|---|---|---|---|---|
| Age (years) | 66.13 (59.21, 73.04) | 60.94 (56.61, 65.27) | 67.73 (62.52, 72.95) | 0.0942 |
| Height (cm) | 168.4 (165.0, 171.8) | 169.0 (166.8, 171.3) | 166.5 (162.9, 170.0) | 0.3761 |
| Weight (kg) | 69.08 (64.23, 73.92) | 72.59 (68.16, 77.02) | 70.31 (64.86, 75.76) | 0.5932 |
| BMI (kg/m2) | 24.45 (22.08, 26.82) | 25.39 (24.03, 26.74) | 25.33 (23.84, 26.82) | 0.6914 |
| Vital sign | ||||
| SBP | 135.0 (126.2, 143.8) | 137.5 (129.9, 145.1) | 136.0 (130.0, 142.0) | 0.8851 |
| DBP‡ | 76.63 (67.65, 85.60) | 84.31 (78.47, 90.16) | 81.33 (75.70, 86.96) | 0.2583 |
P‐value were analyzed by Analysis of variance (ANOVA).
Prostate volume change
Patients with higher mean total prostate volumes and transitional zones were assigned to the herbal medicine group, but the difference was not statistically significant (Table 2). In all three groups, prostate volume increased after treatment compared to that before treatment. Moreover, the transitional volume of the prostate, which is known as a predictor of acute urinary retention, 13 also increased after treatment compared to that before treatment. Concomitant herbal medicines have no effect on volume reduction in BPH.
Table 2.
Comparison of prostate volume, PSA, and uroflowmetric parameters in the study groups
| Control group (n = 8) | HJG group (n = 16) | RST group (n = 15) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | P‐value§ | Baseline | Week 12 | P‐value | Baseline | Week 12 | P‐value | |
| Prostate volume (gm) | 33.38 | 34.5 | 0.5256 | 37.75 | 41.76 | 0.0163* , § | 41.53 | 43.93 | 0.0649§ |
| 0.3610† | 0.7126‡ | ||||||||
| PSA (ng/mL) | 1.5 | 1.63 | 0.6219 | 1.08 | 1.16 | 0.3746§ | 1.89 | 2.22 | 0.7316§ |
| 0.8706† | 0.1481‡ | ||||||||
| Micturition volume (mL) | 278.6 | 281 | 0.9713 | 274.3 | 261.4 | 0.6943§ | 253.8 | 270.2 | 0.6142§ |
| 0.8035† | 0.8222‡ | ||||||||
| Postvoid residual urine volume (mL) | 70.13 | 47.5 | 0.4015 | 43.56 | 53 | 0.4970§ | 65 | 78.03 | 0.3858§ |
| 0.4980† | 0.2004‡ | ||||||||
| Q‐max (mL/s) | 10.35 | 16.45 | 0.9716 | 11.43 | 13.79 | 0.7999§ | 10.85 | 11.54 | 0.8316§ |
| 0.2275† | 0.5632‡ | ||||||||
Indicates P < 0.05.
Least squares mean difference and P‐value were analyzed by analysis of covariance (ANCOVA) with the baseline scores as covariates and group (Control or HJG) as the fixed factor.
Least squares mean difference and P‐value were analyzed by analysis of covariance (ANCOVA) with the baseline scores as covariates and group (Control or RST) as the fixed factor.
Mean difference and P‐value were analyzed using a paired t‐test for the baseline value and week 12.
IPSS
After drug administration, patients in all three groups showed significant improvement in IPSS and QoL index without significant differences among the groups (Fig. 2).
Fig. 2.
