Abstract
Recent studies have shown that sodium/glucose cotransporter 2 (SGLT2) inhibitors might exert favourable changes on cardiac parameters as observed on cardiovascular imaging. We conducted a systematic review and meta-analysis to determine the effects of SGLT2 inhibitors on cardiac imaging parameters. Four electronic databases (PubMed, Embase, Cochrane, Scopus) were searched for studies in which the effects of SGLT2 inhibitors on cardiac imaging parameters were examined. Studies in which a population was administered SGLT2 inhibitors and analysed by echocardiography and/or cardiac magnetic resonance (CMR) imaging were included. Random-effects pair-wise meta-analysis models were utilized to summarize the studies. A total of 11 randomized controlled trials was included with a combined cohort of 910 patients. Comparing patients receiving SGLT2 inhibitors with subjects receiving placebo, the mean change in CMR-measured left ventricular mass (LVM) was −3.87 g (95% confidence interval [CI], −7.77 to 0.04), that in left ventricular end-systolic volume (LVESV) was −5.96 mL (95% CI, −10.52 to −1.41) for combined LVESV outcomes, that in left atrial volume index (LAVi) was −1.78 mL/m2 (95% CI, −3.01 to −0.55) for combined LAVi outcomes, and that in echocardiography-measured E/e′ was −0.73 (95% CI, −1.43 to −0.03). Between-group differences were not observed in LVM and LVESV after indexation. The only between-group difference that persisted was for LAVi. Treatment with SGLT2 inhibitors resulted in reduction in LAVi and E/e′ on imaging, indicating they might have an effect on outcomes associated with LV diastolic function.
Keywords: Echocardiography, Magnetic resonance imaging, Heart failure, Pharmacology
INTRODUCTION
Cardiac imaging is used to evaluate the structure and function of the heart and plays an important role in the diagnosis, treatment, and monitoring of patients with heart diseases.1) Commonly used tests include cardiac magnetic resonance (CMR)2) imaging and echocardiography.3) These modalities generate cardiovascular imaging parameters such as left ventricular ejection fraction (LVEF), left ventricular mass (LVM), left ventricular end-systolic volume (LVESV), and left atrial volume (LAV), which are routinely assessed to determine the patient’s cardiac structure and function.2),3)
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a class of anti-hyperglycaemic drugs increasingly used in managing both diabetic and non-diabetic patients4) and are recognized for their cardioprotective benefits. The EMPA-REG OUTCOME trial5) showed that SGLT2 inhibitors improved cardiovascular outcomes in patients with type 2 diabetes mellitus at high cardiovascular risk. Hence, it is of interest to better understand the underlying pathophysiological changes that can elucidate how SGLT2 inhibitors lead to improved cardiovascular outcomes. In the recently published SUGAR-DM-HF trial6) that included patients with heart failure and reduced ejection fraction (HFrEF) and diabetes mellitus or pre-diabetes, reverse left ventricular (LV) remodelling was observed. In addition, similar findings in patients with type 2 diabetes mellitus and coronary artery disease were reported in the EMPA-HEART CardioLink-6 trial.7)
To the best of our knowledge, the effects of SGLT2 inhibitors on cardiac imaging parameters have not been investigated in a meta-analysis. Therefore, we conducted a systematic review and meta-analysis to determine the effects of SGLT2 inhibitors on cardiac imaging parameters measured using CMR and echocardiography. We hypothesized that treatment with SGLT2 inhibitors was associated with favourable changes in measured cardiovascular imaging parameters compared with placebo.
METHODS
This meta-analysis was conducted according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines.8) Four databases (PubMed, Embase, Cochrane, and Scopus) were searched on February 28, 2021, for articles published from date of inception to February 28, 2021. Literature search was performed using the following terms in combination: (“Empagliflozin” OR “Canagliflozin” OR “Dapagliflozin” OR “Ertugliflozin” OR “Ipragliflozin” OR “Luseogliflozin” OR “Remogliflozin” OR “Sotagliflozin” OR “Licogliflozin”) AND ("trial”).
Studies evaluating the cardiovascular imaging parameters of SGLT2 inhibitors were included. Cardiovascular imaging parameters included left atrial (LA) parameters of LAV, left atrial volume index (LAVi), LA diameter, and LA size; LVM parameters of LVM and left ventricular mass index (LVMi); LV volume parameters of LVESV, left ventricular end-systolic volume index (LVESVi), left ventricular end-diastolic volume (LVEDV), and left ventricular end-diastolic volume index (LVEDVi); LV function parameters of LVEF, left ventricular global longitudinal strain (LVGLS), LV global function index, and stroke volume, as well as extracellular volume fraction, LV internal diameter, aortic root diameter, mitral E velocity, mitral annulus velocity, E/A, E/e′, and pulmonary artery systolic pressure. We included all randomized controlled trials according to the PICOS inclusion and exclusion criteria (Table 1). We excluded all studies in which cardiovascular imaging parameters were not reported.
Table 1. PICOS inclusion criteria and exclusion criteria applied to database search.
| PICOS | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | ||
| Intervention | • SGLT2 inhibitors, including empagliflozin, canagliflozin, dapagliflozin, ertugliflozin, ipragliflozin, luseogliflozin, remogliflozin, sotagliflozin, and licogliflozin | |
| Comparison | • Comparison of SGLT2 inhibitors with a control group (placebo) on their effect on cardiovascular imaging parameters | |
| Outcome | • LA parameters: LAM, LAMi, LAD, and LA size | |
| • LVM parameters: LVM and LVMi | ||
| • LV volume parameters: LVESV, LVESVi, LVEDV, and LVEDVi | ||
| • LV function parameters: LVEF, LVGLS, LVGLSi, and stroke volume | ||
| • Others: extracellular volume fraction, LV internal diameter, aortic root diameter, mitral E velocity, mitral annulus velocity, E/A, E/e′, and pulmonary artery systolic pressure | ||
| Study design | • Articles in English or translated to English | • Mixed methods research, meta-analyses, systematic reviews, cohort studies, case-control studies, cross-sectional studies, and descriptive papers |
| • Randomised controlled trials | • Case reports and series, ideas, editorials, and perspectives | |
| • Conference abstracts or electronic and print information not controlled by commercial publishing, reporting on randomized controlled trials | ||
| • Year of Publication: Date of inception, February 28, 2021 | ||
| • Databases: PubMed, Embase, Cochrane, Scopus |
LA: left atrial, LAD: left atrial diameter, LAM: left atrial mass, LAMi: left atrial mass index, LVEDV: left ventricular end-diastolic volume, LVEDVi: left ventricular end-diastolic volume index, LVEF: left ventricular ejection fraction, LVESV: left ventricular end-systolic volume, LVESVi: left ventricular end-systolic volume index, LVGLS: left ventricular global longitudinal strain, LVM: left ventricular mass, LVMi: left ventricular mass index, PICOS: Populations, Intervention, Comparison, Outcomes, and Study, SGLT2: sodium-glucose cotransporter 2.
