Abstract
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that mostly affects the genital and anal skin areas. Symptoms may vary from pruritis and pain to sexual dysfunction; however, LS can also be asymptomatic. LS occurs at all ages and in both sexes. Approximately 5% of all women affected by vulvar LS will develop vulvar squamous cell carcinoma. Topical treatment is safe but less effective resulting in chronic course in most patients, who suffer from persistent itching and pain. In severe cases of therapy‐resistant LS, there is no adequate treatment. Fat grafting is a novel regenerative therapy to reduce dermal fibrosis. The therapeutic effect of adipose tissue grafts for LS is already investigated in various pioneering studies. This review provides an overview of these studies and the putative mechanisms‐of‐action of fat grafting to treat LS.
Keywords: ageing, dermal, fibrosis, lichen sclerosus, lipoaspirate, regeneration, stem cells, stromal vascular fraction, vulvar
1. INTRODUCTION
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that mostly affects the genital and anal skin areas. LS can be asymptomatic or accompanied by symptoms, for example progressive pruritis, pain, sexual dysfunction and thus, a reduced quality of life (QoL). 1 , 2 LS occurs at all ages and in both sexes, whereby the male‐to‐female ratio ranges between 1:3 and 1:10. The exact prevalence is unclear; however, vulvar LS has been found to affect one in 70 women presenting to a general gynaecology practice. 1 The incidence of histologically proven LS in women was 14.6 per 100.000 woman‐years in 2011 in the Netherlands. Noticeably, approximately 5% of all women affected by LS will develop vulvar squamous cell carcinoma, for which both LS and differentiated vulvar intraepithelial neoplasia (dVIN) are additional risk factors. 3 , 4 Although the aetiology of LS is still unknown, it is hypothesized to be an autoimmune disorder with a genetic component. 3
Currently, treatment of LS is mainly initiated to relieve symptoms of pruritis and pain, to reduce the progression of the skin alterations and eventually decrease the risk of cancer. Conventional treatment of LS consists of local application of corticosteroids, for example clobetasol propionate. 5 In severe cases of therapy‐resistant LS, immunosuppressive agents (e.g. calcineurin inhibitors) can be administered, which inhibit T‐cell activation. 6 , 7 , 8 However, clinical evidence for the efficacy of immunosuppressives as a LS therapeutic is sparse and topical treatment cannot prevent fibrogenesis of the upper dermis. Eventually, progressive fibrosis of the upper dermis culminates in persistent sexual dysfunction and a reduced QoL. 9 Therefore, innovative therapies are considered that aim to control dermal fibrosis due to LS, for example phototherapy, anti‐inflammatory, anti‐biotic and anti‐microbial agents. In some cases, a (temporary) remission of symptoms may be achieved, but long‐term relieve of symptoms is not often accomplished. 10 , 11 , 12 , 13 , 14 , 15 Surgery, for example vulvectomy (with or without a skin graft or gluteal fold flap reconstruction), cryosurgery and laser ablation, is considered to treat severe complications, such as severe vaginal introitus stenosis, urinary retention and synechiae vulvae. 16 Nonetheless, a surgical approach unavoidably creates scars and is characterized by a high recurrence rate of the original pathology.
In the last fifteen years, fat grafting, that is the transplantation of adipose tissue or lipoaspirate, has been developed as a novel autologous treatment modality to reduce skin fibrosis as a result of fibroproliferative diseases or post‐traumatic scarring. 17 Adipose tissue harbours mesenchymal stromal cells, better known as adipose tissue‐derived stromal cells (ASCs), that reside in the stromal vascular fraction (SVF) as progenitor cell types and can differentiate into, for example pericytes and supra‐adventitial cells. 18 , 19 ASCs are able to produce and secrete a variety of growth factors, cytokines and proteins that may act as an immunomodulatory, pro‐angiogenic or anti‐fibrotic agent. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 These paracrine effects may be beneficial in decreasing symptoms and delaying disease progression of LS.
Till date, several pioneering studies and case reports have been published that describe the use of fat grafting as a treatment of LS. 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 This review provides an overview of these studies that have investigated fat grafting as a treatment of LS and summarizes the putative mechanisms‐of‐action of fat grafting to treat LS.
2. MECHANISMS‐OF‐ACTION OF FAT GRAFTING
Autologous fat grafting has been clinically used for many decades. Originally, fat grafting was mainly used to restore volume losses due to congenital or traumatic defects. 41 Later, fat grafting became popular as a regenerative treatment modality to restore tissue damage caused by scarring due to burn wounds or trauma. 42 , 43 The regenerative capacity of the grafted fat can be attributed to the ASCs that reside in the perivascular niche of different tissues. 44 , 45 In 2001, Zuk et al. showed that the adipose tissue contains ASCs with a multilineage differentiation capacity and an extended population doubling capacity with a low occurrence of senescence. 46 Upon cell culture, these ASCs secrete a plethora of paracrine factors, for example growth factors, cytokines, proteins and exosomes that may function either in an (1) immunomodulatory, (2) pro‐angiogenic, (3) anti‐fibrotic or (4) in a combination of the former, fashion. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 ASCs reside in the SVF. SVF harbours all non‐adipocyte cell types that can be divided into blood‐derived and adipose tissue‐derived cells. The blood‐derived cell population of SVF contains granulocytes, macrophages, lymphocytes and hematopoietic stem cells, whereas the adipose tissue‐derived cell population contains supra‐adventitial cells, pericytes, ASCs, fibroblasts and endothelial cells. 47 SVF can be isolated from adipose tissue either by mechanical isolation or by enzymatic isolation. Mechanical isolation utilizes shear stress to disrupt most of the adipocytes while maintaining all trophic factors or bioactive molecules present in the tissue. 19 , 48 Hence, mechanical isolated SVF is named tissue‐SVF (tSVF). In contrast, enzymatic isolation of SVF utilizes the enzymatic digestion of adipose tissue. Enzymatic digestion of SVF yields a cellular‐SVF (cSVF) that is depleted of such trophic factors as during the enzymatic isolation process both intercellular connections and extracellular matrix (ECM) are lost. As ECM is known to bind the majority of trophic factors, 48 , 49 , 50 it is conceivable that the therapeutic capacity of cSVF is lower than that of tSVF. Yet this postulation remains to be investigated by comparative studies and conclusive data are not available.
