Conflicts of interest
MB and VLN are employees of Walgreens Boots Alliance; REBW is in receipt of consultancy fees from Allergan Ltd.; CEMG is a Co‐Founder of CGSkincare Ltd.
Funding sources
This work was funded by Walgreens Boots Alliance.
Dear Editor,
Skin ageing is accelerated during menopause; however, many women and healthcare professionals are insufficiently informed on the impact of menopause on skin. 1 Declining oestrogen detrimentally impacts the skin's extracellular matrix (ECM), which provides strength, elasticity and resilience. 2 The relationship between dermal structural changes and altered skin function has been demonstrated by measuring the biomechanical properties of young, aged and photoaged skin in parallel with histological analyses. 3 Oestrogen levels also influence skin hydration, vascularity and pigmentation 4 and women report a greater incidence of skin dryness and sensitivity in relation to the menstrual cycle and menopause. 5 , 6 However, the specific biological mechanisms underlying these observations are poorly understood, as are the respective contributions of epidermal and dermal changes to skin biomechanics. Therefore, we explored how menopause and hormone replacement therapy (HRT) affect markers of epidermal and dermal ageing in relation to skin structure and biomechanical function, with a view to identifying targets for improvement of skin health at mid‐life. Pre‐menopausal women (Pre; n = 7), post‐menopausal women, with (HRT; n = 10) and without HRT (Post; n = 11), and a group of age‐matched men (n = 29) were recruited to the study. Menopausal status was self‐reported and confirmed by serum oestrogen and follicle stimulating hormone levels. Skin structure was assessed via histological analyses of dermal ECM components (fibrillin, elastin, fibulin and collagen) in our female cohorts, which found few differences between groups, although total elastic fibre abundance was increased in the HRT vs. Post group (P < 0.01; /Fig. 1a). Epidermal thickness was reduced and stratum corneum (SC) thickness increased (Fig. 1b) in the Post vs. Pre group (P < 0.05), while SC thickness was partially normalised in the HRT group (P < 0.05 vs. Post group), supporting regulation of epidermal homeostasis and desquamation by female sex hormones. Cutometry was performed to assess skin biomechanical properties; in the Post group, total skin deformation (R0) was significantly reduced (Fig. 2a) when compared to the Pre group (P < 0.05), indicating a general reduction in elasticity. Increased viscoelastic/elastic ratio (R6) was found in the Post group vs. the Pre group (1.2‐fold, P < 0.01; Fig. 1a) indicating extended stretch or creep following initial elastic deformation, and this again, was partially abrogated by HRT (~10% lower; P < 0.05 vs. Post group). No differences in other elastic parameters were found. Higher R6 correlated with lower serum oestrogen (Fig. 2c) and higher age (Fig. 2b) in women, but no correlation with age was found in men (Fig. 2b), suggesting skin viscoelasticity is specifically influenced by female hormonal ageing. In support of our findings, skin extensibility in peri‐menopausal women has been found previously to increase whilst elasticity decreased, with some prevention by hormone replacement therapies. 7 , 8 No group differences in epidermal or dermal micromechanical stiffness were observed (not shown) when assessed by atomic force microscopy. However, a lower ratio of epidermis/SC thickness (Fig. 1d) was associated with higher R6 (Fig. 1e), suggesting skin viscoelastic changes at menopause may be influenced by alterations to epidermal morphology. Histological expression of epidermal CD44 (a receptor for hyaluronic acid) was also assessed due to its pleiotropic role in epidermal homeostasis and hydration. CD44 expression was reduced in the Post vs. the Pre group (P < 0.05; Fig. 1c), while no change in skin hyaluronic acid expression was found (not shown). As CD44 activation regulates keratinocyte differentiation and lipid biosynthesis, 9 epidermal lipids were quantified using mass spectrometry (UPLC‐MS/MS). Ceramide abundance was found to be lower in Post vs. the Pre group (P < 0.01) and cholesterol was increased vs. Pre and HRT (both P < 0.01; Fig. 1f). This indicates that epidermal lipid synthesis is altered post‐menopause and is susceptible to regulation by HRT. In summary, increased viscoelastic skin distension post‐menopause is reversible by HRT, and may result from altered epidermal homeostasis and dermal morphology. Smaller R6 values are associated with improved skin condition, which is evident where decreased R6 is measured following the application of anti‐ageing skin care routines. 10 Skewed CD44 expression and ceramide synthesis may contribute to declining epidermal barrier function and could represent key targets, beyond ECM rejuvenation, for improving skin function and appearance in mid‐life.