Four reviewers independently performed the literature search and data extraction, and all disagreements were resolved by mutual consensus. The data underlying this article will be shared upon reasonable request to the corresponding author. Reviews on SGLT2 inhibitors were identified from the title and abstract review, and hand search of the references was performed to identify any relevant articles.
In addition to cardiovascular imaging parameters, baseline information of patients was collected including age, sex, diabetes mellitus, body mass index, hypertension, HbA1c, history of coronary artery disease, history of heart failure, New York Heart Association class of heart failure, and drugs used. For the SGLT2 inhibitor regimens, data regarding the drug name, drug dosage, drug frequency, control group, length of intervention, and mean length of follow-up were collected. Data relating to blinding and withdrawals were extracted to assess risk of bias. Quality control was performed by two independent reviewers using the Cochrane Risk of Bias tool (Supplementary Figure 1).9) The quality of pooled evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (Supplementary Table 1).10) A PRISMA checklist8) is included in Supplementary Figure 2.
Statistical analysis
The results were quantitatively combined and analysed using Review Manager (RevMan) Version 5.411) using general approaches according to the Cochrane Handbook.12) In studies without standard deviations, p-values, and confidence intervals (CIs), the square root of weighted mean variance of all other studies was used to estimate the standard deviation.13) In studies without mean and standard deviations, the median and interquartile range were used to estimate mean and standard deviations.14) In studies without standard deviation, the p-value or CI was used to calculate the standard deviations.12) For studies in which changes in mean and standard deviation from baseline are not reported, simple analysis of change scores is used to estimate means and standard deviations, where C was 0.8. For panel data or longitudinal outcomes, pre-intervention baseline imbalances were corrected using the simple analysis of change scores method.12) In studies where the outcome was reported in different scales, a simple unit conversion was performed. Inverse variance was utilized in deriving the combined outcomes. The random-effects model was utilized to account for between-study variance. Between-study heterogeneity was presented using I2 and τ2 statistics. An I2 < 30% was considered to indicate low heterogeneity between studies, 30–60% moderate heterogeneity, and > 60% high heterogeneity. Two-sided p-values < 0.05 were considered to indicate nominal statistical significance. To perform a sensitivity analysis of patients with only diabetes mellitus, the EMPATROPISM trial,15) which reported on patients without diabetes mellitus, was excluded. Subgroup analysis based on baseline LVEF was performed to investigate if there was a tendency for greater improvement in cardiac imaging parameters in patients with reduced LVEF. Network meta-analysis for LAVi was conducted to determine efficacy of the SGLT2 inhibitors dapagliflozin and empagliflozin. Frequentist network meta-analysis of aggregate data was adopted to compare the two SGLT2 inhibitors using Stata 16.0 (StataCorp, College Station, TX, USA).16) The assumption of transitivity was evaluated using a global Wald test of consistency. Consistency models were fitted with restricted maximum likelihood models that assumed a common heterogeneity variance τ2 for all treatment contrasts for each clinical outcome when there was little evidence of inconsistency (p > 0.10 from Wald test). An inconsistency model was utilized for clinical outcomes with evidence of inconsistency (p < 0.10 from Wald test). The geometry of each network plot was visually and numerically inspected for potential biases. The relative ranking probability of the four treatments was estimated from 1,000 draws, and the hierarchy of treatments was analysed using surface under the cumulative ranking (SUCRA) curves. Higher SUCRA values correspond to greater efficacy. Interpretations regarding the relative efficacy of treatments were based on inspection of 95% CIs in interval plots and supported with analysis of ranking probabilities.
RESULTS
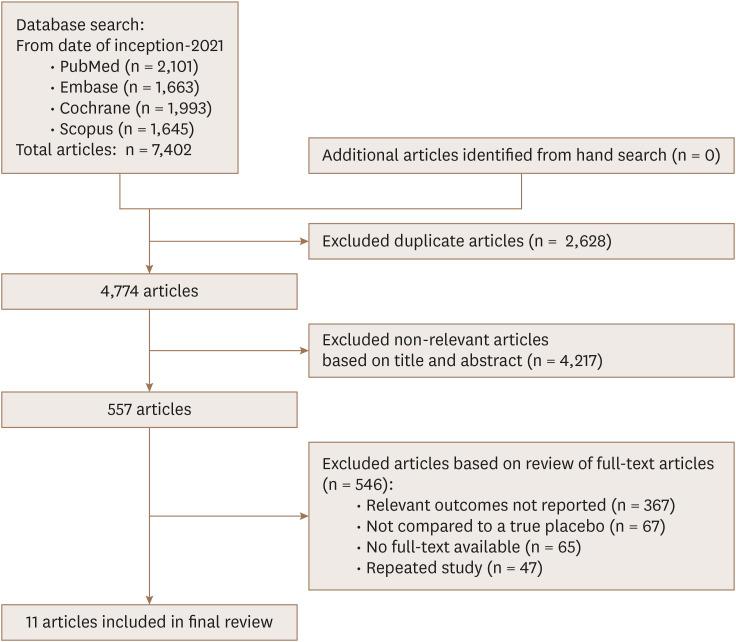
The PRISMA flowchart is presented in Figure 1. Literature search of the four databases (PubMed, Embase, Cochrane, and Scopus) retrieved 7,402 results; 2,628 duplicates were removed. Title and abstract screening excluded a further 4,217 articles because cardiovascular imaging parameters were not evaluated or the study was an inappropriate type. Full text screening excluded 546 articles. Finally, 11 articles were included for the meta-analysis.
