Abstract
Background and aim
Opioid agonist medications for treatment of opioid use disorder (OUD) can improve human immunodeficiency virus (HIV) outcomes and reduce opioid use. We tested whether outpatient antagonist treatment with naltrexone could achieve similar results.
Design
Open‐label, non‐inferiority randomized trial.
Setting
Six US HIV primary care clinics.
Participants
A total of 114 participants with untreated HIV and OUD (62% male; 56% black, 12% Hispanic; positive for fentanyl (62%), other opioids (47%) and cocaine (60%) at baseline). Enrollment halted early due to slow recruitment.
Intervention
HIV clinic‐based extended‐release naltrexone (XR‐NTX; n = 55) versus treatment as usual (TAU) with buprenorphine or methadone (TAU; n = 59).
Measurements
Treatment group differences were compared for the primary outcome of viral suppression (HIV RNA ≤ 200 copies/ml) at 24 weeks and secondary outcomes included past 30‐day use of opioids at 24 weeks.
Findings
Fewer XR‐NTX participants initiated medication compared with TAU participants (47 versus 73%). The primary outcome of viral suppression was comparable for XR‐NTX (52.7%) and TAU (49.2%) [risk ratio (RR) = 1.064; 95% confidence interval (CI) = 0.748, 1.514] at 24 weeks. Non‐inferiority could not be demonstrated, as the lower confidence limit of the RR did not exceed the pre‐specified margin of 0.75 in intention‐to‐treat (ITT) analysis. The main secondary outcome of past 30‐day opioid use was comparable for XR‐NTX versus TAU (11.7 versus 14.8 days; mean difference = −3.1; 95% CI = –8.7, 1.1) in ITT analysis. Among those initiating medication, XR‐NTX resulted in fewer days of opioid use compared with TAU in the past 30 days (6.0 versus 13.6, mean difference = −7.6; 95% CI = –13.8, −0.2).
Conclusions
A randomized controlled trial found supportive, but not conclusive, evidence that human immunodeficiency virus clinic‐based extended‐release naltrexone is not inferior to treatment as usual for facilitating human immunodeficiency virus viral suppression. Participants who initiated extended‐release naltrexone used fewer opioids than those who received treatment as usual.
Keywords: Buprenorphine, extended‐release naltrexone, HIV, methadone, non‐inferiority trial, opioid‐related disorders, randomized controlled trials
INTRODUCTION
Opioid use disorder (OUD) is common in people living with HIV and associated with decreased receipt of antiretroviral therapy (ART), decreased ART adherence and decreased HIV viral suppression [1]. Treatment of substance use disorders can increase engagement in HIV care [2].
Opioid agonist therapy for OUD with either methadone or sublingual buprenorphine decreases HIV transmission risk behaviors [3] and improves HIV[4] and OUD outcomes [5], but access to medications for opioid use disorder treatment (MOUD) remains limited. HIV providers are well positioned to integrate MOUD into HIV treatment settings, but thus far only buprenorphine has been adopted in HIV practice. In the buprenorphine–HIV evaluation and support (BHIVES) collaborative (a demonstration of integrated care for HIV and OUD), people living with HIV and OUD who received buprenorphine from an HIV clinic provider decreased opioid use [6], increased ART use [7] and experienced higher‐quality HIV care [8]. HIV treatment guidelines recommend opioid agonist therapy as a key treatment strategy for engaging people who inject drugs (PWID) in HIV treatment [9]. However, retention on methadone and buprenorphine may be limited due to daily dosing requirements. While the recent emergence of extended‐release formulations may mitigate daily dosing challenges, they are not widely available and some patients prefer alternatives to agonist treatment.
Extended‐release naltrexone (XR‐NTX), a deep muscle opioid antagonist injection that lasts 28 days, may be preferred by some people living with HIV who are seeking a non‐narcotic treatment option and/or once a month dosing, thus potentially increasing the number of people who engage in OUD treatment [10]. In inpatient specialty addiction treatment settings, XR‐NTX decreases opioid use comparable to sublingual buprenorphine after receiving the first XR‐NTX injection while an inpatient [11, 12]. However, induction onto XR‐NTX following medically supervised withdrawal from active opioid use is challenging, and resulted in suboptimal outcomes compared to sublingual buprenorphine in intent‐to‐treat (ITT) analyses [12, 13]. In incarcerated individuals with OUD and HIV, XR‐NTX initiated prior to release increased HIV viral suppression at 6 months compared with those randomized to placebo [14]. Similarly, a long‐acting naltrexone implant achieved greater HIV suppression compared to oral naltrexone in a trial in Russia [15].
Most clinical trials of XR‐NTX for OUD treatment have been conducted in carefully controlled settings, typically with initiation as an inpatient or during incarceration. XR‐NTX improved retention and reduced alcohol use when integrated into outpatient primary care clinics for treatment of alcohol use disorder [16], but has not been tested for treatment of OUD in outpatient HIV clinic settings. A pilot trial in people living with HIV suggested that HIV clinic‐based XR‐NTX was feasible and acceptable to patients with untreated OUD and HIV [17].
The National Drug Abuse Treatment Clinical Trials Network (CTN) Comparing Treatments for HIV‐Infected Opioid Users in an Integrated Care Effectiveness Study (CHOICES; CTN‐0067) compared the effectiveness of HIV clinic‐based XR‐NTX versus TAU in engaging people with an OUD in care to improve HIV viral suppression. We hypothesized that XR‐NTX would be non‐inferior to TAU for the primary outcome of HIV viral suppression at 24 weeks. Secondary aims compared the effectiveness of XR‐NTX versus TAU on opioid use and other secondary outcomes including CD4 count, receipt of ART, ART adherence, HIV clinic visits, overall health and mortality risk, HIV risk behaviors and quality of life at 24 weeks.
METHODS
Study design, setting and participants
The CHOICES study (ClinicalTrials.gov NCT03275350) was an open‐label, randomized, non‐inferiority comparative effectiveness trial of office‐based XR‐NTX for 24 weeks (approximately 6‐monthly injections) versus TAU in people with untreated OUD and HIV at baseline. The Advarra Institutional Review Board (IRB00000971) reviewed and approved the study and served as a single IRB for the study, with participating sites deferring to its regulatory role.