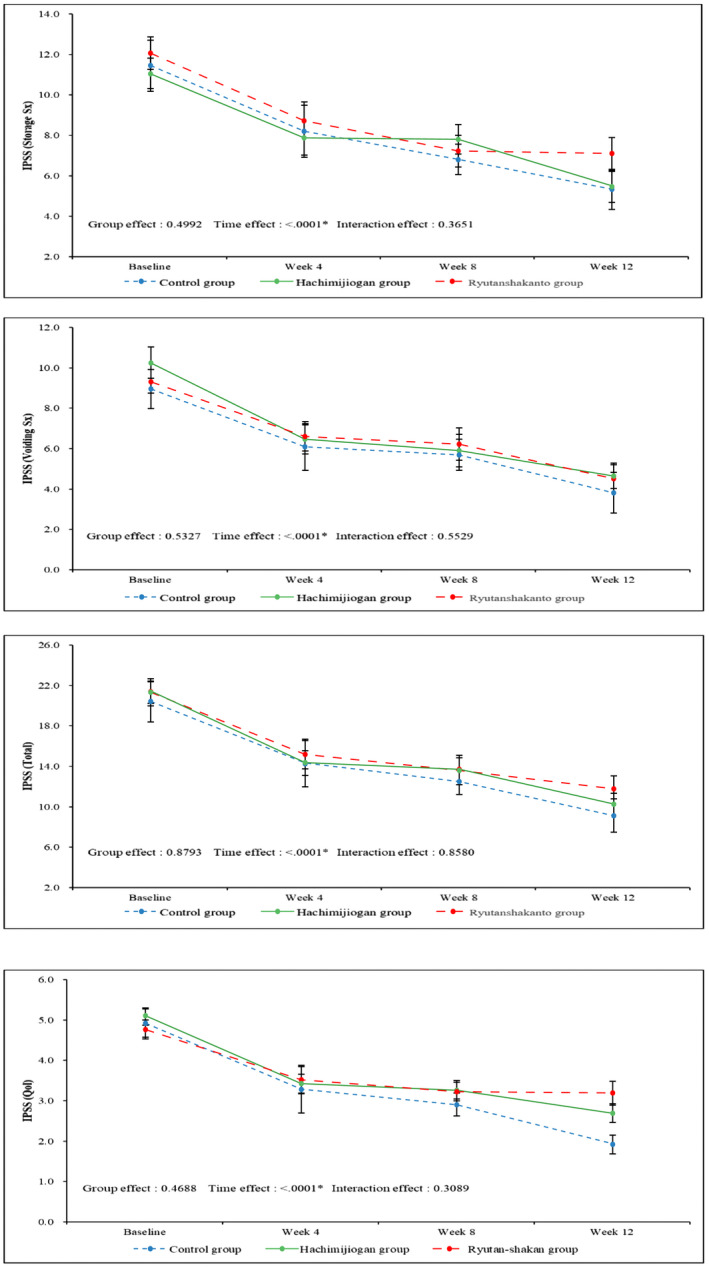
IPSS sub‐score and total score changes and QoL index changes in the study groups: patients in all three groups showed significant improvement in all IPSS and QoL scores over time, however, showed no significant differences among the groups.
Uroflowmetric parameters
There was no difference before and after treatment in uroflowmetric parameters and postvoid residual urine volume. No statistical improvement is observed in all three groups (Table 2).
NIH‐CPSI
The NIH‐CPSI significantly decreased in all three groups before and after treatment, but there was no significant difference among the groups. By subdividing and analyzing the group of patients with more than 80% adherence to medication, the average value of moderate pain in the sub‐score showed a significant average decrease in the tamsulosin + RST group compared to the average decrease in the control group before and after treatment. As the drug is better tolerated, treatment‐associated prostatic pain further improved with RST treatment (Table 3). Furthermore, by analyzing the group of patients with prostate volume over 30 gm, a significant average decrease in the study group compared to the average decrease in the control group before and after treatment (Table S3).
Table 3.
NIH‐CPSI score changes in the study groups
| Control group (n = 8) | HJG group (n = 16) | RST group (n = 15) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | P‐value§ | Baseline | Week 12 | P‐value | Baseline | Week 12 | P‐value | |
| Pain | 2.5 | 0.38 | 0.2102 | 1.23 | 0.92 | 0.8078§ | 4.7 | 0 | 0.0008* , § |
| 0.6126† | P | 0071* , ‡ | |||||||
| Urinary | 6.13 | 2.88 | 0.0009* | 6.69 | 3 | 0.0001* , § | 6.9 | 3.7 | 0.0034* , § |
| 0.9692† | 0.5226‡ | ||||||||
| QoL | 8.38 | 2.75 | <0.0001* | 7.62 | 3.92 | <0.0001* , § | 8.2 | 4.6 | 0.0011* , § |
| 0.0354* , † | 0.0492* , ‡ | ||||||||
| Total | 17 | 6 | 0.0005* | 15.54 | 7.85 | 0.0012* , § | 19.8 | 8.3 | <0.0001* , § |
| 0.3356† | 0.3585‡ | ||||||||
Indicates P < 0.05.
