Abstract
In Saccharomyces cerevisiae, l-malic acid transport is not carrier mediated and is limited to slow, simple diffusion of the undissociated acid. Expression in S. cerevisiae of the MAE1 gene, encoding Schizosaccharomyces pombe malate permease, markedly increased l-malic acid uptake in this yeast. In this strain, at pH 3.5 (encountered in industrial processes), l-malic acid uptake involves Mae1p-mediated transport of the monoanionic form of the acid (apparent kinetic parameters: Vmax = 8.7 nmol/mg/min; Km = 1.6 mM) and some simple diffusion of the undissociated l-malic acid (Kd = 0.057 min−1). As total l-malic acid transport involved only low levels of diffusion, the Mae1p permease was further characterized in the recombinant strain. l-Malic acid transport was reversible and accumulative and depended on both the transmembrane gradient of the monoanionic acid form and the ΔpH component of the proton motive force. Dicarboxylic acids with stearic occupation closely related to l-malic acid, such as maleic, oxaloacetic, malonic, succinic and fumaric acids, inhibited l-malic acid uptake, suggesting that these compounds use the same carrier. We found that increasing external pH directly inhibited malate uptake, resulting in a lower initial rate of uptake and a lower level of substrate accumulation. In S. pombe, proton movements, as shown by internal acidification, accompanied malate uptake, consistent with the proton/dicarboxylate mechanism previously proposed. Surprisingly, no proton fluxes were observed during Mae1p-mediated l-malic acid import in S. cerevisiae, and intracellular pH remained constant. This suggests that, in S. cerevisiae, either there is a proton counterflow or the Mae1p permease functions differently from a proton/dicarboxylate symport.
Previous studies have provided evidence that l-malic acid is metabolized by Saccharomyces cerevisiae only in the presence of an assimilable carbon source (16, 24, 25). Exogenous l-malic acid (3 g/liter) is always consumed to a limited extent (10 to 20%), and the amount of degraded malate depends on the strain and culture conditions. This incomplete consumption of l-malic acid may be due to limited malate uptake and inefficiency of the enzyme systems involved in metabolism of the acid. Indeed, it has been reported that the transport of l-malate is not carrier mediated in S. cerevisiae; the undissociated form of the acid slowly enters the cell by simple diffusion (28). During fermentation in the presence of malate, intracellular malate concentration in this yeast (close to 1 mM) is therefore lower than that in yeasts having a carrier protein for l-malate and able to metabolize l-malic acid completely (10 to 15 mM in Saccharomyces bailii) (20). The constitutive l-malic enzyme, thought to be responsible for the anaerobic metabolism of l-malate in S. cerevisiae, has a high Km for the substrate (50 mM) (16, 17). Given the low levels of intracellular malate, the malic enzyme seems to function to a limited extent. Anaerobically grown S. cerevisiae cells also contain a malate dehydrogenase and at least two isofunctional fumarase enzymes. However, very little malate is degraded via these pathways during alcoholic fermentation: malate dehydrogenase preferentially reduces oxaloacetate to malate and is strongly repressed by glucose (13, 21); fumarase is competitively inhibited by intracellular inorganic and organic phosphate (20).
Elimination of the l-malic acid present in grapes is of considerable technological value in wine making because it results in the deacidification and stabilization of the wine. This substrate is traditionally eliminated by lactic acid bacteria, which carry out malolactic fermentation after alcoholic fermentation. However, l-malic acid degradation is uncertain, as the poor growth of lactic acid bacteria at low pH often delays malolactic fermentation and may even prevent the reaction altogether. Several attempts have been made to improve malate metabolism in the wine yeast S. cerevisiae (6, 33). The bacterial mleS gene, encoding a bifunctional malolactic enzyme that catalyzes the conversion of l-malate into l-lactate and CO2, has been introduced into S. cerevisiae, to improve its ability to consume malate (1, 12). The heterologous enzyme is functional in recombinant strains but increases malate degradation only slightly, and it has been shown that transport is the limiting step in l-malic acid utilization (2).
Two classes of l-malate transporter, one which is repressed by glucose and one which is not, have been described in yeasts and fungi. The dissociated form of the acid has been shown to be transported across the plasma membrane by proton symporters in Neurospora crassa (34), Kluyveromyces lactis (35), Candida utilis (9), Hansenula anomala (10), and Candida sphaerica (11). The transport systems involved are inducible and subject to glucose repression. In contrast, it has been suggested that the uptake of l-malic acid is mediated by a protein and occurs in the presence of glucose in Zygosaccharomyces bailii (3) and in Schizosaccharomyces pombe (22). The heterologous expression of a gene encoding such an l-malic acid permease appeared to be a possible way of improving malate uptake in S. cerevisiae during alcoholic fermentation. Recently, the MAE1 gene, encoding the malate permease of S. pombe, has been cloned (18). The cotransformation of S. cerevisiae cells with the MAE1 gene and either the L. lactis malolactic enzyme gene mleS (4, 31) or the S. pombe malic enzyme gene MAE2 (32) resulted in a marked increase in l-malate utilization. In this study, we found that, in an S. cerevisiae strain expressing the MAE1 gene, l-malate uptake is almost entirely mediated by the heterologous Mae1p permease, due to little or no diffusion of l-malic acid through the S. cerevisiae plasma membrane. The recombinant strain was then used to analyze Mae1p-mediated l-malate transport in detail. Sousa et al. (30) suggested that a proton symport system is involved in l-malate transport in S. pombe, leading to uphill uptake and accumulation. This study provided insight, in physiological and kinetic terms, into the way in which the permease encoded by the MAE1 gene functions. In particular, we established that the Mae1p permease transported the monoanionic form of l-malate, as a function of the transmembrane substrate and pH gradients.
MATERIALS AND METHODS
Microorganism and growth conditions.
We studied the heterologous expression of the MAE1 gene from the wild-type S. cerevisiae V5 strain (MATa ura3), derived from a Champagne wine strain. Yeast strains were maintained in minimal synthetic (SD) medium (1.7 g of yeast nitrogen base without amino acids and ammonium sulfate, 1.4 g of (NH4)2SO4, and 20 g of glucose per liter). Yeasts were grown at 28°C, without agitation, in SD medium containing 200 g of glucose and 6 g of phthalic acid per liter, adjusted to pH 3.5.
Yeast transformation.
The plasmid YEp MAE1, carrying the coding region of the S. pombe malate permease under the regulatory elements of the PGK1 gene, was previously described (4). The strain V5 was transformed by YEp or YEp MAE1 using the lithium acetate method (29).