Figure 1. PRISMA flow diagram of study selection.
PRISMA: Preferred Reporting Items of Systematic Reviews and Meta-Analyses.
Baseline characteristics
The 11 studies comprised a combined cohort of 910 patients.6),7),15),17) – 24) The participant baseline characteristics of the included studies are shown in Table 2.
Table 2. Participant baseline characteristics.
| Study | DM patients/Total patients | Mean age (years) | Males | HTN | Mean BMI (kg/m2) | HbA1c (%) | History of CAD | History of HF | NYHA class of HF | Drugs | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMR | |||||||||||
| Brown et al. (2020)17) | 66/66 (100%) | 65.53 | 38 | 51 | 32.45 | 7.7 | 8 | NR | NR | ACEi (n = 35) ARB (n = 11) beta-blocker (n = 9) | |
| Lee et al. (2020)6) | 82/105 (78.1%) | NR (not specific to DM subgroup) | NR (not specific to DM subgroup) | NR (not specific to DM subgroup) | NR (not specific to DM subgroup) | 7.2 | NR (not specific to DM subgroup) | 105 | II (n = 81) III (n = 24) | ACEi (n = 49) ARB (n = 15) beta-blocker (n = 96) ARNI (n = 36) MRA (n = 63) | |
| Verma et al. (2019)7) | 97/97 (100%) | Cannot be calculated (2 medians) | 90 | 88 | Cannot be calculated (2 medians) | 7.9 (Median) | 97 | 6 | NR | ACEi/ARB (n = 81) beta-blocker (n = 77) | |
| Oldgren et al. (2021)18) | 48/48 (100%) | 64.4 | 25 | 36.48 | NR | 6.7 | 2 | 0 | NR | NR | |
| Singh et al. (2020)20) | 56/56 (100%) | 67.1 | 37 | 40 | 32.5 | 7.7 | 29 | 56 | I (n = 25), II (n = 24), III (n = 7) | ACEi/ARB (n = 50) beta-blocker (n = 46) MRA (n = 23) | |
| Santos-Gallego et al. (2021)15) | 0/84 (0%) | 62 | 54 | 62 | 29.7 | 5.8 | NR | 83 | NR | ACEi/ARB (n = 35) beta-blocker (n = 74) ARNI (n = 36) MRA (n = 28) | |
| Echocardiography | |||||||||||
| de Boer et al. (2020)21) | 124/124 (100%) | All median: Lico 2.5 mg: 70.0 Lico 10 mg: 72.5 Lico 50 mg: 66.0 Empagliflozin 25 mg: 68.5 Placebo: 71.0 | 89 | NR | All median: Lico 2.5 mg: 33.3 Lico 10 mg: 31.9 Lico 50 mg: 32.0 Empagliflozin 25 mg: 31.2 Placebo: 31.3 | NR | NR | 124 | Lico 2.5 mg: II (n = 13), III (n = 2) Lico10 mg: II (n = 14), III (n = 2) Lico 50 mg: II (n = 26), III (n = 3), IV (n = 1) Empagliflozin 25 mg: II (n = 22), III (n = 8) Placebo: II (n = 25), III (n = 8) | ACEi (n = 2) ARB (n = 2) ARNI (n = 1) | |
| Omar et al. (2021)22) | 24/190 (12.6%) | 64 | 162 | 76 | 29 | 5.8 | 103 | 190 | I (n = 12), II (n = 149), III (n = 29) | ACEi/ARB (n = 124) beta-blocker (n = 180) ARNI (n = 58) MRA (n = 125) | |
| Rau et al. (2021)23) | 42/42 (100%) | 62 | 17 | NR | 31.1 | 7.7 | 30 | 18 | NR | Beta-blocker (n = 32) RAAS inhibitors (n = 35) | |
| Eickhoff et al. (2020)24) | 36/40 (90%) | 64 | 32 | NR | 32.8 | 8.9 | 11 | NR | NR | RAAS blockers (n = 40) | |
| Shim et al. (2021)19) | 58/58 (100%) | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
ACEi: angiotensin-converting enzyme inhibitors, ARB: angiotensin receptor blocker, ARNI: angiotensin receptor neprilysin inhibitor, CAD: coronary artery disease, DM: diabetes mellitus, HbA1c: haemoglobin A1c, HF: heart failure, HTN: hypertension, MRA: mineralocorticoid antagonist, NR: not reported, NYHA: New York Heart Association, RAAS: renin-angiotensin-aldosterone system.
For the 11 studies, the SGLT2 inhibitor drug name, dosage, frequency, control group, length of intervention, and length of follow-up were summarized and are attached in Supplementary Table 2. Empagliflozin, dapagliflozin, and licogliflozin were the SGLT2 inhibitors used in 6, 5, and 1 studies, respectively. All regimens were administered once daily and compared with a control group receiving placebo. The length of follow-up ranged from 6 weeks to one year.
