Abstract
BACKGROUND
This study aims to investigate normal changes throughout aging of the heart in cardiac magnetic resonance (CMR) imaging in healthy volunteers. While type 2 diabetes mellitus is a frequent finding in the elderly population, also the influence of this circumstance in otherwise healthy persons is part of our study.
METHODS
In this prospective single-center trial, 75 healthy subjects in distinct age groups and 10 otherwise healthy diabetics were enrolled. All subjects underwent functional, flow sensitive, native T2- and T1-mapping in a 1.5T CMR scanner.
RESULTS
No differences in right and left ventricular ejection fractions were observed between aging healthy groups. Bi-ventricular volumes lowered significantly (p<0.001) between the age groups. There was also a significant decrease in myocardial T1 values, aortic distensibility, and left ventricular peak diastolic strain rates. There were no differences in T2 mapping and the other deformation parameters. Patients with type 2 diabetes mellitus had lower end-diastolic volume indexes; all the other measurements were comparable.
CONCLUSIONS
Aging processes in the healthy heart involve a decrease in ventricular volumes, with ejection fractions remaining normal. Stiffening of the myocardium and aorta and a decrease in T1 values are potential indications of age-related remodeling. Type 2 diabetes mellitus seems to have no major influence on aging processes of the heart.
Trial Registration
EudraCT Identifier: EudraCT 2017-000045-42
Keywords: Cardiac magnetic resonance imaging, Strain imaging, Normal values, Aging, Healthy subjects
INTRODUCTION
It is not a late breaking news that our society in industrialized nations is getting older and the formerly normal age distribution has changed from the shape of a pyramid to a kind of onion. In medicine, this means a wide variety of ‘normal patients’ and demands a distinctive feeling for their individual needs.1),2) Knowledge about physiologic processes of aging and thereby the variety of ‘healthy’ gets more and more important. On one hand, it is crucial to recognize pathological changes even in older subjects and not to dismiss them as supposedly physiological. On the other Hand, physiological aging should not be pathologized, only to achieve ‘positive’ findings in each examination. Individualized medicine in a time of demographic change needs individualized normal ratios.
Cardiac magnetic resonance imaging (CMR) is the gold standard in non-invasive assessment of atrial and ventricular volumes. With a bundle of imaging modalities, it can visualize function, blood velocities, viability, perfusion, and tissue characterization within one radiation free examination. It is therefore a perfect tool to get a deeper understanding of the most universal influencing factor: aging.
With this study, we thereby aim following questions:
a. Is there an age-dependency in left and right ventricular volumes and function, deformation parameters and distensibility of the aortic root?
b. Is aging of the heart different in otherwise healthy patients with diabetes?
METHODS
Study enrollment
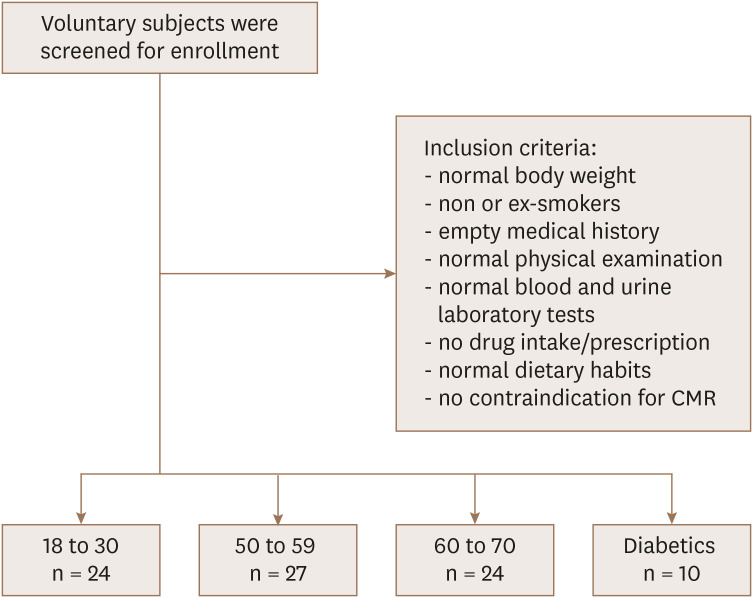