Measurement of l-malic acid exchange.
To simulate enological conditions, most experiments were carried out at pH 3.5. Cells were collected by centrifugation at the beginning of the stationary phase, washed twice with 0.1 M potassium phosphate buffer (appropriate pH), and resuspended in 0.1 M potassium phosphate buffer to a final concentration of about 20 mg (dry weight)/ml. Cell suspensions were stored at 4°C until use for [14C]l-malic acid transport assays. For experiments performed in the presence of glucose, the washing buffer and labeled l-malic acid solutions contained 5 and 133.3 mM glucose, respectively. In experiments involving ionophores, cells were incubated for 15 min at 28°C with the compound being studied before the uptake reaction was started. We checked that ionophores had no effect on cell viability at the concentrations used in these experiments (100 μM carbonyl cyanide m-chlorophenylhydrazone [CCCP] and 1 μM valinomycin).
The cell suspension (170 μl) was preincubated at 28°C for 2 min, 30 μl of labeled l-malic acid solution (about 3 × 105 dpm/μmol) was added to give the required concentration (final concentration of 0.1 to 50 mM), and l-malic acid uptake was measured. At each sampling time, the reaction mixture was diluted with 5 ml of ice-cold 0.1 M potassium phosphate buffer (appropriate pH), immediately filtered through glass microfiber filters (GF/C filters, Whatman), and washed with 10 ml of ice-cold buffer. Samples were taken for initial malate uptake rate determination at 0, 5, and 10 s. Sampling times of 0 to 1 h were used to determine malate accumulation kinetics.
In malate efflux experiments, cells were first loaded by incubating a yeast suspension, prepared as described below, with 2 mM labeled l-malic acid (3 × 105 dpm/μmol) for 45 min (recombinant strain) or 2 h (wild-type strain). To induce malate efflux, 200 μl of the loaded cell suspension was diluted with 1.8 ml of the appropriate buffer and incubated at 28°C. After each sampling, cells were harvested by filtration (GF/C filters) and washed with 10 ml of ice-cold 0.1 M phosphate buffer.
The filters carrying the cells were dried (infrared lamp) and placed in 10 ml of scintillation fluid, and radioactivity was counted with a liquid scintillation counter (LS 6500; Beckman).
Determination of proton fluxes.
Proton fluxes associated with l-malic acid transport were determined using a 718 STAT TITRINO pH meter and recorder system (Metrohm). Yeast suspension (4 mg [dry weight]/ml) was prepared in 10 ml of potassium phosphate buffer (10 mM) or in 10 ml of 10 mM glucose in 10 mM potassium phosphate buffer and placed in a 20-ml vessel fitted with a pH electrode and a magnetic stirrer. The pH was adjusted as required (usually to 3.5), and a baseline was obtained. Transmembrane exchanges were induced by adding l-malic acid (final concentration, 1 or 16.6 mM) in 10 mM potassium phosphate buffer, adjusted to the appropriate pH. Subsequent external alkalinization was assessed by recording the volumes of 50 mM HCl added to maintain a constant pH.
Internal l-malic acid concentration.
To calculate the intracellular l-malic acid concentration, the yeast suspension was characterized as follows. Cells were counted using an electronic particle counter (Z. M., Coultronics). Intracellular volume was estimated with a C256 Channalyser (Coultronics). The data obtained indicated that there was 1.2 μl of intracellular water/mg (dry weight).
Concentrations of the monoanionic and undissociated forms of l-malic acid were calculated with the Henderson-Hasselbach equation, using the following pKa values of l-malic acid: pKa1 = 3.41; pKa2 = 5.05.
Intracellular pH.
We assessed internal pH using a modified version of the method of Eraso et al. (14), based on determination of the distribution of [14C]benzoic acid across the plasma membrane and calculation of internal pH, using Rottenberg's equation (26).
Yeast suspension (170 μl), prepared in potassium phosphate buffer (pH 3.5 or 5), was incubated for 5 min with 10 μl of l-malic acid solution (final concentration of 0 to 20 mM) supplemented with 10 mM glucose (final concentration). [14C]benzoic acid (5 μM, 0.1 μCi/ml) was added to the suspension, which was then incubated for 5 min and diluted in 5 ml of phosphate buffer (0.1 mM, pH 3.5 or 5), rapidly filtered (GF/C glass fiber membrane; Whatman), and washed in 10 ml of buffer. Filters were dried, and radioactivity was counted. Nonspecific binding of labeled benzoic acid was evaluated by adding 10 μl of benzoic acid to 180 μl of the cell suspension (equilibrated with unlabeled l-malic acid), and the mixture obtained was immediately diluted and filtered. Internal pH was then calculated as previously described (14).
Reproducibility of the results.
All experiments were repeated at least four times, and the data reported here correspond to the mean values.
Chemicals.
Radioactively labeled compounds (l-malic acid and benzoic acid) were purchased from Amersham (Little Chalfont, Buckinghamshire, United Kingdom). Other chemicals were obtained from Sigma Chimie (France).
RESULTS
Malate uptake and transmembrane PMF.
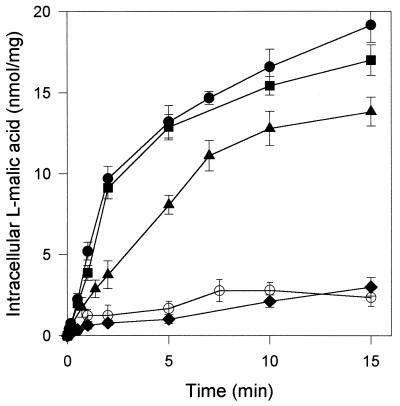
We assessed the transport of l-malic acid at pH 3.5 in the transformed strains V5 YEp and V5 YEp MAE1 (Fig. 1). S. cerevisiae V5 YEp (control strain) took up 16.6 mM l-malic acid slowly, at a rate of 0.46 nmol mg−1 min−1. In the V5 YEp MAE1 transformant, the initial rate of l-malic acid influx was significantly increased (8.7 nmol mg−1 min−1), resulting in a marked accumulation of the 14C-labeled l-malic acid compared to the control strain. Similar results were obtained in experiments performed at pH 5 (data not shown). l-Malic acid uptake in the recombinant strain seems to have been mediated almost entirely by the l-malate permease, Mae1p, due to the efficient expression of the heterologous gene in S. cerevisiae. The initial rate of l-malate uptake was higher in the presence of 10 mM glucose (18.1 nmol mg−1 min−1). This activation may be due to the induction by glucose of proton motive force (PMF), which is generally involved in the control of ionic molecule transport across the plasma membrane. The PMF generated in cells in the presence of glucose was larger than that generated by endogenous substrates.
