Abstract
Backgroundand Aim. Diabetes mellitus is a metabolic disorder that has no known cure with continuous endeavors to find a therapy for the condition. According to some studies, traditional leafy vegetables could prevent and manage diabetes by modifying the carbohydrate and lipid metabolism. In this study, a phytochemical analysis, acute toxicity, as well as antihyperglycemic and antidiabetic activity testing of the methanolic, diethyl ether, and aqueous leaf extracts of Corchorus olitorius L. was performed. Materials and Methods. Methanolic, diethyl ether, and aqueous leaf extracts of Corchorus olitorius L. were prepared by serial extraction. Phytochemical analysis was performed following standard methods. 52 mice were separated into 13 groups (A–M) of 4 and received extracts' doses ranging from 1000 mg/kg to 5000 mg/kg for the acute toxicity testing. For the antihyperglycemic and antidiabetic activities testing, 48 rats were divided into 8 groups of 6 and received 500 mg/kg of each extract. 10 mg/kg of glibenclamide and distilled water were used as controls. Data were analyzed using Prism GraphPad version 8.0.2 (263). Results. Phytochemical analysis revealed the presence of alkaloids, reducing sugars, saponins, and terpenoids. There were no acute toxicity signs observed in this study. Corchorus olitorius L. extracts demonstrated moderate antihyperglycemic and antidiabetic activities. The methanolic extract exhibited the highest degree of antihyperglycemic activity. However, there was no statistically significant difference between the extracts and the negative control (p > 0.05), but with glibenclamide (p < 0.01). Conclusion. Corchorus olitorius L. is a safe and potential postprandial antidiabetic vegetable that could minimize the rise in blood glucose after a meal. We therefore recommend further investigations into the antidiabetic properties of the vegetable using purified extracts.
1. Introduction
Corchorus olitorius L. (C. olitorius), also known as Tossa jute in English, is a traditional medicinal vegetable belonging to the genus Corchorus and the family of Tiliceae [1]. The vegetable is prevalent in northern Australia, tropical Africa, and India [1]. In these places, C. olitorius leaves commonly are served as a vegetable frequently in stews eaten with staple starchy foods [1]. C. olitorius is an annual herb growing to a height of 2.4 m [1]. It has alternating, finely indented margin leaves. C. olitorius has small yellow flowers comprising of five petals that form a brown multiseeded pod later in life [1]. It is proliferated by seeds [2] much as it can be accepted as a wild vegetable in crop fields or cultivated in family gardens [2]. C. olitorius flourishes best throughout rainy seasons, nonetheless it is drought resilient [1, 2]. In West Africa, C. olitorius is used to treat malaria, typhoid fever, female infertility, heart failure, and ulcers [1, 3, 4]. In addition, it is used as a therapy for fevers, colds, constipation, and tumors [5]. The vegetable is also reported to have antiobesity [6, 7], anti-inflammatory [8, 9], antimicrobial [10, 11], and gastroprotective properties [12] along with antidiabetic effects [7, 13, 14].
Diabetes mellitus (DM), a disorder of mainly glucose metabolism, is one of the most common noncommunicable diseases (NCDs). In 2015, DM caused 5 million deaths and an estimated 12% of global expenditure [15–17]. The disease occurs in two types, that is, 1 and 2. Type 1 accounts for 2–10% of all diabetic cases in the world [18] and for >85% of diabetes registered amongst youth below 20 years of age [19]. It occurs as a result of autoimmune destruction of pancreatic beta cells with a subsequent total insulin deficiency [18]. Type 2 results from insensitivity to insulin and/or inadequate compensatory insulin secretion with over 90% of all diabetic cases in the world [15]. In Africa, diabetes occurred among 7.1% of the population in 2014, and the overall cost of DM was USD 19.45 million in 2015 [20]. More than 90% of diabetic cases in Africa are type 2 [20, 21]. In Uganda, the prevalence of diabetes was 1.9% in 2016 [22]. Diabetes is managed by dietary modification, exercise, and pharmacotherapy [23]. However, there is as yet no known cure for DM. This has driven the affected individuals' world over to opt for alternative medication including traditional herbal remedies, while a lot of research is being conducted to evaluate and document plants with glycemic effects [24–31]. In Uganda and other parts of the world, studies have revealed a number of plants that are used by communities in the management of diabetes [32, 33]. According to a study conducted by Momo et al [31], traditional leafy vegetables could prevent and manage diabetes by modifying the carbohydrate and lipid metabolism. C. olitorius has indicated a reduction in serum blood glucose levels in a few studies [34].