This study is a single-center prospective trial. Volunteers were screened for study enrollment between March 2017 and June 2018. A total of 75 healthy probands was planned equally divided into 3 age-dependent groups: 18 to 30, 50 to 59 and 60 to 70 years. To examine an influence of type 2 diabetes mellitus on heart aging, 25 otherwise healthy diabetics between the ages of 50 to 70 years were planned for enrollment according to the criteria of the American Diabetes Association (ADA). Apart from the age and the diabetes status, the inclusion criteria were as follows: 1) male and female healthy Caucasian with a body weight over 50 kg and body mass index (BMI) between 18.0 and 28.0 kg/m2; 2) non or ex-smokers (defined as someone who completely stopped smoking for at least 1 months before the beginning of this study); 3) no clinically relevant findings in the medical history and physical examination, especially with regards to the cardiovascular system, liver and renal function and including repetitive measurements of blood pressure, body temperature and a 12-lead electrocardiogram; 4) normal blood and urine laboratory tests including a negative pregnancy test for female of childbearing potential, negative tests for alcohol consumption or drug abuse and a negative hepatitis serology, the creatine clearance had to be ≥ 80 mL/min for youngest and ≥ 70 mL/min for the other two groups and the diabetic group; 5) No intake of prescribed or not-prescribed drugs within 2 weeks before the investigation; 6) normal dietary habits (e.g. not vegetarian or veganist); 7) no contraindications for CMR including claustrophobia or ferromagnetic implants. For the diabetic cohort, the BMI should be between 18.0 and 35 kg/m2 and a stable medical treatment for the diabetes over the last 3 months was required. Because the low prevalence of these metabolic diabetics with few other comorbidities (50% had hypertension and 20% hypercholesterinemia), the inclusion was very slow and had to be stopped after enrollment of 10 probands only. The evaluation of diabetics can therefore be seen as exploratory and hypothesizing. Diabetic patients were compared with the age-equivalent cohorts of non-diabetic patients between 50 and 70 years of age. Enrollment and inclusion criteria are visualized in Figure 1.
Figure 1. Patient enrollment.
CMR: cardiovascular magnetic resonance.
This trial is approved by the Ethics Committee of the Medical Association of Bavaria (reference number 16111) and registered at EudraCT registry (EudraCT 2017-000045-42). It was funded by Servier (Suresnes, France) and conducted according to the principles of the Declaration of Helsinki.
Cardiac magnetic resonance
Image acquisition
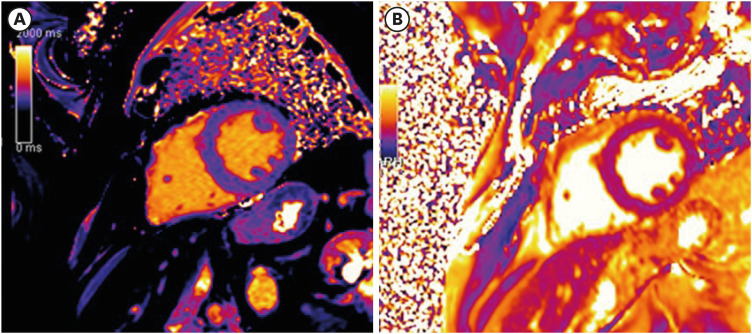
CMR was performed in all subjects using a 1.5T scanner with a 32-channel phased-array cardiac surface coil (MAGNETOM Aera; Siemens, Erlangen, Germany). For volumetric and functional analysis, a balanced steady-state free precision cine sequence (bSSFP) in 2 long-axis views (2- and 4-chamber view orientation) and contiguous short-axis orientation was used (repetition time [TR] 42 ms, echo time [ET] 1.1 ms, slice thickness 8 mm, no interslice gap, acquisition in end-expirational breath-hold). A velocity-encoded flow quantitation sequence was acquired in the ascending aorta for calculation of aortic flow velocities and the aortic area throughout the cardiac cycle. For parametric mapping, the study protocol also consisted of a modified look-locker sequence (MOLLI) for native T1 mapping (TR 280 ms, ET 1.1 ms). T2 mapping was performed using a T2 prepared True Fast Imaging with Steady state Precession (TrueFISP) pulse sequence (TR 103 ms, ET 1.1 ms) with different T2 preparation times (0/25/55 ms) as described earlier.3) Both mapping techniques were obtained in mid-ventricular short axis orientation (Figure 2).
Figure 2. Examples for (A) T1 mapping and (B) T2 mapping in a healthy person with normal values.