2.1. Immunomodulatory actions of fat
One of the key players in inflammation and fibrogenesis is the macrophage. During normal wound healing, macrophages undergo a transition from classical M1 macrophages (pro‐inflammatory phenotype) to M2 macrophages (proliferative phenotype). This phenotypic transition is important because the M1 macrophages play a crucial role in phagocytosis and protection against pathogens, whereas the M2 macrophages are important in the production of collagens and thus tissue repair. Various bioactive molecules and epigenetic factors are involved in the activation of macrophages. The Jak/STAT pathway plays a key role in macrophage transition whereby STAT1 activation promotes transition to the M1 phenotype and STAT6 activation promotes the M2 phenotype. 51 , 52 The M1 phenotype is activated by pro‐inflammatory cytokines such as tumour necrosis factor‐α (TNF‐α), interferon‐γ (IFN‐γ) and interleukin (IL‐6). 51 In contrast, the M2 phenotype is maintained by immunomodulatory cytokines such as IL‐4, IL‐10 and IL‐13. 51 , 53 In chronic wounds or fibroproliferative diseases, the M1‐to‐M2 transition of macrophages is delayed or insufficient, which may cause a prolonged stage of inflammation. Until now, there is no conclusive evidence that LS associates with alterations in M1‐to‐M2 transition; however, in LS tissue, increased levels of pro‐inflammatory cytokines, including IFN‐y, TNF‐α and IL‐1α and IL‐8, are observed. Such data suggests the presence of an inflammatory state contrary to a regulatory or immunomodulatory state in LS tissue. 54 , 55 , 56 , 57 An in vitro study of Sun et al. 52 indicates that ASCs promote M1‐to‐M2 transition of macrophages by downregulating the STAT3 and STAT6 pathway. A study of Zhu et al. showed reduced inflammatory signalling after the application of human SVF subcutaneously in nude mice. On a histological level, this was measured as a decreased expression of the pro‐inflammatory cytokines IL‐6 and TNF‐α and an increased level of the anti‐inflammatory cytokine IL‐10. 58 Collectively, these studies show that the application of ASCs in vivo may lead to a decreased expression of pro‐inflammatory cytokines, suggesting that ASC have an immunomodulatory capacity what might result in a faster M1‐to‐M2 transition of macrophages culminating in enhanced tissue repair.
2.2. Pro‐angiogenic actions of fat
Lichen sclerosus affected tissues show a lower microvessel density compared to normal vulvar tissues, 59 suggestive of tissue hypoperfusion. Insufficient microvascularization culminates in hypoxia of the surrounding tissues. 60 During a state of hypoxia, stabilization of the transcription factor hypoxia‐induced factor‐1 alpha (HIF‐1α) results in the expression and secretion of pro‐angiogenic growth factors, for example vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF), which eventually results in the genesis of microvessels. 61 Stabilization of the microvascularization is promoted by CD34‐positive dermal dendritic cells (DDCs) or pericytes, among others. In systemic sclerosis (SSc) and hypertrophic wound healing, an elevated expression of both VEGF and HIF‐1 is observed compared with healthy tissue. 61 Controversially, consistent HIF‐1 accumulation alone does not result in the formation of sufficient microvasculature. The overexpression of HIF‐1 correlates to an increase in the expression of pro‐fibrotic factors (e.g. TGF‐β1, thrombospondin‐1) by fibroblasts, resulting in excessive production of collagenous matrix. 61 , 62 , 63 Interestingly, vulvar LS tissue shows decreased levels of VEGF 64 and a downregulation of DDCs, 65 which might contribute to a low microvessel density in LS. ASCs are shown to release angiogenic growth factors, for example VEGF, HGF and bFGF, 58 , 66 and they are able to differentiate into pericytes that can stabilize the adjacent microvasculature. 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 Different studies found that increased expression of HIF‐1α promotes ASC proliferation and binding and resulted in increased angiogenesis. 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 Therefore, we postulate that the application of ASCs may result in increased microvessel density and stabilization of the microvasculature in LS, albeit definite proof is lacking.
2.3. Anti‐fibrotic actions of fat
In LS, the expression of pro‐fibrotic growth factors, for example transforming growth factor‐β (TGF‐β) and connective tissue growth factor (CTGF), is increased, culminating in the excessive accumulation and deposition of extracellular matrix proteins (ECM) such as collagens and especially collagen type 1 (Col‐1). 60 , 76 , 77 , 78 In normal wound healing, TGF‐β, CTGF and serum response factor (SRF) are secreted by platelets and macrophages to initiate fibroblast to myofibroblast differentiation and the activation of the ECM remodelling processes. Physiologically, myofibroblast undergo apoptosis and are cleared from the tissue after the remodelling process. However, if myofibroblasts persist in the tissue, myofibroblasts will continue to secrete Col‐1 and excessive scar formation will occur, which is observed in hypertrophic scars. 29 , 79 In vitro studies have shown that the ASCsecretome—a collection term for all factors secreted by ASCsinhibit fibroblast to myofibroblast differentiation in Dupuytren nodules and keloid scar‐derived fibroblasts. 20 , 21 , 80 An in vitro study of Li et al. demonstrated the inhibiting effect of ASCs on the development of hypertrophic scar tissue in both mice and men. Firstly, mice fibroblasts treated with ASC‐conditioned medium (ASC‐CM) showed decreased Col‐1, Col‐3 and alpha‐smooth muscle actin (α‐SMA) levels compared with the control group (no treatment). Secondly, fibroblasts obtained from human hypertrophic scar tissues showed less collagen deposition and faster wound healing after being treated with ASC‐CM. The inhibiting effect on hypertrophic scar formation of ASCs seems to relate to the inhibition of the inflammatory p38/MAPK signalling pathway. 81 A more recent study of Li et al. demonstrated a decrease in the deposition of collagen, downregulated fibroblast to myofibroblast differentiation and a reduction of hypertrophic scar formation by in vitro and in vivo experiments. It was shown that ASCs release epigenetic factors (such as microRNAs) that downregulate IL‐17, a pro‐fibrotic protein that is known to be overexpressed in hypertrophic scar tissues. 82 Similar effects of ASCs, including the diminishment of dermal fibrogenesis and enhanced wound healing in different disease models, for example SSc and Peyronie's disease, were demonstrated by others. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97
In conclusion, ASCs can secrete a plethora of growth factors and cytokines, which may have immunomodulatory, pro‐angiogenic and anti‐fibrotic effects on the surrounding tissues (Figure 1). Since the pathology of LS comprises a state of chronic inflammation and persistent fibrogenesis of vulvar tissues, the application of ASCs to LS affected tissues could be a symptom‐controlling and disease‐delaying therapy for LS.
FIGURE 1.
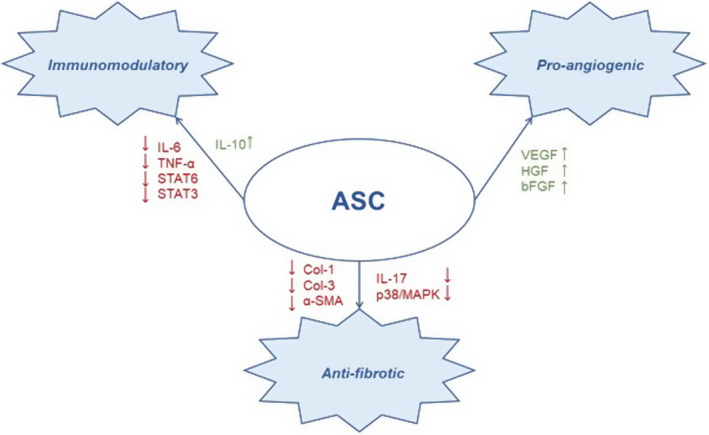
Mechanisms‐of‐action of an ASC; including immunomodulatory, pro‐angiogenic and anti‐fibrotic effects and the corresponding growth factors and cytokines.
