Summary
Objective
Stiripentol (STP; Diacomit®) is an antiepileptic drug indicated for Dravet syndrome that has been identified as a γ‐aminobutyric acid (GABAergic) positive allosteric modulator. Dravet syndrome is characterized by multiple seizure types: generalized tonic–clonic, focal, myoclonic, and absence seizures. In addition to its antiepileptic effects on tonic–clonic seizures, STP has also been reported to reduce the frequency of atypical absence seizures in patients. Our study focused on STP potential effects on absence seizures, to better characterize its full spectrum of mechanisms of action.
Methods
STP effects on absence seizures were quantified by electroencephalographic recording in two animal models: rats treated with a low dose of pentylenetetrazol (20 mg/kg ip) and rats from the WAG/Rij strain. In addition, we characterized STP effects on T‐type calcium channel activity. Peak currents were recorded with manual patch clamp on cells transfected with cDNA encoding for the human isoform for Cav3.1, Cav3.2, and Cav3.3.
Results
STP administered before pentylenetetrazol almost completely abolished the generation of spike‐and‐wave discharges (SWDs) at the dose of 300 mg/kg. At this dose, STP also statistically significantly decreased SWD cumulated duration and number in WAG/Rij rats. Its antiepileptic effect was maintained in WAG/Rij rats, whose seizures were aggravated by the GABA agonist THIP (gaboxadol hydrochloride). Furthermore, electrophysiological recordings showed that STP inhibits T‐type calcium channel peak activity, with a higher specificity for the Cav3.3 subtype.
Significance
In addition to its previously characterized anticonvulsive properties, these data highlight a new mechanism of action of STP on abnormal thalamocortical activity. This strong antiabsence effect on seizures is correlated with an inhibition of T‐type calcium channels. This new mechanism of action could be implicated in the specificity of STP therapeutic effects in Dravet syndrome.
Keywords: absence seizures, Dravet syndrome, epilepsy, spike‐and‐wave discharges, stiripentol, thalamocortical oscillations
Key Points.
Stiripentol, a GABAergic positive allosteric modulator, suppressed absence seizures in two animal models
In vitro recordings have shown that stiripentol inhibits low‐voltage‐activated calcium channels at physiologically relevant concentrations
These data describe a new mechanism of action for stiripentol that could confer antiabsence properties
1. INTRODUCTION
Stiripentol (STP; Diacomit®) is an antiepileptic drug indicated for Dravet syndrome.
Early studies have demonstrated that STP antiepileptic effects are mediated by a potentiation of γ‐aminobutyric acidergic (GABAergic) transmission. STP increases cerebral GABA concentration by two potential mechanisms: an inhibition of the synaptosomal reuptake of GABA and/or an inhibition of GABA‐transaminase, its degradation enzyme. 1 STP has also been identified as a positive allosteric modulator of GABAergic receptors, 2 with a specificity on alpha3 and delta‐containing receptors. 3 , 4 Until recently, when an additional mechanism mediated through lactate dehydrogenase inhibition has been identified, 5 GABAergic potentiation was thus believed to be the main pharmacological mechanism of action of STP.
Dravet syndrome is characterized by the presence of numerous seizure types, namely generalized, focal, myoclonic, and absence seizures. The first seizure recorded in Dravet syndrome is usually hemiclonic or generalized tonic–clonic. 6 , 7 Electroencephalographic (EEG) recordings can later identify the presence of generalized spike‐and‐wave discharges (SWDs) or polyspike‐and‐wave discharges. 8
Whereas alpha2/3/5 agonists have recently been shown to have antiabsence properties in a genetic animal model, 9 historically, a potentiation of GABAergic transmission was found to aggravate absence seizures. 10 A potentiation of tonic extrasynaptic GABAergic current mediated by delta‐containing receptors aggravates absence epilepsy. 11 Several studies have also shown that absence seizures are aggravated by vigabatrin, an irreversible inhibitor of GABA transaminase, increasing GABA extracellular levels. 12 , 13 , 14
Altogether, the pharmacological profile of STP described so far was not in line with antiabsence properties. However, early preclinical or clinical studies had suggested that STP could modulate SWDs. Thus, preclinical studies in the early 1990s showed that STP could be effective in several animal models of absence seizures. Shen and colleagues used the pentylenetetrazol (PTZ) infusion test, a test predictive for antiabsence effects, and showed that STP was effective in increasing the threshold for the first clonic seizures observed. No EEG recording was performed in these studies. 15 In the late 1980s, a study from Micheletti and colleagues found that STP administration decreased the cumulated duration of absence seizures in GAERS rats (unpublished data). Thereafter, an open trial on 10 children with refractory atypical absence seizures showed an average decrease of 70% of seizures after STP addition to the antiepileptic drugs regimen. 16 These studies suggested that STP pharmacological effects could lay beyond a positive GABAergic modulation, as they suggested that STP could have antiabsence properties. Deciphering these mechanisms could not only identify potential new therapeutic applications, but also bring new insights into Dravet syndrome pathological mechanisms.