FIG. 1.
Transport of l-malic acid by S. cerevisiae expressing or not expressing the MAE1 gene. The initial extracellular concentration of total l-malic acid was 16.6 mM in 0.1 M K2PO4 buffer, pH 3.5. ○, V5 YEp strain in the presence of 10 mM glucose; ▴, V5 YEp MAE1 strain without glucose; ●, V5 YEp MAE1 strain in the presence of 10 mM glucose. The dependence of malate uptake on PMF was determined by adding ionophores at the beginning of the transport assays. ⧫, V5 YEp MAE1 strain in the presence of 10 mM glucose and 100 μM CCCP; ■, V5 YEp MAE1 strain in the presence of 10 mM glucose and 1 μM valinomycin.
We investigated the role of PMF and of its constituents, the membrane potential Δψ and the pH gradient ΔpH, on the control of l-malate uptake. Sousa et al. (30) suggested that l-malate transport in S. pombe was dependent on transmembrane PMF. We investigated the role of each of the PMF components in controlling malate uptake by Mae1p (pH 3.5), using the recombinant strain, the protonophore CCCP, and valinomycin (an ionophore) (Fig. 1). In the presence of 100 μM CCCP, which abolished the proton gradient, the initial rate of malate uptake was up to 85% lower and the intracellular accumulation of l-malic acid was prevented, consistent with the observations of Sousa et al. (30). The addition of the valinomycin K+ ionophore to the assay mixture resulted in the collapse of membrane potential in S. cerevisiae (14). The uptake of l-malic acid was not markedly affected by these conditions, in which a considerable pH gradient was maintained, indicating that the transport of l-malic acid by Mae1p does not depend on membrane potential. Thus, ΔpH appears to be the only component of the PMF involved in the control of l-malic acid transport by Mae1p.
Reversibility of transport.
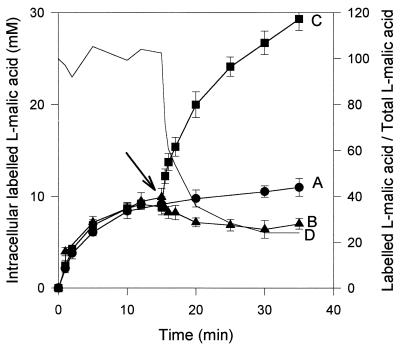
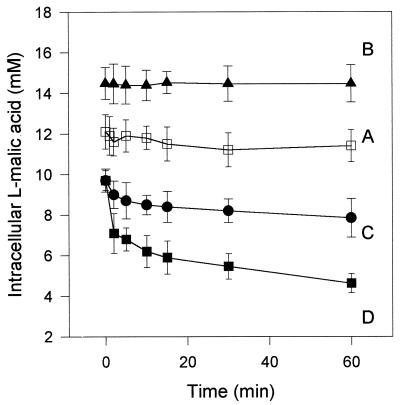
In S. pombe, the l-malate uptake attributed to the permease encoded by MAE1 has been described as accumulative, but the potential of the transport system to excrete l-malate has not been considered. When S. cerevisiae YEp MAE1 cells were incubated for 5 min with 2 mM l-malic acid, the intracellular concentration of labeled compound reached 8 mM (Fig. 2, line A). This confirmed that the MAE1-mediated uptake of total l-malic acid was accumulative at pH 3.5. The reversibility of transport was then studied in the transformant. A pulse of [14C]l-malic acid (final concentration, 35 mM; specific radioactivity, 3 × 105 dpm/μM) during the uptake of 2 mM l-malic acid (3 × 105 dpm/μM) by the recombinant strain resulted in an increase in the intracellular concentration of labeled acid (Fig. 2, line C). In contrast, the addition of unlabeled l-malic acid induced an influx of unlabeled l-malic acid and a weak counterflow of the accumulated labeled substrate, resulting in a fivefold decrease of the specific radioactivity of l-malic acid within the cells (Fig. 2, lines B and C) at the end of the experiment. These observations suggest that the V5 YEp strain is able to excrete l-malic acid. However, this dilution was lower than that of the specific radioactivity of labeled l-malic acid in the external medium (Fig. 2, line D). This may be due to further metabolization of a part of the accumulated l-malic acid. However, such degradation should remain limited due to the low intracellular concentrations of l-malate (below 10 mM), the low substrate affinity, and the limited activity of enzymes involved in l-malic acid degradation in S. cerevisiae (6, 16, 20). In this experiment, the transmembrane gradients of the undissociated and monoanionic forms of l-malic acid did not favor efflux of the organic acid from the cells. The transmembrane gradient concentration of substrate concentration usually controls the reversible transport systems. The limitation of acid excretion, due to an insufficient transmembrane gradient, may thus account for the differences in dilution of specific radioactivity within and outside cells. The dilution of labeled l-malic acid-preloaded S. cerevisiae YEp MAE1 cells in an appropriate buffer induced acid efflux (Fig. 3, lines C and D). No such secretion occurred in cells that did not express the MAE1 gene (S. cerevisiae YEp strain) (Fig. 3, line A). These results indicate that the transport of l-malic acid through the heterologous transporter in S. cerevisiae is reversible and controlled by the transmembrane substrate gradient.
FIG. 2.
Reversibility of Mae1p-mediated l-malate transport in S. cerevisiae. Experiments were performed in 0.1 M K2PO4 buffer, pH 3.5, with an initial extracellular total l-malic acid concentration of 2 mM (specific activity, 3 × 105 dpm/μmol). Line A, accumulation of l-malic acid by the S. cerevisiae strain V5 YEp MAE1 without addition; lines B and C, addition (arrow) of unlabeled l-malic acid or 14C-labeled l-malic acid (specific activity, 3 × 105 dpm/μmol), respectively, to the accumulation assay with the recombinant S. cerevisiae strain at a final concentration of 35 mM. The ratio between the intracellular concentrations of labeled l-malic acid (line B) and total l-malic acid (line C) was calculated (line D).