3. FAT GRAFTING THERAPIES FOR LICHEN SCLEROSUS
Clinically, LS usually presents as white, polygonal papules that coalesce into shiny porcelain‐white plaques in the perivaginal and perianal area. LS can lead to obliteration (fusion) of the labia minora and stenosis of the introitus. Even though most clinicians diagnose LS based on clinical aspects, the gold standard to diagnose LS is punch biopsy followed by histological examination. 98 , 99 , 100 , 101 , 102 , 103 , 104 Histologically, a combination of (A) ectatic (dilated) or sclerotic blood vessels, (B) infiltration of lymphocytes, epidermal atrophy and (C) basement membrane thickening, is found in LS tissue. This could be explained by (A) poor angiogenesis and lack of stabilization of the existing microvasculature, (B) chronic inflammation and (C) increased fibrogenesis. Progression of LS will lead to replacement of the upper dermis by dense fibrotic tissue with insufficient microvasculature. 55 , 76 , 105 , 106 Since adipose tissue has shown to harbour a regenerative potential, that is immunomodulatory, pro‐angiogenic and anti‐fibrotic actions, several clinical studies examined whether fat grafting may ameliorate the pathogenesis of LS, using either (1) fat grafting, (2) a combination of fat grafting with platelet‐rich plasma (PRP), (3) a combination of tSVF with PRP and (4) a combination of ASCs with hyaluronic acid (HA) (Tables 1 and 2).
TABLE 1.
Study Characteristics, Patient Demographics, Intervention Types and Outcomes
| Reference | Study type | Study population | Age (years) | Intervention | Follow‐up | Results | Complications |
|---|---|---|---|---|---|---|---|
| Casabona et al., 2010 32 | Prospective, non‐controlled, non‐blinded, non‐randomized | N = 15, female, histologically proven LS | Range 27–62 |
10–15 cc lipoaspirate Co‐intervention: 5 cc PRP pre‐treated with 0.5 cc of calcium chloride for platelet degranulation Intramucosal, subdermal and submucosal Patients with severe fibrosis underwent one or two extra procedures, after 3 months respectively |
Questionnaires (non‐validated), photographic and physical evaluation up to 24 months postoperative. No specific index parameters were mentioned |
After 15 days symptoms started to improve. Itching and burning disappeared within one month. Four months after surgery, all patients reported total disappearance of pain, regained sexual activity and normal appearance of anatomical features of the vulva. No statistical analysis |
No adverse events were observed. All patients had moderate pain in the treated areas for 10 days after surgery |
| Boero et al., 2015 33 | Prospective, non‐controlled, non‐blinded, non‐randomized | N = 36, female, histologically proven LS | Range 25–80 |
8–15 cc lipoaspirate All perivulvar layers up to the fascia |
Vulvoscopic examination and DLQI and FSFI questionnaires up to 24 months postoperative. Histological evaluation through punch biopsies preoperative and 8 months postoperative |
94% showed improved vulvar trophism of the skin and mucosa. QoL was significantly improved for both DLQI (p < 0.0001) and FSFI (p < 0.0001). On a histological level, reduction of hyperkeratosis (67%), reduction of chronic inflammation (89%), reduction of fibrosis (67%), increased angiogenesis (44%) and reduction of dermal oedema (33%) |
No adverse events were observed. 30% of patients had pain for 10 days after surgery |
| Tamburino et al., 2016 34 | Case report | N = 1, female, histologically proven LS | 48 |
40 cc lipoaspirate Co‐intervention: 20 cc Nanofat Subcutaneous, intradermal |
Questionnaires (not validated) up to 8 months postoperative. No specific index parameters were mentioned |
Decreased symptoms and anatomical features of LS with the greatest improvement in the first two months postoperative and maintained up till 8 months. No specifications were mentioned |
Not described |
| Onesti et al., 2016 35 | Prospective, non‐controlled, non‐blinded, non‐randomized | N = 8, female, 1 with symptoms during menopause, 2 with vulvar scarring due to chronic GVHD and 5 affected by LS | Range 38–75 |
2 cc ASC‐HA solution Subcutaneous of labia minora |
Histological, FSFI questionnaires and physical evaluation up to 24 months postoperative |
All patients mentioned pain reduction and improved sexual function. Histological examination in LS patients showed significantly reduction of dermis sclerosis, less dilated capillaries and reduced inflammatory infiltrate. No statistical analysis |
Not described |
| Kim et al., 2017 36 | Case report | N = 1, female, affected by vaginal atrophy (no histologically proven LS) | 67 |
36 cc lipoaspirate Co‐intervention: 4 cc PRP Subcutaneous |
Physical evaluation up to 12 months postoperative. No specific index parameters were mentioned |
Relieve of vaginal pruritis and irritation, restoration of labia majora contour. White patchy lesions improved | No adverse events were observed |
| Newman et al., 2018 37 | Prospective, non‐controlled, non‐blinded, non‐randomized | N = 111, female, histologically proven LS | Range 20–76 |
8–15 cc tSVF Co‐intervention: 2–3 cc PRP with Lidocaine with Epinephrine 1:100 000. Subcutaneous, intradermal |
Questionnaires (not validated) up to 3 months postoperative. No specific index parameters were mentioned |
All patients experienced improvement of symptoms, with a significant decrease per symptom of 38%–68% (p < 0.001) | No serious complications were described. Side effect, such as swelling, bruising, burning and discomfort were noted |
| Stark et al., 2020 38 | Prospective, non‐controlled, non‐blinded, non‐randomized | N = 10, females affected by vaginal atrophy, vulvovaginal dystrophy, and/or stress urinary incontinence | Range 30–60 |
8.5–32 cc (average of 24 cc) Nanofat Subcutaneous and subepithelial |
Physical evaluation up to 12 months and questionnaires (not validated) up to 24 months. No specific index parameters were mentioned |
All patients reported an improvement of symptoms after 6–16 months, differing from sexual activity, improved vaginal appearance and itching. No statistical analysis |
No adverse events were observed |
| Almadori et al., 2020 39 | Prospective, non‐controlled, non‐blinded, non‐randomized | N = 33, female, histologically proven LS | Range 38–63 |
10 cc lipoaspirate In labia majora, labia minora, clitoral area, posterior fourchette, perianal area |
FSFI, FSDS, VASs, PASS‐20, HADS, RAS and WMQ‐R questionnaires up to a mean follow up of 12.9 months postoperative | Improvement of sexual function (p < 0.001), as well as the distress associated with sexuality (p < 0.0001), romantic relationship (p < 0.05), anxiety (p < 0.0001) and depression (p < 0.0001). Decrease of symptoms such as itching (p < 0.001), burning (p < 0.05), soreness (p < 0.001), pain (p < 0.0001) | Not described |
| Tedesco et al., 2020 40 | Prospective, controlled, non‐blinded, non‐randomized | N = 40, female and male, histologically proven LS | Range 43–78 |
15 cc tSVF Co‐intervention for 20 patients: 4 cc PRP Two procedures per patient distanced over 4‐months. Intradermally |
Physical examination during every visit and DLQI questionnaires up to 6 months follow up | Significant improvement of QoL was observed in both groups after one month. After 6 months the SVF‐group showed a significant improvement of QoL, the SVF‐PRP combination group little improvement compared to pre‐operative QoL | No severe or mild side effects were observed during and after the procedure |
Abbreviations: (t)SVF, (tissue or mechanically isolated) stromal vascular fraction; ASCs, adipose‐derived stromal cells; DLQI, dermatology life quality index; FSDS, female sexual distress scale; FSFI, female sexual function index; GVHD, graft versus host disease; HA, hyaluronic acid; HADS, Hospital Anxiety and Depression Scale; LS, lichen sclerosus; PASS‐20, Pain Anxiety Symptom Scale Short Form 20; PRP, platelet rich plasma; QoL, quality of life; RASS, Richmond Agitation‐Sedation Scale; VASs, Visual Analogue Scale; WMQ‐R, Wound Management Questionnaire.