The aim of this study was to examine the potential antiabsence effect of STP on two rodent models of absence seizures. We chose to use two animal models, whose absence seizures are generated by different mechanisms. 17 One model is a pharmacological model induced by the blockade of GABA‐A receptor, the intraperitoneal PTZ model. 18 , 19 The other model is a well‐characterized genetic model of absence seizures, WAG/Rij rats. 20 Interestingly, a decrease in alpha3 subunits containing GABAergic receptors has been described in this strain. 21 In addition, an administration of gaboxadol hydrochloride (THIP), a GABAergic agonist with a selectivity for delta‐containing receptors, has been shown to induce or aggravate seizures. 22 To examine the role of GABAergic transmission in STP potential antiabsence effects, we studied the effects of STP on spontaneous seizures in the WAG/Rij rat, as well as on aggravated seizures after THIP administration. Finally, we examined the hypothesis that STP could have an antiepileptic effect mediated by a mechanism common to the different models of absence seizures tested, but unrelated to the GABAergic system. An obvious target was T‐type calcium channels, voltage‐gated channels that have a critical role in thalamocortical oscillations. 23 , 24 With this aim, we used electrophysiological recordings to characterize the effect of STP on T‐type channel activity.
2. MATERIALS AND METHODS
2.1. Animals
All experiments were carried out in accordance with the ARRIVE guidelines and the European Union Directive 2010/63/EU on the protection of animals used for scientific purposes. Protocols were approved by an ethical committee (C2EA #72), and experiments have been designed according to the 3R concept.
Sprague Dawley (SD) rats (Charles River, aged 7–9 weeks at the time of experiment) were housed with food and water ad libitum (A04‐SAFE) and on a 12/12‐h light/dark cycle (7:00 a.m./7:00 p.m.). After at least 1‐week acclimatation, they were administered with 20 mg/kg ip PTZ.
Adult male WAG/Rij rats (Charles River, 8–10 weeks old at the time of ordering) were housed three per cage, with food and water ad libitum (A04‐SAFE) and on a 12/12‐h light/dark cycle (7:00 a.m./7:00 p.m.).
2.2. Surgery
In SD rats, electrodes were implanted at 7 weeks of age, approximately 10–11 weeks of age for WAG/Rij rats. Buprenorphine (.015 mg/kg, 5 ml/kg sc) was administered 30 min before surgery, then paracetamol in drinking water (60 mg/kg/24 h), for 2 days after surgery. The animals were anesthetized under isoflurane and placed in a stereotactic frame (David Kopf Instruments). Cortical electrodes were made of a stainless‐steel screw (FST 19010‐00), and an insulated wire soldered to the screw on one end and to a female eight‐contact connector (Harwin, ref. M80‐8890805) on the other end. Four cortical electrodes were placed in contact with the dura above the frontal and parietal cortex (approximately anteroposterior AP +2.0 and mediolateral ML ±2.5 for frontal and AP −5 and ML ±2.5 for parietal cortex according to the Paxinos and Watson coordinates), and one reference electrode was placed above the cerebellum. Electrodes were secured with dental cement.
Animals were allowed to recover for 1 week before the first EEG recording.
2.3. EEG recording and analysis
Cortical activity was recorded (ADInstruments) using frontal and parietal derivations, with a reference electrode positioned above the cerebellum. EEG was amplified (factor of 10 000), sampled at 1 kHz, high‐pass filter at .016 Hz, low‐pass filter at 100 Hz.
In experiments on the PTZ model, a baseline EEG recording was performed to identify potential spontaneous SWDs. On the day of the experiment, basal EEG activity was recorded for 30 min, then treatments were administered, and EEG was recorded for at least 60 min further.
EEG activity in WAG/Rij rats was recorded three times per week, during the light phase (between 9 a.m. and 3 p.m.). At 12 weeks of age, SWDs were visualized, and the effects of STP (150, 300 mg/kg ip) administration were analyzed between 16 and 20 weeks of age. EEG activity recorded on frontal derivations were analyzed for drug effect quantification. As shown previously, SWDs recorded on parietal derivations were shorter and less structured. 25 STP effects were quantified on three parameters: total duration, mean duration, and SWD number.
Quantitative EEG analysis was performed offline in Labchart software (v8, ADInstruments), manually, by an experimenter blinded to animals’ group and treatment. An SWD was typically defined as an activity lasting for at least 2 s, with at least four paroxysmic events and with an amplitude of paroxysmic events of at least three times the amplitude of the baseline. The first 10 min after drug administration were excluded from the analysis to let the animal return to a baseline behavior and to account for the drug absorption time.
2.4. Treatment
STP (Biocodex) was suspended in TWEEN 80 at 2% (vol/vol) in .9% sterile saline and administered intraperitoneally (10 ml/kg).
THIP (gaboxadol hydrochloride, Sigma, T101) and PTZ (Sigma, MKBV0751V) were dissolved in .9% sterile saline solution and administered intraperitoneally (5 ml/kg).
Each rat received all the different treatments in a randomized order (including vehicle of STP; TWEEN 80 at 2% in saline), with at least 3 days between two administrations.
2.5. Electrophysiological recordings
In electrophysiological studies, the effect of STP at seven different concentrations was analyzed on peak currents recorded with manual patch clamp. Cumulative dose–response was recorded. Three recordings were performed for each concentration, three or four cells per concentration. Experiments were performed on Chinese hamster ovary (CHO) cell cultures transiently transfected with cDNA encoding for the human isoform for Cav3.1 and Cav3.3, or in stably transfected HEK393 cells for Cav3.2 channels. Test compounds were dissolved in dimethylsulfoxide (DMSO), and .3% DMSO solution was used as vehicle.