In the current study, we performed a phytochemical analysis, acute toxicity, as well as antihyperglycemic and antidiabetic activity testing of the methanolic, aqueous, and diethyl ether extracts of C. olitorius leaves in laboratory animals. This was done to confirm some of the reports about the vegetable as well as to assess its safety.
2. Materials and Methods
2.1. Laboratory Animals
Laboratory mice and rats from the animal house of the Department of Pharmacology and Therapeutics, Makerere College of Heath Sciences, were used for this study. The animals (male and female) were housed in wooden cages at temperature between 25 and 28°C with a normal 12-hour/12-hour light/dark cycle for at least 2 weeks before the study. They were fed with standard rat feeds and water. Their weight ranged from 18 to 31 g and 128 g to 221 g for mice and rats, respectively. Pregnant and breastfeeding animals were excluded from the study.
2.2. Extract Preparation
The C. olitorius leaves were harvested from Olago village, Lira district (northern Uganda), and packaged in polyethene bags. They were then transported to the Department of Pharmacology and Therapeutics, Makerere University College of Health Sciences, where they were washed clean and spread to air-dry inside the laboratory to a constant weight (900 g). The fully dried leaves were then ground to a course powder and extracted by means of solvents (diethyl ether, methanol, and distilled water) in a serial extraction method as described by Das, Tiwari, and Shrivastava [35]. This was done so as to extract compounds of varied polarity. Briefly, 600 g of C. olitorius leaves powder were soaked in 3 L of diethyl ether (BDH AnalaR) in a flat bottomed conical flask and tightly closed while shaking every 6 hours for two days (48 hours.). The resultant solution was filtered using Whatman filter paper (No. 1), and the filtrate was concentrated by means of a rotary evaporator at 20–30°C. The remains were then spread in the laboratory and allowed to dry. After drying, it was soaked in 3 L of methanol (UltraPure Solutions, Inc. 11485, Commercial Parkway, Castroville, CA 95012), and the process was repeated as with diethyl ether. Last, the dry residue was soaked in 3 L of distilled water while shaking every 6 hours for two days (48 hours). The resultant solution was also filtered using Whatman filter paper (No. 1) and freeze dried. After evaporation of the solvents, the extracts were accordingly labeled and stored in a refrigerator at −80°C. A voucher specimen was kept at a herbarium at the Department of Botany, Makerere University, Kampala, Uganda (# 50906).
2.3. Phytochemical Analysis
The phytochemical analysis was performed as follows.
2.3.1. Tannins
2 mL of 5% FeCl3 were added to 2 mL of aqueous extract and observed for a yellow/brown precipitate formation [36].
2.3.2. Alkaloids
1.5 mL of 1% HCl was added to 2 mL of the methanol extract filtrate, the solution was heated in a waterbath (5 minutes), and then 6 drops of Mayor's reagent was added and observed for an orange precipitate formation [37].
2.3.3. Saponins
2 g powder of the aqueous extract was mixed with few drops of olive oil, shaken vigorously, and observed for formation of a stable persistent froth and an emulsion [38].
2.3.4. Cardiac Glycosides
To 2 mL methanol extract filtrate, 1 mL glacial acetic acid and 1-2 drops of FeCl3 were added followed by 1 mL of concentrated H2SO4. Formation of a brown ring at the interface indicated the presence of deoxy-sugar [39, 40].
2.3.5. Terpenes
To 2 mL of the aqueous extract, 5 mL CHCl3, 2 mL acetic anhydride, and a drop of concentrated H2SO4 were added carefully to form a layer. Formation of a reddish/brown coloration at the interface indicated the presence of terpenes [41].
2.3.6. Reducing Sugars
To 2.5 mL of Benedict's solution was added 0.5 g of the aqueous extract in a test tube. The mixture was warmed over a waterbath for about 5 minutes. Observation of green/red or yellow coloration indicated the presence of reducing sugars [42].
2.3.7. Nonreducing Sugars
3 mL of the aqueous extract was mixed with 2 drops of dilute iodine solution and boiled for 5 minutes. Observation of blue color that disappeared upon boiling and reappeared upon cooling indicated the presence of nonreducing sugar [43].
