Abstract
The neutrophil-to-lymphocyte ratio (NLR) is used as biomarker in malignant diseases showing significant association with poor oncological outcomes. The main research question of the present study was whether NLR has also prognostic value in cholangiocarcinoma patients (CCA). A systematic review was carried out to identify studies related to NLR and clinical outcomes in CCA evaluating the literature from 01/2000 to 09/2021. A random-effects model, pooled hazard ratios (HR) and 95% confidence interval (CI) were used to investigate the statistical association between NLR and overall survival (OS) as well as disease-free survival (DFS). Subgroup analyses, evaluation of sensitivity and risk of bias were further carried out. 32 studies comprising 8572 patients were eligible for this systematic review and meta-analysis. The pooled outcomes revealed that high NLR prior to treatment is prognostic for poor OS (HR 1.28, 95% CI 1.18–1.38, p < 0.01) and DFS (HR 1.39, 95% CI 1.17–1.66, p < 0.01) with meaningful HR values. Subgroup analysis revealed that this association is not significantly affected by the treatment modality (surgical vs. non-surgical), NLR cut-off values, age and sample size of the included studies. Given the likelihood of NLR to be prognostic for reduced OS and DFS, pre-treatment NLR might serve as a useful biomarker for poor prognosis in patients with CCA and therefore facilitate clinical management.
Subject terms: Prognostic markers, Surgical oncology
Introduction
Cholangiocarcinoma (CCA) accounts for 15% of all primary malignant liver tumors and arises from intra- or extrahepatic bile ducts1,2. Due to the anatomical location of the tumor in the extrahepatic (extrahepatic CCA, ECCA) subtype and the usually higher tumor burden in the intrahepatic subtype (intrahepatic CCA, ICCA), clinical outcomes have been reported to be dismal even after radical surgery in comparison to other gastrointestinal tumors3–5. Therefore, the identification of reliable prognostic markers might facilitate patient selection as well as risk-stratification in CCA patients.
Inflammation in the tumor microenvironment plays a well-known and important role in tumor biology. Particularly, carcinogenesis and tumor progression are often linked to systemic inflammatory activation6. Over the past years, several prognostic scores on the basis of laboratory parameters, such as the counts of neutrophiles, lymphocytes as well as C-reactive protein (CRP) levels, have been developed. Based on this, calculated scores e.g. neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and Glasgow Prognostic Score (GPS), have been frequently associated with oncological outcomes in various solid tumors7–10. However, conflicting results have been reported regarding the prognostic value of these preoperative systemic inflammatory parameters in CCA11–13.
Given the prognostic value in other tumor entities, it is hypothesized that NLR has also prognostic value in CCA. Thus, a systematic review and meta-analysis is conducted to further assess the prognostic value of NLR for oncological outcome [overall survival (OS), disease-free survival (DFS)] in CCA patients based on the available evidence.
Material and methods
Literature search
The ex-ante protocol of this systematic review was registered open access in the International Prospective Register of Systematic Reviews (PROSPERO) under the ID: CRD42021271435 and was conducted in line with recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement. PubMed and Google Scholar were systematically searched for articles published between January 2000 and September 2021. The following key search terms were used: “lymphocytes” OR “Neutrophil-to-lymphocyte ratio” AND “Cholangiocarcinoma (CCA)” OR “Biliary tree cancers (BTC)”. Two independent literature searches were carried out by two authors based on the same strategy. Subsequently, no further publications were identified after the reference list and citations search were completed.
Inclusion and exclusion criteria
Inclusion criteria were:
Studies investigating the prognostic value of NLR in CCA.
Reporting of survival data (DFS, OS).
Exclusion criteria were:
No access to the full text.
Reviews, case reports, comments or editorials.
Non-English papers.
Statistical analysis
The statistical analysis was conducted as previously described7. Hazard ratios (HR) and 95% CIs were used to assess the association between NLR and outcomes. Kaplan–Meier curves in combination with Engauge Digitizer version 12.1 were used to extract these information if not directly reported as described previously14. RevMan version 5.4 and R project version 4.1.2 were used to analyze and visualize the results. Measures of statistical heterogeneity between studies were calculated (tau, Q, I value) and assessed using the Chi-squared test and assumed to be significant when I2 > 50% and/or p < 0.05. A random-effect model and subgroup analysis were preferred when heterogeneity existed, while a fixed-effect model was used when no variance was detected in the data set. Subgroup analysis was carried out to investigate heterogeneity in the studies, while sensitivity analyses were performed to determine the stability of the overall effects. Here, one study at a time was excluded to ensure that no single study would dominate and would be solely responsible for a significant result. Baujat plots were used to investigate the contribution of studies to the heterogeneity as well as pooled outcome and funnel plots were utilized to evaluate publication bias15.