TABLE 2.
Intervention Types, Advantages and Disadvantages
| Method | Advantages | Disadvantages |
|---|---|---|
| Monotherapy using fat or nanofat |
|
|
| Combination therapy of fat with PRP |
|
|
| Combination therapy of SVF with PRP |
|
|
| Combination therapy of ASCs with HA |
|
|
Abbreviations: ASCs, adipose‐derived stromal cells; HA, hyaluronic acid; LS, lichen sclerosus; PRP, platelet rich plasma; SVF, stromal vascular fraction.
3.1. Monotherapy using fat or nanofat
Thus far, four studies have investigated the effect of fat grafting or nanofat (an emulsified mixture of fat grafting and infiltration fluid) for therapy‐resistant LS in a total of 60 female patients. 33 , 34 , 38 , 39 The largest study used fat grafting to treat 36 patients suffering from LS. In this study, 94% of patients reported a clinical improvement, which was associated with histopathological improvements. Clinically, 75% of the patients reported improved caliber and elasticity of the vaginal introitus, 50% reported reduced clitoris burying degree, 83% reported increased volumes of labia majora and minora, 94% had a complete disappearance of scratching lesions and 78% of the patients showed complete remission of white lesions on clinical and vulvoscopic evaluation. Improvement in QoL was significant for the Dermatology Life Quality Index (DLQI) and the Female Sexual Function Index (FSFI) after 24 months follow‐up. 33 Two studies in 2020 showed comparable results with the aforementioned study. 38 , 39 Almadori et al. used fat grafting to treat 33 patients suffering from LS with a mean follow‐up of 12.9 months. Symptoms such as itching, burning, soreness and pain were significantly decreased, and sexual function was significantly improved after fat grafting in all patients. Moreover, patients showed significant improvement in intimate contact, anxiety and depression. However, no histological analysis was performed. Moreover, the potential placebo effect cannot be excluded because it was a single‐arm study without a control group. 39 Stark et al. used nanofat to treat 10 patients suffering from vulvar dystrophy caused by LS with a mean follow‐up of 6 to 16 months. All patients reported a decrease of their symptoms without any sequelae. Unfortunately, assessment strategies and statistical analysis are not mentioned in this study, and no patients with histologically proven LS were included. 38 In a case report of Tamburino, one patient suffering from LS was treated with nanofat and showed improvement in texture and elasticity of vulvar skin and mucosa one month after application. This was demonstrated by decreased dryness and dyspareunia measured with a non‐validated questionnaire. 34
3.2. Combination therapy of fat with platelet‐rich plasma (PRP)
Thus far, two studies used a combination therapy of fat grafting with application of PRP to treat a total of 16 female patients suffering from LS. 32 , 36 PRP contains platelets that release growth factors to reduce inflammation and to promote mesenchymal cell proliferation, tissue repair and angiogenesis. 107 , 108 Nevertheless, the number of platelets is highly variable due interpatient variation, intra‐day variation and different reabsorption rates 109 , 110 and PRP might result in hyperplasia. 111 In the study of Casabona et al. 15 patients were treated with a combination of fat grafting and PRP. Symptoms of itching and burning disappeared in all patients within one month. The vulvar skin and mucosa became more elastic and softer with a normal colour as assessed by the investigators. After 4 months, all patients reported total disappearance of pain and regained sexual activity; however, no statistical analysis was performed. 32 Adding to this, a case report of a 67‐year‐old female patient with vaginal atrophy showed decreased sensation of vaginal itching and irritation and improved vulvar contours one year after administration of fat grafting with PRP. 36
3.3. Combination therapy of stromal vascular fraction (SVF) with PRP
Thus far, two studies examined the effect of tSVF with PRP on therapy‐resistant LS in a total of 151 female patients. 37 , 40 In contrast to the combination therapy of the previous paragraph, the relative concentration of ASCs should be higher when SVF is used instead of fat. The largest study treated 111 patients suffering from LS with tSVF whereby most patients were concomitantly treated with PRP. However, the exact number of patients treated with a combination treatment of tSVF and PRP was not mentioned. A significant decrease of symptoms, for example discomfort, pain, fissures, tearing and fusing, was observed after 3 months, varying from 38% to 68% per symptom and all patients showed improvement of one or more symptoms. No validated questionnaires were used and a long‐term follow‐up was not reported for this trial. 37 A smaller study investigated the effect of tSVF with or without PRP in 40 female and male patients with histologically proven LS. Significant improvement of QoL was observed in both groups after one month using the validated DLQI questionnaire. After 6 months, the tSVF‐group (mixed female and male patients) still showed a significant improvement of QoL, but the tSVF and PRP group showed only limited improvement compared with the pre‐operative QoL, 40 suggesting that PRP may mitigate the beneficial effects of tSVF. Willemsen et al. demonstrated a concentration‐related effect of PRP to the regenerative capacity of ASCs. This in vitro study showed that PRP promoted endothelial sprouting and survival in a dose‐dependent manner; however, when a higher concentration of PRP was added to the ASC‐CM, the beneficial effect disappeared or the endothelial sprouting even reduced. 112 More research is necessary to investigate the effects of a combination therapy of tSVF or fat with PRP and which concentration is most effective to treat vulvar LS.