FIG. 3.
l-Malic acid efflux from S. cerevisiae strains. S. cerevisiae V5 YEp cells were preloaded by 2 h of incubation with 2 mM labeled l-malic acid (specific activity, 2 × 105 dpm/μmol). The control experiment was performed by diluting 200 μl of cell suspension containing 12 mM labeled l-malic acid with 2 ml of 0.1 M K2PO4 buffer, pH 6 (line A). S. cerevisiae V5 YEp MAE1 cells were preloaded with 14.45 mM (▴) or 9.4 mM (●, ■) labeled l-malic acid by incubation with 2 or 10 mM labeled l-malic acid, respectively (specific activity; 10 × 105 and 2 × 105 dpm/μmol), and 200 μl of preloaded cell suspension was diluted with 2 ml of 0.1 M potassium phosphate buffer, pH 4 (line B), pH 3.5 (line C), or pH 6 (line D).
Form of l-malic acid transported by Mae1p.
To determine the form of l-malic acid carried by the heterologous transporter Mae1p in S. cerevisiae YEp MAE1, we investigated the secretion by cells of l-malic acid (pKa1, 3.41; pKa2, 5.1) as a function of the transmembrane gradients of undissociated, monoanionic, and dianionic forms. In order to generate varied transmembrane gradients, cells preloaded with labeled substrate at different concentrations were diluted in potassium phosphate buffer at various pH levels (3.5, 4, or 6). The subsequent l-malic acid efflux (Table 1; Fig. 3) was determined for each condition. An unfavorable transmembrane gradient of the undissociated form of acid l-malic was generated by dilution of cells containing 9.4 mM l-malic acid in 2 ml of potassium phosphate buffer (pH 3.5). Under these conditions, a significant efflux of l-malic acid was observed (Fig. 3, line C). This is consistent with transport of the mono- or dianionic forms of l-malic acid. Dilution of the cells in buffer at pH 6 both increased the gradient of the monoanionic form and reduced the gradient of the dianionic form and then resulted in an increase of efflux (Fig. 3, line D). In contrast, if the transmembrane gradient of monoanionic l-malic acid was such that the monoanionic form could not be excreted whereas the dianionic form could (dilution of 14.45 mM l-malic acid-preloaded cells in 2 ml of buffer at pH 6), the acid accumulated in the cells (Fig. 3, line B). These observations demonstrate that the monoanionic form of l-malic acid is transported by Mae1p in S. cerevisiae.
TABLE 1.
Transmembrane gradients of l-malic acid concentrations after dilution of preloaded cellsa
| l-Malic acid form | 2 mM
|
10 mM, pH 4d
|
||||
|---|---|---|---|---|---|---|
| pH 3.5b
|
pH 6c
|
|||||
| IC | EC | IC | EC | IC | EC | |
| Dianionic | 9.04 | 0 | 9.04 | 0.18 | 13.9 | 0.06 |
| Monoanionic | 0.36 | 0.11 | 0.36 | 0.02 | 0.55 | 0.75 |
| Undissociated | 0 | 0.09 | 0 | 0 | 0 | 0.19 |
S. cerevisiae V5 YEp MAE1 cells, preloaded by incubation for 30 min with 2 or 10 mM labeled l-malic acid (specific activity, 10 × 105 or 2 × 105 dpm/μmol, respectively) were diluted with 2 ml of 0.1 M potassium phosphate buffer at the indicated pH. Intracellular and extracellular l-malic acid concentrations (IC and EC, respectively) were determined immediately after dilution. Values are in millimolar units.
Fig. 3, line C.
Fig. 3, line D.
Fig. 3, line B.
Kinetic parameters.
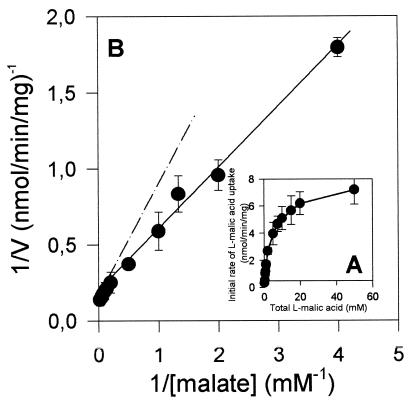
The initial rate of uptake of l-malic acid was determined in the presence of 10 mM glucose, with various substrate concentrations, at pH 3.5. At this pH, the acid was mainly monoanionic (54%) and undissociated (44%). In these conditions, l-malic acid transport was not saturable (Fig. 4A) and was not subject to Michaelis-Menten kinetics, as indicated by the shift in the Lineweaver-Burk plot (Fig. 4B). The biphasic plot obtained is consistent with Mae1p-mediated transport of the monoanionic form of l-malic acid with some simple diffusion of the undissociated form (as previously observed in the control strain, S. cerevisiae V5 YEp). The overall rate of malate uptake in S. cerevisiae strain V5 YEp MAE1 should have a Michaelian component (Mae1p-mediated transport of the monoanionic acid [A−]) and a linear component (diffusion of the undissociated acid [AH]), as follows: V = Vmax · {[A−]/([A−] + Km)} + Kd · [AH]. We calculated the following apparent kinetic parameters: apparent maximal initial rate of monoanionic l-malic acid (Vmax) = 8.7 nmol mg−1 min−1; apparent Michaelis constant (Km) = 1.58 mM; diffusion constant for undissociated l-malic acid (Kd) = 0.057 min−1. These data confirm the low level of undissociated l-malic acid diffusion involved in total acid fluxes.
FIG. 4.
Kinetics of l-malic acid transport by S. cerevisiae V5 YEp MAE1. (A) Initial rate of uptake of labeled l-malic acid as a function of l-malic acid concentration. (B) Lineweaver-Burk plot of 1/V versus 1/S, where V and S are the initial rate of l-malic acid uptake and l-malic acid concentration, respectively.
Substrate specificity.
The substrate selectivity of the Mae1p transporter in S. cerevisiae was determined by measuring the uptake of 2 mM labeled l-malic acid in the presence of other organic acids at a concentration of 50 mM (Table 2). Monocarboxylic acids (pyruvate and lactate) and organic acids with high levels of stearic occupation (polysubstituted C4 diacids or compounds containing more than four carbon atoms) did not markedly affect l-malate transport. In contrast, maleate and malonate, analogs of l-malate, strongly decreased l-malate uptake. Non- or monosubstituted C4 dicarboxylic acids, such as oxaloacetate and (to a lesser extent) succinate and fumarate, competed efficiently with l-malate for entry into the cells. The inhibition of l-malate uptake also occurred when these compounds were used at a concentration of 2 mM. [14C]succinate and [14C]fumarate were transported by S. cerevisiae YEp MAE1, whereas these organic acids did not enter wild-type S. cerevisiae cells (data not shown). It therefore seems that the same carrier transported succinate, fumarate, and malate in the recombinant strain.
