Abstract
The yeast Trichosporon mucoides, grown on either glucose or phenol, was able to transform biphenyl into a variety of mono-, di-, and trihydroxylated derivatives hydroxylated on one or both aromatic rings. While some of these products accumulated in the supernatant as dead end products, the ortho-substituted dihydroxylated biphenyls were substrates for further oxidation and ring fission. These ring fission products were identified by high-performance liquid chromatography, gas chromatography-mass spectrometry, and nuclear magnetic resonance analyses as phenyl derivatives of hydroxymuconic acids and the corresponding pyrones. Seven novel products out of eight resulted from the oxidation and ring fission of 3,4-dihydroxybiphenyl. Using this compound as a substrate, 2-hydroxy-4-phenylmuconic acid, (5-oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid, and 3-phenyl-2-pyrone-6-carboxylic acid were identified. Ring cleavage of 3,4,4′-trihydroxybiphenyl resulted in the formation of [5-oxo-3-(4′-hydroxyphenyl)-2,5-dihydrofuran-2-yl]acetic acid, 4-(4′-hydroxyphenyl)-2-pyrone-6-carboxylic acid, and 3-(4′-hydroxyphenyl)-2-pyrone-6-carboxylic acid. 2,3,4-Trihydroxybiphenyl was oxidized to 2-hydroxy-5-phenylmuconic acid, and 4-phenyl-2-pyrone-6-carboxylic acid was the transformation product of 3,4,5-trihydroxybiphenyl. All these ring fission products were considerably less toxic than the hydroxylated derivatives.
Biphenyl occurs naturally in coal tar, crude oil, and natural gas. It is commonly used as an intermediate in the production of a variety of compounds, as a heat transfer medium in heating fluids, as a dye stuff carrier for textiles and copying paper, as a solvent in pharmaceutical production, and in the preservation of citrus fruits (7; Integrated Risk Information System [IRIS] on 1,1 Biphenyl [91-51-4], U.S. Environmental Protection Agency [http://www.epa.gov/ngispgm3/iris]). Biphenyl is commonly identified in environmental samples since it is formed during the incomplete combustion of mineral oil and coal and is present in the exhaust gases of vehicle traffic and from residential and industrial heating devices. Due to its widespread use, the toxicological properties have been investigated (14, 23). Biphenyl is not considered a human carcinogen and is nonmutagenic in the Salmonella enterica serovar Typhimurium reversion assay with and without mammalian postmitochondrial supernatant (S9) to induce mutations. Negative genotoxicity results were also obtained in several mammalian gene mutation assays. However, chronic human exposure via inhalation can cause muscular weakness, central and peripheral nerve damage, and liver injury. Furthermore, process workers in a paper mill that were exposed to biphenyl-impregnated fruit wrapping paper had symptoms of biphenyl poisoning that included headache, tremors, abdominal pain, hepatic impairment, and neurological abnormalities (15).
The in vitro and in vivo metabolism of unsubstituted biphenyls by eukaryotic organisms has been studied extensively. Mineralization has been proven for the white rot fungus Phanerochaete chrysosporium producing 14CO2 from radioactive labeled biphenyl (34). However, the most common transformation reaction is the hydroxylation of the aromatic ring, leading to a variety of mono-, di- and trihydroxylated intermediates (1, 2, 4, 8, 13, 20, 26, 30). Filamentous fungi and mammals favor initial hydroxylation at the 2 and 4 positions, respectively, followed by further hydroxylation on the second ring (6, 12, 25, 31). Conjugates of these hydroxylated derivatives of biphenyl have also been reported (6, 12, 37). In contrast, some yeasts prefer the same aromatic ring for the second hydroxylation (19). Most of these hydroxylated intermediates accumulated as dead end products. Only a few studies on the isolation and identification of ring cleavage products of biphenyl by eukaryotic organisms have been done. Strains of the fungus Aspergillus were able to form 3-arylmuconolactones from 4,4′-dihydroxybiphenyl (21) and the ascomycetous yeast Debaryomyces vanrijiae produced 4-phenyl-2-pyrone-6-carboxylic acid from 3,4-dihydroxybiphenyl (19). Our objectives were (i) to determine the pathway of biphenyl up to cleavage of the aromatic ring system by the anamorphic basidiomycetous yeast Trichosporon mucoides, the structure of ring cleavage products, and the chemical intermediates through which the ring cleavage occurs and (ii) to investigate the toxicity of these ring cleavage products.
MATERIALS AND METHODS
Microorganisms, media, and incubation conditions.
T. mucoides was isolated from a dioxin-contaminated soil sample (16). The strain was deposited as T. mucoides SBUG 801 into the strain collection of the Department of Biology of the University of Greifswald (SBUG) and as DSM 12017 into the Deutsche Sammlung fuer Mikroorganismen und Zellkulturen, Braunschweig, Germany. Saccharomyces cerevisiae SBUG 118 was obtained from the collection of strains in the Department of Biology at the University of Greifswald.
Biphenyl transformation experiments involved T. mucoides SBUG 801 cells grown under two different conditions. The cultivation of glucose-grown cells was carried out in 500-ml Erlenmeyer flasks containing 100 ml of mineral salts medium (MM), pH 5.4, supplemented with 2% glucose and 1 ml of vitamin solution (35) according to a method described previously (16). Cultures were incubated for 48 h on a rotary shaker at 30°C and 180 rpm. For the cultivation of T. mucoides cells with phenol, cells were first grown in 100 ml of MM supplemented with 1% glucose and 0.2% yeast extract. After 24 h of cultivation, 5-ml cell suspensions were transferred into 500-ml Erlenmeyer flasks containing 100 ml of MM supplemented with 0.05% phenol and 0.2% yeast extract. After 16 h of cultivation, cells were induced again with 0.05% phenol for an additional 3 h.