Image analysis
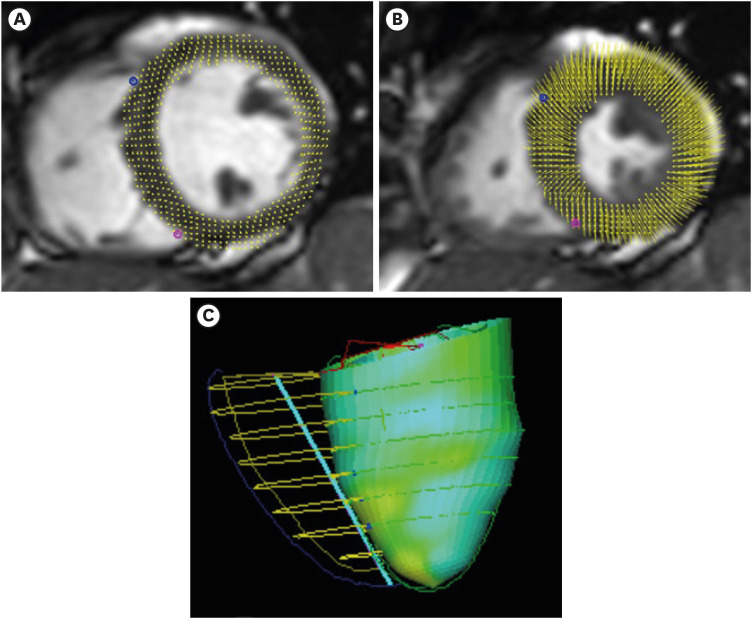
All images were evaluated by 2 experienced examiners in consensus using an established software (cmr42; Circle, Calgary, Canada). Left and right ventricular volumetry as well as myocardial mass were evaluated, respectively, and ejection fractions were calculated, correspondingly. Deformation parameters were determined using feature tracking analysis (Figure 3). After contouring of the endothelial layer, the aortic area was measured in the velocity-encoded flow quantitation sequence. Afterwards, aortic distensibility was calculated according to following formula:
Figure 3. Visualization of feature tracking at (A) end-diastole and (B) end-systole. (C) Example of visualisation of the regional left ventricular longitudinal strain in a 3D modell (values collor-coded).
Native myocardial T1 and T2 values were obtained after contouring endo- and epicardial layers in the corresponding sequences.
Statistical analysis
Descriptive analysis is reported as mean ± standard deviation. Age-dependency was checked using an analysis of covariance (ANCOVA) analysis with the gender as independent covariate. For comparison of differences between diabetic and non-diabetic subjects, a Shapiro-Wilk test of normal distribution was done. In case of normally distributed variables, a two-tailed Student’s t-test was applied. In case of a non-normally distribution in the Shapiro-Wilk-test, a Mann-Whitney U test is used. These results are specially marked. Comparisons of gender distribution as a nominally distributed variable were performed using χ2 test. Statistical significance was assumed at a p-value < 0.05.
RESULTS
Healthy subjects
75 healthy probands were enrolled. More men than women were included (women without type 2 diabetes mellitus 26 [34.7%]) with a different distribution across age groups (Table 1). Therefore, an adjustment was made for the covariate sex in the comparison of age groups. Overall, women had smaller left ventricles (left ventricular end-diastolic volume index [LVEDVI]: 76.4 ± 14.3 mL/m2 vs. 69.8±10.1 mL/m2, p = 0.044) with comparable global function (left ventricular ejection fraction [LVEF]: 62.4 ± 6.1% vs. 63.2 ± 5.8%, p = 0.582). Analysis of deformation parameters revealed equal values for radial and circumferential strain with slightly better values of left and right ventricular longitudinal strain in favor of females (left ventricular [LV] global longitudinal strain −19.2 ± 2.2% vs. −20.7 ± 2.5%, p = 0.010; right ventricular [RV] global longitudinal strain −23.2 ± 3.8% vs. −25.1 ± 3.4%, p = 0.047). Parametric mapping revealed higher native T1 values in women (945 ± 43 ms vs. 988 ± 85 ms, p = 0.006) with comparable T2. Aortic distensibility showed no difference between men and women.
Table 1. CMR results by the age-group in healthy subjects.