HIV primary care clinics in six geographically diverse US HIV clinics (Baltimore, MD; Chicago, IL; Lexington, KY; Miami, FL; Tarzana, CA; Washington, DC) served as study sites. Clinics were selected based on the availability of office‐based buprenorphine or methadone as TAU and community prevalence of untreated OUD and HIV. All participants were offered ART and other routine HIV and primary care. Participants completed written informed consent and passed a consent comprehension quiz prior to enrollment. Translated consent forms and surveys were available in Spanish and English.
Eligible participants included people living with HIV and DSM‐5 moderate or severe OUD, who had uncontrolled HIV disease [RNA polymerase chain reaction (PCR) > 200 copies/ml], were aged at least 18 years, willing to establish HIV care, be randomized to antagonist‐based therapy or TAU, able to provide written informed consent and, if female, to take at least one evidence‐based contraceptive measure. Participants were excluded for severe medical, psychiatric or other substance use disorder that, in the opinion of the study physician, would make study participation hazardous, aspartate aminotransferase or alanine aminotransferase greater than five times the upper limit of normal, INR > 1.5 or platelet count < 100 000; known allergy or sensitivity to naloxone or naltrexone, anticipated surgery; chronic pain requiring ongoing opioid analgesics; body habitus that precluded safe intramuscular injection; receipt of methadone, buprenorphine or XR‐NTX in the 4 weeks prior to consent; taking investigational drugs; currently incarcerated or pending legal action; and, if female, were pregnant or breastfeeding or planning to conceive.
Randomization and masking
A centralized data coordinating center randomized participants 1:1 to office‐based XR‐NTX or TAU using a permuted block design with randomly sized blocks. Randomization was stratified by study clinic. The study was not blinded.
Procedures
The HIV clinic care team and research staff recruited participants from their HIV clinics and interacted with community and hospital‐based outreach services to identify and engage potential participants outside the HIV clinic. Potential participants received an overview of opioid agonist and antagonist therapies and, with verbal consent, completed a pre‐screening interview to assess initial eligibility. Potentially eligible participants then completed written informed consent and completed screening, including laboratory testing, and were randomized to treatment condition, if eligible.
Participants assigned to office‐based XR‐NTX underwent medically supervised withdrawal and naltrexone induction in outpatient or residential settings in accordance with the package insert and published outpatient protocols [18, 19, 20]. XR‐NTX (4 ml, 380 mg of naltrexone base) was administered as a gluteal intramuscular injection (alternating sides monthly) at induction (week 0) and at treatment weeks 4, 8, 12, 16 and 20 for a maximum of six doses.
Participants assigned to the TAU group were offered the standard treatment for OUD provided at each HIV clinic. All clinics offered opioid agonist treatment services (buprenorphine or methadone), with the schedule of medical care and behavioral support determined by the treating provider. Study clinicians provided all participants medical management, a brief counseling intervention delivered by medical providers to improve patient responses to MOUD treatments delivered in medical settings [21].
Participants completed urine drug screens (UDS) and surveys regarding substance use and HIV measures at baseline and weeks 0, 4, 8, 12, 16, 20 and 24. HIV viral load and CD4 count were assessed at baseline, 12 and 24 weeks. Adverse events were elicited at each study visit.
Outcomes
We compared treatment groups for the primary outcome of HIV viral suppression, defined as HIV‐1 RNA ≤ 200 copies/ml at 24 weeks from time of randomization. Treatment groups were compared for the main secondary outcomes of proportion with any opioid use in the past 30 days by self‐report, proportion with any opioid use in the past 30 days by UDS, and number of days of use by time‐line follow‐back at 24 weeks as well as CD4 count, VACS index (a marker of overall health and mortality risk in HIV‐infected patients) [22], receipt of ART, ART adherence (100% prescribed ART in past month), retention in HIV care (proportion of participants with at least one HIV primary care visit in the past 12 weeks) measured at week 24, HIV risk behaviors (past 30‐day injection drug use, unprotected sex, multiple sexual partners) [23], quality of life visual analog scale (EuroQol Group 5D[24]) at 24 weeks.
Analysis
Determination of non‐inferiority margin and sample size
Virological suppression in people living with HIV receiving buprenorphine in the 2004–09 BHIVES collaborative was 57% at 6 months [7], and we assumed that in the absence of any treatment for OUD, the suppression rate would be approximately 15%. Thus, the risk difference compared to placebo would be 57–15% = 42%. A clinically reasonable preserved fraction was chosen as ⅔, implying a non‐inferiority margin in the risk difference of 42% × ⅓ = 14%. Therefore, if the true suppression probability for TAU was approximately 57% (as for BHIVES), any true XR‐NTX suppression probability greater than 43% would indicate that XR‐NTX is not inferior to TAU. On the risk ratio (RR) scale, the margin was thus set at 0.43/0.57 = 0.754. We estimated that a sample size of 350 (175/arm) would grant at least 80% power for the non‐inferiority conclusion using a random‐assignment bootstrap approach, calculating the point estimate from bootstrapped participant data observed in the pilot study [17]. Enrollment was halted at 33% of target sample size due to slow recruitment resulting from advances in HIV treatment (e.g. widespread uptake of potent, one‐pill, once‐daily integrase inhibitors and increased treatment of PWID), giving rise to an effective power level of approximately 37% based on our a priori assumptions.
Statistical analyses
Participant demographic and clinical characteristics were summarized overall and by treatment arm. Study site and baseline alcohol use disorder were included as pre‐specified covariates in all models, with the two smallest‐enrolling sites combined in order to achieve model convergence. In a preliminary analysis there was no evidence in these data for a site × intervention interaction (intervention effect heterogeneity, P < 0.38), implying that using fixed parameters for sites would closely approximate using a random site effect. Therefore, given the relatively small number of sites and limited data, fixed site effects were used to provide stable estimates. The primary analysis was a non‐inferiority comparison of HIV viral suppression rates between treatment arms at 24 weeks with a pre‐specified non‐inferiority margin of 0.75. The binary repeated measure of viral load (RMVL) model [25] was used to predict the log of the RR for suppression via a generalized estimating equation (GEE) model with an exchangeable covariance structure. This longitudinal model incorporated two post‐randomization time‐points for each patient (12 and 24 weeks), with a contrast used to estimate the treatment effect at 24 weeks. The primary analysis was conducted under ITT principals with missing data imputed as unsuppressed [i.e. missing data treated as ‘not at random’ (MNAR)]. As a sensitivity analysis, the model was re‐estimated in the per‐protocol population (those who received at least one dose of their assigned study medication); several other sensitivity analyses were also conducted (Supporting information, Table S1). We conducted a Bayes factor analysis comparing the likelihood of the results under the non‐inferiority versus inferiority hypotheses [26]. We estimated subgroup effects for sex, race and ethnicity with treatment effect by characteristic interactions.