The cells were continuously maintained in and passaged in sterile culture flasks containing a 1:1 mixture of Dulbecco modified Eagle medium and nutrient mixture F‐12 (DMEM/F‐12 with L‐glutamine for HEK293 cells or Ham's F‐12 with L‐glutamine for CHO cells) supplemented with 10% fetal bovine serum and 1.0% penicillin/streptomycin solution.
All solutions were maintained at room temperature (19°C–30°C). After formation of a gigaohm seal between the patch electrodes and an individual cell (pipette resistance range = 2.5–6.0 MΩ, seal resistance range = >1 GΩ, EPC‐10, HEKA Electronics), the cell membrane across the pipette tip was ruptured to assure electrical access to the cell interior (whole‐cell patch configuration). Currents were clamped to −100 mV and CaV3.1/3.2/3.3 currents were recorded upon depolarization of the cell membrane to −25 mV for a duration of 250 ms at a frequency of .1 Hz.
If current amplitude was judged to be too low for measurement, another cell was recorded.
Once control recordings had been accomplished, cells were continuously perfused with the test item solutions, .3% DMSO or mibefradil chosen as positive reference. During wash‐in of the compound, the voltage protocol indicated above was run continuously until the steady‐state level of block was reached.
For the IC50 determination, as calcium peak currents were inhibited by the highest test item concentration by >30%, a dose–response curve was generated, and the IC50 and Hill coefficient were estimated (Sigma Plot 11.0., SPSS).
The IC50 and the Hill coefficient were estimated by fitting the dose–response curve with a two‐parameter logistic function (amax = 100%):
currentpeakrelative = 100 / 1 + 10 ([LOGIC 50 − X] H)
where X is the drug concentration and IC50 is the concentration of drug at half maximal inhibition.
2.6. Statistical analysis
Normality of the data distribution and homogeneity of variances were tested, and the effect of treatment was assessed with a two‐way analysis of variance (ANOVA) for repeated measures (factor treatment and factor period), followed by a Holm–Sidak multiple comparison post hoc test for parametric data, or by a Kruskal–Wallis multiple comparison test followed by a Dunn test for nonparametric data. Significance was set at p < .05 (Sigma Stat, v3.5, SPSS).
Data are represented as the mean ± SEM for the quantitative analysis per period (Figure 1B). For the one‐period analysis (10–50 min after administration; Figure 1C), data are represented as boxplot with median, first and third quartile, and minimum and maximum value. Individual values and the value for group means are also represented. The effect of treatment was assessed with a one‐way ANOVA for repeated measures, followed by a Holm–Sidak multiple comparison post hoc test for parametric data, or by a Tukey test for nonparametric data. For mean duration comparisons, data in the 300‐mg/kg group were missing (due to complete suppression of SWDs in most of the animals), and comparison was only made between vehicle and 150mg/kg treatment (paired t test for parametric data). Comparison between the two treatments THIP and THIP with STP in the WAG/Rij rats was assessed with paired t test (Figure 3B).
FIGURE 1.

(A) Stiripentol (STP) decreased spike‐and‐wave discharge (SWD) duration at the dose of 150 mg/kg and suppressed SWD occurrence at 300 mg/kg. Representative electroencephalographic traces depict cortical activity recordings 10–15 min after administration of (a) STP (300 mg/kg ip), (b) pentylenetetrazol (PTZ; 20 mg/kg sc), (c) STP (150 mg/kg ip) and PTZ (20 mg/kg sc), and (d) STP (300 mg/kg ip) and PTZ (20 mg/kg sc). Scale bar = 1 s; amplitude = .2 mV. (B) STP suppressed SWDs induced after PTZ administration. Quantification of SWDs in 20‐min periods after PTZ (20 mg/kg ip) and vehicle (veh) or STP (150, 300 mg/kg) is shown. Data are represented as the mean and SEM; n = 9, 7, 10/group; *p < .05 compared to vehicle, two‐way analysis of variance for repeated measures (factor treatment and factor period), followed by a Holm–Sidak multiple comparison test for parametric data. (C) Quantification of SWDs in a 40‐min period after PTZ and STP administration. Mean values are represented as stars; n = 9, 7, 10/group; *p < .05 compared to vehicle, Kruskal–Wallis nonparametric comparison followed by a post hoc Dunn test. LV, liquid vehicle
FIGURE 3.