Glucose- and phenol-grown cells were harvested by centrifugation at 3,100 × g for 5 min at 4°C, washed twice in sterile MM, and resuspended in 5 to 10 ml of MM. Biphenyl was added to sterile 200-ml Erlenmeyer flasks as a diethyl ether solution in a final concentration of 250 μg ml−1 (1.62 mmol liter−1). After the evaporation of the diethyl ether in about 12 h, 40 ml of MM was added to each flask and flasks were shaken for 24 h at 30°C and 180 rpm to reach saturation of the compound in the liquid medium. Transformation products of biphenyl used as substrates were added to a final concentration of 20 or 100 μg ml−1. Then, the cell suspension was added until an optical density (A600) of 6.0 was reached. Cultures were incubated with biphenyl or transformation products for 120 h on a rotary shaker at 30°C and 180 rpm. Flasks with cell suspension in MM without substrate, or with biphenyl and transformation products without cells, and autoclaved cells were used as controls.
Detection and identification of transformation products.
At intervals during the incubation, 1 ml of cell suspension was removed aseptically and centrifuged to remove cells. Then, 100-μl aliquots of the supernatant were analyzed by high-performance liquid chromatography (HPLC) for biotransformation products. Experiments were replicated at least three times, and the standard deviation of each metabolite concentration was not more than 12%.
To purify products for further characterization, larger amounts of cell suspensions were used. For that, 10 500-ml Erlenmeyer flasks containing 100 ml of cell suspension with the different substrates were incubated as mentioned earlier. Supernatants were extracted three times with an equal volume of ethyl acetate, pH 7, and then extracted again in the same manner after acidification of the aqueous phase to pH 2 with 6 N HCl. Extracts were dried over anhydrous sodium sulfate and concentrated by rotary evaporation. The residues obtained were dissolved in methanol and analyzed by HPLC and gas chromatography-mass spectrometry (GC-MS) analysis for detection and characterization of the transformation products.
Chemical analysis and identification of products.
HPLC was performed on a Hewlett Packard (Bad Homburg, Germany) HPLC model 1050 M equipped with a quaternary pump system, a diode array detector (1040 M series I) set at 220 and 254 nm, and an HP Chemstation. Transformation products were separated on a LiChroCart 125-4 RP-18 end-capped (5 μm) column (Merck, Darmstadt, Germany) using a solvent system of acetonitrile and phosphoric acid (0.1% [wt/vol]) with a linear gradient from 20 to 90% acetonitrile within 14 min at a flow rate of 1 ml min−1.
For the purification of products, a semipreparative HPLC was conducted on a LiChrospher 100 RP-18 end-capped column (16 by 250 mm, 10 μm; Knauer, Berlin, Germany) by using methanol—0.1% acetic acid (80:20 [vol/vol] for neutral extracts and 50:50 [vol/vol] for acidic extracts) as the mobile phase at a flow rate of 8 ml min−1.
For GC-MS analyses, a coupled system consisting of a GC 8000 gas chromatograph (Fisons Instruments, Mainz, Germany) equipped with a 30-m DB5-ms column (0.25-mm-by-0.33-μm film; J and W Scientific, Folsom, Calif.) and a mass selective detector MD 800 (Fisons Instruments) operating at 70 eV or a TSQ 700 (Finnigan Corp., San Jose, Calif.) triple quadrupole mass spectrometer operated in a single quadrupole mode (Q1) was used. Separation on the column was achieved by using a temperature program from 80 to 300°C (10°C/min). Acid extracts were derivatized by methylation with diazomethane (5).
Nuclear magnetic resonance (NMR) spectra were obtained at 500 MHz with a Bruker AM500 spectrometer (Bruker Instruments, Billerica, Mass.) at 28°C. The products were dissolved in methanol-d3 (99.96 atom% 2H). 1H NMR spectra of product I were recorded on a 500-MHz Inova spectrometer Varian (Palo Alto, Calif.) at 27°C. The sample was dissolved in dimethyl sulfoxide. Assignments of resonance signals to specific proton and carbon atoms are based on their chemical shifts, integrals, and data from selective homonuclear decoupling experiments (correlated spectroscopy [COSY] and nuclear Overhauser effect spectroscopy [NOESY]) as well as data from heteronuclear experiments (heteronuclear multiple bond correlation [HMBC] and heteronuclear multiple quantum coherence [HMQC]).
Toxicity test.
The toxicity of biphenyl transformation products was estimated by their influence on the growth of eukaryotic cells with glucose in MM (28) for T. mucoides SBUG 801 and S. cerevisiae SBUG 118. The cultivation of cells was carried out in 500-ml Erlenmeyer flasks containing 100 ml of MM supplemented with 2% glucose and 1 ml of vitamin solution (T. mucoides) as well as 2% glucose and 0.2% yeast extract (S. cerevisiae) as described above. Cultures were incubated for 24 h at 30°C with rotary shaking (180 rpm).
For the tests, various concentrations of biphenyl as well as transformation products (10, 50, 100, and 250 μg ml−1) were added to 200-ml Erlenmeyer flasks containing 40 ml of MM. Flasks were shaken for 24 h at 30°C and 180 rpm so the compounds could reach saturation in the liquid medium. After 24 h, 1% glucose and 0.2 ml of vitamin solution (T. mucoides) and 1% glucose and 0.2% yeast extract (S. cerevisiae), respectively, were added to the flasks as well as an equal amount of the culture described above to reach an optical density (A600) of 0.2. Flasks were shaken for 48 h at 30°C and 180 rpm, and samples were removed at intervals for spectrometric analysis (optical density at 600 nm) to determine the cell growth. Supplemented flasks without biphenyl or transformation products were used as controls.
All experiments described were carried out in duplicate.
Chemicals.