| Variables | Age group (yr) | ||||
|---|---|---|---|---|---|
| 18 to 30 (n = 24) | 50 to 59 (n = 27) | 60 to 70 (n = 24) | p-value | ||
| Age (yr) | 26.0 ± 3.3 | 54.2 ± 6.2 | 63.8 ± 2.9 | - | |
| Women | 13 (54.2) | 6 (22.2) | 7 (29.2) | 0.045* | |
| Left and right ventricular volumetry | |||||
| LVEDV (mL) | 155.4 ± 33.0 | 137.0 ± 24.8 | 131.3 ± 24.5 | < 0.001 | |
| LVEDVI (mL/m2) | 83.0 ± 13.6 | 70.9 ± 11.0 | 68.6 ± 11.0 | < 0.001 | |
| LVESV (mL) | 58.0 ± 17.2 | 53.2 ± 13.5 | 46.9 ± 10.4 | < 0.001 | |
| LVEF (%) | 62.9 ± 5.5 | 61.4 ± 6.3 | 64.0 ± 6.1 | 0.305 | |
| Stroke volume (mL) | 97.4 ± 21.1 | 83.8 ± 16.1 | 84.4 ± 18.6 | < 0.001 | |
| Stroke volume indexed (mL/m2) | 52.4 ± 9.2 | 43.3 ± 8.5 | 44.0 ± 8.4 | 0.004 | |
| LV mass (g) | 113.9 ± 32.0 | 111.4 ± 27.0 | 107.6 ± 22.1 | 0.012 | |
| LV mass indexed (g/m2) | 60.9 ± 13.6 | 57.3 ± 12.6 | 55.9 ± 8.6 | 0.007 | |
| LV mass to volume ratio (mg/mL) | 724.7 ± 74.6 | 817.7 ± 155.7 | 828.5 ± 149.5 | 0.089 | |
| RVEDV (mL) | 146.8 ± 32.9 | 126.7 ± 25.1 | 125.1 ± 30.5 | < 0.001 | |
| RVEDVI (mL/m2) | 78.3 ± 13.5 | 65.2 ± 12.9 | 65.5 ± 14.0 | < 0.001 | |
| RVESV (mL) | 59.1 ± 19.0 | 54.1 ± 13.8 | 49.4 ± 14.9 | < 0.001 | |
| RVEF (%) | 60.0 ± 7.2 | 57.3 ± 7.1 | 60.7 ± 6.0 | 0.246 | |
| Parametric mapping | |||||
| Native T1 (ms) | 993 ± 63 | 950 ± 45 | 938 ± 72 | 0.031 | |
| T2 (ms) | 44.8 ± 1.7 | 45.9 ± 2.7 | 47.2 ± 2.7 | 0.185 | |
| Measurement of the aortic stiffness | |||||
| Aortic distensibility (mmHg−1) | 9.1 ± 3.3 | 5.1 ± 2.3 | 4.5 ± 1.8 | < 0.001 | |
| Deformation parameters | |||||
| LV global radial strain (%) | 41.5 ± 6.1 | 41.4 ± 8.2 | 43.2 ± 6.5 | 0.431 | |
| LV peak systolic radial strain rate (/sec) | 2.39 ± 0.5 | 2.28 ± 0.5 | 2.34 ± 0.5 | 0.797 | |
| LV peak diastolic radial strain rate (/sec) | −2.87 ± 1.4 | −2.47 ± 1.0 | −2.06 ± 0.5 | 0.070 | |
| LV global circumferential strain (%) | −20.2 ± 2.0 | −20.6 ± 2.6 | −21.0 ± 2.5 | 0.263 | |
| LV peak systolic circumferential strain rate (/sec) | −1.20 ± 0.2 | −1.15 ± 0.2 | −1.13 ± 0.2 | 0.433 | |
| LV peak diastolic circumferential strain rate (/sec) | 1.39 ± 0.3 | 1.12 ± 0.4 | 0.96 ± 0.2 | < 0.001 | |
| LV global longitudinal strain (%) | −20.1 ± 2.5 | −19.7 ± 2.5 | −19.3 ± 2.1 | 0.763 | |
| LV peak systolic longitudinal strain rate (/sec) | −1.04 ± 0.2 | −1.05 ± 0.2 | −0.99 ± 0.2 | 0.443 | |
| LV peak diastolic longitudinal strain rate (/sec) | 1.33 ± 0.3 | 1.05 ± 0.2 | 1.00 ± 0.2 | < 0.001 | |
| RV global longitudinal strain (%) | −24.2 ± 3.9 | −22.8 ± 3.9 | −24.8 ± 3.2 | 0.203 | |
| RV peak systolic longitudinal strain rate (/sec) | −0.87 ± 1.4 | −1.51 ± 0.7 | −1.14 ± 0.9 | 0.062 | |
| RV peak diastolic longitudinal strain rate (/sec) | 1.55 ± 0.9 | 1.30 ± 0.4 | 1.37 ± 0.4 | 0.791 | |
| Biomarkers | |||||
| IL-6 (pg/mL) | 0.42 ± 0.14 | 0.51 ± 0.23 | 0.62 ± 0.32 | 0.026 | |
| TNF-α (pg/mL) | 2.10 ± 0.94 | 2.15 ± 0.47 | 2.46 ± 0.74 | 0.223 | |
| hsCRP (mg/L) | 1.70 ± 2.44 | 1.21 ± 1.66 | 1.33 ± 1.84 | 0.965 | |
Variables are expressed as means ± standard deviations or number (%). All values are adjusted for gender in the ANCOVA analysis. *The χ2 test used.
ANCOVA: analysis of covariance, CMR: cardiac magnetic resonance imaging, hsCRP: high-sensitive C-reactive protein, IL-6: interleukin 6, LV: left ventricular, LVEDV: left ventricular end-diastolic volume, LVEDVI: left ventricular end-diastolic volume indexed, LVEF: left ventricular ejection fraction, RV: right ventricular, RVEDV: right ventricular end-diastolic volume, RVEDVI: right ventricular end-diastolic volume indexed, RVEF: right ventricular ejection fraction, RVESV: right ventricular endsystolic volume, TNF-α: tumor necrosis factor α.