Least squares mean difference and P‐value were analyzed by analysis of covariance (ANCOVA) with the baseline scores as covariates and group (Control or HJG) as the fixed factor.
Least squares mean difference and P‐value were analyzed by analysis of covariance (ANCOVA) with the baseline scores as covariates and group (Control or RST) as the fixed factor.
Mean difference and P‐value were analyzed using a paired t‐test for the baseline value and weak 12.
Laboratory tests
Regarding the hematologic chemistry tests, urinalysis, and BSW questionnaire analysis, there were no significant differences among the groups before and after treatment, and stable laboratory results were observed after treatment. In the case of PSA, which increased and decreased according to the size of the prostate, an increase in overall prostate volume was observed after 12 weeks, but it was not significant in all three groups and showed a slight increase (Table 2).
Adverse events
A total of 11 adverse reactions occurred, but they were all mild (grade 1) and were not related to the study drugs.
Discussion
Despite advances in medicine, the number of patients with LUTS/BPH is increasing, and thus either is the number of patients who are both irresponsive to medical treatment and ineligible for surgical intervention. Several studies have been conducted to determine the efficacy of traditional herbal medicine, but the sample sizes have been small. 14 This study is the first to identify the efficacy and safety of the combination therapy of alpha‐blockers and traditional herbal medicines for LUTS/BPH.
Prostate volume usually correlates with the severity of LUTS, and transitional zone volume is the main contributor to the total prostate volume growth, which is suggested to be closely related to the severity of LUTS/BPH. 15 , 16 Although the assigned patients in the study group had a higher average prostate volume, the comparable effectiveness was shown in IPSS and QoL index with the control group scores. This result suggests that the HJG and RST combination do not exhibit inferior efficacy to alpha‐blocker monotherapy. However, in future, statistical analysis will be required to verify drug efficacy by comparing patients with similar prostate size or dividing subgroups for patients with mild prostatic hyperplasia suitable for medical treatment, accompanied by a long‐term study with more patients.
In a previous study in Japan, the combination of an alpha‐blocker and HJG had a positive effect on improving the detrusor overactivity induced by cold environment, which is mediated through a sensitive C‐fiber sensory nerve pathway involving α1‐adrenergic receptors. 17 Lee et al. reported that in patients with ketamine‐induced cystitis, HJG could improve overactivity by inhibiting neuroreceptor upregulation and reducing inflammatory mediators. 18 Yoshimura et al. also reported that low‐dose HJG significantly improves dysuria in LUTS/BPH patients, while improving Q‐max and voiding scores of the IPSS. 7 In the present study, even though the change in IPSS was inferior to that in the control group, the HJG group also showed a comparable decrease in storage symptom score of IPSS, which was comparable to the control group. These results suggest that HJG may affect bladder afferent sensory receptors and improve the storage symptoms of LUTS/BPH patients. In another study in Japan, RST administration was suggested to improve the severity and frequency of postoperative pain and discomfort after TURP. 9 In the present study, RST also showed a significant effect on improving the pain score in NIH‐CPSI. Moreover, as the drug is better tolerated, the treatment effect on prostatic pain shows further improvement. As a result, this suggests that the combination of HJG and RST can be added to patients with chronic pelvic pain syndrome who are not responsive to conventional medical treatment.
Park et al. suggested that RST water extract may decrease the volume of prostate in a rat model induced by testosterone and that the extract had an inhibitory effect on the proliferation of BPH‐1 cells by cell‐cycle arrest and anti‐inflammatory activities in a dose‐dependent manner. 19 , 20 In another study, the administration of HJG was suggested to decrease serum testosterone levels, especially at low dosages. 21 Based on these studies, we initially expected to reduce the volume of the prostate with the herbal medicine combination. However, the prostate volume and transitional volume of all patients increased in this study. To identify for herbal medicines to have effect on reducing prostate volume, an optimal dosage adjustment will be needed in the future study.
Dizziness, ejaculatory dysfunction, and impotence are the major adverse events associated with tamsulosin. In a 4‐year study to establish the safety of tamsulosin, up to 26% of patients experienced some drug‐related adverse events. 6 Mirabegron, a β3‐adrenoceptor agonist that is usually added to tamsulosin for patients with OAB, also showed 3.9% of adverse events. 22 In the present study, a total of 11 adverse events occurred, three in the control group and five and three in the HJG and RST groups, respectively. However, they were not drug‐related adverse events without severe adverse events, but only with grade 1 events. Therefore, HJG and RST add‐on to tamsulosin can be safe supplements for patients with adverse events on taking other medications or high operation risk.