We assessed opioid use at 24 weeks using two self‐report metrics—the number of days used and any use in the past month (collected with time‐line follow‐back)—as well as UDS positivity. The number of days of opioid use was analyzed with negative binomial regression. Any self‐reported use and UDS positivity were analyzed with logistic regression. Treatment effect estimates were contrasts of expected marginal counts/probabilities under two competing counterfactual scenarios: if everyone in the study were treated with XR‐NTX versus everyone in the study treated with TAU. Missing data were imputed as opioid‐positive; a multiple imputation model analyzed days of use with parameters set to the observed values among the subset of participants reporting ≥ 1 day of use. Confidence intervals (CIs) were obtained by bootstrapping.
Linear mixed models compared changes in mean VACS index score, CD4 cell count and quality of life between randomization and 24 weeks by treatment arm using superiority hypothesis testing. Fixed effects in the model were time (baseline, 24 weeks), treatment arm and their interaction, geographic site and baseline AUD. Subject‐level random intercepts were included to account for repeated measurements. Change in the binary outcomes of ART prescription using and sexual risk behaviors were analyzed using generalized linear mixed models with a similar strategy. Rates of 100% ART adherence and HIV care engagement at 24 weeks were compared between treatment arms using χ2 tests. Analyses were conducted using SAS version 9.4 and R version 3.6.2.
Role of the funding source
The US National Institutes of Health, National Institute on Drug Abuse (NIDA) funded the study. The study received donated extended‐release naltrexone 380 mg injections from Alkermes (Waltham, MA, USA), which played no role in study design, analysis, manuscript development or publication decisions. The CTN is a cooperative agreement. Study investigators and NIDA personnel collaborated in the development, implementation, analysis and manuscript preparation. Emmes Co. (Rockville, MD, USA), with a NIDA contract, provided independent review of study implementation, supported web‐based data collection, and conducted the primary outcome analysis. The corresponding author had full access to all the data following Emmes’ Company primary outcome analysis and had final responsibility for the decision to submit for publication.
RESULTS
Of 376 individuals screened for potential participation, 262 were ineligible, with HIV viral suppression being the leading reason for exclusion (Figure 1). Among participants who were not included, the average age was 47 years, 60% were male, 11% were Hispanic, 62% were black and 22% were white. Of 114 randomized participants (n = 55 XR‐NTX; n = 59 TAU), 98 (86%) were retained in the study at 24 weeks (n = 47 XR‐NTX; n = 51 TAU). At baseline, the majority of participants were male (62%), black (56%) or Latino/Hispanic (12%), with at least a high school education (52%). Only 4% were employed; 86% reported a history of incarceration and 39% reported at least 1 day of homelessness in the past month. At baseline, the mean log10 HIV viral load was 4.0 copies/ml [standard deviation (SD) = 0.97, median = 4.2], mean CD4 count was 412 cells/mm3 (SD = 298) and 61% of participants were hepatitis C antibody‐positive. Substance use by UDS was common among participants at baseline, with 44% meeting criteria for alcohol use disorder, 62% UDS positive for fentanyl, 47% positive for other opioids and 61% positive for cocaine; 57% reported a history of overdose. Baseline demographic and clinical characteristics were comparable by treatment arm (Table 1).
FIGURE 1.
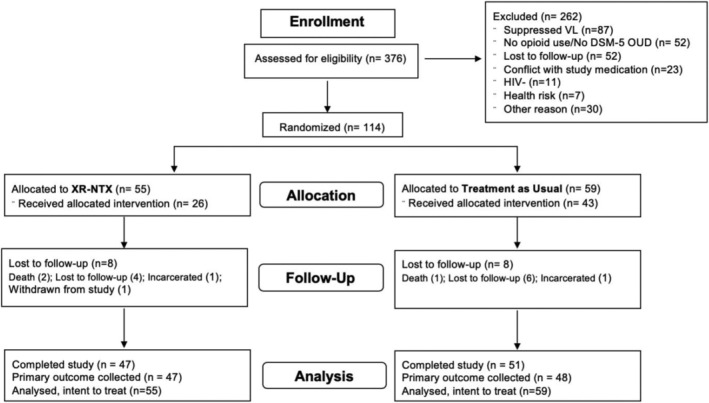
CONSORT diagram
TABLE 1.
Participant characteristics by treatment arm, n = 114 CHOICES participants
| Characteristic | Overall | XR‐NTX n = 55 | TAU n = 59 |
|---|---|---|---|
| Mean age (SD) | 47 (11.1) | 48 (10.8) | 46 (11.5) |
| Male gender | 71 (62.3%) | 32 (58.2%) | 39 (66.1%) |
| Race | |||
| Black | 64 (56.2%) | 30 (54.5%) | 34 (57.6%) |
| White | 42 (36.8%) | 19 (34.5%) | 23 (39%) |
| Other | 8 (7%) | 6 (10.9%) | 2 (3%) |
| Hispanic ethnicity | 14 (12.3%) | 9 (16.4%) | 5 (8.5%) |
| < High school education | 59 (51.8%) | 29 (52.7%) | 30 (50.8%) |
| Employed | 5 (4.4%) | 4 (7.3%) | 1 (1.7%) |
| Homeless in past 30 days | 44 (38.6%) | 24 (43.6%) | 20 (33.9%) |
| HIV viral load (log10 copies/ml) (SD) | 4.0 (1.0) | 4.0 (0.9) | 4.0 (1.1) |
| Mean CD4 cells/mm3 (SD) (n = 112) | 411.9 (298.2) | 380.7 (248.2) | 439.8 (336.6) |
| HCV antibody‐positive | 69 (60.5%) | 32 (58.2%) | 37 (62.7%) |
| History of incarceration | 98 (86.0%) | 46 (83.6%) | 52 (88.1%) |
| Self‐reported previous overdose | 65 (57.0%) | 31 (56.4%) | 34 (57.6%) |
| Baseline alcohol use disorder | 50 (43.9%) | 25 (45.5%) | 25 (42.4%) |
| UDS | |||
| Opioids (other than fentanyl) | 54 (47.4%) | 27 (49.1%) | 27 (45.8%) |
| Fentanyl | 71 (62.3%) | 36 (65.5%) | 35 (59.3%) |
| Methamphetamine/amphetamines | 8 (7%) | 3 (5.5%) | 5 (8.5%) |
| Cocaine | 69 (60.5%) | 36 (65.5%) | 33 (55.9%) |
SD = standard deviation; CHOICES = Comparing Treatments for HIV‐Infected Opioid Users in an Integrated Care Effectiveness Study; UDS = urine drug screen; XR‐NTX = extended‐release naltrexone; TAU = treatment as usual.