As seen in Table 1, sizes of the left and the right ventricle (volume and mass) decreased with the age (left ventricular end-diastolic volume [LVEDV], LVEDVI, left ventricular end systolic volume [LVESV], right ventricular end-diastolic volume [RVEDV], right ventricular end-diastolic volume index [RVEDVI], and right ventricular end systolic volume [RVESV]; all p < 0.001) as did the LV stroke volume, while the systolic function remains normal at a global level (LVEF and right ventricular ejection fraction [RVEF]) as well as at the level of deformation analysis (LV global radial, circumferential and longitudinal strain and RV longitudinal strain). LV peak diastolic strain rates, in particular the circumferential and longitudinal, statistically decreased with increasing age (both p < 0.001). All right ventricular deformation parameters stayed unchanged in all age groups. Interestingly, there was also a significant decrease in native T1 mapping and a trend towards an increase in T2 mapping. The aortic distensibility became lower (p < 0.001), suggesting a higher stiffness at higher ages.
No difference regarding inflammation parameters, except for a slight but significant difference in interleukin (IL)-6 with higher values in the older cohorts, was observed.
Aging and diabetes
Because of a very slow enrollment of the relatively healthy diabetics according to the inclusion criteria, only 10 patients with type 2 diabetes mellitus could be included into our study (1 woman). Thereby our data have exploratory and hypothesizing character. Comparison was done with all healthy volunteers in the elderly cohorts (age 50 to 70 years). There was no age difference between the 2 groups (without diabetes 58.7 ± 6.9 vs. with diabetes 61.4 ± 3.9, p = 0.093). Due to the adjusted inclusion criteria for diabetics, body mass index was significantly higher in this cohort than in non-diabetics (25.4 ± 2.0 kg/m2 vs. 28.7 ± 3.3 kg/m2, p = 0.011). The diabetic status had no influence on RVEF and LVEF. In comparison of subjects with and without type 2 diabetes mellitus, lower values for LVEDVI (69.8 ± 10.9 mL/m2 vs. 58.8 ± 8.1 mL/m2, p = 0.006), RVEDVI (65.3 ± 13.3 vs. 57.3 ± 9.7, p = 0.040) and the indexed LV stroke volume (43.6 ± 8.4 vs. 35.7 ± 3.8, p = 0.004) were found in the diabetic cohort. No differences were seen regarding to end-systolic volumes, mapping values, aortic distensibility and essential deformation parameters. There was just a difference comparing results for LV peak systolic radial strain rate favoring the diabetic group (2.31 ± 0.5 vs. 2.87 ± 1.1, p = 0.013), most likely due to the small sample size. No difference was seen for IL-6, tumor necrosis factor (TNF)-α and high-sensitive C-reactive protein (hsCRP). More details are provided in Table 2.
Table 2. CMR results by diabetic status.
| Variables | Without diabetes (n = 51) | Type 2 diabetes mellitus (n = 10) | p-value | |
|---|---|---|---|---|
| Age (yr) | 58.7 ± 6.9 | 61.4 ± 3.9 | 0.093 | |
| Women | 13 (25.5) | 1 (10) | 0.287* | |
| Left and right ventricular volumetry | ||||
| LVEDV (mL) | 134.4 ± 24.6 | 126.0 ± 17.4 | 0.258† | |
| LVEDVI (mL/m2) | 69.8 ± 10.9 | 58.8 ± 8.1 | 0.006† | |
| LVESV (mL) | 50.4 ± 12.5 | 49.2 ± 12.5 | 0.789 | |
| LVEF (%) | 62.6 ± 6.3 | 61.3 ± 6.3 | 0.460† | |
| Stroke volume (mL) | 84.0 ± 17.1 | 76.8 ± 9.5 | 0.200 | |