This study has limitations. The number of patients was small, and some assigned patients had a relatively large prostate, with less response to treatment and requiring surgery. Although this study provides meaningful results, in future, long‐term studies with large number of patients with prostate volumes of 35 gm or less which the previously reported best cutoff value for small prostate volume in 2003, 23 will be required to verify drug efficacy to use as add‐on treatments to patients previously treated by alpha‐blocker monotherapy.
Author contributions
Chung Lyul Lee: Conceptualization; Data curation; Formal analysis; Writing – original draft; Writing – review and editing;. Hyeun‐Kyoo Shin: Conceptualization; Funding acquisition; Investigation; Methodology; Supervision; Validation. Ji Yong Lee: Conceptualization; Writing – original draft; Writing – review and editing. Ojin Kwon: Data curation; Formal analysis; Software; Validation. Chang‐Seob Seo: Data curation; Formal analysis; Investigation; Software; Supervision; Validation. Ae‐Ran Kim: Data curation; Investigation; Resources; Software. Bok‐Nam Seo: Data curation; Investigation. Seung Woo Yang: Investigation; Supervision. Ki Hak Song: Conceptualization; Supervision. Jae Sung Lim: Conceptualization; Supervision. Jong Mok Park: Investigation; Supervision. Yong Gil Na: Conceptualization; Project administration; Supervision. Ju Hyun Shin: Conceptualization; Data curation; Project administration; Supervision.
Conflict of interest
None declared.
Approval of the research protocol by an Institutional Reviewer Board
IRB 2018‐11‐015‐025.
Informed consent
All patients provided written informed consent before enrollment.
Registry and the Registration No. of the study/trial
Korea Food and Drug Administration 1AA‐1902‐197854: 2019‐02‐28.
Animal studies
Not applicable.
Supporting information
Table S1. Timeline of clinical visits and assessments.
Table S2. Sub‐analysis with the prostate volume over 30 gm of prostate volume and uroflowmetric parameters.
Table S3. NIH‐CPSI score changes in the sub‐analysis group with the prostate volume over 30 gm.
Table S4. IPSS changes in the sub‐analysis group with the prostate volume over 30 gm.
Acknowledgments
This research was supported by grants from the Korea Institute of Oriental Medicine (grant numbers KSN1812240, KSN2013310 and KSN2021310).
References
- 1. Vuichoud C, Loughlin KR. Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can. J. Urol. 2015; 22: 1–6. [PubMed] [Google Scholar]
- 2. Maserejian NN, Chen S, Chiu GR et al. Incidence of lower urinary tract symptoms in a population‐based study of men and women. Urology 2013; 82: 560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parsons JK, Bergstrom J, Silberstein J, Barrett‐Connor E. Prevalence and characteristics of lower urinary tract symptoms in men aged > or = 80 years. Urology 2008; 72: 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McConnell JD, Roehrborn CG, Bautista OM et al. The long‐term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N. Engl. J. Med. 2003; 349: 2387–98. [DOI] [PubMed] [Google Scholar]
- 5. Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long‐term outcome of medical therapy for BPH. Eur. Urol. 2007; 51: 1522–33. [DOI] [PubMed] [Google Scholar]
- 6. Narayan P, Tunuguntla HS. Long‐term efficacy and safety of tamsulosin for benign prostatic hyperplasia. Rev. Urol. 2005; 7(Suppl 4): S42–8. [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshimura K, Terai A, Arai Y. Two‐week administration of low‐dose Hachimi‐jio‐gan (Ba‐Wei Di‐Huang‐Wan) for patients with benign prostatic hyperplasia. Hinyokika Kiyo 2003; 49: 509–14. [PubMed] [Google Scholar]
- 8. Yagi H, Nishio K, Sato R et al. Effect of Hachimijiogan for male lower urinary tract symptoms. Kampo Med. 2015; 66: 49–53. [Google Scholar]