LA parameters
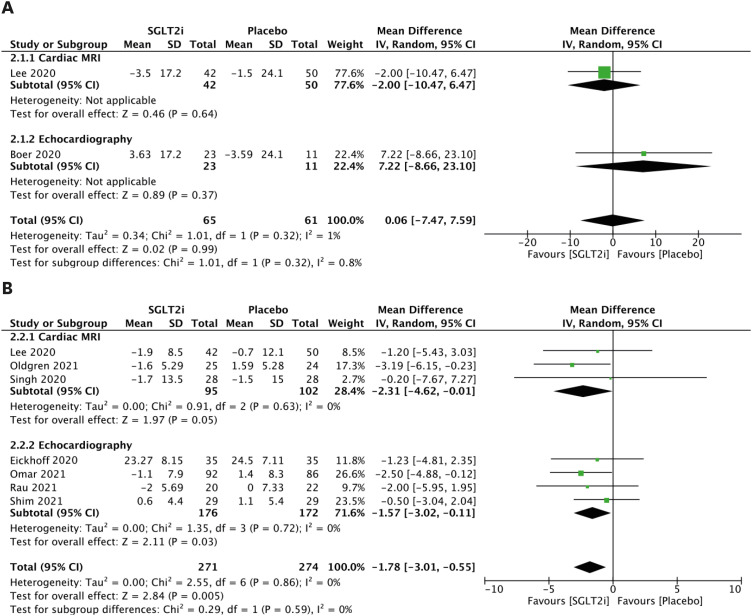
There was no significant change in LAV between groups (Figure 2A). The analysis of LAVi is presented in Figure 2B. Comparing patients receiving SGLT2 inhibitors to patients who did not, the random effects model demonstrated a mean reduction in LAVi of −2.31 mL/m2 (95% CI, −4.62 to −0.01) using CMR (Figure 2B), a mean reduction in LAVi of −1.57 mL/m2 (95% CI, −3.02 to −0.11; Figure 2B) using echocardiography, and a mean reduction in combined LAVi outcome of −1.78 mL/m2 (95% CI, −3.01 to −0.55; Figure 2B).
Figure 2. LAV parameters. (A) Forest plot of mean change in LAV in mL. (B) Forest plot of mean change in LAVi in mL/m2.
CI: confidence interval, IV: interval variable, LAV: left atrial volume, LAVi: left atrial volume index, MRI: magnetic resonance imaging, SD: standard deviation, SGLT2i: sodium-glucose cotransporter 2 inhibitor.
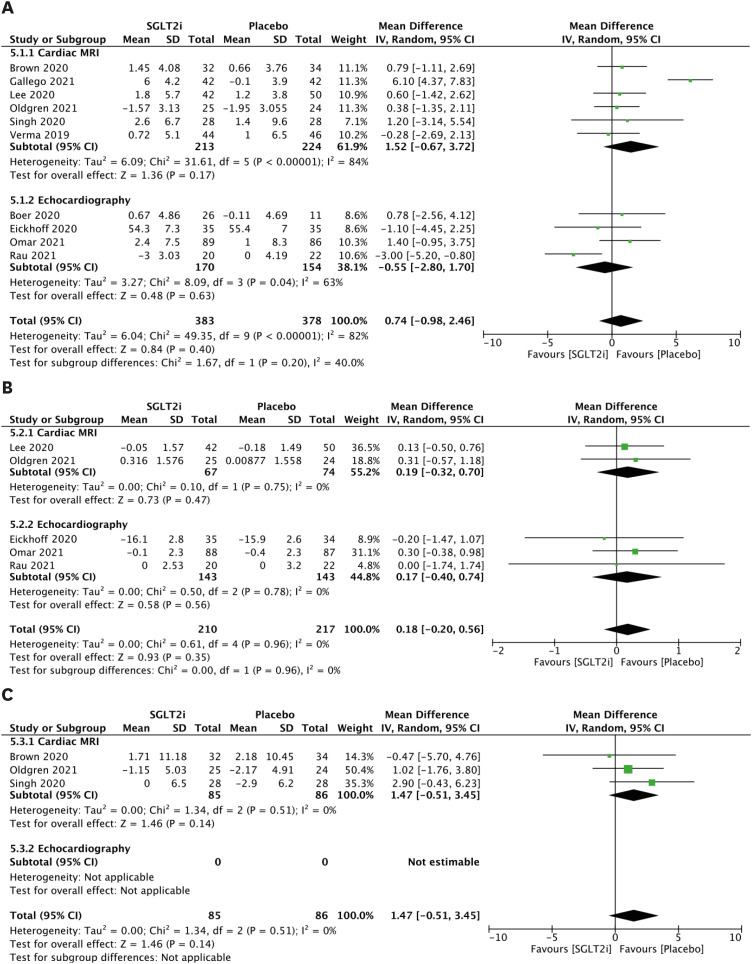
LVM parameters
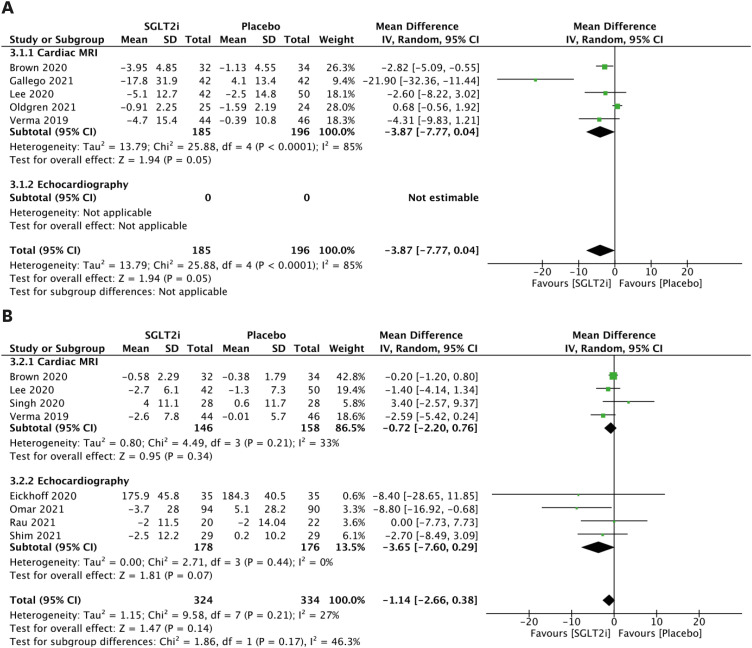
The LVM parameters are presented in Figure 3A. When comparing patients receiving SGLT2 inhibitors with patients who did not, the random effects model demonstrated a mean reduction in LVM of −3.87 g (95% CI, −7.77 to 0.04; Figure 3A) using CMR. There are no previous studies reporting LVM for echocardiography. Although LVMi was investigated in 4 CMR and 4 echocardiography studies, there was no significant mean change based on meta-analysis (Figure 3B).
Figure 3. LVM parameters. (A) Forest plot of mean change in LVM in g. (B) Forest plot of mean change in LVMi in g/m2.