3.4. Combination therapy of ASCs with hyaluronic acid (HA)
One study used a combination therapy of ASCs with synthetic HA to treat eight female patients suffering from vulvar discomfort, which were affected by LS (n = 5), or had vulvar scarring do to chronic graft versus host disease (GVHD) (n = 2) or symptoms during their menopause (n = 1). 35 HA is a natural glycosaminoglycan believed to function as both a filler and a scaffold that provides support for cell ingrowth. 113 Furthermore, the transplantation of cells in hydrogels is thought to result in long‐term protection of the cells in vivo. 114 From obtained lipoaspirate, SVF was isolated and subsequently ASCs were cultured and added to HA. Application of ASC‐HA solution into the subcutaneous plane of labia minora resulted in pain reduction and improvement of sexual function in all patients, which was assessed using the validated FSFI questionnaire. This effect was maintained up to two years postoperative. Furthermore, postoperative biopsy specimens indicated that sclerosis of the dermis was reduced, capillaries were less dilated and the inflammatory infiltrate was reduced compared with pre‐operative histological examinations. However, the time period of the obtained postoperative biopsy specimens was not mentioned, and the histological findings were not objectively measured. 35 It would be interesting to get a better understanding of the percentages of reduction of dermal sclerosis and quantity and quality of microvasculature, for example.
4. CONCLUSIONS
Fat grafting is a novel regenerative treatment modality to treat therapy‐resistant LS, wherein the endogenous regenerative capacity of the fat tissue is employed to mitigate the mechanisms underlying the pathophysiology of LS. Various pioneering studies have already applied fat grafts to vulvar LS tissue, and they all report promising results regarding the relieve of clinical symptoms and an improvement of QoL. The proposed mechanisms‐of‐action of the fat grafting to mitigate the pathophysiology of LS include immunomodulatory, pro‐angiogenic and anti‐fibrotic effects; however, in these clinical studies, these proposed mechanisms have hardly been investigated. Therefore, we recommend that future studies should validate these initial findings and should use validated outcome measurements and histological examinations before and after treatment, in order to elucidate the mechanisms‐of‐action, which are currently elusive.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
NS, ECAHS and JD performed the research. ECAHS, GK, BL and MHMO designed the research study. Nobody contributed essential reagents or tools, since this article is a review article. NS, ECAHS, GK and JD analysed the data. NS wrote the paper. All authors have read and approved the final manuscript. Anyone who contributed to the study and/or manuscript preparation, but who does not meet the authorship criteria is listed in the acknowledgments.
ACKNOWLEDGEMENTS
We thank prof. dr. Ate G.J. van der Zee (Onco‐Gynecologist of University Medical Center Groningen, the Netherlands) and dr. Joost Bart (Pathologist with expertise in Gynecopathology of University Medical Center Groningen, the Netherlands) for their expert advice. We thank the plastic surgeons of Medisch Spectrum Twente, the Netherlands, for useful discussions during the writing period of our article.
van der Sluis N, Scheers ECAH, Krenning G, van der Lei B, Oonk MHM, van Dongen JA. Autologous lipoaspirate as a new treatment of vulvar lichen sclerosus: A review on literature. Exp Dermatol. 2022;31:689–699. doi: 10.1111/exd.14561
Nanouk van der Sluis and Esther C.A.H. Scheers are equally shared first author.
DATA AVAILABILITY STATEMENT
The data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Goldstein AT, Marinoff SC, Christopher K, Srodon M. Prevalence of vulvar lichen sclerosus in a general gynecology practice. J Reprod Med. 2005;50(7):477‐480. [PubMed] [Google Scholar]
- 2. Leibovitz A, Kaplun VV, Saposhnicov N, Habot B. Vulvovaginal examinations in elderly nursing home women residents. Arch Gerontol Geriatr. 2000;31(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 3. Krapf JM, Mitchell L, Holton MA, Goldstein AT. Vulvar lichen sclerosus: current perspectives. Int. J Womens Health. 2020;12:11‐20. Published 2020 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bleeker MC, Visser PJ, Overbeek LI, van Beurden M, Berkhof J. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25(8):1224‐1230. [DOI] [PubMed] [Google Scholar]
- 5. Chi CC, Kirtschig G, Baldo M, Brackenbury F, Lewis F, Wojnarowska F. Topical interventions for genital lichen sclerosus. Cochrane Database Syst Rev. 2011;2011(12):CD008240. Published 2011 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hengge UR, Krause W, Hofmann H, et al. Multicentre, phase II trial on the safety and efficacy of topical tacrolimus ointment for the treatment of lichen sclerosus. Br J Dermatol. 2006;155(5):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 7. Nissi R, Eriksen H, Risteli J, Niemimaa M. Pimecrolimus cream 1% in the treatment of lichen sclerosus. Gynecol Obstet Invest. 2007;63(3):151‐154. [DOI] [PubMed] [Google Scholar]
- 8. Oskay T, Sezer HK, Genç C, Kutluay L. Pimecrolimus 1% cream in the treatment of vulvar lichen sclerosus in postmenopausal women. Int J Dermatol. 2007;46(5):527‐532. [DOI] [PubMed] [Google Scholar]
- 9. Van de Nieuwenhof HP, Meeuwis KA, Nieboer TE, Vergeer MC, Massuger LF, De Hullu JA. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31(4):279‐284. [DOI] [PubMed] [Google Scholar]
- 10. Teodoro MC, Scibilia G, Lomeo E, Pecorino B, Galia A, Scollo P. Carbon dioxide laser as a new valid treatment of lichen sclerosus. Clin Exp Obstet Gynecol. 2019;46(2):206‐210. [Google Scholar]
- 11. Belotto RA, Chavantes MC, Tardivo JP, et al. Therapeutic comparison between treatments for Vulvar Lichen Sclerosus: study protocol of a randomized prospective and controlled trial. BMC Womens Health. 2017;17(1):61. Published 2017 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maździarz A, Osuch B, Kowalska M, Nalewczyńska A, Śpiewankiewicz B. Photodynamic therapy in the treatment of vulvar lichen sclerosus. Photodiagnosis Photodyn Ther. 2017;19:135‐139. [DOI] [PubMed] [Google Scholar]
- 13. Borghi A, Corazza M, Minghetti S, Virgili A. Topical tretinoin in the treatment of vulvar lichen sclerosus: an advisable option? Eur J Dermatol. 2015;25(5):404‐409. [DOI] [PubMed] [Google Scholar]
- 14. Joura EA, Zeisler H, Bancher‐Todesca D, Sator MO, Schneider B, Gitsch G. Short‐term effects of topical testosterone in vulvar lichen sclerosus. Obstet Gynecol. 1997;89(2):297‐299. [DOI] [PubMed] [Google Scholar]