2.4. Acute Toxicity Testing
The extracts were orally administered following the protocol laid down by the Organization for Economic Cooperation and Development [44], where the dose of the extract per animal body weight was calculated and administered to the animals suspended in distilled water. The animals were observed for mortality, behavioral changes, physical appearance, injury, and pain after 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, and once daily for 13 days. The control group was only given distilled water. Observations were recorded per group of animals. The experiment was performed as follows: a total of 52 mice (average weight 25 g) were divided into thirteen (13) groups (A–M) of four (4). Groups A–D, E–H, and I–L received 1000 mg/kg, 2000 mg/kg, 4000 mg/kg, and 5000 mg/kg of the diethyl ether, methanol, and aqueous extracts, respectively. Last, group M received 1 mL of distilled water.
2.5. The Antihyperglycemic and Antidiabetic Tests
This was performed as described by Mushtaq et al. [45]with minor modifications as follows. The rats were fasted overnight and randomly selected and distributed into eight groups (n = 6). To one of the groups, 1 mL of distilled water was orally administered. To three of the groups (for the antihyperglycemic test), the methanolic, diethyl ether, and aqueous extracts of C. olitorius (500 mg/kg body weight for each extract) were orally administered. After one hour, the animals were fed with glucose (2 g/kg body weight) [45]. To another set of three groups (for the antidiabetic test), alloxan (60 mg/kg; i.v) was given prior (3 days) to the study in order to induce diabetes. These too were given in 500 mg/kg of body weight of each of the extracts. The last group received glibenclamide (10 mg/kg body weight). A blood sample was then collected by pricking the tail vein of the rat using a scalp vein needle. Blood was gently milked, dropped on a glucose strip, and read using a glucometer (Code-free blood glucose test strips, Biosenser, Inc, Korea and Code free, Yeongtong-dong Yeontong-du, Suwoni-si, Kyonggi-go, Korea). Blood was withdrawn from the tail vein at 0, 30, 60, 90, 120, and 150 minutes of glucose administration for the antihyperglycemic test and the same time interval following test drug administration for the antidiabetic test. The glucose level is recorded in a table as indicated by the glucometer.
2.6. Data Management
Data was analyzed by Prism GraphPad version 8.0.2 (263) and expressed as mean + standard deviation. Significance level was set at p ≤ 0.05 at 95% confidence interval (CI). The results are presented as tables and figures.
3. Results
3.1. Phytochemical Analysis
Phytochemical analysis revealed the presence of alkaloids, reducing sugars, and saponins among others (Table 1).
Table 1.
Phytochemical analysis of C. olitorius.
| Phytochemicals | Present (+)/absent (−) |
|---|---|
| Alkaloid | + |
| Glycoside | + |
| Reducing sugar | + |
| Nonreducing sugar | − |
| Saponins | + |
| Tannins | + |
| Terpenoids | + |
3.2. Acute Toxicity Testing
There were no observed acute toxicity effects of the C. olitorius extracts at the doses tested. Neither behavioral or appearance changes nor mortality was observed in all the groups. All the animals appeared and behaved normally (Tables 2 and 3).
Table 2.
Extract doses given.
| Group | Extract | Dose (mg/kg) |
|---|---|---|
| A | Methanol | 1000 |
| B | Methanol | 2000 |
| C | Methanol | 4000 |
| D | Methanol | 5000 |
| E | Diethyl ether | 1000 |
| F | Diethyl ether | 2000 |
| G | Diethyl ether | 4000 |
| H | Diethyl ether | 5000 |
| I | Aqueous | 1000 |
| J | Aqueous | 2000 |
| K | Aqueous | 4000 |
| L | Aqueous | 5000 |
| M | Distilled water | 0 |
Table 3.
Acute toxicity of C. olitorius extracts.
| Groups | Dose | Mortality (#) | Behavioral change (#) | Change in physical appearance (#) | Expression of pain (#) |
|---|---|---|---|---|---|
| A, E, I | 1000 mg/kg | 0 | 0 | 0 | 0 |
| B, F, J | 2000 mg/kg | 0 | 0 | 0 | 0 |
| C, G, K | 4000 mg/kg | 0 | 0 | 0 | 0 |
| D, H, L | 5000 mg/kg | 0 | 0 | 0 | 0 |
| M | 0 mg/kg | 0 | 0 | 0 | 0 |
#Number.
3.3. Antihyperglycemic and Antidiabetic Activities Testing
To assess the antihyperglycemic activity of C. olitorius, the methanolic, diethyl ether, and aqueous extracts of C. olitorius leaves along with distilled water and glibenclamide were administered to rats an hour before glucose administration. The blood glucose level was then taken at different time intervals using a glucometer. For the antidiabetic test, the test animals were given alloxan three days before the experiment. C. olitorius extracts moderately exhibited antihyperglycemic and antidiabetic effects, although not comparable to glibenclamide (Table 4 and Figure 1).