TABLE 2.
Specificity of malate transport in the S. cerevisiae strain V5 YEp MAE1a
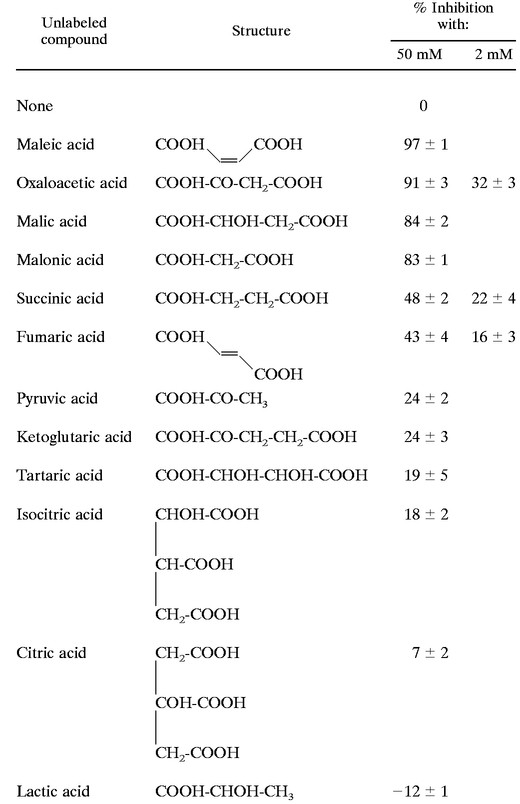 |
The initial rate of [14C]l-malic acid uptake (2 mM; specific activity, 3 × 105 dpm/μmol) was determined in 0.1 M K2PO4 buffer, pH 3.5, supplemented with 50 or 2 mM unlabeled organic acid. The values correspond to percent reductions in the rate of uptake of labeled l-malic acid measured without organic acids.
These results indicate that substrate specificity depends on the three-dimensional structure of the compound transported. Mae1p seems to transport preferentially C3 or C4 dicarboxylic acids containing no more than one hydroxyl or ketonyl group.
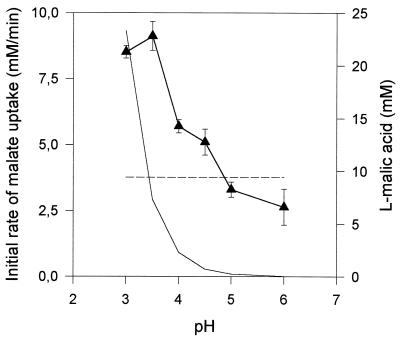
pH and l-malic acid uptake.
For a given total l-malic acid content, the concentration of the monoanionic (and undissociated) form of l-malic acid decreased as the pH increased and may have led to substrate limitation. To prevent such limitation occurring, we determined the optimum pH for malate uptake in S. cerevisiae strain V5 YEp MAE1, using a constant concentration of monoanionic l-malate (9.4 mM) at various pH levels (Fig. 5). The initial rate of l-malate uptake was optimal at pH 3.5, whereas the monoanionic form of the acid was preferentially transported by Mae1p, with some simple diffusion of the undissociated form. The rate of l-malate uptake decreased above pH 4. This decrease in l-malate transport with increasing pH indicated inhibition of the Mae1p transporter, due to a simple effect of pH on carrier activity or to the lower proton gradient at a high external pH.
FIG. 5.
Effect of pH on l-malic acid uptake in S. cerevisiae V5 YEp MAE1. The initial rates were determined using a constant monoanionic l-malic acid concentration of 9.4 mM (▴). Variations in the concentrations of the monoanionic (– –) and the undissociated (––) forms of the acid are reported.
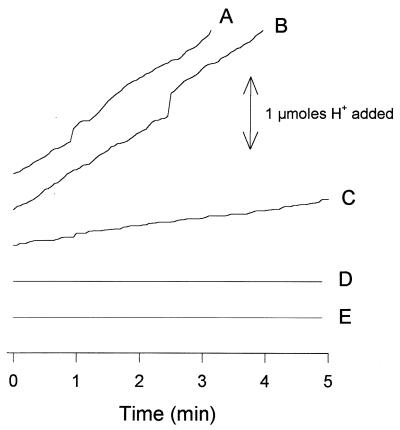
Finally, changes in intracellular pH and external alkalinization were measured during l-malic acid uptake in the S. cerevisiae V5 YEp MAE1 and S. pombe (control experiment) strains. In the S. pombe strain, malate uptake was accompanied by a proton influx (Fig. 6, lines A and B) and generated an intracellular acidification (Table 3). The range of the proton flux is related to external malate concentration (Fig. 6, lines B and C). This exchange is consistent with the presence of an l-malate/proton symporter in S. pombe, as previously suggested (31). Surprisingly, intracellular pH remained constant and external alkalinization was not observed during malate uptake in the recombinant S. cerevisiae strain, regardless of the experimental conditions used (Fig. 6, lines D and E; Table 3). This may be due to a H+ counterflow in S. cerevisiae during Mae1p-mediated malate uptake by a H+/dicarboxylate symport mechanism, resulting in a null proton exchange. However, we cannot exclude the possibility that the Mae1p transporter functions as a uniport system coupled with a counterion exchange. Further work is required to clarify this point.
FIG. 6.
Proton fluxes associated with l-malic acid uptake in S. pombe and the S. cerevisiae V5 YEp MAE1 strain. (Line A) S. pombe in the presence of 16.6 mM labeled l-malic acid; (line B) S. pombe in the presence of 16.6 mM labeled l-malic acid and 10 mM glucose; (line C) S. pombe in the presence of 1 mM labeled l-malic acid and 10 mM glucose; (line D) S. cerevisiae in the presence of 16.6 mM labeled l-malic acid and 10 mM glucose; (line E) S. cerevisiae in the presence of 16.6 mM labeled l-malic acid.
TABLE 3.