- 15. Renaud‐Vilmer C, Cavelier‐Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus: effect of long‐term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140(6):709‐712. [DOI] [PubMed] [Google Scholar]
- 16. Abramov Y, Elchalal U, Abramov D, Goldfarb A, Schenker JG. Surgical treatment of vulvar lichen sclerosus: a review. Obstet Gynecol Surv. 1996;51(3):193‐199. [DOI] [PubMed] [Google Scholar]
- 17. Krastev TK, Schop SJ, Hommes J, Piatkowski A, van der Hulst RRWJ. Autologous fat transfer to treat fibrosis and scar‐related conditions: a systematic review and meta‐analysis. J Plast Reconstr Aesthet Surg. 2020;73(11):2033‐2048. [DOI] [PubMed] [Google Scholar]
- 18. Spiekman M, van Dongen JA, Willemsen JC, Hoppe DL, van der Lei B, Harmsen MC. The power of fat and its adipose‐derived stromal cells: emerging concepts for fibrotic scar treatment. J Tissue Eng Regen Med. 2017;11(11):3220‐3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Dongen JA, Stevens HP, Parvizi M, van der Lei B, Harmsen MC. The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound Repair Regen. 2016;24(6):994‐1003. [DOI] [PubMed] [Google Scholar]
- 20. Guisantes E, Fontdevila J, Rodríguez G. Autologous fat grafting for correction of unaesthetic scars. Ann Plast Surg. 2012;69(5):550‐554. [DOI] [PubMed] [Google Scholar]
- 21. You D, Jang MJ, Kim BH, et al. Comparative study of autologous stromal vascular fraction and adipose‐derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl Med. 2015;4(4):351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lam MT, Nauta A, Meyer NP, Wu JC, Longaker MT. Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng Part A. 2013;19(5‐6):738‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee SH, Lee JH, Cho KH. Effects of human adipose‐derived stem cells on cutaneous wound healing in nude mice. Ann Dermatol. 2011;23(2):150‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uysal CA, Tobita M, Hyakusoku H, Mizuno H. The effect of bone‐marrow‐derived stem cells and adipose‐derived stem cells on wound contraction and epithelization. Adv Wound Care. 2014;3(6):405‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zonari A, Martins TMM, Paula ACC, et al. Polyhydroxybutyrate‐co‐hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomater. 2015;17:170‐181. [DOI] [PubMed] [Google Scholar]
- 26. Yun IS, Jeon YR, Lee WJ, et al. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012;38(10):1678‐1688. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG. Intralesional injection of adipose‐derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6(1):145. Published 2015 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castiglione F, Hedlund P, Van der Aa F, et al. Intratunical injection of human adipose tissue‐derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease [published correction appears in Eur Urol. 2013 Jul;64(1):e21]. Eur Urol. 2013;63(3):551‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229(2):298‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Semon JA, Zhang X, Pandey AC, et al. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl Med. 2013;2(10):789‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Bellows CF, Kolonin MG. Adipose tissue‐derived progenitor cells and cancer. World J Stem Cells. 2010;2(5):103‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Casabona F, Priano V, Vallerino V, Cogliandro A, Lavagnino G. New surgical approach to lichen sclerosus of the vulva: the role of adipose‐derived mesenchymal cells and platelet‐rich plasma in tissue regeneration. Plast Reconstr Surg. 2010;126(4):210e‐211e. [DOI] [PubMed] [Google Scholar]
- 33. Boero V, Brambilla M, Sipio E, et al. Vulvar lichen sclerosus: a new regenerative approach through fat grafting. Gynecol Oncol. 2015;139(3):471‐475. [DOI] [PubMed] [Google Scholar]
- 34. Tamburino S, Lombardo GA, Tarico MS, Perrotta RE. The role of nanofat grafting in vulvar lichen sclerosus: a preliminary report. Arch Plast Surg. 2016;43(1):93‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giuseppina Onesti M, Carella S, Ceccarelli S, Marchese C, Scuderi N. The use of human adipose‐derived stem cells in the treatment of physiological and pathological vulvar dystrophies. Stem Cells Int. 2016;2016:2561461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim SH, Park ES, Kim TH. Rejuvenation using platelet‐rich plasma and lipofilling for vaginal atrophy and lichen sclerosus. J Menopausal Med. 2017;23(1):63‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Newman N, Rogowski RN, Newman D, Talavera‐Adame D. Autologous adipose‐derived stem cell treatment for women with genital lichen sclerosus. Gynecol Reprod Health. 2018;2(4):1‐4. [Google Scholar]
- 38. Stark L, Razzaque M, Yoon J, Aref‐Adib M, Banwell M, Beski S. Safety and feasibility of autologous micro‐fragmented adipose tissue injections for the treatment of vaginal atrophy, vulvovaginal dystrophy, and stress urinary incontinence: an observational case series. EMJ Urol. 2020;8(1):29‐37. [Google Scholar]
- 39. Almadori A, Hansen E, Boyle D, et al. Fat grafting improves fibrosis and scarring in vulvar lichen sclerosus: results from a prospective cohort study. J Low Genit Tract Dis. 2020;24(3):305‐310. [DOI] [PubMed] [Google Scholar]
- 40. Tedesco M, Bellei B, Garelli V, et al. Adipose tissue stromal vascular fraction and adipose tissue stromal vascular fraction plus platelet‐rich plasma grafting: new regenerative perspectives in genital lichen sclerosus. Dermatol Ther. 2020;33(6):e14277. [DOI] [PubMed] [Google Scholar]
- 41. Billings E Jr, May JW Jr. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83(2):368‐381. [DOI] [PubMed] [Google Scholar]
- 42. Katz AJ, Llull R, Hedrick MH, Futrell JW. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26(4):587‐603. [PubMed] [Google Scholar]
- 43. Bauer‐Kreisel P, Goepferich A, Blunk T. Cell‐delivery therapeutics for adipose tissue regeneration. Adv Drug Deliv Rev. 2010;62(7‐8):798‐813. [DOI] [PubMed] [Google Scholar]
- 44. Ergün S, Tilki D, Klein D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal. 2011;15(4):981‐995. [DOI] [PubMed] [Google Scholar]
- 45. Zannettino A, Paton S, Arthur A, et al. Multipotential human adipose‐derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214(2):413‐421. [DOI] [PubMed] [Google Scholar]
- 46. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng. 2001;7(2):211‐228. [DOI] [PubMed] [Google Scholar]
- 47. Ghiasloo M, Lobato RC, Díaz JM, Singh K, Verpaele A, Tonnard P. Expanding clinical indications of mechanically isolated stromal vascular fraction: a systematic review. Aesthet Surg J. 2020;40(9):NP546‐NP560. [DOI] [PubMed] [Google Scholar]