Table 4.
The mean values of the plasma glucose level per group with the standard deviations.
| T | M1 | DE1 | A1 | M2 | DE2 | A2 | Gl | W |
|---|---|---|---|---|---|---|---|---|
| 0 | 6.850 ± 0.797 | 5.967 ± 0.625 | 5.317 ± 0.688 | 6.160 ± 0.953 | 5.900 ± 0.490 | 5.920 ± 0.746 | 3.633 ± 1.098 | 6.538 ± 1.327 |
| 30 | 7.767 ± 0.441 | 11.467 ± 0.644 | 9.983 ± 1.057 | 7.600 ± 0.797 | 5.460 ± 0.783 | 6.820 ± 1.062 | 9.600 ± 1.534 | 17.383 ± 1.326 |
| 60 | 7.583 ± 1.186 | 7.033 ± 1.529 | 8.333 ± 1.537 | 7.360 ± 1.555 | 5.860 ± 1.106 | 5.560 ± 0.757 | 4.367 ± 0.413 | 7.567 ± 0.378 |
| 90 | 6.783 ± 1.091 | 6.483 ± 1.592 | 6.450 ± 0.907 | 6.940 ± 1.001 | 6.620 ± 0.746 | 7.720 ± 1.893 | 3.167 ± 1.303 | 6.217 ± 1.353 |
| 120 | 5.583 ± 0.542 | 6.617 ± 0.571 | 6.217 ± 1.530 | 7.220 ± 0.576 | 6.680 ± 0.847 | 7.760 ± 0.358 | 2.500 ± 1.081 | 5.683 ± 1.118 |
| 150 | 5.267 ± 0.799 | 5.467 ± 1.549 | 5.317 ± 0.854 | 6.440 ± 0.777 | 7.400 ± 0.812 | 6.440 ± 0.643 | 2.517 ± 1.300 | 5.733 ± 0.668 |
T, time in minutes; M1, DE1, and Aq1, methanol, diethyl ether, and aqueous for extracts antihyperglycemic test; M2, DE2, and Aq2, methanol, diethyl ether, and aqueous extracts for the antidiabetic test; Gl, glibenclamide; W, distilled water.
Figure 1.
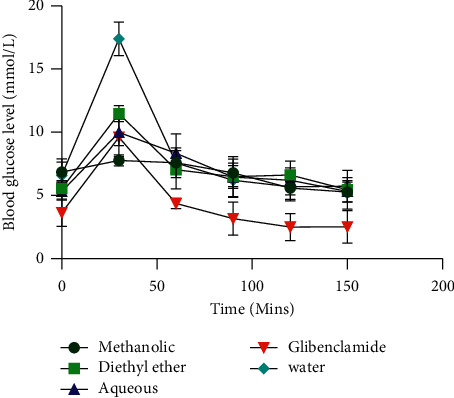
Blood glucose levels for the antihyperglycemic test.
P value <0.05 was compared with positive control but >0.05 with negative control for the antihyperglycemic test; P value = <0.0001 was compared with water in the antidiabetic test (unpaired t-test with Welch's correction) for all the extracts. The overall P value calculation using the one-way ANOVA and multiple t-test was below 0.05 showing a statistically significant difference between the C. olitorius extracts and glibenclamide, whereas it was above 0.05 in comparison with water indicating no significant difference. However, the multiple t-test per time interval revealed a statistically significant difference between C. olitorius extracts and water at 30 minutes of glucose administration (P value <0.05).