Biphenyl and 2-hydroxybiphenyl were obtained from Merck, 3-hydroxybiphenyl and 4-hydroxybiphenyl were from Aldrich (Steinheim, Germany), and 2,3-dihydroxybiphenyl was from Wako Chemicals (Neuss, Germany). 3,4-Dihydroxybiphenyl was purchased from Promochem (Wesel, Germany). 2,3,4-Trihydroxybiphenyl, 3,4,5-trihydroxybiphenyl, 3,4,4′-trihydroxybiphenyl, and 4- phenyl-2-pyrone-6-carboxylic acid were synthesized by methods described by Gesell et al. (11).
NMR solvents were purchased from Cambridge Isotope Labs (Andover, Mass.). All other chemicals were of reagent grade or were the highest available purity.
RESULTS
Glucose-grown as well as phenol-grown cells of T. mucoides SBUG 801 were able to hydroxylate biphenyl into a large variety of mono-, di-, and trihydroxylated products. Hydroxylation occurred on one or both aromatic rings. About 30% of the biphenyl used (1.62 mmol liter−1) based on the molar yield was transformed by glucose-grown cells to products within 120 h, quantified by gas chromatographic analysis (27). Most of the transformation products accumulated in the culture supernatant. While 2,3- and 3,4-dihydroxybiphenyl as well as 3,4,4′-trihydroxybiphenyl were isolated as transformation products of biphenyl, 2,3,4- and 3,4,5-trihydroxybiphenyl were not detected but were transformed if used as substrates. Phenol-grown cells oxidized biphenyl and its transformation products more rapidly, and the resulting products were produced in higher quantity. Thus, for instance, after 24 h of incubation with biphenyl, the amount of product I (see below) was 3.75 times higher with phenol-grown cells than with glucose-grown cells. However, in general, the transformation pathway of biphenyl by T. mucoides was independent of the previous mode of cultivation. The only exception was observed in the case of 2,3-dihydroxybiphenyl, which was transformed only by phenol-grown cells. We recently reported that hydroxylated biphenyls with at least two hydroxy groups adjacent to each other underwent further transformation, revealing transformation products with unknown chemical structures (27). Therefore, such di- and trihydroxylated derivatives of biphenyl were used as substrates in this investigation to determine the identification of ring cleavage products and the pathway of biphenyl transformation.
Biotransformation of ortho-substituted dihydroxylated derivatives of biphenyl.
The 3,4-dihydroxybiphenyl was oxidized completely by glucose- as well as phenol-grown cells into 3,4,4′-trihydroxybiphenyl (found in trace amounts and detectable only by GC-MS analysis) and four unknown products named I, II, III, and IV. For instance, phenol-grown cells transformed 3,4-dihydroxybiphenyl (108 μmol liter−1) completely within 24 h, accumulating product I (80% of the substrate added), product II (4%), and product IV (15%). Product III was found only in trace amounts.
Product I was extracted at pH 7 and pH 2. The other three transformation products (II, III, and IV) were enriched only by extraction with ethyl acetate at pH 2. Acidic extracts suggest that ring fission products were formed. This assumption was verified for product IV, identified by HPLC and GC-MS analysis as 4-phenyl-2-pyrone-6-carboxylic acid by comparison with the available standard (19). The other products extracted at pH 2 (I, II, and III) were separated by semipreparative HPLC and identified by extensive GC-MS and NMR analysis.
2,3-Dihydroxybiphenyl was transformed only by phenol-grown cells into product II, extractable with ethyl acetate at pH2.
Identification of product I.
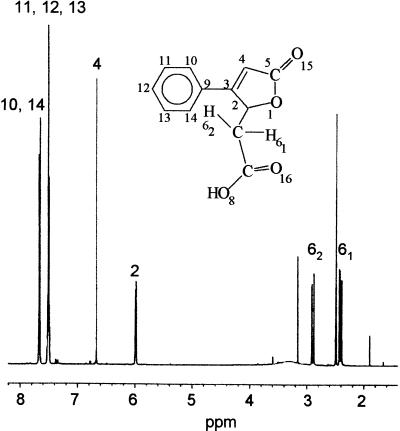
Spectra received by 1H NMR (Fig. 1), 13C NMR, and 1H1H-correlated NMR spectroscopy as well as long-range coupling experiments like HMQC and NOESY identified one methylene group, a carboxyl group, a hydrofuranyl ring, and a monosubstituted phenyl ring for product I. The data were as follows: δ 2.42 (dd, 1H, J6-1/6-2 = 16.5 Hz, J6-1/2 = 8.8 Hz (v)2, H6-1), 2.88 (dd, 1 H, J6-1/6-2=16.5 Hz, J6-2/2=3.03 Hz, H6-2), 5.99 (dq, 1H, J2/6-1 = 8.8 Hz, J2/6-2 = 3.03 Hz, J2/4 = 1.6 Hz, H2), 6.67 (d, 1H, J4/2 = 1.6 Hz, H4), 7.51 (m, 3H, H11; H12; H13), and 7.66 (m, 2 H, H10; H14) ppm. The proton-proton spin coupling of product I showed a high similarity to 1H NMR spectra reported for 3-arylmuconolactones (21). GC-MS analysis of product I showed a molecular mass (m/z) of 218. By high-resolution mass spectral analysis, the result was verified (218.058200, 218.057909). The fragmentation pattern of this compound (Table 1) indicated the loss of two carboxylic groups and ethylene. The data obtained by GC-MS and NMR analysis led to the identification of product I as (5-oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid.
FIG. 1.
1H NMR spectrum of (5-oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid (product I).
TABLE 1.