| Stroke volume indexed (mL/m2) | 43.6 ± 8.4 | 35.7 ± 3.8 | 0.004† | |
| LV mass (g) | 109.7 ± 24.8 | 113.2 ± 17.5 | 0.606† | |
| LV mass indexed (g/m2) | 56.7 ± 10.9 | 52.5 ± 6.3 | 0.111 | |
| RVEDV (mL) | 126.0 ± 27.4 | 123.3 ± 20.3 | 0.903† | |
| RVEDVI (mL/m2) | 65.3 ± 13.3 | 57.3 ± 9.7 | 0.040 | |
| RVESV (mL) | 52.0 ± 14.4 | 55.3 ± 8.3 | 0.334 | |
| RVEF (%) | 58.8 ± 6.8 | 54.9 ± 4.2 | 0.086 | |
| Parametric mapping | ||||
| Native T1 (ms) | 945 ± 59 | 952 ± 74 | 0.777 | |
| T2 (ms) | 46.7 ± 2.7 | 45.5 ± 2.8 | 0.255 | |
| Measurement of the aortic stiffness | ||||
| Aortic distensibility | 4.8 ± 2.1 | 5.1 ± 2.0 | 0.710 | |
| Deformation parameters | ||||
| LV global radial strain (%) | 42.2 ± 7.4 | 43.3 ± 8.7 | 0.951† | |
| LV peak systolic radial strain rate (/sec) | 2.31 ± 0.5 | 2.87 ± 1.1 | 0.013 | |
| LV peak diastolic radial strain rate (/sec) | −2.28 ± 0.8 | −2.07 ± 0.3 | 0.192 | |
| LV global circumferential strain (%) | −20.8 ± 2.6 | −21.3 ± 2.4 | 0.773† | |
| LV peak systolic circumferential strain rate (/sec) | −1.14 ± 0.2 | −1.31 ± 0.4 | 0.128† | |
| LV peak diastolic circumferential strain rate (/sec) | 1.05 ± 0.3 | 1.02 ± 0.1 | 0.699 | |
| LV global longitudinal strain (%) | −19.5 ± 2.3 | −18.6 ± 3.4 | 0.773† | |
| LV peak systolic longitudinal strain rate (/sec) | −1.02 ± 0.2 | −1.13 ± 0.2 | 0.184† | |
| LV peak diastolic longitudinal strain rate (/sec) | 1.03 ± 0.2 | 1.09 ± 0.2 | 0.275† | |
| RV global longitudinal strain (%) | −23.7 ± 3.7 | −24.8 ± 4.1 | 0.447 | |
| RV peak systolic longitudinal strain rate (/sec) | −1.34 ± 0.8 | −1.21 ± 1.0 | 0.685 | |
| RV peak diastolic longitudinal strain rate (/sec) | 1.33 ± 0.4 | 1.37 ± 0.5 | 0.810 | |
| Biomarkers | ||||
| IL-6 (pg/mL) | 0.56 ± 0.29 | 0.61 ± 0.16 | 0.627 | |
| TNF-α (pg/mL) | 2.31 ± 0.63 | 2.30 ± 0.37 | 0.987 | |
| hsCRP (mg/L) | 1.15 ± 1.44 | 1.46 ± 1.25 | 0.527 | |
Variables are expressed as means ± standard deviations.
ANCOVA: analysis of covariance, CMR: cardiac magnetic resonance imaging, hsCRP: high-sensitive C-reactive protein, IL-6: interleukin 6, LV: left ventricular, LVEDV: left ventricular end-diastolic volume, LVEDVI: left ventricular end-diastolic volume indexed, LVEF: left ventricular ejection fraction, RV: right ventricular, RVEDV: right ventricular end-diastolic volume, RVEDVI: right ventricular end-diastolic volume indexed, RVEF: right ventricular ejection fraction, RVESV: right ventricular endsystolic volume, TNF-α: tumor necrosis factor α.
*The χ2 test used. †Mann-Whitney-U test was used because of a non-normal distribution in the Shapiro-Wilk-Test.
DISCUSSION
The main findings of this trial are: In healthy subjects, 1) age-dependent decrease of left and right ventricular volumes and increase of ventricular and aortic stiffness occur; 2) T1 values decrease with age, while T2 values remain stable; and 3) the diabetic status seems to have an influence only on LV and RV volumes, not on ventricular function.
Due to an unequal distribution of the sexes between the different age groups, it was not possible to make a sufficient and valid statement about gender-specific differences in aging. However, since a difference between women and men could be identified or at least is controversial for many CMR measurements, an adjustment was made to the covariate gender.