4. Discussion
Phytochemical analysis revealed the presence of alkaloids, glycosides, saponins, tannins, terpenoids, and reducing sugars (Table 1). This finding was consistent with a related study in India [46] and Bangladesh [47] but not in West Africa [48]. In another related study, alkaloids, tannins, and resins were found [34]. The discrepancy in phytochemical composition could be attributed to the differences in the climate of the regions. In addition, there was no observed acute toxicity effect of C. olitorius extracts seen in the current study (Table 3). This finding is similar to a set of related studies in Bangladesh and Nigeria where there were no observed acute toxicity effects in doses comparable to those of this study [47, 49]. In another related study, C. olitorius did not show any toxicity using enzyme markers in alloxan-induced diabetic rats [34]. This finding also supports the results of the present study. This implies that the vegetable is safe for consumption. To assess the antihyperglycemic activity of C. olitorius leaf extracts, the controls and the C. olitorius extracts were administered an hour before glucose ingestion. Glibenclamide registered the lowest blood glucose at 0 hour of glucose administration. This was partly in agreement with the findings of Maxwell et al. in Nigeria [14] who tested the antidiabetic potential of the ethanolic seed extract of C. olitorius and found glibenclamide to have a higher blood glucose lowering potential than the C. olitorius extract in normal glycemic rats. Meanwhile, there was no significant difference between the blood glucose levels of the C. olitorius extracts in comparison with the negative control at 0 hour. However, the blood glucose level for the methanolic extract was slightly higher than that of the negative control at 0 hour (Table 4). This indicates that the extracts have a minimal effect on baseline blood glucose. In an oral glucose tolerance test (OGTT) performed by Maxwell et al. [14], the ethanolic C. olitorius seed extract demonstrated a significant decrease in the blood glucose level from 30 minutes to 2 hours (P < 0.001). Similarly, in the current study, the different C. olitorius extracts demonstrated significant antihyperglycemic activity at 30 minutes of glucose ingestion (p < 0.01). Nevertheless, the overall p value (p > 0.05) in comparison with the negative control for the entire test was not statistically significant. This could be attributed to the fact that they (Maxwell et al.) [14] used the C. olitorius seed extract, yet the current study used leaves. There is also a probability that C. olitorius seeds exhibit a higher antihyperglycemic potential than the leaves. Comparison between the C. olitorius extracts and glibenclamide revealed that glibenclamide had a higher antihyperglycemic activity (p < 0.01) except with the aqueous extract at 30 minutes (p=0.63). This too was similar to the findings of Maxwell et al. [14]. Their glibenclamide group had the highest reduction in blood glucose level among the test animals. This could be justified by the fact that glibenclamide is an already purified compound unlike the extracts. In another study conducted by Arise et al. [13], to establish the antidiabetic properties of C. olitorius in alloxan-induced diabetic rats, the ethanolic leaf extract exhibited antidiabetic activity. This finding relates to the outcome of the current study, although a methanolic extract was used in the current study. In addition, according to a study that tested the hypoglycemic effect of C. olitorius in albino rats, the methanolic extract demonstrated hypoglycemic activity, thereby, supporting the findings of the current study [7]. In a related study conducted by Sanjida et al. in Bangladesh [47], C. olitorius showed a dose-dependent reduction in the blood glucose level in an oral glucose tolerance test (OGTT) among test animals. This as well is in agreement with the first one hour findings of the current study. Furthermore, a study was conducted by Oboh et al. [50] to identify the antidiabetic potential of C. olitorius, and the plant demonstrated antidiabetic activity in type 2 diabetic rats. This outcome also is in agreement with the findings of the current study, although the current effect was minimal. All the extracts in the present study moderated the rise in plasma glucose an hour after oral ingestion of glucose by the test animals. The methanolic extract showed the greatest antihyperglycemic activity within the first one hour. However, after the first one hour, there was no significant difference between the plasma glucose level of the extracts and water signifying a short-lived antihyperglycemic activity of the extracts. They therefore cannot be considered for long-term control of high blood sugar. In addition, the antihyperglycemic and antidiabetic activities of the extracts were less when compared to those of glibenclamide. This is attributable to the fact that glibenclamide is a pure compound. The antihyperglycemic and antidiabetic activities of C. olitorius are ascribed to the presence of alkaloids, which are believed to induce glucose uptake by pancreatic beta cells [51].
5. Conclusion and Recommendation
C. olitorius L. has moderate antihyperglycemic and antidiabetic activities with no toxic effects. Further in-depth studies to investigate the antihyperglycemic, antidiabetic, pharmacodynamics, and the pharmacokinetic properties of C. olitorius L. leaf extracts as a potential nutraceutical amongst diabetic patients should be performed using purified extracts.
Acknowledgments
This study was supported by Lira University and the Department of Pharmacology and Therapeutics, Makerere University College of Health Sciences.
Data Availability
The data used to support this study are available from the corresponding author upon request.
Ethical Approval
Ethical approval to conduct the study was provided by the Research and Ethics Committee (REC) of Mbarara University of Science and Technology, the Uganda National Council for Science and Technology (UNCST-HS2589), and the concerned laboratory administrators.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
RN conceptualized and designed the study, collected and analyzed data, and drafted the manuscript. AL performed laboratory tests and reviewed the manuscript. PA and JO supervised and reviewed the manuscript.