Ring cleavage products produced during the biotransformation of hydroxylated biphenyls by T. mucoides SBUG 801 as revealed by GC-MS analyses
| Compoundsa | Identification | Characteristic mass ions (m/z) (relative abundance [%]) |
|---|---|---|
| I | (5-Oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid | 218 [M+] (32), 172 [M]-HCOOH (56), 131 [M]-CH2COOH-CO (56), 103 [M]-CH2COOH-2CO (100), 77 C6H5 (32) |
| II | 3-Phenyl-2-pyrone-6-carboxylic acid | 230 [M+] (20), 202 [M]-CO (6), 171 [M]-COOCH3 (100), 115 [M]-COOCH3-2CO (95) |
| III | 2-Hydroxy-4-phenylmuconic acid | 276 [M+] (2), 261 [M]-CH3 (2), 245 [M]-OCH3 (5), 217 [M]-COOCH3 (100), 202 [M]-CH3-COOCH3 (5), 185 [M]-COOCH3-CH3OH (12), 115 [M]-COOCH3-2CO (28), 59 (COOCH3+) (8) |
| IV | 4-Phenyl-2-pyrone-6-carboxylic acid | 230 [M+] (30), 202 [M]-CO (4), 171 [M]-COOCH3 (72), 115 [M]-COOCH3-2CO (100) |
| V | 2-Hydroxy-5-phenylmuconic acid | 276 [M+] (4), 245 [M]-OCH3 (6), 217 [M]-COOCH3 (100), 202 [M]-CH3-COOCH3 (14), 185 [M]-COOCH3-CH3OH (4), 115 [M]-COOCH3-2CO (52), 59 [COOCH3+] (18) |
| VI | [5-Oxo-3-(4′-hydroxyphenyl)-2,5-dihydrofuran- 2-yl]acetic acid | 262 [M+] (30), 234 [M]-CO (35), 189 [M]-CH2COOCH3 (20), 161 [M]-CH2COOCH3-CO (45), 133 [M]-CH2COOCH3-2CO (100), 132 [C9H8O+] (75) |
| VII | 4-(4′-Hydroxyphenyl)-2-pyrone-6-carboxylic acid | 260 [M+] (22), 232 [M]-CO (5), 201 [M]-COOCH3 (80), 145 [M]-COOCH3-2CO (100), 102 (45) |
| VIII | 3-(4′-Hydroxyphenyl)-2-pyrone-6-carboxylic acid | 260 [M+] (35), 232 [M]-CO (10), 201 [M]-COOCH3 (100), 145 [M]-COOCH3-2CO (70), 102 (32) |
Compounds II to VIII were completely methylated
Identification of product II.
GC-MS analysis of the methylated compound II showed a peak in the chromatogram with a molecular weight of m/z 230 (Table 1). The fragmentation pattern of the compound was very similar to the one obtained for 4-phenyl-2-pyrone-6-carboxylic acid. Only the intensities of the fragment ions varied. The loss of a carboxyl group (M+ -59) and a quinone group (M+ -28) were strong hints for the occurrence of a lactone structure. The 1H NMR spectrum of product II showed the presence of seven protons [δ 7.21 (d, 1 H, J3 = 7.0 Hz, H5), 7.45 (m, 3H, H3′; H4′; H5′), 7.64 (d, 1H, J3 = 7.0 Hz, H4), 7.68 (m, 2H, H2′; H6′) ppm]. One triplet and two multiplets indicated a nonsubstituted aromatic ring, and two coupling doublets presented the other ring system. By 13C NMR analysis, the presence of 12 carbons was demonstrated. By using extensive NMR techniques like HMQC and HMBC as well as GC-MS analysis, product II was identified as 3-phenyl-2-pyrone-6-carboxylic acid.
Identification of product III.
The mass spectrum of the methylated compound consisted of a base peak at m/z 276 and a major fragment ion at m/z 217 [M+]-COOCH3 (Table 1). These mass spectral data corresponded to a product isolated and characterized after incubation of the imperfect fungus Paecilomyces lilacinus with 3,4-dihydroxybiphenyl (11), and thus product III was identified as 2-hydroxy-4-phenylmuconic acid.
Biotransformation of trihydroxylated derivatives of biphenyl.
Similar to incubation experiments with 2,3-dihydroxybiphenyl, the carbon source of cultivation of the yeast cells had a great influence on the transformation of 2,3,4-trihydroxybiphenyl. This substrate (99 μmol liter−1) was transformed completely by phenol-grown cells into the novel product V within 6 h of incubation. Glucose-grown cells produced the same product only in very small quantities, and the 2,3,4-trihydroxybiphenyl decreased only insignificantly.
Using 3,4,5-trihydroxybiphenyl as a substrate, HPLC analysis showed the formation of 4-phenyl-2-pyrone-6-carboxylic acid (product IV) as a major product as well as two other products with UV spectra similar to those of products I and II. Phenol-grown cells transformed 3,4,5-trihydroxybiphenyl (99 μmol liter−1) completely within 6 h, accumulating 4-phenyl-2-pyrone-6-carboxylic acid (26 μmol liter−1) and the products mentioned above, whereas glucose-grown cells did not totally transform the substrate within 120 h of incubation and less 4-phenyl-2-pyrone-6-carboxylic acid (6.5 μmol liter−1) was produced.
HPLC analysis of the incubation assay with 3,4,4′-trihydroxybiphenyl revealed the formation of two additional products (VI and VII). GC-MS analysis of the ethyl acetate extract showed three products, VI, VII, and VIII. While product VI was extractable with ethyl acetate at pH 7 and pH 2, products VII and VIII were recovered from the organic phase only after acidification of the supernatant and were purified by semipreparative HPLC analysis. This distribution of the products was similar to that obtained by using 3,4-dihydroxybiphenyl as a substrate.
Identification of product V.
GC-MS analysis of the methylated compound V revealed two peaks in the chromatogram with masses of m/z 230 and m/z 276. The compound with the mass m/z 230 had a fragmentation pattern identical to the one obtained for product II (Table 1). The electron impact mass spectrum of the peak (m/z 276) indicated the loss of two carboxylic groups and one methylated hydroxy group. Product II was identified as 3-phenyl-2-pyrone-6-carboxylic acid. Together with the fact that lactones can result from muconic acids (9, 10), we assumed that product V was the methylated derivative of 2-hydroxy-5-phenylmuconic acid. The 1H NMR spectrum of product V showed one multiplet, two doublets of doublets indicating a nonsubstituted aromatic ring, and two coupled doublets [δ 6.40 (d, 1H, J3 = 9.5 Hz, H3), 7.33 (m, 1H, H4′), 7.36 (dd, 2H, J3 = 6.8 Hz, H3′; H5′), 7.42 (d, 2H, J3 = 8.0 Hz, J4 = 1.3 Hz, H2′; H6′), 7.65 (d, 1H, H4) ppm]. An additional 13C NMR analysis proved the presence of 12 carbons. Based on these NMR and GC-MS data, the product V was identified as 2-hydroxy-5-phenylmuconic acid.