Internal acidification induced by l-malic acid uptake in S. pombe and S. cerevisiae V5 YEp MAE1a
| l-Malic acid concn (mM) | Intracellular pH
|
|||
|---|---|---|---|---|
|
S. pombe
|
S. cerevisiae
|
|||
| External pH 3.5 | External pH 5 | External pH 3.5 | External pH 5 | |
| 0 | 6.0 ± 0.01 | 6.39 ± 0.07 | 6.54 ± 0.01 | 6.66 ± 0.05 |
| 1 | 5.96 ± 0.06 | 6.16 ± 0.03 | 6.59 ± 0.02 | 6.63 ± 0.03 |
| 10 | 5.54 ± 0.03 | 5.98 ± 0.02 | 6.52 ± 0.02 | 6.44 ± 0.03 |
| 20 | 5.38 ± 0.03 | 5.87 ± 0.03 | 6.60 ± 0.03 | 6.42 ± 0.02 |
Intracellular pH was evaluated after incubation of the cell suspensions for 5 min with unlabeled l-malic acid in 0.1 M K2PO4 buffer, pH 3.5 or 5, by measurement of the distribution of [14C]benzoic acid across the plasma membrane. Values are the means of at least four repeated experiments.
Malate utilization by S. cerevisiae V5 YEp MAE1.
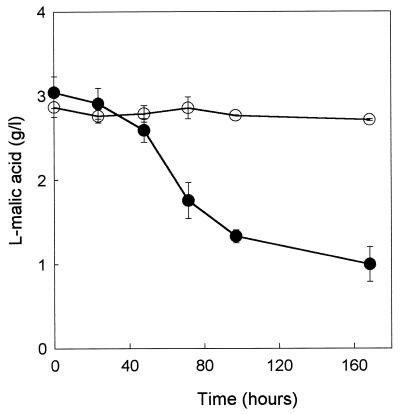
The ability of the recombinant strain expressing the MAE1 gene to degrade l-malic acid was tested. Fermentation experiments were conducted with strains V5 YEp and V5 YEp MAE1 in SD medium with a high sugar concentration and an acidity (glucose, 180 g/liter; pH 3.5) simulating enological conditions and containing 3 g of l-malic acid per liter. While l-malic acid was scarcely consumed by the control strain, the expression of the MAE1 gene resulted in a significant increase of malate utilization: up to 2 g of malate per liter was degraded by the recombinant strain during fermentation (Fig. 7). It also appeared that the increase in malate consumption did not affect the growth or the fermentative characteristics of the recombinant yeast (data not shown). These results confirm that the uptake of malic acid is limiting for malate utilization in S. cerevisiae.
FIG. 7.
Malic acid degradation during fermentation. S. cerevisiae V5 YEp (○) and S. cerevisiae V5 YEp MAE1 (●) strains were grown on SD medium, pH 3.5, containing 180 g of glucose per liter and 3 g of l-malic acid per liter.
DISCUSSION
It has been suggested that the S. pombe l-malic acid transporter is a carboxylate/proton symport system, dependent on the plasma membrane proton gradient (22). The MAE1 gene, encoding the permease for l-malic acid and other organic acids, has been cloned (18). However, the mechanism of transport through this permease has been only partially described. To further characterize the S. pombe malate transporter, we introduced the MAE1 gene into S. cerevisiae strain V5. We found that, in the resulting recombinant strain, the Mae1p permease was almost entirely responsible for the observed uptake of l-malic acid, due to (i) the absence of an endogenous carrier-mediated transport system and (ii) the low efficiency of free diffusion of the undissociated form of l-malic acid through the plasma membrane in S. cerevisiae, even at low pH (as observed in the control strain). Exchanges of l-malic acid in the recombinant S. cerevisiae strain were investigated at pH 3.5, under conditions similar to those encountered in wine making.
In the recombinant S. cerevisiae, there was an influx or efflux of l-malic acid, depending on the transmembrane gradient of the monoanionic form of the acid. Gradients of undissociated or dianionic forms did not affect l-malate fluxes through the plasma membrane. This control of malate exchange demonstrates that the substrate of the Mae1p transport system is the monoanionic form of the acid, as previously suggested in S. pombe (30). This is the first time that the excretion of l-malic acid by the Mae1p permease has been reported. However, due to the transmembrane gradient in monoanionic l-malate encountered in physiological conditions (pH up to 3.3), the Mae1p transporter functions in vivo mainly by acid influx. l-Malate uptake, induced by a favorable gradient, occurred until a balance between intra- and extracellular concentrations of monoanionic l-malic acid was reached, leading to the intracellular accumulation of l-malic acid in its dianionic form (intracellular pH around 6.8 [19]). The observed intracellular accumulation of (total) l-malic acid, previously observed by Osothsilp and Subden and by Sousa et al. (22, 30), appears to result from the transport, by the Mae1p permease, of the monoanionic l-malate form. Finally, the kinetic data confirmed that Mae1p mediated transport of the monoanionic l-malic acid, which was accompanied by low-level simple diffusion of the undissociated acid (Kd = 0.057 min−1) in the recombinant S. cerevisiae strain at pH 3.5. The apparent Km for malate transport by S. pombe (3.7 mM), determined by Osothsilp and Subden (22), is slightly higher than the affinity constant for monoanionic malate uptake in recombinant S. cerevisiae (1.6 mM). The model used for our calculations, taking into account the possible diffusion of l-malic acid in S. pombe omission, may account for the difference between the apparent Km values.
We investigated the relationship between the inhibition of Mae1p-mediated malate uptake in the recombinant S. cerevisiae strain by maleic, oxaloacetic, malonic, succinic, and fumaric acids and the three-dimensional structure of these dicarboxylic acids. The carrier did not transport monocarboxylic acids or organic acids with high levels of stearic occupation. We found that α-ketoglutaric acid, consistent with its structure, was weakly transported by the Mae1p permease in S. cerevisiae. This result supports the observations of Grobler et al. (18), but it is contrary to the findings of Sousa et al. (30) with S. pombe. We also demonstrated glucose activation of l-malate transport via the Mae1p permease. The properties of MAE1 permease (glucose activation, selectivity related to the three-dimensional structure of transported organic acids, and apparent affinity) differ from those of other malate transport systems in yeast and fungi: C. utilis (9), C. sphaerica (11), and H. anomala (10). These glucose-repressed carriers have a higher apparent affinity for their substrates and specifically transport all the dicarboxylates of the Krebs cycle, including α-ketoglutaric acid (malate analogs, such as maleic and malonic acids, are not transported). The existence of two classes of malate transporter may reflect different fates of the malate taken up in vivo: in C. utilis, C. sphaerica, and H. anomala, l-malic acid (and other organic acids, Krebs cycle intermediates) is used as the sole source of carbon and energy once the glucose supply is exhausted, whereas in S. pombe, l-malate degradation occurs only in the presence of another carbon source (glucose, glycerol, or fructose).