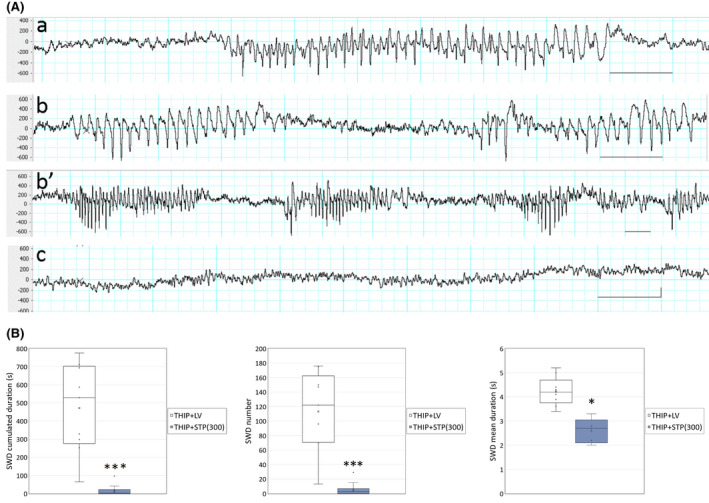
(A) Gaboxadol hydrochloride (THIP) increased spike‐and‐wave discharges (SWDs) in the WAG/Rij rat, and stiripentol (STP) retained its suppressive effect on SWDs at the dose of 300 mg/kg. Representative electroencephalographic traces depict cortical activity recordings 10–15 min after administration of (a) vehicle, (b and b’) THIP, or (c) THIP and STP. Scale bar = 1 s; amplitude = .2 mV. (B) Quantification of SWDs in a 40‐min period after THIP and STP administration. Mean values are represented as stars; n = 10/group; *p < .05, ***p < .001 compared to vehicle, paired t test. LV, liquid vehicle
For electrophysiological patch clamp experiments, relative current amplitudes from cells treated with the test item were compared to the current amplitudes measured in steady‐state conditions under .3% DMSO treatment. Mean current amplitudes were compared with a one‐way ANOVA followed by a Dunnett post hoc comparison test. Significance was set at p < .05 (Sigma Stat, v3.5, SPSS).
3. RESULTS
3.1. STP inhibits SWDs in the low‐dose PTZ model
A baseline EEG was recorded for 2 h at minimum before the day of PTZ administration to screen for spontaneous SWDs. No spontaneous SWDs were recorded in these series of animals, and all the rats were included for further experiments. PTZ administration (20 mg/kg ip) induced SWD starting 2–3 min after injection (Figure 1A). These SWDs were constant in frequency and mean duration during the first 40 min, with a frequency of 5.2 ± .2 Hz, mean duration 1.8 ± .1 s in the 0–20‐min period after PTZ administration, and 5.3 ± .2 Hz, mean duration 1.7 ± .2 s during the 20–40‐min period after PTZ. Their number rapidly declined afterward (Figure 1B). Animals were under continuous visual observation, and no myoclonic jerks were noted.
STP administered before PTZ (20 mg/kg ip) almost completely abolished the generation of SWD at the maximal dose of 300 mg/kg (Figure 1B,C). At the dose of 150 mg/kg, STP administration statistically significantly decreased the number of discharges and their cumulated duration during the first 20 min of recording (p < .05, n = 9/7/10 per group; Kruskal–Wallis nonparametric comparison followed by a post hoc Dunn test; Figure 1B). The mean frequency (5.0 ± .2 Hz) and mean duration (1.7 ± .2 s) of the discharges after 150‐mg/kg STP administration are not different from the vehicle group.
3.2. STP inhibits SWDs in WAG/Rij rats
Baseline EEG recording was performed twice per week for approximately 1 h. SWDs were observed as early as 12 weeks of age (Figure 2A). Their mean duration and hourly number increased over time, as previously described. 20 , 26 , 27 At 16 weeks of age, we observed a cumulated duration of 51.1 s, a mean duration of approximately 4.6 s, and a mean number of 11.3 discharges in 40 min (Figure 2B, mean values represented as stars). STP administered in young adult WAG/Rij rats (16–20 weeks old) almost completely suppressed SWD generation at the maximal dose of 300 mg/kg. At this dose, it statistically significantly decreased SWD cumulated duration and number (p < .001 and p < .01 respectively, one‐way ANOVA for repeated measured, followed by a Holm–Sidak comparison, n = 7 per group; Figure 2B). The lower dose of 150 mg/kg STP did not statistically change the occurrence of SWDs, with 11.3 SWDs in 40 min after vehicle administration compared to 8.3 SWDs after 150 mg/kg STP administration, and a cumulated duration of 51.1 s in vehicle compared to 29.4 after STP.
FIGURE 2.

(A) Stiripentol (STP) decreased spike‐and‐wave discharge (SWD) duration at the dose of 150 mg/kg and suppressed SWD occurrence at 300 mg/kg in the WAG/Rij rat. Representative electroencephalographic traces depict cortical activity recordings 10–15 min after administration of (a) vehicle, (b) STP (150 mg/kg ip), and (c) STP (300 mg/kg ip). Scale bar = 1 s; amplitude .2 mV. (B) Quantification of SWDs in a 40‐min period after STP administration. Mean values are represented as stars; n = 7/group; *p < .05, **p < .01, ***p < .001, compared to vehicle, one‐way analysis of variance for repeated measures followed by a Holm–Sidak multiple comparison test for parametric data. LV, liquid vehicle
However, SWD durations were statistically significantly shorter after 150‐mg/kg STP administration than in control animals, with a mean duration of 3.3 s after STP administration (p < .05, Wilcoxon rank test, median value of 4.8 s for the vehicle group and 3.3 s for the STP group; Figure 2B).
3.3. STP inhibits SWDs aggravated after administration of THIP in WAG/Rij rats
THIP is a positive allosteric modulator of GABA receptors with a specific affinity for receptors containing delta subunits. 28 In this new series of experiments, the animals were the same age as in the previous set of experiments (16–20 weeks old), and each animal was its own control, with a washout period of at least 5 days between the two treatment conditions. Only the maximal dose of 300 mg/kg of STP was tested to avoid potential long‐term effects of the treatments on SWD circuit plasticity.