CI: confidence interval, IV: interval variable, LVM: left ventricular mass, LVMi: left ventricular mass index, MRI: magnetic resonance imaging, SD: standard deviation, SGLT2i: sodium-glucose cotransporter 2 inhibitor.
LV volume parameters
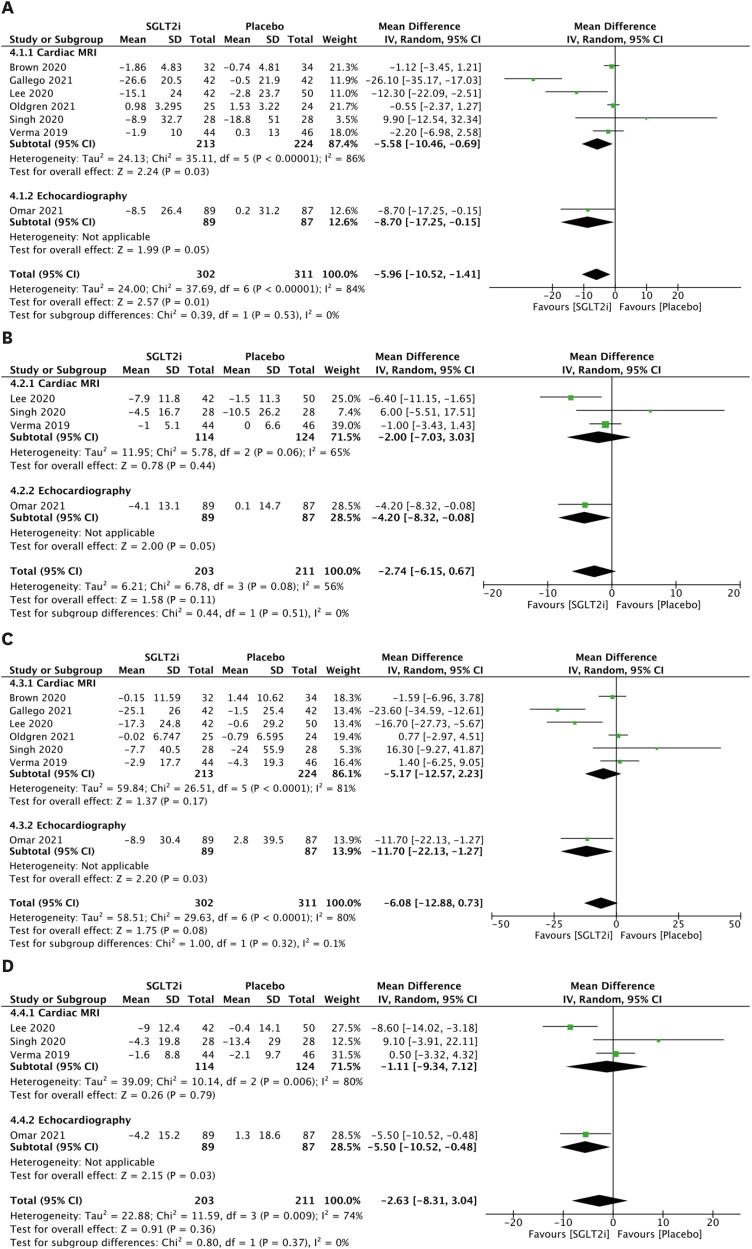
The LVESV parameters are presented in Figure 4A. Comparing patients receiving SGLT2 inhibitors to patients without, the random effects model demonstrated a mean reduction in LVESV of −5.58 mL (95% CI, −10.46 to −0.69; Figure 4A) using CMR, a mean reduction in LVESV of −8.70 mL (95% CI, −17.25 to −0.15; Figure 4A) using echocardiography, and a mean reduction in combined LVESV outcome of −5.96 mL (95% CI, −10.52 to −1.41; Figure 4A). However, after indexing, there was no significant mean change in LVESVi (Figure 4B). In addition, significant mean change was not observed in LVEDV (Figure 4C) or LVEDVi (Figure 4D).
Figure 4. LV volume parameters. (A) Forest plot of mean change in LVESV in mL. (B) Forest plot of mean change in LVESVi in mL/m2. (C) Forest plot of mean change in LVEDV in mL. (D) Forest plot of mean change in LVEDVi in mL/m2.
CI: confidence interval, IV: interval variable, LV: left ventricular, LVEDV: left ventricular end-diastolic volume, LVEDVi: left ventricular end-diastolic volume index, LVESV: left ventricular end-systolic volume, LVESVi: left ventricular end-systolic volume index, MRI: magnetic resonance imaging, SD: standard deviation, SGLT2i: sodium-glucose cotransporter 2 inhibitor.
LV function parameters
Significant change was not observed in LVEF (Figure 5A), LVGLS (Figure 5B), or stroke volume (Figure 5C).
Figure 5. LV function parameters. (A) Forest plot of mean change in LVEF in %. (B) Forest plot of mean change in LVGLS in %. (C) Forest plot of mean change in stroke volume in mL.
CI: confidence interval, IV: interval variable, LV: left ventricular, LVEF: left ventricular ejection fraction, LVGLS: left ventricular global longitudinal strain, MRI: magnetic resonance imaging, SD: standard deviation, SGLT2i: sodium-glucose cotransporter 2 inhibitor.
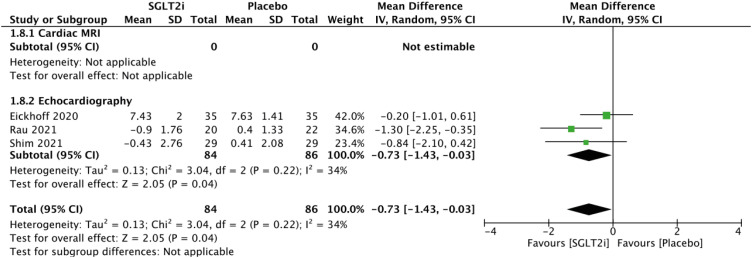
Other cardiovascular imaging parameters
The E/e′ parameters are presented in Figure 6. Comparing patients receiving SGLT2 inhibitors with patients who did not, the random effects model demonstrated a mean reduction in E/e′ of −0.73 (95% CI, −1.43 to −0.03; Figure 6) using echocardiography. The E/A, mitral E velocity, and pulmonary artery systolic pressure parameters are presented in Supplementary Figures 3, 4, 5, respectively. There were no statistically significant changes in the combined outcomes of the abovementioned parameters.