- 9. Furuya S, Takahashi K. Clinical investigation of the efficacy of Ryutan‐shakan‐to in the treatment of postoperative pain and discomfort following transurethral resection of the prostate. Kampo Med. 2003; 54: 183–9. [Google Scholar]
- 10. Jin SE, Ha H, Shin HK. Effects of herbal formulas Bojungikgi‐tang and Palmijihwang‐hwan on inflammation in RAW 264.7 cells and the activities of drug‐metabolizing enzymes in human hepatic microsomes. J. Med. Food 2018; 21: 1173–87. [DOI] [PubMed] [Google Scholar]
- 11. Kim JH, Shin HK, Seo CS. Development of a quantitative analysis method for the 12 marker compounds in Palmijihwang‐hwan, a herbal formula, using a reversed‐phase C18 column and an amino column by HPLC. Anal. Methods 2014; 6: 3763–71. [Google Scholar]
- 12. Seo CS, Shin HK. Simultaneous quantification of eight marker compounds in Yongdamsagan‐tang using a high‐performance liquid chromatography equipped with photodiode array detector. J. Chromatogr. Sci. 2017; 55: 926–33. [DOI] [PubMed] [Google Scholar]
- 13. Milonas D, Trumbeckas D. Prostate‐specific antigen and transition zone index ‐ powerful predictors for acute urinary retention in men with benign prostatic hyperplasia. Medicina. 2003; 39: 1071–7. English, Lithuanian. [PubMed] [Google Scholar]
- 14. Moriyama MT, Ikeda R, Suzuki K. Ryutan‐shakan‐to for the treatment of urination difficulty. J. Tradit. Med. 2003; 20: 230–4. [Google Scholar]
- 15. Song Y, Chen G, Huang P, Hu C, Liu X. Effects of tamsulosin combined with solifenacin on lower urinary tract symptoms: evidence from a systematic review, meta‐analysis, and trial sequential analysis of randomized controlled trials. Front. Pharmacol. 2020; 11: 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5alpha‐reductase inhibitors on prostate volume and symptoms in men with BPH: a prospective study. Urology 2009; 73: 802–6. [DOI] [PubMed] [Google Scholar]
- 17. Imamura T, Ishizuka O, Nishizawa O. Cold stress induces lower urinary tract symptoms. Int. J. Urol. 2013; 20: 661–9. [DOI] [PubMed] [Google Scholar]
- 18. Lee WC, Tain YL, Chuang YC, Tsai CN, Yu CC, Su CH. Ba‐Wei‐Die‐Huang‐Wan (Hachimi‐jio‐gan) can ameliorate ketamine‐induced cystitis by modulating neuroreceptors, inflammatory mediators, and fibrogenesis in a rat model. Neurourol. Urodyn. 2019; 38: 2159–69. [DOI] [PubMed] [Google Scholar]
- 19. Park E, Lee MY, Seo CS, Jeon WY, Shin HK. Yongdamsagan‐tang, a traditional herbal formula, inhibits cell growth through the suppression of proliferation and inflammation in benign prostatic hyperplasia epithelial‐1 cells. J. Ethnopharmacol. 2017; 14: 230–5. [DOI] [PubMed] [Google Scholar]
- 20. Park E, Lee MY, Jeon WY, Lee N, Seo CS, Shin HK. Inhibitory effect of Yongdamsagan‐Tang water extract, a traditional herbal formula, on testosterone‐induced benign prostatic hyperplasia in rats. Evid. Based Complement. Alternat. Med. 2016; 2016: 1428923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Murayama C, Michihara S, Saito TR, Norimoto H. Effects of Hachimijiogan, a Kampo powder, on epididymidis sperm characteristics in healthy male rats. Reprod. Med. Biol. 2014; 14: 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kakizaki H, Lee KS, Yamamoto O et al. Mirabegron add‐on therapy to tamsulosin for the treatment of overactive bladder in men with lower urinary tract symptoms: a randomized, placebo‐controlled study (MATCH). Eur. Urol. Focus 2020; 6: 729–37. [DOI] [PubMed] [Google Scholar]
- 23. Hong SJ, Ko WJ, Kim SI, Chung BH. Identification of baseline clinical factors which predict medical treatment failure of benign prostatic hyperplasia: an observational cohort study. Eur. Urol. 2003; 44: 94–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Timeline of clinical visits and assessments.
Table S2. Sub‐analysis with the prostate volume over 30 gm of prostate volume and uroflowmetric parameters.
Table S3. NIH‐CPSI score changes in the sub‐analysis group with the prostate volume over 30 gm.
Table S4. IPSS changes in the sub‐analysis group with the prostate volume over 30 gm.