Overall, 69 of 114 (61%) participants received at least one dose of assigned study medication, including 26 of 55 XR‐NTX participants (47%) and 43 of 59 TAU participants (73%). Among XR‐NTX participants who received at least one injection, the mean number of injections was 3.3 (SD = 2.1, median = 3) and seven participants received all six possible injections (27% of those with at least one). Among TAU participants, 37 of 43 (86%) initiated buprenorphine, three of 43 (7%) initiated methadone and three of 43 (7%) initiated oral naltrexone. Among TAU participants receiving at least one dose of buprenorphine or methadone, the mean number of months was 2.9 (SD = 1.7). Five of 43 (12%) TAU participants received opioid agonist treatment for all 6 months. Excluding 10 participants who only received one prescription (and thus probably never received more than an induction dose), the mean daily dose of buprenorphine was 14.6 mg. The mean daily dose of methadone was 73.3 mg. Of the 92 of 114 participants (81%) who received ART during the study, 84 (91%) received an integrase‐inhibitor‐based regimen, typically as a single daily pill. Receipt of ART was not correlated with initiation of MOUD (83% of those initiating MOUD also started ART versus 78% not initiating MOUD, P = 0.5).
Table 2 and Figure 2 present the results of the primary study analyses. Of 114 randomized participants, 83.3% had HIV viral load collected at week 24, including 48 of 59 TAU participants (81.4%) and 47 of 55 XR‐NTX participants (85.5%). With missing viral load data treated as unsuppressed, 29 of 55 participants (52.7%) in the XR‐NTX arm and 29 of 59 participants (49.2%) in the TAU arm achieved HIV viral suppression at 24 weeks (RR = 1.064; 95% CI = 0.748, 1.514). As the lower confidence limit of the RR of 0.748 did not exceed the pre‐specified non‐inferiority margin of 0.754, we were unable to conclude that XR‐NTX was non‐inferior to TAU for achieving viral suppression.
TABLE 2.
Non‐inferiority analyses of HIV viral suppression by treatment arm (n = 114)
| Overall | XR‐NTX | TAU | RR (95% CI) | |
|---|---|---|---|---|
| Intent‐to‐treat (n = 114) | ||||
| Missing imputed as unsuppressed | ||||
| 12 weeks | 44/114 (38.6%) | 23/55 (41.8%) | 21/59(35.6%) | 1.15 (0.73, 1.81) |
| 24 weeks a | 58/114 (50.9%) | 29/55 (52.7%) | 29/59 (49.2%) | 1.06 (0.748, 1.51) |
| Per‐protocol b (n = 69) | ||||
| Missing as unsuppressed | ||||
| 12 weeks | 27/69 (39%) | 11/26 (42.3%) | 16/43 (37.2%) | 1.18 (0.68, 2.04) |
| 24 weeks | 38/69 (55%) | 15/26 (57.7%) | 23/43 (53.5%) | 1.03 (0.69, 1.55) |
Primary study outcome;
per‐protocol population includes 69 participants who received at least one dose of their assigned study medication.
RR = relative risk; XR‐NTX = extended‐release naltrexone; TAU = treatment as usual; CI = confidence interval.
The critical value for the 95% lower confidence limit (LCL) used to test the primary non‐inferiority hypothesis was 0.75. If the LCL > 0.75, we conclude that XR‐NTX is non‐inferior to TAU for achieving HIV viral suppression.
FIGURE 2.
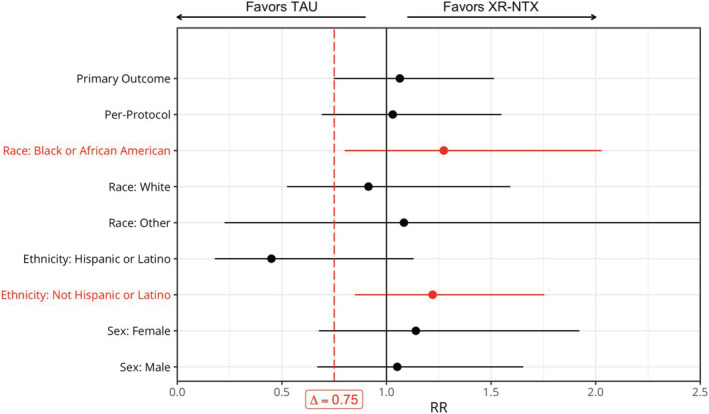
HIV viral suppression at 24 weeks by treatment arm, overall and by pre‐specified subgroups (n = 114), with 95% confidence limits [truncated at (0, 2.5)]. Δ = 0.75 is the pre‐specified non‐inferiority margin; if the lower confidence limit of the RR > Δ, we conclude that XR‐NTX is non‐inferior to TAU for achieving HIV viral suppression. These significant findings are noted in red. TAU = treatment as usual; XR‐NTX = extended‐release naltrexone
Given the limited sample size accrued in the trial, similar observed rates of viral suppression between the two arms, and an underpowered hypothesis test, we performed a Bayes factor analysis comparing the relative likelihood of the observed results under non‐inferiority versus inferiority of XR‐NTX versus TAU (at the pre‐specified non‐inferiority margin of 14%). The Bayes factor comparing these hypotheses was 31.1, suggest that non‐inferiority of XR‐NTX is 31.1 times more likely than inferiority, given the observed data. Applying a suggested cut‐off of Bayes factor ≥ 3 to quantify ‘substantial’ evidence in favor of one hypothesis over an alternative [27], our data provide substantial evidence that XR‐NTX has no more than a 3% absolute reduction in efficacy compared to TAU for achieving viral suppression.