References
- 1.Loumerem M., Alercia A. Descriptors for jute (Corchorus olitorius L.) Genetic Resources and Crop Evolution . 2016;63(7):1103–1111. doi: 10.1007/s10722-016-0415-y. [DOI] [Google Scholar]
- 2.Begum T., Kumar D. Usefulness of morphological characteristics for DUS testing of jute (Corchorus olitorius L. and C. capsularis L.) Spanish Journal of Agricultural Research . 2011;9(2):p. 473. doi: 10.5424/sjar/20110902-203-10. [DOI] [Google Scholar]
- 3.Adebo H. O., Ahoton L. E., Quenum F. J. B., Adoukonou-Sagbadja H., Bello D. O., Chrysostome C. A. A. M. Ethnobotanical knowledge of jute (Corchorus olitorius L.) in Benin. European Journal of Medicinal Plants . 2018;26:1–11. doi: 10.9734/ejmp/2018/43897. [DOI] [Google Scholar]
- 4.Nyadanu D., Adu Amoah R., Kwarteng A. O., et al. Domestication of jute mallow (Corchorus olitorius L.): ethnobotany, production constraints and phenomics of local cultivars in Ghana. Genetic Resources and Crop Evolution . 2017;64(6):1313–1329. doi: 10.1007/s10722-016-0438-4. [DOI] [Google Scholar]
- 5.Islam M. M. Biochemistry, medicinal and food values of jute (Corchorus capsularis L. and C. olitorius L.) leaf: a review. International Journal of Enhanced Research In Science Technology & Engineering . 2013;2(11):135–144. [Google Scholar]
- 6.Wang L., Yamasaki M., Katsube T., Sun X., Yamasaki Y., Shiwaku K. Antiobesity effect of polyphenolic compounds from molokheiya (Corchorus olitorius L.) leaves in LDL receptor-deficient mice. European Journal of Nutrition . 2011;50(2):127–133. doi: 10.1007/s00394-010-0122-y. [DOI] [PubMed] [Google Scholar]
- 7.Airaodion A. I., Akinmolayan J. D., Ogbuagu E. O., Airaodion E. O., Ogbuagu U., Awosanya O. O. Effect of methanolic extract of Corchorus olitorius Leaves on hypoglycemic and hypolipidaemic activities in albino rats. Asian Plant Research Journal . 2019;2:1–13. doi: 10.9734/aprj/2019/v2i430054. [DOI] [Google Scholar]
- 8.Handoussa H., Hanafi R., Eddiasty I., et al. Anti-inflammatory and cytotoxic activities of dietary phenolics isolated from Corchorus olitorius and Vitis vinifera. Journal of Functional Foods . 2013;5(3):1204–1216. doi: 10.1016/j.jff.2013.04.003. [DOI] [Google Scholar]
- 9.Yan Y.-Y., Wang Y. W., Chen S. L., Zhuang S. R., Wang C. K. Anti-inflammatory effects of phenolic crude extracts from five fractions of Corchorus olitorius L. Food Chemistry . 2013;138(2-3):1008–1014. doi: 10.1016/j.foodchem.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 10.Ilhan S., Savaroğlu F., Çolak F. Antibacterial and antifungal activity of Corchorus olitorius L.(Molokhia) extracts. International Journal of Natural and Engineering Sciences . 2007;1(3) [Google Scholar]
- 11.Zakaria Z. A., Somchit M. N., Zaiton H., et al. The in vitro antibacterial activity of Corchorus olitorius extracts. International Journal of Pharmacology . 2006;2(2):213–215. doi: 10.3923/ijp.2006.213.215. [DOI] [Google Scholar]
- 12.Nakaziba R. Traditional uses of Corchorus olitorius L in Oyam District, northern uganda: a cross-sectional ethnobotanical survey. 2020. https://www.researchgate.net/publication/340189765_Traditional_uses_of_Corchorus_olitorius_L_in_Oyam_District_Northern_Uganda_A_cross-sectional_ethnobotanical_survey .