Identification of product VI.
Derivatization with diazomethane was necessary to analyze product VI by GC-MS, showing a fragmentation pattern in the mass spectrum similar to the one of the methylated product I (m/z 232). The methylated derivative revealed a molecular ion at m/z 262 and significant fragment ions (Table 1) at m/z 132 and m/z 133, indicating the loss of two methylated carboxyl groups and ethylene. UV-Vis spectra, HPLC retention time, and GC-MS data were identical with those of [5-oxo-3-(4′-hydroxyphenyl)-2,5-dihydrofuran-2-yl]acetic acid characterized after incubation of P. lilacinus with 3,4,4′-trihydroxybiphenyl (11).
Identification of products VII and VIII.
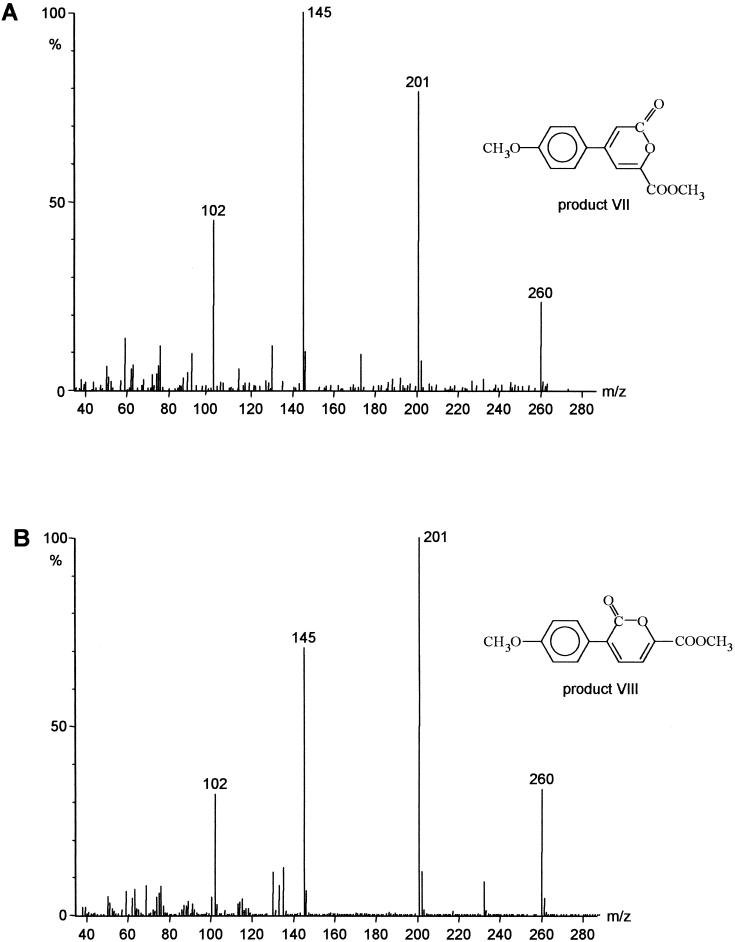
To analyze products VII and VIII by GC-MS, derivatization with diazomethane was necessary. Both products showed molecular masses of m/z 260 (Fig. 2). The high similarity of the mass spectral data to those of product II, as well as to an already identified product within the transformation of biphenyl by P. lilacinus, resulted in the identification of product VII as 4-(4′-hydroxyphenyl)-2-pyrone-6-carboxylic acid. This structure was confirmed by high-resolution mass spectral data and 1H NMR analysis (11).
FIG. 2.
Mass spectra of methylated 4-(4′-hydroxyphenyl)-2-pyrone-6-carboxylic acid (product VII) (A) and methylated 3-(4′-hydroxyphenyl)-2-pyrone-6-carboxylic acid (product VIII) (B).
The fragmentation pattern of product VIII resembled the one of 4-(4′-hydroxyphenyl)-2-pyrone-6-carboxylic acid (product VII). The only difference consisted in the varying intensities of the fragment ions. Behavior such as that of the intensities of the fragment ions within the same fragmentation pattern can also be seen for product II (Table 1). Therefore, product VIII can be postulated to be 3-(4′-hydroxyphenyl)-2-pyrone-6-carboxylic acid.
Toxicity of biphenyl and products formed during the transformation of biphenyl.