Table 2 and Figure 2 present pre‐specified sensitivity and subgroup analyses. Non‐inferiority was not demonstrated in the per‐protocol population. In subgroup analyses, XR‐NTX was non‐inferior to TAU in black and non‐Hispanic participants. Among black participants, 56.7% assigned to XR‐NTX and 41.2% assigned to TAU achieved viral suppression (RR = 1.27; 95% CI = 0.80, 2.03). Among non‐Hispanic participants, 56.5% assigned to XR‐NTX and 46.3% assigned to TAU achieved viral suppression (RR = 1.22; 95% CI = 0.85, 1.75). Additional sensitivity analyses are presented in Supporting information, Table S1.
Opioid use outcomes at 24 weeks are presented in Table 3. The mean adjusted number of days of self‐reported opioid use in the past 30 days was 11.7 in the XR‐NTX arm and 14.8 in the TAU arm [mean difference (MD) = –3.1; 95% CI = –8.73, 1.05], indicating no significant difference between treatment arms in ITT analysis. However, in the per‐protocol population, there were fewer adjusted days of self‐reported opioid use in the XR‐NTX group compared to TAU (6.0 versus 13.6 days; MD = –7.55; 95% CI = –13.8, −0.22). Similarly, self‐reported use of any opioid was comparable between groups in ITT analysis and XR‐NTX participants were less likely to report any opioid use in the per‐protocol population. Biomarkers confirmed findings of self‐reported opioid use (Table 3).
TABLE 3.
Opioid use at 24 weeks by treatment arm (n = 114)
| Population | XR‐NTX Mean/% | TAU | MD/RR (95% CI) |
|---|---|---|---|
| Mean days of self‐reported use (last 30 days) | |||
| Intent‐to‐treat | 11.73 | 14.81 | −3.07 (−8.73, 1.05) |
| Per‐protocol | 6.02 | 13.58 | −7.55 (−13.78, −0.22) |
| Probability of any self‐reported use (last 30 days) | |||
| Intent‐to‐treat | 64.2% | 68.9% | 0.93 (0.72, 1.20) |
| Per‐protocol | 39.6% | 66.9% | 0.59 (0.30, 0.99) |
| UDS positivity | |||
| Intent‐to‐treat | 62.2% | 69.1% | 0.90 (0.69, 1.18) |
| Per‐protocol | 38.5% | 70.0% | 0.55 (0.29, 0.92) |
MD = mean difference; RR = relative risk; CI = confidence interval; UDS = urine drug screen; XR‐NTX = extended‐release naltrexone; TAU = treatment as usual.
Secondary outcomes of VACS index, receipt of ART prescription, ART adherence, HIV clinic visits, HIV risk behaviors and quality of life were similar between treatment arms (i.e. no comparisons met the statistical threshold for significance) (Supporting information, Tables S2–S5).
Twenty‐one participants experienced at least one mild‐to‐moderate adverse event [10 of 55 (18.2%) XR‐NTX; 11 of 59 (18.6%) TAU] and 12 participants experienced at least one serious adverse event [five of 55 (9.1%)] XR‐NTX; seven of 59 (11.9%) TAU] (Table 4). There were three deaths [two of 55 (3.6%) XR‐NTX; one of 59 (1.7%) TAU]. No serious adverse events or deaths were related to study medication.
TABLE 4.
CHOICES adverse events and serious adverse events
| XR‐NTX (n = 55) | TAU (n = 59) | |
|---|---|---|
| Treatment emergent adverse events (mild or moderate) | ||
| Participants with one or more adverse events | 10 (18.2%) | 11 (18.6%) |
| Number of adverse events | 17 | 11 |
| Type of adverse event | ||
| Infections | 2 (3.6%) | 5 (8.5%) |
| Injury, poisoning and procedural complications | 1 (1.8%) | 2 (3.4%) |
| Gastrointestinal disorders | 3 (5.5%) | 0 (0.0%) |
| Respiratory disorders | 2 (3.6%) | 1 (1.7%) |
| Psychiatric disorders | 2 (3.6%) | 1 (1.7%) |
| Nervous system disorders | 2 (3.6%) | 0 (0.0%) |
| Vascular disorders | 1 (1.8%) | 1 (1.7%) |
| Neoplasms benign, malignant and unspecified | 0 (0.0%) | 1 (1.7%) |
| Musculoskeletal and connective tissue disorders | 1 (1.8%) | 0 (0.0%) |
| Metabolism and nutrition disorders | 1 (1.8%) | 0 (0.0%) |
| Transaminases increased | 1 (1.8%) | 0 (0.0%) |
| Skin and subcutaneous tissue disorders | 1 (1.8%) | 0 (0.0%) |
| Treatment emergent serious adverse events | ||
| Participants with one or more serious adverse events | 5 (9.1%) | 7 (11.9%) |
| Number of serious adverse events | 6 | 7 |
| Type of serious adverse event | ||
| Infections | 2 (3.6%) | 3 (5.1%) |
| Psychiatric disorders | 2 (3.6%) | 1 (1.7%) |
| Injury, poisoning and procedural complications | 0 (0.0%) | 2 (3.4%) |
| Vascular disorders | 1 (1.8%) | 0 (0.0%) |
| Respiratory disorders | 1 (1.8%) | 0 (0.0%) |
| Neoplasms benign, malignant and unspecified | 0 (0.0%) | 1 (1.7%) |
| Mortality events | ||
| All deaths | 2 (3.6%) | 1 (1.7%) |
| Overdose deaths | 0 | 0 |
| AIDS‐related deaths | 1 | 0 |
| Pneumonia | 0 | 1 |
| Cardiac | 1 | 0 |
XR‐NTX = extended‐release naltrexone; TAU = treatment as usual; CHOICES = Comparing Treatments for HIV‐Infected Opioid Users in an Integrated Care Effectiveness Study.
DISCUSSION
The CHOICES study informs the utility of providing long‐acting opioid antagonist treatment to people with untreated HIV and OUD in six geographically diverse US cities. The study documents marked and similar improvements in HIV viral suppression at 24 weeks with both XR‐NTX and TAU. The trial did not meet criteria for non‐inferiority of XR‐NTX versus TAU, due to limited power. Bayes factor analysis, however, suggests that the observed data increases our confidence × 31 that the treatment groups did not substantially differ in HIV viral suppression. Thus, study findings may be interpreted as overall supportive, but not conclusive, of the non‐inferiority of HIV clinic‐based XR‐NTX versus TAU for facilitating HIV viral suppression. Pre‐specified subgroup analyses demonstrated that XR‐NTX was non‐inferior to TAU among participants who identified as black/African American and non‐Hispanic ethnicity. Secondary outcomes of VACS index, receipt of ART prescription, ART adherence, HIV clinic visits, HIV risk behaviors and quality of life did not differ by treatment arm.