Figure 6. Forest plot of mean change in E/e′.
CI: confidence interval, IV: interval variable, MRI: magnetic resonance imaging, SD: standard deviation, SGLT2i: sodium-glucose cotransporter 2 inhibitor.
Sensitivity analysis
The EMPATROPISM trial15) was excluded from analysis due to its substantial heterogeneity for statistically significant outcome LVESV as well as LVM, which trends towards statistically significant mean reduction (see Limitations). After sensitivity analysis, heterogeneity for LVM using CMR decreased (I2 = 69%), and heterogeneity for LVESV using CMR (I2 = 38%) and combined outcomes (I2 = 47%) decreased; however, the mean reductions in LVM and LVESV were not statistically significant.
Subgroup analysis
Subgroup analysis was performed based on baseline LVEF to determine if there was a tendency for greater improvement in cardiac imaging parameters in patients with reduced LVEF. Five studies in which reduced/mildly reduced LVEF6),15),20),22),23) was reported were included in the subgroup analysis. The LAVi, LVM/LVMi, LVESV/LVESVi/LVEDV/LVEDVi, and LVEF/LVGLS parameters are presented in Supplementary Figures 6, 7, 8, 9, respectively. The mean reduction in LAVi (Supplementary Figure 6) and LVESV (Supplementary Figure 8A) remained statistically significant, the mean reduction in LVEDV (Supplementary Figure 8C) changed from not statistically non-significant to statistically significant, and the mean reduction in LVM (Supplementary Figure 7A), LVMi (Supplementary Figure 7B), LVESVi (Supplementary Figure 8B), LVEDVi (Supplementary Figure 8D), LVEF (Supplementary Figure 9A), and LVGLS (Supplementary Figure 9B) remained not statistically significant. Comparing patients with reduced/mildly reduced LVEF with patients in the main analysis, there was a greater mean decrease in LAVi (−2.03 mL/m2; 95% CI, −3.82 to −0.25; Supplementary Figure 6 vs. −1.78 mL/m2; 95% CI, −3.01 to −0.55; Figure 2B), in LVESV (−11.92 mL; 95% CI, −23.25 to −0.59; Supplementary Figure 8A, vs. −5.96 mL; 95% CI, −10.52 to −1.41; Figure 4A), and in LVEDV (−13.04 mL; 95% CI, −23.97 to −2.11; Supplementary Figure 8C vs. −6.08 mL; 95% CI, −12.88 to 0.73; Figure 4C) in patients with reduced/mildly reduced LVEF. Furthermore, we performed subgroup analysis of five studies with preserved LVEF.7),17),18),19),24) The mean reduction in outcomes analysed, such as LAVi, LVM, LVMi, LVESV, LVEDV, LVEF, LVGLS, stroke volume, E/A, and E/e′, was not statistically significant.
Network meta-analysis
Because the mean reduction in LAVi was statistically significant, network meta-analysis was performed to compare the effects of the SGLT2 inhibitors dapagliflozin and empagliflozin on LAVi. We could not detect a significant difference in effect estimate between dapagliflozin and empagliflozin on LAVi. Comparing patients receiving dapagliflozin with patients receiving empagliflozin, the mean change in LAVi was 0.89 mL/m2 (95% CI, −2.26 to 4.03; Supplementary Figure 10). Licogliflozin was not considered in the analysis because this drug was used in only one study.
DISCUSSION
In this pair-wise meta-analysis of 11 randomized-controlled trials, patients treated with SGLT2 inhibitor had mean reduction in LAVi and E/e′ compared with those administered placebo. Although mean reduction was observed in LVM and LVESV, statistically significant change was not found after indexing. There were no differences in any other parameters examined.
The proposed mechanisms by which SGLT2 inhibitors exert their effects include diuresis and natriuresis.25) This help decrease preload and afterload through reduction in intravascular volume and increase in sodium excretion as well as reducing arterial stiffness and vascular resistance26) and, when combined, can lead to a mean reduction in LVM. Furthermore, SGLT2 inhibitors might provide an alternative and potentially more efficient source of myocardial adenosine by increasing circulating ketone triphosphate level.27) In addition, and changes in myocardial substrate utilization might exert changes on myocardial structure and function. Last, SGLT2 inhibitors have been thought to reduce inflammatory cytokines28),29),30) which help decrease extracellular matrix turnover and fibrosis.
Heart failure is a rapidly growing public health issue, with an estimated prevalence of 64.3 million cases globally.31) Thus, to reduce the significant burden of heart failure, effective treatment is needed. In recent clinical trials such as IDDIA,19) addition of the SGLT2 inhibitor dapagliflozin to standard antihyperglycemic treatment in patients with type 2 diabetes mellitus was associated with a significant improvement in LV diastolic dysfunction based on diastolic stress echocardiography. In this meta-analysis, treatment with SGLT2 inhibitors compared with placebo was similarly associated with favourable changes in cardiac imaging parameters reflective of LV diastolic function, such as LAVi and E/e′. This is clinically important because LV diastolic dysfunction has been associated with risk of heart failure32) and affects prognosis in heart failure patients.33) Therefore, the findings of our meta-analysis indicate that SGLT2 inhibitors have a role in prevention and treatment of heart failure. The size of the atrial myocardium (LAV) could reflect the increased LV diastolic dysfunction present due to increased stretch from increased LV filling pressure.34) To better understand this relationship, the exact mechanisms of the effect of SGLT2 inhibitors on the atrial myocardium should be investigated in future studies.