Negatively charged molecules are transported across the plasma membrane by anion exchange, cotransport with cations or protons involving a single mediator, or separate electrically coupled carriers. Thus, the transmembrane PMF plays an important role in these transport activities. We demonstrate here that pH exerts two levels of control on Mae1p-mediated l-malate uptake in S. cerevisiae. Firstly, the transport of monoanionic l-malate by the Mae1p permease is dependent on the presence of a transmembrane pH gradient, as indicated by the abolition of l-malate transport by the addition of a protonophore, CCCP (reference 19 and this work). Such regulation of organic acid transport in yeast has been reported: dicarboxylate transport in C. utilis and H. anomala (9, 10), citric acid transport in C. utilis (8), and transport of short-chain monocarboxylic acids in Torulaspora delbrueckii (7). We found that membrane potential, another component of the transmembrane PMF, did not affect the exchange. Secondly, for a given monoanionic l-malate concentration, we found that pH values above 4 inhibited malate uptake in recombinant S. cerevisiae, reducing the initial rate. Similarly, increasing pH had a negative effect on intracellular malate accumulation by Mae1p, the rate of accumulation being higher at pH 3.5 than at pH 5 (data not shown)
It has been suggested that in S. pombe, monoanionic l-malic acid enters the cells via a proton/dicarboxylate symport system (30). Consistent with this observation, we demonstrated that intracellular acidification and proton fluxes occur during malate uptake in this yeast. The opposite was observed with the S. cerevisiae strain. The lack of transient external alkalinization and changes in intracellular pH were consistent with the absence of net proton fluxes during Mae1p-mediated malate transport in the recombinant yeast. Assuming that a dicarboxylate/proton symport mechanism is involved in Mae1p-mediated malate uptake, then the observed null proton exchange could be explained by the presence of an active H+ counterflow in S. cerevisiae. Such proton extrusion could be ensured by (i) cotransport with an anion or exchange with another cation (e.g., H+/K+ exchange, as recently observed in S. cerevisiae using plasma membrane vesicles [5, 23]) or by (ii) active secretion involving plasma membrane H+-ATPase activity, which differs between the two strains: Haworth and Fliegel demonstated that the level of internal alkalinization due to H+-ATPase activation is higher in S. cerevisiae than in S. pombe (19). On the other hand, uniport systems have been described for lactic acid transport in Kluyveromyces marxianus (15) and for monoanionic malate uptake in Leuconostoc oenos (27). The possibility that the Mae1p permease functions as a malate uniport system was ruled out because such a system would depend on Δψ, which we have shown not to be the case.
Finally, we have shown that malate utilization by S. cerevisiae is limited due to the lack of a malate transporter. Indeed, expression of the MAE1 gene significantly improves malate consumption. This is of interest for wine making, because the removal of malic acid, one of the main organic acids of grape must, is essential for the quality and stability of wine. However, to achieve complete degradation of malate present in high amounts in wine (up to 8 g/liter), the efficiency of intracellular malate assimilation must be improved. This can be done by replacing the native malic enzyme by the S. pombe enzyme, which has a higher affinity for malate, in a strain expressing MAE1 (32) or by coexpression of the L. lactis mleS gene, coding for the malolactic enzyme, and the S. pombe mleS gene (4, 31).
REFERENCES
- 1.Ansanay V, Dequin S, Blondin B, Barre P. Cloning sequence and expression of the gene encoding the malolactic enzyme from Lactococcus lactis. FEBS Lett. 1993;332:74–80. doi: 10.1016/0014-5793(93)80488-g. [DOI] [PubMed] [Google Scholar]
- 2.Ansanay V, Dequin S, Camarasa C, Schaeffer V, Grivet J P, Blondin B, Salmon J M, Barre P. Malolactic fermentation by engineered Saccharomyces cerevisiae as compared with engineered Schizosaccharomyces cerevisiae. Yeast. 1996;12:215–225. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C215::AID-YEA903%3E3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Baranowski K, Radler F. The glucose-dependent transport of l-malate in Zygosaccharomyces baili. Antonie Leeuwenhoek. 1984;50:37–42. doi: 10.1007/BF00394646. [DOI] [PubMed] [Google Scholar]
- 4.Bony M, Bidard F, Camarasa C, Ansanay V, Dulau L, Barre P, Dequin S. Metabolic analysis of S. cerevisiae engineered for malolactic fermentation. FEBS Lett. 1997;410:452–456. doi: 10.1016/s0014-5793(97)00637-6. [DOI] [PubMed] [Google Scholar]
- 5.Camarasa C, Prieto S, Ros R, Salmon J M, Barre P. Evidence for a selective and electroneutral K+/H+-exchange using Saccharomyces cerevisiae plasma membrane vesicles. Yeast. 1996;12:1301–1313. doi: 10.1002/(SICI)1097-0061(199610)12:13%3C1301::AID-YEA18%3E3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Carrau J L, Azevedo J L, Siedbery P, Campbell D. Methods for recovering fusion products among oenological strains of Schizosaccharomyces pombe and Saccharomyces cerevisiae. Rev Bras Genet. 1983;1:221–226. [Google Scholar]
- 7.Casal M, Leao C. Utilization of short-chain monocarboxylic acids by the yeast Torulaspora delbrueckii: specificity of the transport systems and their regulation. Biochim Biophys Acta. 1995;1267:122–130. doi: 10.1016/0167-4889(95)00067-3. [DOI] [PubMed] [Google Scholar]
- 8.Cassio F, Leao C. Low- and high-affinity transport systems for citric acid in the yeast Candida utilis. Appl Environ Microbiol. 1991;57:3623–3628. doi: 10.1128/aem.57.12.3623-3628.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassio F, Leao C. A comparative study on the transport of (l)-malic acid and other short-chain carboxylic acids in the yeast Candida utilis; evidence for a general organic acid permease. Yeast. 1993;9:743–752. doi: 10.1002/yea.320090708. [DOI] [PubMed] [Google Scholar]