Our findings advance those of previous studies of the impact of MOUD on HIV clinical outcomes in people with uncontrolled HIV disease and OUD, and in particular the potential effect of XR‐NTX on HIV viral suppression. Remarkably, half of participants in this high‐needs study population, overall, achieved HIV viral suppression by 24 weeks. In a US trial randomizing incarcerated individuals with HIV and OUD to XR‐NTX versus placebo injection prior to release, 30% of those with non‐suppressed HIV at baseline who received XR‐NTX achieved HIV viral suppression at 6 months [14]. In a trial in Russia, people with OUD and untreated HIV were randomized to receive long‐acting naltrexone implants or daily oral naltrexone dosing. At 24 weeks, 38% of those assigned to long‐acting naltrexone and 35% of those assigned to oral naltrexone achieved an HIV viral load < 400 copies/ml [15]. HIV viral suppression rates of 52.7% at 24 weeks among those randomized to XR‐NTX in the CHOICES trial exceeds viral suppression observed in these prior studies, as well as a systematic review of 32 mainly observational studies that reported a 45% increase in HIV viral suppression attributed to opioid agonist treatment [4]. Our results suggest that initiating XR‐NTX in a non‐addiction specialty outpatient setting facilitates HIV viral suppression on a level comparable to initiating opioid agonist treatment. While OUD treatment adherence most probably mediates the effect of XR‐NTX on HIV viral suppression [15], XR‐NTX may also mitigate risky decision‐making and improve treatment adherence through central effects on the reward pathway [28].
The proportion of participants with successful outpatient induction on XR‐NTX was lower than that reported in previous trials in which induction occurred in more controlled environments or where eligibility criteria excluded people with active opioid use at the time of enrollment [14, 29]. In one trial demonstrating efficacy of XR‐NTX for community‐dwelling, criminal justice‐involved adults with OUD, participants were required to be seeking non‐opioid agonist treatment and be opioid‐free on UDS prior to randomization; 95% successfully completed outpatient XR‐NTX induction [29]. In another trial, 100% of incarcerated people with OUD who had abstained from opioids received their first XR‐NTX injection in the week prior to release [14]. In a large comparative effectiveness trial of XR‐NTX versus buprenorphine for treatment of OUD, participants were recruited from inpatient medically supervised withdrawal facilities and initiated treatment in the inpatient setting—treatment initiation was 72% for XR‐NTX and 94% for sublingual buprenorphine [12]. These studies suggest that a controlled setting for XR‐NTX induction is important. Engaging non‐treatment‐seeking patients with untreated OUD through community‐based outreach for XR‐NTX treatment in a less controlled, real‐life outpatient primary care setting probably requires more intensive supports for XR‐NTX induction. For example, qualitative interviews with clinical staff involved in the CHOICES trial identified limited access to inpatient facilities for medically managed opioid withdrawal as a barrier to XR‐NTX induction [30]. Treatment models where patients interested in opioid antagonist treatment receive their first dose of XR‐NTX in an inpatient withdrawal setting followed by linkage to primary care for subsequent doses may increase the feasibility of XR‐NTX in primary care.
Despite limited XR‐NTX uptake, all CHOICES participants experienced marked reductions in opioid use that compare favorably with changes in opioid use in previous trials. In ITT analysis, self‐reported opioid use and UDS opioid positivity decreased comparably in participants assigned to the XR‐NTX arm compared to those assigned to TAU. Among those who initiated OUD treatment, reductions in opioid use were greater in the XR‐NTX than the TAU arm. Our findings contrast with those of a large comparative effectiveness trial of XR‐NTX versus sublingual buprenorphine [12], which demonstrated higher rates of return to opioid use at 24 weeks for XR‐NTX (65%) than for buprenorphine [57%; hazard ratio (HR) = 1·36; 95% CI = 1·10–1·68] in the ITT analysis, and comparable rates of return to use in the per protocol analysis [12]. In a second trial of XR‐NTX versus sublingual buprenorphine conducted in a specialty addiction treatment setting in Norway, XR‐NTX was non‐inferior to buprenorphine for the primary outcome of the proportion of UDS negative for opioids at 12 weeks, and superior to buprenorphine for opioid craving [11]. People with OUD who initiated XR‐NTX in the CHOICES trial may have benefited from the additional wrap‐around primary care, HIV and social services offered in outpatient HIV primary care clinics. Another notable difference between previous trials and the CHOICES study is the high prevalence of fentanyl in the study population. Fentanyl was the most common opioid detected on baseline UDS in the CHOICES study and previous studies were conducted prior to the upsurge in fentanyl use. While fentanyl may decrease the utility of XR‐NTX due to initiation challenges, it is possible that, once started, XR‐NTX might be more effective than buprenorphine in reducing opioid cravings, as suggested by the Norwegian trial [11]. Further research is required to assess the role of fentanyl in starting MOUD treatment. Recently approved long‐acting injectable buprenorphine may also serve as a more equivalent comparator than daily dosed formulations.
The CHOICES trial was limited by a slow pace of recruitment. Enrollment ceased after achieving only 33% of the targeted sample size, which limited power to meet pre‐determined thresholds for statistical significance. The primary reason for slow recruitment was an eligibility requirement of uncontrolled HIV disease, the leading reason for study exclusion. At the time the trial was conceived, achieving virological suppression among people with OUD was still a considerable challenge in many HIV clinics. During the study’s development, highly potent, once‐daily, single‐pill combination formulations of integrase inhibitor‐based regimens became first‐line therapy. National guidelines also emerged encouraging HIV providers to initiate ART in people actively using drugs. Together, these secular trends increased the proportion of Ryan White‐funded clinic patients with HIV viral suppression from 65% in 2010 to 87.5% in 2018 [31], reducing the pool of eligible participants in HIV clinics and transforming study recruitment into more of a ‘seek, test and treat’ approach to OUD and HIV engagement and linkage to care. Top‐recruiting study sites hired peer recovery support specialists to conduct direct community outreach and assist with study engagement and retention [30]. It is possible that the additional support received through study participation contributed to HIV outcomes, apart from MOUD treatment. Diagnosing and treating HIV in people who use drugs remains essential to eliminate HIV. The CHOICES study reinforces the need to expand community‐based outreach and engagement efforts beyond the walls of HIV clinics. Further research on the role of peer recovery support specialists and other engagement strategies are urgently needed.