Our meta-analysis also demonstrated trends for reduction in LVM parameters (LVM and LVMi), reduction in LV volume parameters (LVESV, LVESVi, LVEDV, and LVEDVi), and increase in LV function parameters (LVEF, LVGLS, and stroke volume). These findings could be used in future studies to further determine the effects of SGLT2 inhibitors on cardiac imaging parameters. In addition, our meta-analysis did not demonstrate a significant reduction of LVESVi or LVMi, which might be due to the lack of reporting of these outcomes in trials such as the EMPATROPISM,15) in which positive findings in LVESV and LVM were reported. Future imaging studies should report the indexed values of these parameters because anthropometric measurements can influence interpretation.35)
In addition, there was no mean change in LV function parameters LVEF, LVGLS, and stroke volume. The implies that drugs used to improve ejection fraction in heart failure, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and angiotensin receptor neprilysin inhibitors, as well as revascularization are still required as part of heart failure treatment. Although an imperfect measure, LVEF cut-offs are the basis of current guidelines, and efforts should be made to improve LVEF to ensure that only eligible patients receive advanced therapies as indicated (e.g., implantable cardiac defibrillators).36),37)
To the best of our knowledge, this is the first study to review comprehensively the effects of SGLT2 inhibitors on cardiac imaging parameters. However, the results should be interpreted with consideration of the limitations. First, SGLT2 inhibitors were only administered for a maximum of one year, and favourable cardiac remodelling in response to pharmacological therapy might require a longer follow-up period. Second, among statistically significant outcomes, there was substantial heterogeneity for mean change in LVM (I2 = 85%) using CMR, mean change in LVESV (I2 = 86%) using CMR, and for combined outcomes (I2 = 84%). Future studies are necessary to elucidate if the difference in cardiac structure benefits is attributable to diabetic status in our meta-analysis cohort because recent trials have shown that the cardiovascular benefits of SGLT2 inhibitors extend to both diabetic and non-diabetic patients. Third, reverse cardiac remodelling can occur in approximately 30–40% of HFrEF patients receiving guideline-directed medical treatment.38) Therefore, the favourable effects on cardiac imaging parameters might be misattributed to SGLT2 inhibitors in certain circumstances. Fourth, type of SGLT2 inhibitors might affect cardiac imaging parameters differently. In the 11 included studies, only three SGLT2 inhibitors (empagliflozin, dapagliflozin, and licogliflozin) were used. Future studies should be conducted to examine the differential effects of various SGLT2 inhibitors on cardiac imaging parameters and determine if the findings of our meta-analysis can be applied to all types of SGLT2 inhibitors.
CONCLUSION
In this meta-analysis, treatment with SGLT2 inhibitors compared with placebo was associated with favourable change in LAVi and E/e′ on imaging. Future research on the effects of treatment with SGLT2 inhibitors on cardiovascular imaging parameters compared with other commonly used heart failure drugs could clarify the utility of SGLT2 inhibitors in overall heart failure management, including prognosis and treatment.
Footnotes
Funding: CS was supported by the Junior Academic Faculty Scheme of the National University of Singapore Yong Loo Lin School of Medicine.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Wee CF, Teo YH, Teo YN, Syn NL, Sia CH.
- Data curation: Wee CF, Teo YH, Teo YN, Syn NL, See RM, Leong S, Yip ASY, Ong ZX.
- Formal analysis: Wee CF, Teo YH, Teo YN, Syn NL, See RM, Leong S, Yip ASY, Ong ZX, Sia CH.
- Methodology: Wee CF, Teo YH, Teo YN, Syn NL.
- Software: Wee CF.
- Supervision: Wee CF, Teo YH, Lee CH, Chan MYY, Poh KK, Ong CC, Teo LL, Singh D, Tan BY, Yeo LL, Kong WK, Yeo TC, Wong RC, Chai P, Sia CH.
- Validation: Lee CH, Chan MYY, Poh KK, Ong CC, Teo LL, Singh D, Tan BY, Yeo LL, Kong WK, Yeo TC, Wong RC, Chai P, Sia CH.
- Writing - original draft: Wee CF.
- Writing - review & editing: Wee CF, Teo YH, Syn NL, Lee CH, Chan MYY, Poh KK, Ong CC, Teo LL, Singh D, Tan BY, Yeo LL, Kong WK, Yeo TC, Wong RC, Chai P, Sia CH.
SUPPLEMENTARY MATERIALS
Outcome characteristics evaluated using the GRADE system
Intervention characteristics in the 11 studies including SGLT2 inhibitor drug name, dosage, frequency, control group, length of intervention, and length of follow-up
Cochrane risk of bias graph.
PRISMA 2020 checklist.
Forest plot of mean change in E/A.
Forest plot of mean change in mitral E velocity in cm/s.
Forest plot of mean change in pulmonary artery systolic pressure in mmHg.
Forest plot of mean change in LAVI in mL/m2 in patients with reduced/mildly reduced LVEF.
Parameters of LVM in patients with reduced/mildly reduced LVEF. (A) Forest plot of mean change in LVM in g. (B) Forest plot of mean change in LVMi in g/m2.
Parameters of LV volume in patients with reduced/mildly reduced LVEF. (A) Forest plot of mean change in LVESV in mL. (B) Forest plot of mean change in LVESVi in mL/m2. (C) Forest plot of mean change in LVEDV in mL. (D) Forest plot of mean change in LVEDVi in mL/m2.
Parameters of LV function in patients with reduced/mildly reduced LVEF. (A) Forest plot of mean change in LVEF (%). (B) Forest plot of mean change in LVGLS (%).
Network meta-analysis comparing the effects of dapagliflozin and empagliflozin on LAVi.