Quality assessment of selected studies
The quality of the included studies was structurally evaluated by 2 reviewers (DL and JB) using the Newcastle–Ottawa scale16. The Newcastle–Ottawa scale is composed of the following three quality indicators: outcome assessment, comparability, and selection. Each paper was scored from 0 to 9 based on these parameters.
Results
Literature search
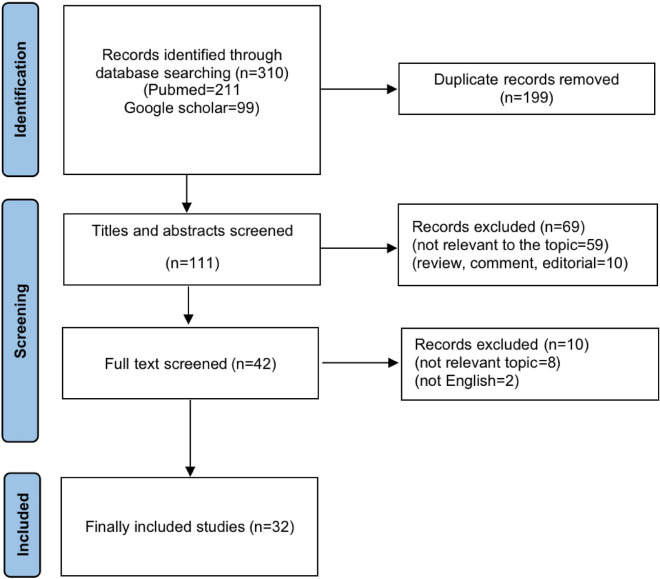
The process of selecting publications is depicted in Fig. 1. Initially, 310 articles were identified searching two databases. Subsequently, 199 duplicate records were detected and eliminated. The remaining 111 studies were further assessed for eligibility after titles and abstracts were reviewed and subsequently, a full-text screening was conducted for 42 publications of which finally 32 studies were eligible to be included in this meta-analysis 17–48.
Figure 1.
Flowchart of study selection for this study.
Study characteristics and quality assessment
The key characteristics of the 32 publications analyzed in this manuscript are depicted in Table 1. All publications included were retrospective cohort studies comprising a total of 8572 patients, 6427 of whom had liver resection and 2145 of whom had undergone non-surgical therapy. The mean age of the study populations was 56 to 70 years with males accounting for 31% to 79% of the patients in the investigated data set. NLR cut-off values were different between the studies and obtained using various approaches. Regarding the investigated entities, 19 studies focused on ICCA, 8 on ECCA and 5 studies analyzed both ICCA and ECCA. While all studies reported a correlation between OS and NLR, only 14 reported a correlation between DFS and NLR.
Table 1.
Characteristics of included studies.