Figure 1.
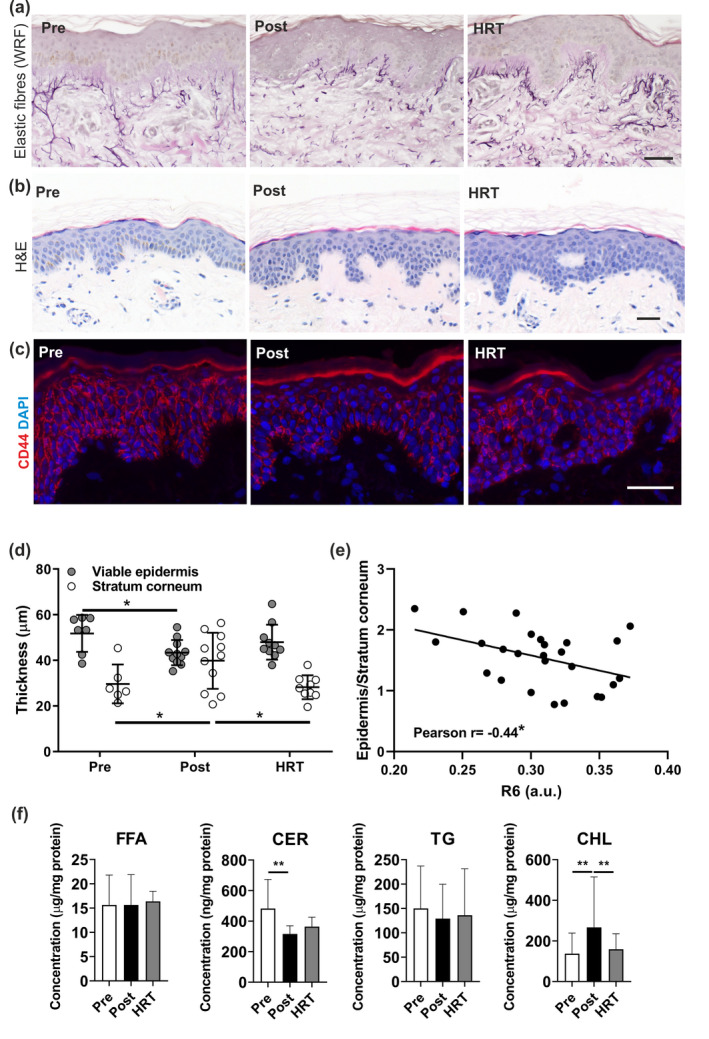
The dermal elastic fibre network was not significantly altered in post‐menopausal women but epidermal morphology, CD44 expression and lipid biosynthesis was impacted by menopause and HRT. (a) The dermal elastic fibre network was assessed in biopsies from photoprotected skin of pre‐menopausal (Pre; n = 7), post‐menopausal (Post; n = 11) and post‐menopausal women taking hormone replacement therapy (HRT; n = 10) groups using immunohistochemistry and immunofluorescence. Total elastic fibres were visualised using Weigert's resorcin fuchsin (WRF). (b) Epidermal morphology was assessed in H&E stained skin sections from photoprotected buttock skin of Pre (n = 6), Post (n = 11) and HRT (n = 10) groups and (d) thickness measurements of viable epidermis (nucleated cells) and stratum corneum were taken separately, and (e) the ratio calculated for each individual before correlation with the R6 cutometry parameter. (c) CD44 expression was assessed by immunofluorescence and (f) epidermal lipids quantified using UPLC‐MS/MS. Data presented as mean ± SD. Statistical analysis by one‐way ANOVA with Holm‐Sidak's multiple comparisons and Pearson's correlation. *P < 0.05, **P < 0.01. Scale bar 50 µm.
Figure 2.