In WAG/Rij rats, the administration of THIP (5 mg/kg ip) dramatically increased the cumulated duration of SWDs (Figure 3A) in a 40‐min period, with a cumulated duration of 471.4 s and an occurrence of 113.3 SWDs (Figure 3B), compared to 49.1 s and 11 SWDs during the baseline period recorded just before THIP administration (these numbers are extrapolated from the pretreatment recording period of approximately 20 min). The mean duration of the discharges was not modified by THIP administration, with a duration of 4.4 s before and 4.2 s after THIP.
STP administration 15 min before THIP completely blocked THIP effect on SWDs, and still displayed antiabsence properties, with a residual number of 5.7 SWDs and a cumulated duration of 17.5 s in the 40‐min recording period (p < .001, n = 10, paired t test; Figure 3B).
3.4. STP inhibits voltage‐dependent calcium channels in vitro
The effects of seven concentrations of STP on the voltage‐gated calcium channels Cav3.1, Cav3.2, and Cav3.3 were analyzed by means of manual patch‐clamp. Mibefradil (10 µmol·L–1) as calcium channel blocker was used as the reference compound to assure the accuracy of the protocol. The observed inhibitions of the peak current amplitude with mibefradil for the three channels were in agreement with published values, showing ≥95% inhibition. 29 Concentrations of STP between 5 μmol·L–1 and 300 μmol·L–1 were tested. STP significantly blocked the peak current amplitudes at concentrations of ≥50 μmol·L–1 for CaV3.1 and at concentrations of ≥15 μmol·L–1 for CaV3.2 and Cav3.3 (Figure 4). The IC50 values were determined to be 69.16 μmol·L–1 for CaV3.1 (Hill coefficient = 1.71), 64.35 μmol·L–1 for CaV3.2 (Hill coefficient = 1.68), and 36.6 μmol·L–1 for Cav 3.3 (Hill coefficient = 1.19).
FIGURE 4.

Stiripentol (STP) inhibits voltage‐dependent calcium channels. (A) Representative current traces from Chinese hamster ovary cells expressing Cav3.1 and Cav3.3 or human embryonic kidney cells expressing Cav3.2. Green = 5 µmol·L–1; red = 50 µmol·L–1. (B) Concentration–effect curve for the inhibition of maximal current induced by STP at steady state. Each point represents the mean ± SEM (n = 3 cells per concentration)
4. DISCUSSION
Our results have shown that STP administration prevented SWDs evocative of absence seizures. Interestingly, the antiabsence effect was observed in both animal models studied, even though these two models implicate different mechanisms underlying absence seizures.
In the PTZ model, SWDs are generated by a blockade of GABA‐A receptors. 18 Depending on the route of administration, several PTZ tests are identified, screening for distinct antiepileptic activities. For example, levetiracetam and pregabalin are anticonvulsant in the intravenous PTZ test but are ineffective in the subcutaneous PTZ test. 30 STP is strongly anticonvulsant in the subcutaneous PTZ test 31 but also delayed the first clonic movement in the intravenous test. 15 In addition, we have shown in this study that STP also has antiabsence properties in the intraperitoneal PTZ model. It is noteworthy that a nonspecific antiabsence effect due to an increased level of locomotor activity was excluded. 32 STP induced a slight but statistically significant decrease in the distance traveled in the open field test in SD and Wistar rats, starting at the dose of 250 mg/kg (p < .05, data not shown) but did not induce any other modification in the animals’ behavior.
These results suggest that STP has several pharmacological targets, resulting in its large spectrum of antiepileptic effects.
Historical data have shown that STP's mechanism of action was mainly mediated through a positive GABAergic potentiation. A higher potency of STP for receptors containing delta and alpha3 subunits was demonstrated in electrophysiological studies. 3 , 4 Alpha3 subunits are more abundant in the immature brain, 33 and this selectivity suggested that STP would have a stronger antiepileptic effect in young patients. This was confirmed in animal models of status epilepticus, where STP protective effects were stronger in juvenile animals. 31 , 34 In the adult rodent brain, the GABAergic alpha3 subunit is expressed in the cortex and thalamus, and is the most abundant subunit in the reticular thalamic nucleus (RTN). 35 However, a specific loss of alpha3‐subunits containing receptors was found in the RTN in WAG/Rij rats. 21 Inhibition of absence seizures at the thalamic level is mediated through a decreased inhibition of thalamic relay nuclei by RTN projections. 36 A direct inhibition of RTN GABAergic neurons, as seen after alpha3 subunit‐containing receptor potentiation, would decrease SWD occurrence. 21 Conversely, a potentiation of thalamic relay nuclei inhibition increases SWD occurrence. Local infusion in the thalamus of tiagabine, a GABA reuptake inhibitor, also increased the number and duration of SWDs in the WAG/Rij strain. 37
We cannot exclude the possibility that STP antiabsence effects are mediated through alpha3 subunit‐containing GABAergic receptors. 9 An inhibition of these GABAergic receptors in the somatosensory cortex, 38 a site involved in seizure generation, would probably have antiepileptic effects. However, considering that these studies were performed in SD adult rats, in which general expression of alpha3 subunits is decreased, and in WAG/Rij animals, a strain that displays a specific decrease of this subunit in the RTN, 21 a key structure in the thalamocortical circuit, we hypothesized that another mechanism of action could contribute to this antiabsence effect.