- 13.Olusanya A. R., Ifeoluwa B. S., Aboyewa A. J., Khadijat B. Antidiabetic and safety properties of ethanolic leaf extract of corchorus olitorius in alloxan-induced diabetic rats. Diabetes Food Plan . 2018;6:p. 57. doi: 10.5772/intechopen.71529. [DOI] [Google Scholar]
- 14.Egua M. O., Etuk E. U., Belloc S. O., Hassan S. W. Anti-diabetic activity of ethanolic seed extract of Corchorus olitorius. International Journal of Sciences: Basic and Applied Research . 2013;12(1):8–21. [Google Scholar]
- 15.Zheng Y., Ley S. H., Hu F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology . 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 16.Roglic G. WHO Global report on diabetes: a summary. International Journal of Noncommunicable Diseases . 2016;1(1):p. 3. doi: 10.4103/2468-8827.184853. [DOI] [Google Scholar]
- 17.Ogurtsova K., da Rocha Fernandes J., Huang Y., et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice . 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Maahs D. M., West N. A., Lawrence J. M., Mayer-Davis E. J. Epidemiology of type 1 diabetes. Endocrinology and Metabolism Clinics of North America . 2010;39(3):481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence J. M., Standiford D. A., Loots B., et al. Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics . 2006;117(4):1348–1358. doi: 10.1542/peds.2005-1398. [DOI] [PubMed] [Google Scholar]
- 20.Atun R., Davies J. I., Gale E. A. M., et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes & Endocrinology . 2017;5(8):622–667. doi: 10.1016/S2213-8587(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 21.Pastakia S. D., Pekny C., Manyara S., Fischer L. Diabetes in sub-Saharan Africa – from policy to practice to progress: targeting the existing gaps for future care for diabetes. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy . 2017;10:247–263. doi: 10.2147/dmso.s126314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahendeka S., Wesonga R., Mutungi G., Muwonge J., Neema S., Guwatudde D. Prevalence and correlates of diabetes mellitus in Uganda: a population-based national survey. Tropical Medicine and International Health . 2016;21(3):405–416. doi: 10.1111/tmi.12663. [DOI] [PubMed] [Google Scholar]
- 23.Madhu S., Srivastava S. Diabetes mellitus: diagnosis and management guidelines. Journal International Medical Sciences Academy . 2015;1:47–50. [Google Scholar]
- 24.Tripathi P., Srivatava R., Pandey A., Pandey R., Goswami S. Alternative therapies useful in the management of diabetes: a systematic review. Journal of Pharmacy and BioAllied Sciences . 2011;3(4):p. 504. doi: 10.4103/0975-7406.90103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan E. A., Pick M. E., Marceau C. Use of alternative medicines in diabetes mellitus. Diabetic Medicine . 2001;18(3):242–245. doi: 10.1046/j.1464-5491.2001.00450.x. [DOI] [PubMed] [Google Scholar]
- 26.Hjelm K., Mufunda E. Zimbabwean diabetics’ beliefs about health and illness: an interview study. BMC International Health and Human Rights . 2010;10(1):p. 7. doi: 10.1186/1472-698x-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salihu Shinkafi T., Bello L., Wara Hassan S., Ali S. An ethnobotanical survey of antidiabetic plants used by Hausa–Fulani tribes in Sokoto, Northwest Nigeria. Journal of Ethnopharmacology . 2015;172:91–99. doi: 10.1016/j.jep.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Al-Kindi R. M., Al-Mushrafi M., Al-Rabaani M., Al-Zakwani I. Complementary and alternative medicine use among adults with diabetes in Muscat region, Oman. Sultan Qaboos University Medical Journal . 2011;11(1):62–68. [PMC free article] [PubMed] [Google Scholar]
- 29.Kesari A. N., Kesari S., Singh S. K., Gupta R. K., Watal G. Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. Journal of Ethnopharmacology . 2007;112(2):305–311. doi: 10.1016/j.jep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Dey L., Attele A. S., Yuan C.-S. Alternative therapies for type 2 diabetes. Alternative Medicine Review: A Journal of Clinical Therapeutic . 2002;7(1):45–58. [PubMed] [Google Scholar]
- 31.Momo C. E., Oben J. E., Tazoo D., Dongo E. Antidiabetic and hypolipidemic effects of laportea ovalifolia (urticaceae) in alloxan induced diabetic rats. African Journal of Traditional, Complementary and Alternative Medicines . 2005;3(1):36–43. doi: 10.4314/ajtcam.v3i1.31137. [DOI] [Google Scholar]