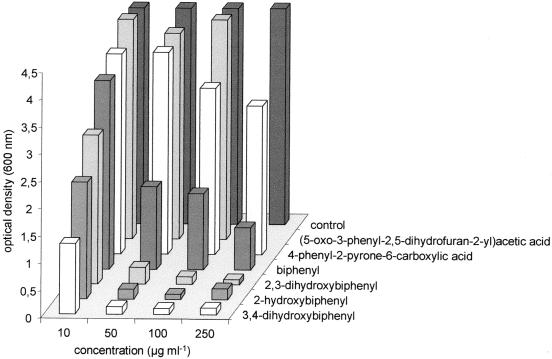
Biphenyl, 2-, 3-, and 4-hydroxybiphenyl, and 2,3- and 3,4-dihydroxybiphenyl, as well as the ring fission products 4-phenyl-2-pyrone-6-carboxylic acid and a mixture of (5-oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid and 3-phenyl-2-pyrone-6-carboxylic acid, were tested for their influence on glucose-grown cells of T. mucoides (Fig. 3). Cultures in the presence of 4-phenyl-2-pyrone-6-carboxylic acid and the mixture of (5-oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid and 3-phenyl-2-pyrone-6-carboxylic acid showed only slightly reduced growth (93 to 95%) compared with the control experiment. At the end of cultivation (48 h), the optical densities of all cultures and of the control were similar. In contrast, cultures grown in the presence of biphenyl and monohydroxylated intermediates reached the same optical density only at the lowest concentration of the compound (10 μg ml−1). The same dependency between the concentration of the aromatic compound and the inhibition of growth was observed in the presence of 3,4-dihydroxybiphenyl, but the growth was already reduced at the lowest concentration used. 2,3-Dihydroxybiphenyl inhibited growth at increasing concentrations. These results were confirmed by growth inhibition experiments done with the yeast S. cerevisiae, which does not have the capability to transform hydroxylated biphenyls. A strong inhibition was found in case of 4-hydroxybiphenyl and 3,4-dihydroxybiphenyl. On the other hand, the addition of the same amount of 4-phenyl-2-pyrone-6-carboxylic acid and the mixture of (5-oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid and 3-phenyl-2-pyrone-6-carboxylic acid (20 μg ml−1) showed little or no effect on the growth rate of S. cerevisiae.
FIG. 3.
Influence of biphenyl and selected products formed during the biotransformation of biphenyl by T. mucoides SBUG 801 on the growth of this organism with 1% glucose by measuring the optical density (A600) after 12 h. Cells grown with 1% glucose without the presence of aromatic compounds were used as a control. (5-Oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid was in a mixture with 3-phenyl 1-2-pyrone-6-carboxylic acid (1:0.01). There are no data available for the influence of 250 μg of this mixture ml−1 on the growth of the yeast cells because of the limited amount of this mixture enriched by semipreparative HPLC.
DISCUSSION
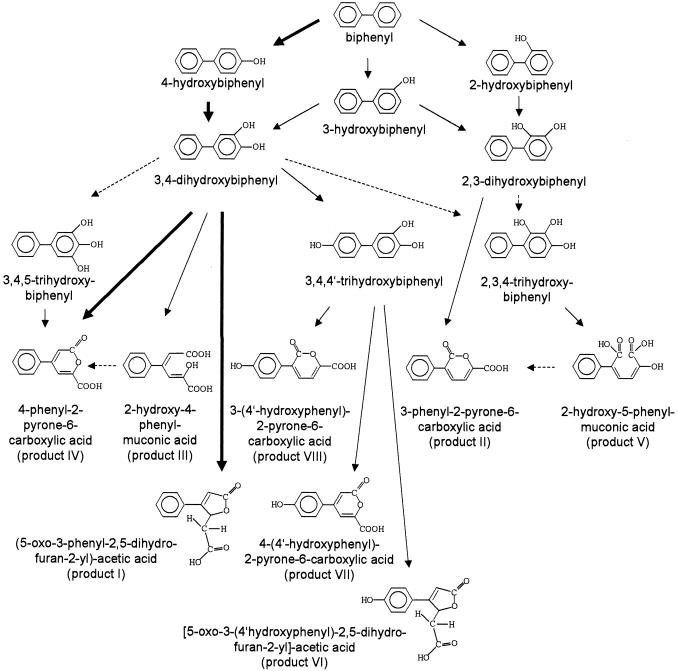
In this study, we extended previous studies on the transformation of biphenyl by T. mucoides SBUG 801 with special emphasis on novel ring cleavage products. Most fungi and mammals initially hydroxylate biphenyl into mono-, di-, and trihydroxylated intermediates. The same process was observed for T. mucoides. However, the kinetic data show that all 4-hydroxybiphenyl produced was transformed via 3,4-dihydroxybiphenyl to the main ring cleavage products 4-phenyl-2-pyrone-6-carboxylic acid and (5-oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid. A large variety of further ring cleavage products (Fig. 4) was produced from other ortho-di- and trihydroxylated biphenyls via ortho-ring fission, which is unique on this scale for eukaryotic organisms until now. Ring fission occurred as well as from substrates hydroxylated on one or both aromatic rings, leading to hydroxymuconic acids as well as the corresponding lactones. Previously, products with hydroxymuconic acid structure were also described for the transformation of dibenzofuran by T. mucoides (16) and diphenyl ether by Trametes versicolor (17) as well as for the metabolism of phloroglucinol by Fusarium solani (36). Trihydroxylated biphenyls with all hydroxy groups on one aromatic ring were supposed to be the preliminary stage for the lactones produced. Although these products were not detected, this assumption was confirmed by using them as a substrate. The possible ortho-fission of a trihydroxylated transformation product was first assumed in the metabolism of diphenyl ether by Trichosporon domesticum (formerly as Trichosporon beigelii), but such a product had not been detected as an intermediate (24).
FIG. 4.
Proposed pathway for the transformation of biphenyl by T. mucoides SBUG 801 via 2-, 3-, and 4-hydroxybiphenyl, resulting in ring cleavage products. Other oxidation products of biphenyl formed by this yeast strain (27) were not included in this scheme.→, Proved product formation; - - - ▸, assumed product formation.
The genus Trichosporon is known for its ability to cleave the ring systems of aromatic compounds. Thus, T. mucoides produced a hydroxymuconic acid derivative with dibenzofuran as a substrate (16) and incubation of T. domesticum SBUG 752 with diphenyl ether led to a lactone as the major product (24). Because of the fact that ring fission of compounds with two or more aromatic ring systems is not common in eukaryotic organisms, the toxicity of transformation products of biphenyl produced by the yeast T. mucoides was investigated. Only the ring fission products 4-phenyl-2-pyrone-6-carboxylic acid and the mixture of (5-oxo-3-phenyl-2,5-dihydrofuran-2-yl)acetic acid and 3-phenyl-2-pyrone-6-carboxlic acid, obtained by semipreparative HPLC, hardly showed any effect on the growth of the yeast cells independent of the concentration used. In contrast, biphenyl as well as mono- and dihydroxylated transformation products inhibited the growth, with the response increasing when higher concentrations were tested.