Delta subunit‐containing GABAergic receptors are highly expressed in the thalamic nuclei. They are abundant in relay nuclei but almost absent in the RTN. 35 , 39 They are preferentially located at extrasynaptic sites and mediate tonic inhibition. 40 In different models of absence seizures, an increased tonic GABA‐A inhibition has been found. 11
STP shows a strong affinity for delta subunit‐containing GABAergic receptors. 3 In our in vivo experiments, the potentiation of these receptors might interfere with the antiabsence effects of the alpha3 subunit‐containing GABAergic receptors. Potentiation of GABAergic tonic inhibition in relay nuclei aggravate absence seizures. 40 This was also confirmed in our experiments, where THIP administration in WAG/Rij rats dramatically increased SWD occurrence. Surprisingly, STP retained its antiepileptic effect in the THIP‐induced aggravation of SWDs in WAG/Rij rats.
These results suggest that STP pharmacological effects are not mediated through GABAergic delta receptors in this model, as it would aggravate even more THIP‐induced SWD. 41
We hypothesized that an additional mechanism could be implicated in the antiabsence effects observed, and an obvious target was T‐type calcium channels. A previous study had shown that the modulation of voltage‐dependent calcium channels could be involved in the neuroprotective properties of STP in different models of neuronal injury. 42 T‐type voltage‐gated channels (Cav3.1, Cav3.2, Cav3.3) are strongly implicated in thalamocortical oscillations. 36 , 43 , 44 They generate low‐threshold burst firing that sustain SWDs in absence seizures. 45 , 46 , 47 Their critical role in SWDs is also demonstrated by the effect of ethosuximide and mibefradil in rats. 48 These two compounds antagonized thalamocortical rhythmic burst firing and attenuated SWDs in the WAG/Rij rat model.
In vitro patch clamp experiments showed that STP inhibits Cav3.1, Cav3.2, and Cav3.3 at concentrations in the same range (37–69 µmol·L–1) as circulating concentrations found at therapeutic doses. 16 , 49 Plasma concentrations of 4–22 mg/L (17–94 µmol·L–1) are found at the therapeutic dose of 50 mg/kg in children, and >40 mg/L in rats after an administration of 350 mg/kg ip. 26 That the brain‐to‐plasma concentration ratio of STP in rats is close to unity 15 allows us to consider that the effective concentrations on calcium channels are clinically relevant. Receptor occupancy and region selectivity regarding STP effects have not been studied. The chemical properties of STP make it highly insoluble in physiological solutions, and local intracerebral injections are not feasible. Therefore, the respective involvement of GABAergic alpha3 subunit‐containing receptors and of low‐threshold calcium channel modulation is difficult to assess, but considering that effective concentrations for both mechanisms are in close range, one can hypothesize that they are both involved in the antiepileptic mechanisms of action of STP. Interestingly, we also observed that STP at the lower dose of 150 mg/kg did not modify the mean number of seizures per recording period but decreased their mean duration. These results suggest that STP at this dose did not modulate seizure initiation circuits, but it could modify circuits involved in their maintenance and termination. The somatosensory cortex is involved in seizure initiation in the two models used, WAG/Rij rats and low‐dose PTZ, 50 whereas the RTN is rather involved in maintaining the oscillation to sustain the seizure. 51 This result suggests that in these models, STP at low doses could preferentially target the thalamus and decrease seizure duration through an inhibition of T‐type channels in the RTN.
Dravet syndrome is part of the developmental epileptic encephalopathy spectrum, where the respective role of genetic anomalies and epileptic seizures in developmental delays is difficult to apprehend. Retrospective studies have analyzed the correlation between epileptic activity and cognitive scores. In 2011, Ragona 52 found that cognitive scores are related to nonconvulsive seizures (myoclonus and absence) in the population aged >3 years, where cognitive delays are the strongest. In the study from Nabbout and collaborators, 53 the correlation between intelligence quotient/intellectual disability score and epileptic activity was established with myoclonic or focal seizures. However, the quantification of focal seizures with loss of consciousness or atypical absence seizures is particularly challenging in this population, which could explain the differences between the two studies. These studies suggest that other than generalized convulsive seizures, nonconvulsive seizures could be detrimental for the cognitive and developmental score in Dravet children, and that controlling these abnormal electrical activities, including SWDs, could be important.
In conclusion, the present results have shown that STP, in addition to its positive allosteric modulation of GABAergic receptor properties, is also an inhibitor of T‐type calcium channels, and that this spectrum of pharmacological effects could confer antiabsence properties. Short sporadic absence seizures are considered benign. However, recurrent abnormal thalamocortical oscillations can have strong detrimental effects in a developing brain and, even more so, when other underlying dysfunctions are present, as in Dravet syndrome. Recent studies have also found than 60% of children with absence seizures presented severe comorbidities. 50 In this context, new therapeutic approaches including STP could be relevant.
CONFLICT OF INTEREST
V.R., I.H., L.R., and M.V. are full‐time employees at Biocodex. H.S.H. has no conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENT
None.