- 48. van Dongen JA, Tuin AJ, Spiekman M, Jansma J, van der Lei B, Harmsen MC. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review. J Tissue Eng Regen Med. 2018;12(1):e261‐e274. [DOI] [PubMed] [Google Scholar]
- 49. van Dongen JA, Stevens HP, Harmsen MC, van der Lei B. Mechanical micronization of lipoaspirates: squeeze and emulsification techniques. Plast Reconstr Surg. 2017;139(6):1369e‐1370e. [DOI] [PubMed] [Google Scholar]
- 50. Mescher LA. Junqueira’s Basic Histology Text and Atlas, 12th ed. The McGraw‐Hill Companies; 2010:480 p. [Google Scholar]
- 51. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun M, Sun L, Huang C, Chen BC, Zhou Z. Induction of macrophage M2b/c polarization by adipose tissue‐derived mesenchymal stem cells. J Immunol Res. 2019;2019:7059680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Essandoh K, Li Y, Huo J, Fan GC. MiRNA‐mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46(2):122‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15(7):1429‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Terlou A, Santegoets LAM, van der Meijden WI, et al. An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: a Th1 response and high levels of microRNA‐155. J Invest Dermatol. 2012;132(3 Pt 1):658‐666. [DOI] [PubMed] [Google Scholar]
- 56. Farrell AM, Dean D, Millard PR, Charnock FM, Wojnarowska F. Cytokine alterations in lichen sclerosus: an immunohistochemical study. Br J Dermatol. 2006;155(5):931‐940. [DOI] [PubMed] [Google Scholar]
- 57. Corazza M, Oton‐Gonzalez L, Scuderi V, et al. Tissue cytokine/chemokine profile in vulvar lichen sclerosus: an observational study on keratinocyte and fibroblast cultures. J Dermatol Sci. 2020;100(3):223‐226. [DOI] [PubMed] [Google Scholar]
- 58. Zhu M, Dong Z, Gao J, et al. Adipocyte regeneration after free fat transplantation: promotion by stromal vascular fraction cells. Cell Transplant. 2015;24(1):49‐62. [DOI] [PubMed] [Google Scholar]
- 59. Saravanamuthu J, Reid WMN, George DS, et al. The role of angiogenesis in vulvar cancer, vulvar intraepithelial neoplasia, and vulvar lichen sclerosus as determined by microvessel density analysis. Gynecol Oncol. 2003;89(2):251‐258. [DOI] [PubMed] [Google Scholar]
- 60. Oh CK, Lee J, Jang BS, et al. Treatment of atrophies secondary to trilinear scleroderma en coup de sabre by autologous tissue cocktail injection. Dermatol Surg. 2003;29(10):1073‐1075. [DOI] [PubMed] [Google Scholar]
- 61. Hong WX, Hu MS, Esquivel M, et al. The role of hypoxia‐inducible factor in wound healing. Adv Wound Care. 2014;3(5):390‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Choi JJ, Min DJ, Cho ML, et al. Elevated vascular endothelial growth factor in systemic sclerosis. J Rheumatol. 2003;30(7):1529‐1533. [PubMed] [Google Scholar]
- 63. Distler JHW, Jüngel A, Pileckyte M, et al. Hypoxia‐induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 2007;56(12):4203‐4215. [DOI] [PubMed] [Google Scholar]
- 64. Li YZ, Wu Y, Zhang QH, Wang Y, Zhen JH, Li SL. Hypoxia‐ischaemia is involved in the pathogenesis of vulvar lichen sclerosus. Clin Exp Dermatol. 2009;34(8):e531‐e536. [DOI] [PubMed] [Google Scholar]
- 65. Hussein MRA. Immunohistological Analysis of CD34‐Positive Dermal Dendritic Cells and Microvessel Density in the Genital and Extragenital Lichen Sclerosus [published online ahead of print, 2021 May 18]. Actas Dermosifiliogr (Engl Ed). 2021;S1578‐2190(21): 00173‐6. [DOI] [PubMed] [Google Scholar]
- 66. Zhang D, Lv FL, Wang GH. Effects of HIF‐1α on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci. 2018;22(16):5071‐5076. [DOI] [PubMed] [Google Scholar]
- 67. Chen G, Zhang W, Zhang K, et al. Hypoxia‐induced mesenchymal stem cells exhibit stronger tenogenic differentiation capacities and promote patellar tendon repair in rabbits. Stem Cells Int. 2020;2020:8822609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Buravkova LB, Grinakovskaia OS, Andreeva EP, Zhambalova AP, Kozionova MP. Characteristics of human lipoaspirate‐isolated mesenchymal stromal cells cultivated under a lower oxygen tension. Tsitologiia. 2009;51(1):5‐11. [PubMed] [Google Scholar]
- 69. Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358(3):948‐953. [DOI] [PubMed] [Google Scholar]
- 70. Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia‐inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9(3):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hwa Cho H, Bae YC, Jung JS. Role of toll‐like receptors on human adipose‐derived stromal cells. Stem Cells. 2006;24(12):2744‐2752. [DOI] [PubMed] [Google Scholar]
- 72. Hassan WU, Greiser U, Wang W. Role of adipose‐derived stem cells in wound healing. Wound Repair Regen. 2014;22(3):313‐325. [DOI] [PubMed] [Google Scholar]
- 73. Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14(11):1569‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim W‐S, Park B‐S, Sung J‐H, et al. Wound healing effect of adipose‐derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15‐24. [DOI] [PubMed] [Google Scholar]
- 75. Cerqueira MT, Pirraco RP, Santos TC, et al. Human adipose stem cells cell sheet constructs impact epidermal morphogenesis in full‐thickness excisional wounds. Biomacromol. 2013;14(11):3997‐4008. [DOI] [PubMed] [Google Scholar]
- 76. Gambichler T, Skrygan M, Czempiel V, et al. Differential expression of connective tissue growth factor and extracellular matrix proteins in lichen sclerosus. J Eur Acad Dermatol Venereol. 2012;26(2):207‐212. [DOI] [PubMed] [Google Scholar]
- 77. Yesudian PD, Sugunendran H, Bates CM, O'Mahony C. Lichen sclerosus. Int J STD AIDS. 2005;16(7):465‐474. [DOI] [PubMed] [Google Scholar]
- 78. Yasar S, Mumcuoglu CT, Serdar ZA, Gunes P. A case of lichen sclerosus et atrophicus accompanying bullous morphea. Ann Dermatol. 2011;23(Suppl 3):S354‐S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Spiekman M, Przybyt E, Plantinga JA, Gibbs S, van der Lei B, Harmsen MC. Adipose tissue‐derived stromal cells inhibit TGF‐β1‐induced differentiation of human dermal fibroblasts and keloid scar‐derived fibroblasts in a paracrine fashion. Plast Reconstr Surg. 2014;134(4):699‐712. [DOI] [PubMed] [Google Scholar]
- 81. Li Y, Zhang W, Gao J, et al. Adipose tissue‐derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li Y, Zhang J, Shi J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR‐192‐5p/IL‐17RA/Smad axis. 2021 Sep 3;12(1):490. Stem Cell Res Ther. 2021;12(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bollero D, Pozza S, Gangemi EN, et al. Contrast‐enhanced ultrasonography evaluation after autologous fat grafting in scar revision. G Chir. 2014;35(11‐12):266‐273. [PMC free article] [PubMed] [Google Scholar]
- 84. Griffin MF, Almadori A, Butler PE. Use of lipotransfer in scleroderma. Aesthet Surg J. 2017;37(suppl 3):S33‐S37. [DOI] [PubMed] [Google Scholar]