- 10.Corte-Real M, Leao C. Transport of malic acid and other dicarboxylic acids in the yeast Hansenula anomala. Appl Environ Microbiol. 1990;56:1109–1113. doi: 10.1128/aem.56.4.1109-1113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corte-Real M, Leao C, Van Uden N. Transport of L(-)malic acid and other dicarboxylic acids in the yeast Candida sphaerica. Appl Microbiol Biotechnol. 1989;31:551–555. [Google Scholar]
- 12.Denayrolles M, Aigle M, Lonvaud-Funel A. Functional expression in Saccharomyces cerevisiae of the Lactococcus lactis mleS gene encoding the malolactic enzyme. FEMS Microbiol Lett. 1995;125:37–43. doi: 10.1111/j.1574-6968.1995.tb07332.x. [DOI] [PubMed] [Google Scholar]
- 13.Duntze W, Neumann D, Holzer H. Glucose-induced inactivation of malate dehydrogenase in intact yeast cells. Eur J Biochem. 1968;3:326–331. doi: 10.1111/j.1432-1033.1968.tb19533.x. [DOI] [PubMed] [Google Scholar]
- 14.Eraso P, Mazon M J, Gancedo M J. Internal acidification and cAMP increase are not correlated in Saccharomyces cerevisiae. Eur J Biochem. 1989;165:671–674. doi: 10.1111/j.1432-1033.1987.tb11493.x. [DOI] [PubMed] [Google Scholar]
- 15.Fonseca A, Spencer-Martins I, Van Uden N. Transport of lactic acid in Kluyveromyces marxianus: evidence for a monocarboxylate uniport. Yeast. 1991;7:775–780. doi: 10.1002/yea.320070803. [DOI] [PubMed] [Google Scholar]
- 16.Fuck E, Radler F. Malic acid metabolism of Saccharomyces. 1. The anaerobic decomposition of malic acid by Saccharomyces cerevisiae. Arch Microbiol. 1972;87:149–164. [PubMed] [Google Scholar]
- 17.Fuck E, Stark G, Radler F. Malic acid metabolism of Saccharomyces. 2. Partial purification and characterization of a malic enzyme. Arch Microbiol. 1973;89:223–231. [PubMed] [Google Scholar]
- 18.Grobler J, Bauer F, Subden R E, Van Vuuren H J J. The MAE1 gene of Schizosaccharomyces pombe encodes a permease for malate and other C4 dicarboxylic acids. Yeast. 1995;11:1485–1491. doi: 10.1002/yea.320111503. [DOI] [PubMed] [Google Scholar]
- 19.Haworth R S, Fliegel L. Intracellular pH in Schizosaccharomyces pombe. Comparison with Saccharomyces cerevisiae. Mol Cell Biochem. 1993;124:131–140. doi: 10.1007/BF00929205. [DOI] [PubMed] [Google Scholar]
- 20.Kuczynski J T, Radler F. The anaerobic metabolism of malate of Saccharomyces bailii and the partial purification and characterization of malic enzyme. Arch Microbiol. 1982;131:266–270. doi: 10.1007/BF00405891. [DOI] [PubMed] [Google Scholar]
- 21.Neeff J, Mecke D. In vivo and in vitro studies on the glucose-dependent inactivation of yeast cytoplasmic malate dehydrogenase. Arch Microbiol. 1977;115:55–60. doi: 10.1007/BF00427845. [DOI] [PubMed] [Google Scholar]
- 22.Osothsilp C, Subden R E. Malate transport in Schizosaccharomyces pombe. J Bacteriol. 1986;168:1439–1443. doi: 10.1128/jb.168.3.1439-1443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez L, Pena A, Montero-Lomeli L. H+/K+ exchange in reconstituted yeast plasma membranes vesicles. Biochim Biophys Acta. 1996;1215:175–182. doi: 10.1016/s0005-2736(96)00153-8. [DOI] [PubMed] [Google Scholar]
- 24.Rankine B C. Decomposition of l-malic acid by wine yeast. J Sci Food Agric. 1966;17:312–316. doi: 10.1002/jsfa.2740170707. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez S B, Thornton R J. Factors influencing the utilisation of l-malate by yeasts. FEMS Microbiol Lett. 1990;72:17–22. doi: 10.1016/0378-1097(90)90337-p. [DOI] [PubMed] [Google Scholar]
- 26.Rottenberg H. The measurement of membrane potential and pH in cells, organelles and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 27.Salema M, Poolman B, Lolkema J S, Dias M C, Konings W N. Uniport of monoanionic l-malate in membranes vesicles from Leuconostoc oenos. Eur J Biochem. 1994;255:289–295. doi: 10.1111/j.1432-1033.1994.00289.x. [DOI] [PubMed] [Google Scholar]
- 28.Salmon J M. l-Malic acid permeation in resting cells of anaerobically grown Saccharomyces cerevisiae. Biochim Biophys Acta. 1987;901:30–34. doi: 10.1016/0005-2736(87)90253-7. [DOI] [PubMed] [Google Scholar]
- 29.Schiestl R H, Gietz R G. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 30.Sousa M J, Mota M, Leao C. Transport of malic acid in the yeast Schizosaccharomyces pombe: evidence for a proton dicarboxylate symport. Yeast. 1992;8:1025–1031. doi: 10.1002/yea.320081205. [DOI] [PubMed] [Google Scholar]
- 31.Volschenk H, Viljoen M, Grobler J, Bauer F, Lonvaud A, Denayrolles M, Subden R, Van Vuuren H. Malolactic fermentation in grape musts by a genetically engineered strain of Saccharomyces cerevisiae. Am J Enol Vitic. 1997;18:193–197. [Google Scholar]
- 32.Volschenk H, Viljoen M, Grobler J, Petzold B, Bauer F, Subden R, Young R, Lonvaud A, Denayrolles M, Van Vuuren H. Engineering pathways for malate degradation in Saccharomyces cerevisiae. Nat Biotechnol. 1997;15:253–257. doi: 10.1038/nbt0397-253. [DOI] [PubMed] [Google Scholar]
- 33.Williams A S, Hodges R A, Strike T L, Snow R S, Kunkee R E. Cloning the gene for the malolactic fermentation from Lactobacillus delbrueckii in Escherichia coli and yeasts. Appl Environ Microbiol. 1984;47:288–293. doi: 10.1128/aem.47.2.288-293.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolinburger L, Kay W W. Transport of C4-dicarboxylic acids in Neurospora crassa. Biochim Biophys Acta. 1973;307:243–257. doi: 10.1016/0005-2736(73)90041-2. [DOI] [PubMed] [Google Scholar]
- 35.Zmjewski M J, MacQuillan A M. Dual effect of glucose on dicarboxylic acid transport in Kluyveromyces lactis. Can J Microbiol. 1975;175:473–480. doi: 10.1139/m75-066. [DOI] [PubMed] [Google Scholar]