An additional limitation is that excluding people with untreated OUD and already suppressed viral loads may have increased study internal validity at the expense of generalizability to people engaged in HIV care with untreated OUD, who remain at risk for overdose. Alternative primary outcomes such as maintenance of viral suppression have been used in other studies that included such participants [14], but would have required a larger sample size which was not feasible and could have biased the study results toward non‐inferiority.
One strength of the current study was its ability to recruit and retain a highly vulnerable population of people on the margins of the health‐care system who had high rates of unemployment, homelessness, history of overdose, and criminal justice involvement. These are people who may be most likely to benefit from long‐acting medication formulations and an essential group required for achieving HIV elimination. A second strength of the current study was the high proportion of participants from racial and ethnic minority groups and inclusion of monolingual Spanish‐speaking participants. Pre‐planned subgroup analyses demonstrated that XR‐NTX was non‐inferior to TAU in the ITT analysis for achieving HIV viral suppression among African American/black participants and among those of non‐Hispanic ethnicity. Further research is urgently needed to explore differences in the effects of and to improve access to MOUD treatment in people of minority race/ethnicity. A third strength was the novel primary outcome of HIV viral suppression, as previous studies focused on OUD outcomes.
Overall, the CHOICES trial affirms the importance of medications for OUD treatment for achieving HIV viral suppression in people who use drugs. The study suggests, but does not definitively demonstrate, that uptake of XR‐NTX in HIV clinics may improve HIV viral suppression and decrease opioid use as well as opioid agonist treatment. Outpatient initiation of XR‐NTX for the treatment of OUD among people with uncontrolled HIV disease is challenging. Better induction strategies are needed to successfully integrate XR‐NTX into HIV primary care settings. Based on the experiences of clinical staff in the CHOICES trial, one strategy may require development of systems of care that increase the availability of inpatient induction services with linkage to outpatient continuation services. Once initiated on XR‐NTX, patients may then benefit from decreased opioid use that otherwise hinders ART adherence and HIV disease control and stabilization.
CLINICAL TRIAL REGISTRATION
The study was registered with ClinicalTrials.gov, NCT03275350, https://clinicaltrials.gov/ct2/show/NCT03275350.
DECLARATION OF INTERESTS
P.T.K. reports awards from the NIH National Institute on Drug Abuse and serves as principal investigator for NIH‐funded studies that accept donated study medication from Indivior (buprenorphine) and Alkermes (XR‐NTX). Alkermes donated XR‐NTX for CHOICES study participants. H.T. reports grants from Gilead Sciences. Other authors report no conflicts of interest. This article was prepared while P.J. was employed at National Institute on Drug Abuse. P.L. was substantially involved in a design and implementation of CTN0067 (CHOICES) study supported by UG1DA015815, UG1DA013732, UG1DA01372, consistent with her role as Scientific Officer. She had no substantial involvement in the other cited grants. The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy or position of the US Department of Health and Human Services or any of its affiliated institutions or agencies.
AUTHOR CONTRIBUTIONS
Philip Korthuis: Conceptualization; funding acquisition; investigation; methodology; project administration. Ryan Cook: Formal analysis; methodology. Paula Lum: Conceptualization; investigation; methodology; project administration. Elizabeth Waddell: Investigation; project administration. Hansel Tookes: Investigation. Pamela VERGARA‐RODRIGUEZ: Investigation. Lynn Kunkel: Project administration. Gregory Lucas: Investigation. Allan Rodriguez: Investigation. Sarann Bielavitz: Project administration. Laura Fanucchi: Investigation. Kim Hoffman: Project administration. Ken Bachrach: Project administration. Elizabeth Payne: Data curation; formal analysis; methodology. Julia Collins: Investigation. Abigail Matthews: Data curation; formal analysis; methodology. Neal Oden: Formal analysis; methodology. Petra Jacobs: Methodology; project administration. Eve Jelstrom: Project administration. James Sorensen: Project administration. Dennis McCarty: Conceptualization; methodology; project administration.
DATA SHARING
De‐identified data from the study will be available to researchers free of charge at the NIDA Data Share website (http://datashare.nida.nih.gov/) within 18 months of study completion and data analysis, per CTN data‐sharing policy. Users must register for NIDA Data Share by providing a name and valid e‐mail address in order to download data, and agree to use those data in accordance with the NIDA Data Share Agreement detailed on the website.
Supporting information
Table S1. Additional sensitivity analyses for the primary outcome of HIV viral suppression.
Table S2. VACS Index, CD4 cell count, and ART prescriptions, over time by treatment arm, N = 114 CHOICES participants.
Table S3. 100% ART adherence and HIV care visits at 24 weeks by treatment group, N = 114 CHOICES participants.
Table S4. Sexual risk behaviors over time by treatment arm, N = 114 CHOICES participants.
Table S5. Overall quality of life (Equation 5‐D visual analogue scale) over time by treatment arm, N = 114 CHOICES participants.
ACKNOWLEDGEMENTS
This research was supported through cooperative agreements and grants from the US National Institutes of Health National Institute on Drug Abuse (UG1DA015815, UG1DA013732, UG1DA01372, K24DA035684) and Agency for Healthcare Research and Quality (K12HS026370). The authors wish to thank the study participants, HIV clinic staff and outreach workers who contributed to the study’s success.