A shrinkage of the ventricles while aging was described before with an equally decrease of end-diastolic and end-systolic volumes while maintaining LVEF and RVEF.4),5) In the Multi-Ethnic Study of Atherosclerosis (MESA) study, 3,014 participants underwent a baseline and a follow-up CMR examination with an interval of 10 years.4),6) A progressive decrease in volume and an increase in a ratio between mass and volume was thereby seen as a result of ventricular remodeling in a concentric manner. There was only a trend towards a higher mass to volume ratio with age in our study. While the MESA study was a big longitudinal trial, it has the advantage of a low risk for a selection bias. In contrast, the strict inclusion criteria of our cohort offer the possibility of a consideration without comorbidities or confounders. As seen in another sub-analysis of the MESA study, comorbidities and cardiovascular risk factors have an impact on ventricular volumes and mass.7) Either because of the lack of cardiovascular risk factors or the lower case number the increase in the mass to volume ratio did not reach significance in our cohort. A higher physical activity is not to be seen as possible uncontrolled confounder, since a very low influence on the mass to volume ratio is reported otherwise.8)
We have not found differences in LV or RV systolic deformation parameters at different ages, which could be of importance, since strain is an interesting tool for monitoring diseases in different entities and aging could be excluded as confounder.9),10) In a study of Andre and colleagues, the radial strain increased by age, circumferential and longitudinal strain remained unchanged.11) Looking at the absolute change of the LV peak radial strain, they found a change from 35.5 ± 7.3% in the youngest group over 39.9 ± 7.8% to 38.2 ± 9.1% at the oldest. Similar to our results, there was a statistically significant decrease of all diastolic parameters. Thereby, an age-dependent stiffening of the left ventricle and a concordant increase of left ventricular filling pressures can be assumed.12) Interestingly, there is a big difference between the diastolic parameters reported by Andre et al and ours. In that way, our probands reach nearly pathological results in accordance to their calculations. In our opinion, this was mostly due to a different software vendor.13) Strain measurements also show a certain variability between the studies of different study groups.14) Another study postulates a quadratic curve of the LV global circumferential strain, while longitudinal and radial strain remain stable.15)
According to a stiffening of the left ventricle, the aorta stiffens measured by the aortic distensibility. These results were consistent with other studies.5)
An inverse relationship between age and myocardial native T1 was surprising, as there was none in other trials.16),17) In fact, an increase of native T1 would be more likely due to a higher grade of fibrosis.18) Although we can only hypothesize, an intracellular increase in triglycerides on the one hand and deposits such as lipofuscin on the other hand could be causative. Such histochemical changes are reported before.19),20),21),22) In a trial with Singaporean volunteers, the mean native T1 value at 1.5 T was 1,013 ± 27 ms using the same scanner vendor and the same software like in our study. They could only see a trend towards a lower T1 value and only in the male subgroup.17) For T2 measurements, other authors found a positive correlation to age.23) In contrast to our study, they scanned a population with a broader age distribution. Otherwise, there was no restriction for cardiovascular risk factors, leading to a high percentage of hypertensive and smoking probands. The authors again discuss an influence of triglyceride deposits as explanation for the increasing T2.
Unfortunately, the inclusion of a proper number of diabetics failed due to the strict inclusion criteria. The data should therefore be seen as exploratory and hypothesizing. The advantage of only a very small number of comorbidities stands again in contrast to the MESA study, where diabetics had a higher LV mass and a lower stroke volume.7) Additionally, the incidence of new diabetes mellitus was threefold higher among participants whose baseline levels of C-reactive protein or IL-6 were in the fourth versus the first quartile.24) In accordance, the worsening of LV function and the diabetic status in the MESA cohort were possibly only coincident rather than causative, since a connection between heart failure and inflammation is already known.25) In fact, inflammation parameters were all normal in our diabetic cohort with no difference to the non-diabetic. This may be due to the good health status and the lack of comorbidities because of the strict inclusion criteria. Another study implicates that the duration of diabetes has an influence on the ventricular function.26) The time since diagnosis of type 2 diabetes mellitus was not recorded in our study, so this remains a possible confounder. A recent trial by Cao et al.27) otherwise confirmed our results with a preservation of ventricular function, while they did not report a shrinkage of ventricular volumes. Because our diabetic cohort had a significantly higher body mass index, comparisons with indexed volumes are insufficient to deduce a change in ventricular volumes. Interestingly, Cao et al.27) found a correlation between HbA1c levels and native T1. Since our diabetic cohort had a good and stable drug setting, this could be explanatory for our mapping results.
In the current study, we show that age-related remodeling in the absence of other confounders consists of a decrease in ventricular volumes and mass with stable systolic function, including deformation parameters. There is evidence for stiffening of the myocardium as well as the aorta. Parametric mapping provides evidence for a change in myocardial composition with a decrease in T1 value with stable T2. With reference to patients with type 2 diabetes mellitus, it can be hypothesized that under circumstances of good drug therapy and the absence of other comorbidities, there is no major influence on aging processes. However, larger prospective studies are needed to validate our results in patients with type 2 diabetes mellitus. Likewise, prospectively obtained values at other field strengths such as 3 Tesla are of further interest.
Footnotes
Funding: The trial was funded by a research grant of Servier.
Conflict of Interest: MB and BT are employed by the sponsor of the study (Servier). The other authors declare that they have no competing interests.
- Conceptualization: Bouly M, Tyl B, Bernhardt P.
- Formal analysis: Kersten J.
- Funding acquisition: Bouly M, Tyl B.
- Investigation: Kersten J.