The hydroxylation of hydrophobic aromatic compounds by eukaryotic organisms is very often linked to the formation of relatively toxic products, as described above. The formation of glucuronide conjugates (29) is generally considered a mechanism for detoxification and rapid excretion of transformation products of biphenyl and polycyclic aromatic hydrocarbons (PAHs) in filamentous fungi and mammals. The filamentous fungus Cunninghamella elegans produces glucuronide conjugates during biphenyl metabolism (6) and glucoside conjugates during the transformation of PAHs (22), as does Phanerochaete chrysosporium (32). Mammals and fungi also produce glucuronide and sulfate conjugates (3, 12, 37). Rhizoctonia solani was even able to produce xylose conjugates with anthracene (33). The formation of xylose as well as glucose conjugates was also described for the transformation of triclosan (2,4,4′-trichloro-2′-hydroxydiphenyl ether) by T. versicolor (18). In contrast, conjugates with hydroxylated intermediates were not found during biphenyl transformation by T. mucoides. A second possible reaction is a further transformation of these toxic hydroxylated derivatives via cleavage of one of the aromatic ring systems. In the case of the transformation of biphenyl by T. mucoides, the resulting ring fission products were considerably less toxic.
It seems that there is at least one more mechanism for the detoxification of foreign aromatic compounds with two or more aromatic ring systems in eukaryotic organisms. The inability to cleave the aromatic ring might be an explanation for the production of conjugates with the hydroxylated intermediates as a common mechanism of detoxification of those kinds of aromatic compounds by the organisms described above. T. mucoides did not produce conjugates but formed less-toxic ring fission products from hydroxylated biphenyls as an alternative detoxification mechanism. Nevertheless, only a few of all the different hydroxylated products seemed to be transformed by such a mechanism which depends on the enzyme pattern and specificity of the organism.
ACKNOWLEDGMENTS
This study was supported by the Studienstiftung des deutschen Volkes.
We specially thank B. Shoulders (University of Texas at Austin) for carrying out extensive NMR analysis and helping with structure elucidation. M. Kindermann and S. Siegert (University of Greifswald) are acknowledged for recording NMR data, as is B. Schneider (Max-Planck-Institute of Jena) for carrying out high-resolution mass spectrometry. The help of A. Schäfer with the interpretation of NMR data is gratefully acknowledged.
REFERENCES
- 1.Block W D, Cornish H H. Metabolism of biphenyl and 4-chlorobiphenyl in the rabbit. J Biol Chem. 1959;234:3301–3302. [Google Scholar]
- 2.Cerniglia C E, Crow S A. Metabolism of aromatic hydrocarbons by yeasts. Arch Microbiol. 1981;129:9–13. [Google Scholar]
- 3.Cerniglia C E, Freeman J P, Mitchum R K. Glucuronide and sulfate conjugation in the fungal metabolism of aromatic hydrocarbons. Appl Environ Microbiol. 1982;43:1070–1075. doi: 10.1128/aem.43.5.1070-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox J C, Golbeck J H. Hydroxylation of biphenyl by Aspergillus parasiticus: Approaches to yield improvement in fermenter cultures. Biotechnol Bioeng. 1985;27:1395–1402. doi: 10.1002/bit.260271002. [DOI] [PubMed] [Google Scholar]
- 5.De Boer T D, Backer H J. Diazomethane. Org Synth. 1956;36:14–16. [Google Scholar]
- 6.Dodge R H, Cerniglia C E, Gibson D T. Fungal metabolism of biphenyl. Biochem J. 1979;178:223–230. doi: 10.1042/bj1780223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert J W. Control of postharvest diseases. In: Siegel M R, Sisler H D, editors. Antifungal compounds, vol. 1. Discovery, development, and uses. New York, N.Y: Marcel Dekker, Inc; 1977. pp. 269–352. [Google Scholar]
- 8.Fry J R. A comparison of biphenyl 4-hydroxylation and 4-methoxybiphenyl o-demethylation in rat liver microsomes. Biochem Pharmacol. 1981;30:1915–1919. doi: 10.1016/0006-2952(81)90199-4. [DOI] [PubMed] [Google Scholar]
- 9.Gaal A, Neujahr H Y. Metabolism of phenol and resorcinol in Trichosporon cutaneum. J Bacteriol. 1979;137:13–21. doi: 10.1128/jb.137.1.13-21.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaal A, Neujahr H Y. cis,cis-Muconate cyclase from Trichosporon cutaneum. Biochem J. 1980;191:37–43. doi: 10.1042/bj1910037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gesell M, Hammer E, Specht M, Francke W, Schauer F. Biotransformation of biphenyl by Paecilomyces lilacinus and characterization of ring cleavage products. Appl Environ Microbiol. 2001;67:1551–1557. doi: 10.1128/AEM.67.4.1551-1557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golbeck J H, Albaugh S A, Radmer R. Metabolism of biphenyl by Aspergillus toxicarius: induction of hydroxylating activity and accumulation of water-soluble conjugates. J Bacteriol. 1983;156:49–57. doi: 10.1128/jb.156.1.49-57.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golbeck J H, Cox J C. The hydroxylation of biphenyl by Aspergillus toxicarius: conditions for a bench scale fermentation process. Biotechnol Bioeng. 1984;26:434–441. doi: 10.1002/bit.260260506. [DOI] [PubMed] [Google Scholar]