- 85. Strong AL, Rubin JP, Kozlow JH, Cederna PS. Fat grafting for the treatment of scleroderma. Plast Reconstr Surg. 2019;144(6):1498‐1507. [DOI] [PubMed] [Google Scholar]
- 86. Daumas A, Magalon J, Delaunay F, et al. Fat grafting for treatment of facial scleroderma. Clin Plast Surg. 2020;47(1):155‐163. [DOI] [PubMed] [Google Scholar]
- 87. Guillaume‐Jugnot P, Daumas A, Magalon J, et al. State of the art. Autologous fat graft and adipose tissue‐derived stromal vascular fraction injection for hand therapy in systemic sclerosis patients. Curr Res Transl Med. 2016;64(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 88. Magalon G, Daumas A, Sautereau N, Magalon J, Sabatier F, Granel B. Regenerative approach to scleroderma with fat grafting. Clin Plast Surg. 2015;42(3):353‐364. [DOI] [PubMed] [Google Scholar]
- 89. Almadori A, Griffin M, Ryan CM, et al. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS One. 2019;14(7):e0218068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Del Papa N, Di Luca G, Andracco R, et al. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: results of a monocentric randomized controlled study. Arthritis Res Ther. 2019;21(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Scuderi N, Ceccarelli S, Onesti MG, et al. Human adipose‐derived stromal cells for cell‐based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013;22(5):779‐795. [DOI] [PubMed] [Google Scholar]
- 92. Granel B, Daumas A, Jouve E, et al. Safety, tolerability and potential efficacy of injection of autologous adipose‐derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open‐label phase I trial. Ann Rheum Dis. 2015;74(12):2175‐2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen BO, Wang X, Long X, et al. Supportive use of adipose‐derived stem cells in cell‐assisted lipotransfer for localized scleroderma. Plast Reconstr Surg. 2018;141(6):1395‐1407. [DOI] [PubMed] [Google Scholar]
- 94. Gheisari M, Ahmadzadeh A, Nobari N, Iranmanesh B, Mozafari N. Autologous fat grafting in the treatment of facial scleroderma. Dermatol Res Pract. 2018;2018:6568016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Segna E, Pucciarelli V, Beltramini GA, et al. Parry Romberg Syndrome and linear facial scleroderma: management in pediatric population. J Biol Regul Homeost Agents. 2017;31(2 Suppl 1):131‐138. [PubMed] [Google Scholar]
- 96. Song JI, Volz S, Liodaki ME, Mailänder P, Kalousis K. Stem cells therapy: the future in the management of systemic sclerosis? A case report. Hell J Nucl Med. 2017;20(Suppl):164. [PubMed] [Google Scholar]
- 97. Del Papa N, Caviggioli F, Sambataro D, et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transplant. 2015;24(1):63‐72. [DOI] [PubMed] [Google Scholar]
- 98. Baldo M, Bailey A, Bhogal B, Groves RW, Ogg G, Wojnarowska F. T cells reactive with the NC16A domain of BP180 are present in vulval lichen sclerosus and lichen planus. J Eur Acad Dermatol Venereol. 2010;24(2):186‐190. [DOI] [PubMed] [Google Scholar]
- 99. Zollinger T, Mertz KD, Schmid M, Schmitt A, Pfaltz M, Kempf W. Borrelia in granuloma annulare, morphea and lichen sclerosus: a PCR‐based study and review of the literature. J Cutan Pathol. 2010;37(5):571‐577. [DOI] [PubMed] [Google Scholar]
- 100. Cooper SM, Ali I, Baldo M, Wojnarowska F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: a case‐control study. Arch Dermatol. 2008;144(11):1432‐1435. [DOI] [PubMed] [Google Scholar]
- 101. Kreuter A, Kryvosheyeva Y, Terras S, et al. Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta Derm Venereol. 2013;93(2):238‐241. [DOI] [PubMed] [Google Scholar]
- 102. Bjekić M, Šipetić S, Marinković J. Risk factors for genital lichen sclerosus in men. Br J Dermatol. 2011;164(2):325‐329. [DOI] [PubMed] [Google Scholar]
- 103. Günthert AR, Faber M, Knappe G, Hellriegel S, Emons G. Early onset vulvar Lichen Sclerosus in premenopausal women and oral contraceptives. Eur J Obstet Gynecol Reprod Biol. 2008;137(1):56‐60. [DOI] [PubMed] [Google Scholar]
- 104. Sherman V, McPherson T, Baldo M, Salim A, Gao XH, Wojnarowska F. The high rate of familial lichen sclerosus suggests a genetic contribution: an observational cohort study. J Eur Acad Dermatol Venereol. 2010;24(9):1031‐1034. [DOI] [PubMed] [Google Scholar]
- 105. Kaya G, Augsburger E, Stamenkovic I, Saurat JH. Decrease in epidermal CD44 expression as a potential mechanism for abnormal hyaluronate accumulation in superficial dermis in lichen sclerosus et atrophicus. J Invest Dermatol. 2000;115(6):1054‐1058. [DOI] [PubMed] [Google Scholar]
- 106. Morrel B, Ewing‐Graham PC, van der Avoort IAM, Pasmans SGMA, Damman J. Structured analysis of histopathological characteristics of vulvar lichen sclerosus in a juvenile population. Hum Pathol. 2020;106:23‐31. [DOI] [PubMed] [Google Scholar]
- 107. Goldstein AT, Mitchell L, Govind V, Heller D. A randomized double‐blind placebo‐controlled trial of autologous platelet‐rich plasma intradermal injections for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2019;80(6):1788‐1789. [DOI] [PubMed] [Google Scholar]
- 108. Eshtiaghi P, Sadownik LA. Fact or fiction? Adipose‐derived stem cells and platelet‐rich plasma for the treatment of vulvar lichen sclerosus. J Low Genit Tract Dis. 2019;23(1):65‐70. [DOI] [PubMed] [Google Scholar]
- 109. Dhurat R, Sukesh M. Principles and methods of preparation of platelet‐rich plasma: a review and author's perspective. J Cutan Aesthet Surg. 2014;7(4):189‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet‐rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Casabona F, Gambelli I, Casabona F, et al. Autologous platelet‐rich plasma (PRP) in chronic penile lichen sclerosus: the impact on tissue repair and patient quality of life. Int Urol Nephrol. 2017;49:573‐80. [DOI] [PubMed] [Google Scholar]
- 112. Willemsen JCN, Spiekman M, Stevens HPJ, van der Lei B, Harmsen MC. Platelet‐rich plasma influences expansion and paracrine function of adipose‐derived stromal cells in a dose‐dependent fashion. Plast Reconstr Surg. 2016;137(3):554e‐565e. [DOI] [PubMed] [Google Scholar]
- 113. Altman AM, Abdul Khalek FJ, Seidensticker M, et al. Human tissue‐resident stem cells combined with hyaluronic acid gel provide fibrovascular‐integrated soft‐tissue augmentation in a murine photoaged skin model. Plast Reconstr Surg. 2010;125(1):63‐73. [DOI] [PubMed] [Google Scholar]
- 114. Hamilton M, Harrington S, Dhar P, Stehno‐Bittel L. Hyaluronic acid hydrogel microspheres for slow release stem cell delivery. ACS Biomater Sci Eng. 2021;7(8):3754‐3763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing is not applicable to this article as no new data were created or analysed in this study.