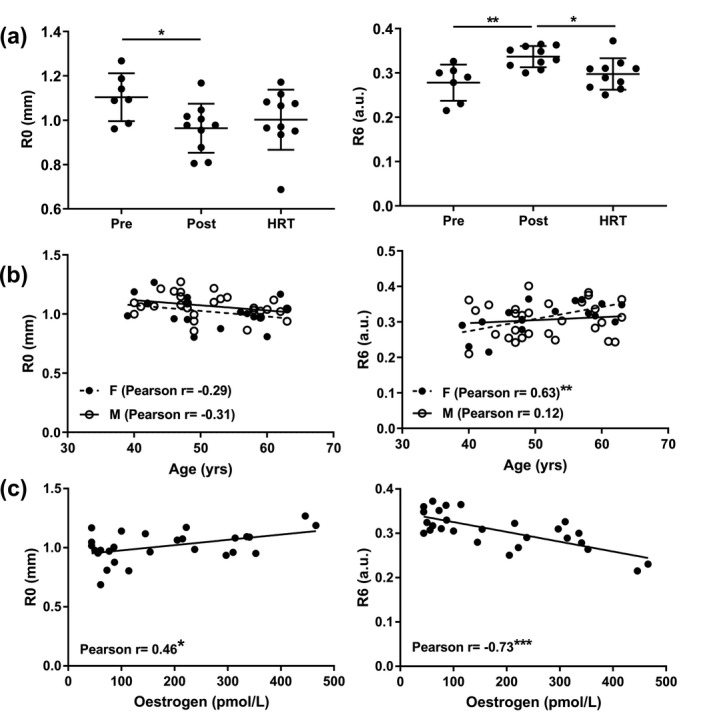
Increased skin viscoelasticity post‐menopause is associated with increasing age and decreasing oestrogen levels. Using a Cutometer and 4‐mm diameter probe the biomechanical properties of photoprotected buttock skin were measured and (a) mean time‐strain curves generated for each group (Pre, n = 7; Post, n = 10; HRT, n = 10) prior to calculation of the different elastic measures (R parameters). From the curves: (a) R0 (total deformation) and R6 (viscoelasticity) were calculated and; (b) associations between age and cutometry parameters, R0 and R6, were assessed in females (F; n = 17: Pre n = 7 and Post n = 10) and in a group of age‐matched males (M; n = 29). (c) Associations between serum oestradiol levels and R0 and R6 were also assessed in females (n = 27: Pre n = 7, Post n = 10, and HRT n = 10). Data presented as mean ± SD. Statistical analysis by Kruskal–Wallis test with Dunn's multiple comparisons (R0), one‐way ANOVA with Holm‐Sidak's multiple comparisons (R6) and Pearson's correlation, *P < 0.05, **P < 0.01, ***P < 0.001.
Acknowledgements
We thank Walgreens Boots Alliance for funding this work, Nigel Hodson for help with the microscopy and Neil O'Hara and NonLinear Dynamics/Waters for technical support. The Bioimaging Facility microscopes used were purchased with grants from BBSRC, Wellcome and the University of Manchester Strategic Fund. We acknowledge the NIHR Manchester Biomedical Research Council and Manchester Institute for Collaborative Research on Ageing for supporting CEMG and REBW.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. LePillouer‐Prost A, Kerob D, Nielsen M et al. Skin and menopause: women's point of view. J Eur Acad Dermatol Venereol 2020; 34: e267–e269. [DOI] [PubMed] [Google Scholar]
- 2. European Commission, Eurostat . In, Vol. Life Expectancy across European Regions. URL https://ec.europa.eu/eurostat/web/products‐eurostat‐news/‐/edn‐20200930‐1 (last accessed: 22 February 2021).
- 3. Asavasupreechar T, Saito R, Miki Y et al. Systemic distribution of progesterone receptor subtypes in human tissues. J Steroid Biochem Mol Biol 2020; 199: 105599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt JB, Lindmaier A, Spona J. Hormone receptors in pubic skin of premenopausal and postmenopausal females. Gynecol Obstet Invest 1990; 30: 97–100. [DOI] [PubMed] [Google Scholar]
- 5. Pelletier G, Ren L. Localization of sex steroid receptors in human skin. Histol Histopathol 2004; 19: 629–636. [DOI] [PubMed] [Google Scholar]
- 6. Huang AH, Chien AL. Photoaging: a review of current literature. Curr Dermatol Rep 2020; 9: 22–29. [Google Scholar]
- 7. Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas 2011; 69: 249–256. [DOI] [PubMed] [Google Scholar]
- 8. Thornton MJ. Human skin: a mirror for estrogen action? Menopause (New York, N.Y.) 2016; 23: 119–120. [DOI] [PubMed] [Google Scholar]
- 9. Bourguignon LY, Ramez M, Gilad E et al. Hyaluronan‐CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J Invest Dermatol 2006; 126: 1356–1365. [DOI] [PubMed] [Google Scholar]
- 10. Tran D, Townley JP, Barnes TM et al. An antiaging skin care system containing alpha hydroxy acids and vitamins improves the biomechanical parameters of facial skin. Clin Cosmet Investig Dermatol 2014; 8: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