- 32.Ssenyange C. W., Namulindwa A., Oyik B., Ssebuliba J. Plants used to manage type II diabetes mellitus in selected districts of central Uganda. African Health Sciences . 2015;15(2):p. 496. doi: 10.4314/ahs.v15i2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meresa A., Gemechu W., Basha H., et al. Herbal medicines for the management of diabetic mellitus in Ethiopia and Eretria including their phytochemical constituents. AJADD . 2017;5(01):040–058. [Google Scholar]
- 34.Mohammed A., Luka C. D., Ngwen A. L., Omale O. F. R., Yaknan B. J. Evaluation of the effect of aqueous leaf extract of jute mallow corchorus olitorius on some biochemical parameters in alloxan-induced diabetic rats. European Journal of Pharmaceutical and Medical research . 2019;6(10):652–658. [Google Scholar]
- 35.Das K., Tiwari R., Shrivastava D. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. Journal of Medicinal Plants Research . 2010;4(2):104–111. [Google Scholar]
- 36.Parekh J., Chanda S. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turkish Journal of Biology . 2007;31(1):53–58. [Google Scholar]
- 37.Shellard E. African Medicinal Plants, Proceeding of a Conference . University of Ife press; 1979. The significance of research into medicinal plants. [Google Scholar]
- 38.Sofowora A. Screening Plants for Bioactive Agents. Medicinal Plants and Traditional Medicinal in Africa . Ibadan, Nigeria: Spectrum Books Ltd, Sunshine House; 1993. [Google Scholar]
- 39.Evans W. C. Trease and Evans’ Pharmacognosy E-Book . Elsevier Health Sciences; 2009. [Google Scholar]
- 40.Trease G., Evans W. Pharmacognosy . London, UK: Bailliere Tindall; 1989. [Google Scholar]
- 41.Harborne J. B. Phytochemical Methods . Berlin, Germany: Springer; 1973. Phenolic compounds. [Google Scholar]
- 42.Salman S. M. Preliminary phytochemical, essential element analysis and antimicrobial activities of ethanolic extract of Lotus corniculatus. International Journal of Biosciences . 2015;7(2):106–115. [Google Scholar]
- 43.Talele P. Isolation of starch from Ginger rhizome (Zingiber officinale) Journal of Pharmacognosy and Phytochemistry . 2015;3(6):157–162. [Google Scholar]
- 44.In O. Acute oral toxicity-Acute oral toxic class method. Guideline 423. Eleventh Addendum to the OECD Guidelines for the Testing . 2001 [Google Scholar]
- 45.Mushtaq A., Akbar S., Zargar M. A., et al. Phytochemical screening, physicochemical properties, acute toxicity testing and screening of hypoglycaemic activity of extracts of Eremurus himalaicus baker in normoglycaemic Wistar strain albino rats. BioMed Research International . 2014;2014:1–6. doi: 10.1155/2014/867547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadat A., Hore M., Chakraborty K., Roy S. Phytochemical analysis and antioxidant activity of methanolic extract of leaves of corchorus olitorius. International Journal of Current Pharmaceutical Research . 2017;9(5):59–63. doi: 10.22159/ijcpr.2017v9i5.22138. [DOI] [Google Scholar]
- 47.Parvin S., Marzan’s M., Rahman S., Kumer Das A., Haque S., Rahmatullah M. Preliminary phytochemical screening, antihyperglycemic, analgesic and toxicity studies on methanolic extract of aerial parts of Corchorus olitorius L. Journal of Applied Pharmaceutical Science . 2015;5(9):68–71. [Google Scholar]
- 48.Adjatin A., Hounkpatin A., Assogba F., et al. Phytochemical screening, antioxidant and cytotoxic activity of different morphotypes of Corchorus olitorius L. leaves in the central region of Benin Republic (West Africa) Journal of Pharmacognosy and Phytotherapy . 2018;10(12):195–203. doi: 10.5897/jpp2018.0525. [DOI] [Google Scholar]
- 49.Orieke D., Ohaeri O., Ijeh I., Ijioma S. Identification of phytocomponents and acute toxicity evaluation of Corchorus olitorius leaf extract. European Journal of Medicinal Plants . 2018;23:1–16. doi: 10.9734/ejmp/2018/38739. [DOI] [Google Scholar]
- 50.Saliu J. A., Oboh G., Schetinger M. R., Stefanello N., Rocha J. B. T. Antidiabetic potentials of jute leaf (Corchorus olitorius) on type-2 diabetic rats. Journal of Emerging Trends in Engineering and Applied Sciences . 2015;6(7):223–230. [Google Scholar]
- 51.Tiong S. H., Looi C., Hazni H., et al. Antidiabetic and antioxidant properties of alkaloids from Catharanthus roseus (L.) G. Don. Molecules . 2013;18(8):9770–9784. doi: 10.3390/molecules18089770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support this study are available from the corresponding author upon request.