Korthuis PT, Cook RR, Lum PJ, Waddell EN, Tookes H, Vergara‐Rodriguez P, et al. HIV clinic‐based extended‐release naltrexone versus treatment as usual for people with HIV and opioid use disorder: a non‐blinded, randomized non‐inferiority trial. Addiction. 2022;117:1961–1971. 10.1111/add.15836
Funding information Agency for Healthcare Research and Quality, Grant/Award Number: K12HS026370; National Institute on Drug Abuse, Grant/Award Numbers: K24DA035684, UG1DA01372, UG1DA013732, UG1DA015815
REFERENCES
- 1. Roux P, Carrieri MP, Villes V, Dellamonica P, Poizot‐Martin I, Ravaux I, et al. The impact of methadone or buprenorphine treatment and ongoing injection on highly active antiretroviral therapy (HAART) adherence: evidence from the MANIF2000 cohort study. Addiction. 2008;103:1828–36. [DOI] [PubMed] [Google Scholar]
- 2. Lucas GM, Chaudhry A, Hsu J, Woodson T, Lau B, Olsen Y, et al. Clinic‐based treatment of opioid‐dependent HIV‐infected patients versus referral to an opioid treatment program: a randomized trial. Ann Intern Med. 2010;152:704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edelman EJ, Chantarat T, Caffrey S, Chaudhry A, O'Connor P.G., Weiss L, et al. The impact of buprenorphine/naloxone treatment on HIV risk behaviors among HIV‐infected, opioid‐dependent patients. Drug Alcohol Depend. 2014;139:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, et al. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta‐analysis. Clin Infect Dis. 2016;63:1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta‐analysis of cohort studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB, et al. Drug treatment outcomes among HIV‐infected opioid‐dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56:S33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altice FL, Bruce RD, Lucas GM, et al. HIV treatment outcomes among HIV‐infected, opioid‐dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56:S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korthuis PT, Fiellin DA, Fu R, Lum PJ, Altice FL, Sohler N, et al. Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J Acquir Immune Defic Syndr. 2011;56:S83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the international antiviral society—USA panel. JAMA. 2012;308:387–402. [DOI] [PubMed] [Google Scholar]
- 10. Solli KK, Kunoe N, Latif ZE, Sharma‐Haase K, Opheim A, Krajci P, et al. Availability of extended‐release naltrexone may increase the number of opioid‐dependent individuals in treatment: extension of a randomized clinical trial. Eur Addict Res. 2019;25:303–9. [DOI] [PubMed] [Google Scholar]
- 11. Tanum L, Solli KK, Latif ZE, Benth JS, Opheim A, Sharma‐Haase K, et al. Effectiveness of injectable extended‐release naltrexone versus daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended‐release naltrexone versus buprenorphine–naloxone for opioid relapse prevention (X:BOT): a multicentre, open‐label, randomised controlled trial. Lancet. 2018;391:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, et al. Extended‐release injectable naltrexone for opioid use disorder: a systematic review. Addiction. 2018;113:1188–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Springer SA, Di Paola A, Azar MM, Barbour R, Biondi BE, Desabrais M, et al. Extended‐release naltrexone improves viral suppression among incarcerated persons living with HIV with opioid use disorders transitioning to the community: results of a double‐blind, placebo‐controlled randomized trial. J Acquir Immune Defic Syndr. 2018;78:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krupitsky E, Blokhina E, Zvartau E, Verbitskaya E, Lioznov D, Yaroslavtseva T, et al. Slow‐release naltrexone implant versus oral naltrexone for improving treatment outcomes in people with HIV who are addicted to opioids: a double‐blind, placebo‐controlled, randomised trial. Lancet HIV. 2019;6:e221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JD, Grossman E, Huben L, Manseau M, McNeely J, Rotrosen J, et al. Extended‐release naltrexone plus medical management alcohol treatment in primary care: findings at 15 months. J Subst Abuse Treat. 2012;43:458–62. [DOI] [PubMed] [Google Scholar]
- 17. Korthuis PT, Lum PJ, Vergara‐Rodriguez P, Ahamad K, Wood E, Kunkel LE, et al. Feasibility and safety of extended‐release naltrexone treatment of opioid and alcohol use disorder in HIV clinics: a pilot/feasibility randomized trial. Addiction. 2017;112:1036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, Woody G. Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse. 2012;38:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bisaga A, Mannelli P, Yu M, Carpenter KM, Mariani JJ, Levin FR, et al. Outpatient transition to extended‐release injectable naltrexone for patients with opioid use disorder: a phase 3 randomized trial. Drug Alcohol Depend. 2018;187:171–8. [DOI] [PubMed] [Google Scholar]
- 20. Rudolf G, Walsh J, Plawman A, Gianutsos P, Alto W, Mancl L, et al. A novel non‐opioid protocol for medically supervised opioid withdrawal and transition to antagonist treatment. Am J Drug Alcohol Abuse. 2018;44:302–9. [DOI] [PubMed] [Google Scholar]
- 21. Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O'Connor PG, et al. Counseling plus buprenorphine–naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365–74. [DOI] [PubMed] [Google Scholar]
- 22. Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, et al. Predictive accuracy of the veterans aging cohort study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, et al. Preparations for AIDS vaccine trials. An automated version of the risk assessment battery (RAB): enhancing the assessment of risk behaviors. AIDS Res Hum Retroviruses. 1994;10:S281–3. [PubMed] [Google Scholar]
- 24. The EuroQuol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 25. Rose CE, Gardner L, Craw J, Girde S, Wawrzyniak AJ, Drainoni ML, et al. A comparison of methods for analyzing viral load data in studies of HIV patients. PLOS ONE. 2015;10:e0130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Ravenzwaaij D, Monden R, Tendeiro JN, Ioannidis JPA. Bayes factors for superiority, non‐inferiority, and equivalence designs. BMC Med Res Methodol 2019;19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee MD, Wagenmakers EJ. Bayesian Cognitive Modeling: A Practical Course. Cambridge, UK: Cambridge University Press; 2014. [Google Scholar]
- 28. Kohno M, Dennis LE, McCready H, Schwartz DL, Hoffman WF, Korthuis PT. A preliminary randomized clinical trial of naltrexone reduces striatal resting state functional connectivity in people with methamphetamine use disorder. Drug Alcohol Depend. 2018;192:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, et al. Extended‐release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med. 2016;374:1232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoffman KA, Baker R, Kunkel LE, Waddell EN, Lum PJ, McCarty D, et al. Barriers and facilitators to recruitment and enrollment of HIV‐infected individuals with opioid use disorder in a clinical trial. BMC Health Serv Res. 2019;19:862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Health Resources and Services Administration . Ryan White HIV/AIDS Program Annual Client‐Level Data Report. Available at: http://hab.hrsa.gov/data/data-reports 2018. Accessed 7 Dec 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Additional sensitivity analyses for the primary outcome of HIV viral suppression.
Table S2. VACS Index, CD4 cell count, and ART prescriptions, over time by treatment arm, N = 114 CHOICES participants.
Table S3. 100% ART adherence and HIV care visits at 24 weeks by treatment group, N = 114 CHOICES participants.
Table S4. Sexual risk behaviors over time by treatment arm, N = 114 CHOICES participants.
Table S5. Overall quality of life (Equation 5‐D visual analogue scale) over time by treatment arm, N = 114 CHOICES participants.