- 14.Gosselin R E, Smith R P, Hodge H D. Clinical toxicology of commercial products 5th ed, p. II–152. Baltimore, Md: Williams and Wilkins; 1984. [Google Scholar]
- 15.Häkkinen I, Siltanen E, Hernberg S, Seppäläinen A M, Karli P, Vikkula E. Diphenyl poisoning in fruit paper production. Arch Environ Health. 1973;26:70–74. doi: 10.1080/00039896.1973.10666226. [DOI] [PubMed] [Google Scholar]
- 16.Hammer E, Krowas D, Schäfer A, Specht M, Francke W, Schauer F. Isolation and characterization of a dibenzofuran-degrading yeast: identification of oxidation and ring cleavage products. Appl Environ Microbiol. 1998;64:2215–2219. doi: 10.1128/aem.64.6.2215-2219.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hundt K, Jonas U, Hammer E, Schauer F. Transformation of diphenyl ethers by Trametes versicolor and characterization of ring cleavage products. Biodegradation. 1999;10:279–286. [Google Scholar]
- 18.Hundt K, Martin D, Hammer E, Jonas U, Kindermann M K, Schauer F. Transformation of triclosan by Trametes versicolor and Pycnoporus cinnabarinus. Appl Environ Microbiol. 2000;66:4157–4160. doi: 10.1128/aem.66.9.4157-4160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange J, Hammer E, Specht M, Francke W, Schauer F. Biodegradation of biphenyl by the ascomycetous yeast Debaryomyces vanrijiae. Appl Microbiol Biotechnol. 1998;50:364–368. doi: 10.1007/s002530051305. [DOI] [PubMed] [Google Scholar]
- 20.Meyer T, Scheline R R. The metabolism of biphenyl. II. Phenolic metabolites in the rat. Acta Pharmacol Toxicol. 1976;39:419–432. doi: 10.1111/j.1600-0773.1976.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 21.Mobley D P, Finkbeiner H L, Lockwood S H, Spivack J. Synthesis of 3-arylmuconolactones using biphenyl metabolism in Aspergillus. Tetrahedron. 1993;49:3273–3280. [Google Scholar]
- 22.Pothuluri J V, Freeman J P, Evans F E, Cerniglia C E. Fungal transformation of fluoranthene. Appl Environ Microbiol. 1990;56:2974–2983. doi: 10.1128/aem.56.10.2974-2983.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandmeyer E E. –1982. Aromatic hydrocarbons. In: Clayton G D, Clayton F E, editors. Patty's Industrial Hygiene and Toxicology. 2A. Toxicology. New York, N.Y: John Wiley Sons; 1981. pp. 3253–3258. [Google Scholar]
- 24.Schauer F, Henning K, Pscheidl H, Wittich R M, Fortnagel P, Wilkes H, Sinnwell V, Francke W. Biotransformation of diphenyl ether by the yeast Trichosporon beigelii SBUG 752. Biodegradation. 1995;6:173–180. doi: 10.1007/BF00695348. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz R D, Williams A L, Hutchinson D B. Microbial production of 4,4′-dihydroxybiphenyl: biphenyl hydroxylation by fungi. Appl Environ Microbiol. 1980;39:702–708. doi: 10.1128/aem.39.4.702-708.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz R D. A novel reaction: meta hydroxylation of biphenyl by an actinomycete. Enzyme Microb Technol. 1981;3:158–159. [Google Scholar]
- 27.Sietmann R, Hammer E, Moody J, Cerniglia C E, Schauer F. Hydroxylation of biphenyl by the yeast Trichosporon mucoides. Arch Microbiol. 2000;174:353–361. doi: 10.1007/s002030000219. [DOI] [PubMed] [Google Scholar]
- 28.Singer-Bohne B, Hofeneder M, Koch H P. Der Hefetest: Eine Ergänzungsmethode zur Bestimmung der akuten Toxizität von Arzneistoffen und Umweltgiften. BIOforum. 1993;16:244–248. [Google Scholar]
- 29.Smith R L, Williams R T. Implications of the conjugation of drugs and other exogenous compounds. In: Dutton G J, editor. Glucuronic acid: free and combined. London, United Kingdom: Academic Press; 1966. pp. 457–491. [Google Scholar]
- 30.Smith R V, Rosazza J P. Microbial models of mammalian metabolism. Aromatic hydroxylation. Arch Biochem Biophys. 1974;161:551–558. doi: 10.1016/0003-9861(74)90338-5. [DOI] [PubMed] [Google Scholar]
- 31.Smith R V, Davis P J, Clark A M, Glover-Milton S. Hydroxylations of biphenyl by fungi. J Appl Bacteriol. 1980;49:65–73. doi: 10.1111/j.1365-2672.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland J B, Selby A L, Freeman J P, Evans F E, Cerniglia C E. Metabolism of phenanthrene by Phanerochaete chrysosporium. Appl Environ Microbiol. 1991;57:3310–3316. doi: 10.1128/aem.57.11.3310-3316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland J B. Detoxification of polycyclic aromatic hydrocarbons by fungi. J Ind Microbiol. 1992;9:53–62. doi: 10.1007/BF01576368. [DOI] [PubMed] [Google Scholar]
- 34.Thomas D R, Carswell K S, Georgiou G. Mineralization of biphenyl and PCBs by the white rot fungus Phanerochaete chrysosporium. Biotechnol Bioeng. 1992;40:1395–1402. doi: 10.1002/bit.260401114. [DOI] [PubMed] [Google Scholar]
- 35.van der Walt J P. Criteria and methods used in classification. In: Lodder J, editor. The yeasts, a taxonomic study. Amsterdam, The Netherlands: North-Holland Publishing Co.; 1970. pp. 34–113. [Google Scholar]
- 36.Walker J R L, Taylor B G. Metabolism of phloroglucinol by Fusarium solani. Arch Microbiol. 1983;134:123–126. [Google Scholar]
- 37.Wiebkin P, Fry J R, Jones C A, Lowing R, Bridges J W. The metabolism of biphenyl by isolated viable rat hepatocytes. Xenobiotica. 1976;6:725–743. doi: 10.3109/00498257609151390. [DOI] [PubMed] [Google Scholar]