References
- 1.Blankstein R. Cardiology patient page. Introduction to noninvasive cardiac imaging. Circulation. 2012;125:e267–e271. doi: 10.1161/CIRCULATIONAHA.110.017665. [DOI] [PubMed] [Google Scholar]
- 2.Cavalcante JL, Lalude OO, Schoenhagen P, Lerakis S. Cardiovascular magnetic resonance imaging for structural and valvular heart disease interventions. JACC Cardiovasc Interv. 2016;9:399–425. doi: 10.1016/j.jcin.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Shibayama K, Watanabe H. Clinical use of echocardiography in structural heart disease. Gen Thorac Cardiovasc Surg. 2016;64:365–372. doi: 10.1007/s11748-016-0649-9. [DOI] [PubMed] [Google Scholar]
- 4.Teo YH, Teo YN, Syn NL, et al. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc. 2021;10:e019463. doi: 10.1161/JAHA.120.019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 6.Lee MM, Brooksbank KJ, Wetherall K, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF) Circulation. 2021;143:516–525. doi: 10.1161/CIRCULATIONAHA.120.052186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140:1693–1702. doi: 10.1161/CIRCULATIONAHA.119.042375. [DOI] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. place unknown: The GRADE Working Group; 2013. [Google Scholar]
- 11.The Cochrane Collaboration. ReviewManager (RevMan). Version 5.4. London: The Cochrane Collaboration; 2020. [Google Scholar]
- 12.Higgins JP, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. London: The Cochrane Collaboration; 2019. [Google Scholar]
- 13.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243–255. doi: 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. In: Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1. Higgins JPT, Thomas J, Chandler J, et al., editors. London: The Cochrane Collaboration; 2020. Chapter 11: Undertaking network meta-analyses. [Google Scholar]
- 17.Brown AJ, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J. 2020;41:3421–3432. doi: 10.1093/eurheartj/ehaa419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldgren J, Laurila S, Åkerblom A, et al. Effects of 6 weeks of treatment with dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on myocardial function and metabolism in patients with type 2 diabetes: a randomized, placebo-controlled, exploratory study. Diabetes Obes Metab. 2021;23:1505–1517. doi: 10.1111/dom.14363. [DOI] [PubMed] [Google Scholar]
- 19.Shim CY, Seo J, Cho I, et al. Randomized, controlled trial to evaluate the effect of dapagliflozin on left ventricular diastolic function in patients with type 2 diabetes mellitus: the IDDIA trial. Circulation. 2021;143:510–512. doi: 10.1161/CIRCULATIONAHA.120.051992. [DOI] [PubMed] [Google Scholar]
- 20.Singh JS, Mordi IR, Vickneson K, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. 2020;43:1356–1359. doi: 10.2337/dc19-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer RA, Núñez J, Kozlovski P, Wang Y, Proot P, Keefe D. Effects of the dual sodium-glucose linked transporter inhibitor, licogliflozin vs placebo or empagliflozin in patients with type 2 diabetes and heart failure. Br J Clin Pharmacol. 2020;86:1346–1356. doi: 10.1111/bcp.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omar M, Jensen J, Ali M, et al. Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the empire HF randomized clinical trial. JAMA Cardiol. 2021;6:836–840. doi: 10.1001/jamacardio.2020.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rau M, Thiele K, Hartmann NK, et al. Empagliflozin does not change cardiac index nor systemic vascular resistance but rapidly improves left ventricular filling pressure in patients with type 2 diabetes: a randomized controlled study. Cardiovasc Diabetol. 2021;20:6. doi: 10.1186/s12933-020-01175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eickhoff MK, Olsen FJ, Frimodt-Møller M, et al. Effect of dapagliflozin on cardiac function in people with type 2 diabetes and albuminuria - a double blind randomized placebo-controlled crossover trial. J Diabetes Complications. 2020;34:107590. doi: 10.1016/j.jdiacomp.2020.107590. [DOI] [PubMed] [Google Scholar]
- 25.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 Inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–1339. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 28.Iannantuoni F, M de Marañon A, Diaz-Morales N, et al. The SGLT2 inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. J Clin Med. 2019;8:1814. doi: 10.3390/jcm8111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heerspink HJ, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154–1166. doi: 10.1007/s00125-019-4859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leng W, Wu M, Pan H, et al. The SGLT2 inhibitor dapagliflozin attenuates the activity of ROS-NLRP3 inflammasome axis in steatohepatitis with diabetes mellitus. Ann Transl Med. 2019;7:429. doi: 10.21037/atm.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little WC, Oh JK. Echocardiographic evaluation of diastolic function can be used to guide clinical care. Circulation. 2009;120:802–809. doi: 10.1161/CIRCULATIONAHA.109.869602. [DOI] [PubMed] [Google Scholar]
- 34.Tamura H, Watanabe T, Nishiyama S, et al. Increased left atrial volume index predicts a poor prognosis in patients with heart failure. J Card Fail. 2011;17:210–216. doi: 10.1016/j.cardfail.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Hense HW, Gneiting B, Muscholl M, et al. The associations of body size and body composition with left ventricular mass: impacts for indexation in adults. J Am Coll Cardiol. 1998;32:451–457. doi: 10.1016/s0735-1097(98)00240-x. [DOI] [PubMed] [Google Scholar]
- 36.Writing Committee Members. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 37.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 38.Reis Filho JR, Cardoso JN, Cardoso CM, Pereira-Barretto AC. Reverse cardiac remodeling: a marker of better prognosis in heart failure. Arq Bras Cardiol. 2015;104:502–506. doi: 10.5935/abc.20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Outcome characteristics evaluated using the GRADE system
Intervention characteristics in the 11 studies including SGLT2 inhibitor drug name, dosage, frequency, control group, length of intervention, and length of follow-up
Cochrane risk of bias graph.
PRISMA 2020 checklist.
Forest plot of mean change in E/A.
Forest plot of mean change in mitral E velocity in cm/s.
Forest plot of mean change in pulmonary artery systolic pressure in mmHg.
Forest plot of mean change in LAVI in mL/m2 in patients with reduced/mildly reduced LVEF.
Parameters of LVM in patients with reduced/mildly reduced LVEF. (A) Forest plot of mean change in LVM in g. (B) Forest plot of mean change in LVMi in g/m2.
Parameters of LV volume in patients with reduced/mildly reduced LVEF. (A) Forest plot of mean change in LVESV in mL. (B) Forest plot of mean change in LVESVi in mL/m2. (C) Forest plot of mean change in LVEDV in mL. (D) Forest plot of mean change in LVEDVi in mL/m2.
Parameters of LV function in patients with reduced/mildly reduced LVEF. (A) Forest plot of mean change in LVEF (%). (B) Forest plot of mean change in LVGLS (%).
Network meta-analysis comparing the effects of dapagliflozin and empagliflozin on LAVi.