- Project administration: Tyl B, Bernhardt P.
- Resources: Hackenbroch C.
- Supervision: Bernhardt P.
- Validation: Hackenbroch C.
- Visualization: Kersten J, Hackenbroch C.
- Writing - original draft: Kersten J.
- Writing - review & editing: Hackenbroch C, Bouly M, Tyl B, Bernhardt P.
References
- 1.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard JR, Officer A, de Carvalho IA, et al. The world report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoneyama K, Venkatesh BA, Bluemke DA, McClelland RL, Lima JA. Cardiovascular magnetic resonance in an adult human population: serial observations from the multi-ethnic study of atherosclerosis. J Cardiovasc Magn Reson. 2017;19:52. doi: 10.1186/s12968-017-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heckbert SR, Post W, Pearson GD, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkbey EB, Jorgensen NW, Johnson WC, et al. Physical activity and physiological cardiac remodelling in a community setting: the Multi-Ethnic Study of Atherosclerosis (MESA) Heart. 2010;96:42–48. doi: 10.1136/hrt.2009.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kersten J, Güleroglu AM, Rosenbohm A, et al. Myocardial involvement and deformation abnormalities in idiopathic inflammatory myopathy assessed by CMR feature tracking. Int J Cardiovasc Imaging. 2021;37:597–603. doi: 10.1007/s10554-020-02020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stiermaier T, Lange T, Chiribiri A, et al. Left ventricular myocardial deformation in Takotsubo syndrome: a cardiovascular magnetic resonance myocardial feature tracking study. Eur Radiol. 2018;28:5160–5170. doi: 10.1007/s00330-018-5475-2. [DOI] [PubMed] [Google Scholar]
- 11.Andre F, Steen H, Matheis P, et al. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2015;17:25. doi: 10.1186/s12968-015-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–1383. doi: 10.1161/CIRCULATIONAHA.106.662882. [DOI] [PubMed] [Google Scholar]
- 13.Schuster A, Stahnke VC, Unterberg-Buchwald C, et al. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol. 2015;70:989–998. doi: 10.1016/j.crad.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vo HQ, Marwick TH, Negishi K. MRI-derived myocardial strain measures in normal subjects. JACC Cardiovasc Imaging. 2018;11:196–205. doi: 10.1016/j.jcmg.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RJ, Moody WE, Umar F, et al. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015;16:871–881. doi: 10.1093/ehjci/jev006. [DOI] [PubMed] [Google Scholar]
- 16.Dabir D, Child N, Kalra A, et al. Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2014;16:69. doi: 10.1186/s12968-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulluck H, Bryant JA, Tan JZ, et al. Gender differences in native myocardial T1 in a healthy Chinese volunteer cohort. Cardiovasc Imaging Asia. 2017;1:110–115. [Google Scholar]
- 18.Obas V, Vasan RS. The aging heart. Clin Sci (Lond) 2018;132:1367–1382. doi: 10.1042/CS20171156. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer RW, Rijzewijk LJ, Diamant M, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J. 2008;29:1516–1522. doi: 10.1093/eurheartj/ehn207. [DOI] [PubMed] [Google Scholar]
- 20.Roffe C. Ageing of the heart. Br J Biomed Sci. 1998;55:136–148. [PubMed] [Google Scholar]
- 21.Klausner SC, Schwartz AB. The aging heart. Clin Geriatr Med. 1985;1:119–141. [PubMed] [Google Scholar]
- 22.Nyman K, Granér M, Pentikäinen MO, et al. Cardiac steatosis and left ventricular function in men with metabolic syndrome. J Cardiovasc Magn Reson. 2013;15:103. doi: 10.1186/1532-429X-15-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bönner F, Janzarik N, Jacoby C, et al. Myocardial T2 mapping reveals age- and sex-related differences in volunteers. J Cardiovasc Magn Reson. 2015;17:9. doi: 10.1186/s12968-015-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertoni AG, Burke GL, Owusu JA, et al. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2010;33:804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Linthout S, Tschöpe C. Inflammation - cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14:251–265. doi: 10.1007/s11897-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Yang ZG, Gao Y, et al. Left ventricular subclinical myocardial dysfunction in uncomplicated type 2 diabetes mellitus is associated with impaired myocardial perfusion: a contrast-enhanced cardiovascular magnetic resonance study. Cardiovasc Diabetol. 2018;17:139. doi: 10.1186/s12933-018-0782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y, Zeng W, Cui Y, et al. Increased myocardial extracellular volume assessed by cardiovascular magnetic resonance T1 mapping and its determinants in type 2 diabetes mellitus patients with normal myocardial systolic strain. Cardiovasc Diabetol. 2018;17:7. doi: 10.1186/s12933-017-0651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]