Riban V, Heulard I, Reversat L, Si Hocine H, Verleye M. Stiripentol inhibits spike‐and‐wave discharges in animal models of absence seizures: A new mechanism of action involving T‐type calcium channels. Epilepsia. 2022;63:1200–1210. 10.1111/epi.17201
REFERENCES
- 1. Poisson M, Huguet F, Savattier A, Bakri‐Logeais F, Narcisse G. A new type of anticonvulsant, stiripentol. Pharmacological profile and neurochemical study. Arzneimittelforschung. 1984;34:199–204. [PubMed] [Google Scholar]
- 2. Quilichini PP, Chiron C, Ben‐Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA‐A receptor channels. Epilepsia. 2006;47:704–16. [DOI] [PubMed] [Google Scholar]
- 3. Fisher JL. The anti‐convulsant stiripentol acts directly on the GABA(A) receptor as a positive allosteric modulator. Neuropharmacology. 2009;56:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher JL. Interactions between modulators of the GABA(A) receptor: stiripentol and benzodiazepines. Eur J Pharmacol. 2011;654:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–7. [DOI] [PubMed] [Google Scholar]
- 6. Dravet C, Bureau M, Dalla Bernardina B, Guerrini R. Severe myoclonic epilepsy in infancy (Dravet syndrome) 30 years later. Epilepsia. 2011;52(Suppl 2):1–2. [DOI] [PubMed] [Google Scholar]
- 7. Li W, Schneider AL, Scheffer IE. Defining Dravet syndrome: an essential pre‐requisite for precision medicine trials. Epilepsia. 2021;62:2205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brunklaus A, Ellis R, Reavey E, Forbes GH, Zuberi SM. Prognostic, clinical and demographic features in SCN1A mutation‐positive Dravet syndrome. Brain. 2012;135:2329–36. [DOI] [PubMed] [Google Scholar]
- 9. Duveau V, Buhl DL, Evrard A, Ruggiero C, Mandé‐Niedergang B, Roucard C, et al. Pronounced antiepileptic activity of the subtype‐selective GABAA‐positive allosteric modulator PF‐06372865 in the GAERS absence epilepsy model. CNS Neurosci Ther. 2019;25:255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vergnes M, Marescaux C, Micheletti G, Depaulis A, Rumbach L, Warter JM. Enhancement of spike and wave discharges by GABAmimetic drugs in rats with spontaneous petit‐mal‐like epilepsy. Neurosci Lett. 1984;44:91–4. [DOI] [PubMed] [Google Scholar]
- 11. Cope DW, Di Giovanni G, Fyson SJ, Orbán G, Errington AC, Lorincz ML, et al. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coenen AM, Drinkenburg WH, Inoue M, van Luijtelaar EL . Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epilepsy Res. 1992;12:75–86. [DOI] [PubMed] [Google Scholar]
- 13. Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg—a review. J Neural Transm Suppl. 1992;35:37–69. [DOI] [PubMed] [Google Scholar]
- 14. Bouwman BM, Suffczynski P, Midzyanovskaya IS, Maris E, van den Broek PL , van Rijn CM . The effects of vigabatrin on spike and wave discharges in WAG/Rij rats. Epilepsy Res. 2007;76:34–40. [DOI] [PubMed] [Google Scholar]
- 15. Shen DD, Levy RH, Moor MJ, Savitch JL. Efficacy of stiripentol in the intravenous pentylenetetrazol infusion seizure model in the rat. Epilepsy Res. 1990;7:40–8. [DOI] [PubMed] [Google Scholar]
- 16. Farwell JR, Anderson GD, Kerr BM, Tor JA, Levy RH. Stiripentol in atypical absence seizures in children: an open trial. Epilepsia. 1993;34:305–11. [DOI] [PubMed] [Google Scholar]
- 17. Pitkänen A, Schwartzkroin PA, Moshé SL, editors. Models of seizures and epilepsy. Burlington, MA: Elsevier Academic Press; 2006. [Google Scholar]
- 18. Marescaux C, Micheletti G, Vergnes M, Depaulis A, Rumbach L, Warter JM. A model of chronic spontaneous petit mal‐like seizures in the rat: comparison with pentylenetetrazol‐induced seizures. Epilepsia. 1984;25:326–31. [DOI] [PubMed] [Google Scholar]
- 19. Snead OC. Pharmacological models of generalized absence seizures in rodents. J Neural Transm Suppl. 1992;35:7–19. [DOI] [PubMed] [Google Scholar]
- 20. Coenen AML, van Luijtelaar ELJM. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–55. [DOI] [PubMed] [Google Scholar]
- 21. Liu X‐B, Coble J, van Luijtelaar G, Jones EG. Reticular nucleus‐specific changes in alpha3 subunit protein at GABA synapses in genetically epilepsy‐prone rats. Proc Natl Acad Sci USA. 2007;104:12512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fariello RG, Golden GT. The THIP‐induced model of bilateral synchronous spike and wave in rodents. Neuropharmacology. 1987;26:161–5. [DOI] [PubMed] [Google Scholar]
- 23. Cain SM, Tyson JR, Choi H‐B, Ko R, Lin PJC, LeDue JM, et al. CaV 3.2 drives sustained burst‐firing, which is critical for absence seizure propagation in reticular thalamic neurons. Epilepsia. 2018;59:778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCafferty C, David F, Venzi M, Lőrincz ML, Delicata F, Atherton Z, et al. Cortical drive and thalamic feed‐forward inhibition control thalamic output synchrony during absence seizures. Nat Neurosci. 2018;21:744–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sitnikova E, van Luijtelaar G. Electroencephalographic characterization of spike‐wave discharges in cortex and thalamus in WAG/Rij Rats. Epilepsia. 2007;12:2296–311. [PubMed] [Google Scholar]
- 26. van Luijtelaar G, Zobeiri M. Progress and outlooks in a genetic absence epilepsy model (WAG/Rij). Curr Med Chem. 2014;21:704–21. [DOI] [PubMed] [Google Scholar]
- 27. Coenen AM, van Luijtelaar EL. The WAG/Rij rat model for absence epilepsy: age and sex factors. Epilepsy Res. 1987;1:297–301. [DOI] [PubMed] [Google Scholar]
- 28. Winsky‐Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABA(A)delta‐subunit‐containing receptors. Eur J Neurosci. 2007;25:1893–9. [DOI] [PubMed] [Google Scholar]
- 29. Martin RL, Lee JH, Cribbs LL, Perez‐Reyes E, Hanck DA. Mibefradil block of cloned T‐type calcium channels. J Pharmacol Exp Ther. 2000;295:302–8. [PubMed] [Google Scholar]
- 30. Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazol infusion test: a comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure. 2007;16:636–44. [DOI] [PubMed] [Google Scholar]
- 31. Auvin S, Lecointe C, Dupuis N, Desnous B, Lebon S, Gressens P, et al. Stiripentol exhibits higher anticonvulsant properties in the immature than in the mature rat brain. Epilepsia. 2013;54:2082–90. [DOI] [PubMed] [Google Scholar]
- 32. van Luijtelaar G, van Oijen G. Establishing drug effects on electrocorticographic activity in a genetic absence epilepsy model: advances and pitfalls. Front Pharmacol. 2020;11:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grosenbaugh DK, Mott DD. Stiripentol in refractory status epilepticus. Epilepsia. 2013;54(Suppl 6):103–5. [DOI] [PubMed] [Google Scholar]
- 35. Fritschy JM, Mohler H. GABAA‐receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–94. [DOI] [PubMed] [Google Scholar]
- 36. Huguenard JR, Prince DA. A novel T‐type current underlies prolonged Ca(2+)‐dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992;12:3804–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D'Amore V, von Randow C, Nicoletti F, Ngomba RT, van Luijtelaar G . Anti‐absence activity of mGlu1 and mGlu5 receptor enhancers and their interaction with a GABA reuptake inhibitor: effect of local infusions in the somatosensory cortex and thalamus. Epilepsia. 2015;56:1141–51. [DOI] [PubMed] [Google Scholar]
- 38. Meeren HKM, Pijn JPM, van Luijtelaar ELJM, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. [DOI] [PubMed] [Google Scholar]
- 40. Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–501. [DOI] [PubMed] [Google Scholar]
- 41. Vergnes M, Marescaux C, Micheletti G, Rumbach L, Warter JM. Blockade of "antiabsence" activity of sodium valproate by THIP in rats with petit mal‐like seizures. Comparison with ethosuximide. J Neural Transm. 1985;63:133–41. [DOI] [PubMed] [Google Scholar]
- 42. Verleye M, Buttigieg D, Steinschneider R. Neuroprotective activity of stiripentol with a possible involvement of voltage‐dependent calcium and sodium channels. J Neurosci Res. 2016;94:179–89. [DOI] [PubMed] [Google Scholar]
- 43. Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low‐threshold calcium currents in thalamic relay cells. J Neurosci. 1998;18:3574–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Errington AC, Renger JJ, Uebele VN, Crunelli V. State‐dependent firing determines intrinsic dendritic Ca2+ signaling in thalamocortical neurons. J Neurosci. 2010;30:14843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurogibol. 1998;55:27–57. [DOI] [PubMed] [Google Scholar]
- 46. D'Arcangelo G, D'Antuono M, Biagini G, Warren R, Tancredi V, Avoli M. Thalamocortical oscillations in a genetic model of absence seizures. Eur J Neurosci. 2002;16:2383–93. [DOI] [PubMed] [Google Scholar]
- 47. Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. [DOI] [PubMed] [Google Scholar]
- 48. Broicher T, Seidenbecher T, Meuth P, Munsch T, Meuth SG, Kanyshkova T, et al. T‐current related effects of antiepileptic drugs and a Ca2+ channel antagonist on thalamic relay and local circuit interneurons in a rat model of absence epilepsy. Neuropharmacology. 2007;53:431–46. [DOI] [PubMed] [Google Scholar]
- 49. Chiron C, Marchand MC, Tran A, Rey E, d'Athis P, Vincent J, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo‐controlled syndrome‐dedicated trial. Lancet. 2000;356:1638–42. [DOI] [PubMed] [Google Scholar]
- 50. Crunelli V, Lőrincz ML, McCafferty C, Lambert RC, Leresche N, Di Giovanni G , et al. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain. 2020;143:2341–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lüttjohann A, van Luijtelaar G. Dynamics of networks during absence seizure's on‐ and offset in rodents and man. Front Physiol. 2015;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ragona F. Cognitive development in children with Dravet syndrome. Epilepsia. 2011;52(Suppl 2):39–43. [DOI] [PubMed] [Google Scholar]
- 53. Nabbout R, Chemaly N, Chipaux M, Barcia G, Bouis C, Dubouch C, et al. Encephalopathy in children with Dravet syndrome is not a pure consequence of epilepsy. Orphanet J Rare Dis. 2013;8:176. [DOI] [PMC free article] [PubMed] [Google Scholar]