| Author | Year published | Country | Tumor type | Sample size | Stage | Age (median) | Male (%) | Treatment | Follow-up (months, median) | Endpoint | Cut-off value (high expression) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | Zhao JP | 2021 | China | ICCA | 468 | NR | 58 | 60.30% | Surgery | NR | OS | NLR ≥ 3 |
| 18 | Ma B | 2021 | China | ICCA | 174 | I–IV | 58 | 55.90% | Surgery | 25.1 | OS/DFS | NLR ≥ 3 |
| 19 | Zhang ZY | 2020 | China | ICCA | 128 | I–III | 56 | 55.00% | Surgery | NR | OS/DFS | NLR ≥ 3 |
| 20 | Tsilimigras DI | 2020 | USA | ICCA | 688 | I–III | 57 | 60.50% | Surgery | 22.3 | OS | NLR ≥ 5 |
| 21 | Ohira M | 2020 | Japan | ICCA | 52 | I–IV | 58 | 78.84% | Surgery | NR | OS | NLR ≥ 1.93 |
| 22 | Ji F | 2020 | China | ECCA | 59 | I–IV | 57 | 55.93% | Surgery | NR | OS | NLR ≥ 2.93 |
| 23 | Huh G | 2020 | Korea | ICCA | 137 | III–IV | 64 | 60.60% | Non-surgery | 9.9 | OS/DFS | NLR ≥ 5 |
| 24 | Filippi L | 2020 | Latina | ICCA | 20 | NR | 65 | 45.00% | Non-surgery | 12.5 | OS | NLR ≥ 2.7 |
| 25 | Zhang Y | 2019 | China | ICCA | 322 | I–IV | 57 | 60.25% | Surgery | 44 | OS/DFS | NLR ≥ 3 |
| 26 | Wu YH | 2019 | China | ICCA | 123 | I–IV | 57 | 54.47% | Surgery | 29.1 | OS | NLR ≥ 2.05 |
| 27 | Sellers CM | 2019 | USA | ICCA | 131 | I–IV | 65 | 51.90% | Surgery | 13 | OS | NLR ≥ 3.96 |
| 28 | Lin J | 2019 | China | ICCA | 218 | I–IV | 60 | 56.90% | Surgery | NR | OS | NLR ≥ 2.94 |
| 29 | Hu HJ | 2019 | China | ECCA | 134 | I–IV | 60 | 63.01% | Surgery | NR | OS | NLR ≥ 3 |
| 30 | Hoshimoto S | 2019 | Japan | ECCA | 53 | I–IV | 70 | 58.00% | Surgery | 18 | OS/DFS | NLR ≥ 1.97 |
| 31 | Buettner S | 2018 | Netherlands | ICCA | 991 | I–IV | 59 | 54.10% | Surgery | 29 | OS | NLR ≥ 5 |
| 32 | Yoh T | 2017 | Japan | ICCA | 141 | I–IV | 65 | 63.00% | Surgery | NR | OS | NLR ≥ 5 |
| 33 | Omichi K | 2017 | USA | ICCA | 119 | I–IV | 58 | 57.14% | Non-surgery | NR | OS/DFS | NLR ≥ 3 |
| 34 | Nam K | 2017 | Korea | ICCA | 377 | I–IV | 60 | 69.00% | Surgery | NR | OS | NLR ≥ 2.7 |
| 35 | Kitano Y | 2017 | Japan | ECCA | 120 | I–IV | 58 | 68.33% | Surgery | NR | OS/DFS | NLR ≥ 2.8 |
| 36 | Cho H | 2017 | Korea | ICCA | 305 | III–IV | 59 | 61.50% | Non-surgery | 25 | OS/DFS | NLR ≥ 2.8 |
| 37 | Okuno M | 2016 | Japan | ECCA | 219 | III–IV | 65 | 58.45% | Non-surgery | 80.4 | OS | NLR ≥ 5 |
| 38 | Okuno M | 2016 | Japan | ECCA | 534 | I–IV | 66 | 62.92% | Surgery | 78 | OS | NLR ≥ 3 |
| 39 | Lin GH | 2016 | China | ICCA | 102 | I–IV | 58 | 64.71% | Surgery | NR | OS/DFS | NLR ≥ 3 |
| 40 | Lee BS | 2016 | Korea | CCA | 221 | III–IV | 62 | 69.20% | Non-surgery | NR | OS/DFS | NLR ≥ 5 |
| 41 | Ha H | 2016 | Korea | CCA | 534 | III–IV | 60 | 65.20% | Non-surgery | 95.3 | OS | NLR ≥ 3.49 |
| 42 | Beal EW | 2016 | USA | ECCA | 525 | I–IV | 68 | 50.67% | Surgery | NR | OS/DFS | NLR ≥ 5 |
| 43 | Chen Q | 2016 | China | ICCA | 322 | I–IV | 58 | 60.25% | Surgery | NR | OS/DFS | NLR ≥ 2.49 |
| 44 | Chen Q | 2015 | China | ICCA | 322 | I–IV | 58 | 60.25% | Surgery | NR | OS/DFS | NR |
| 45 | McNamara MG | 2014 | Canada | CCA | 864 | I–IV | 65 | 51.39% | Mix* | 14.4 | OS | NLR ≥ 3 |
| 46 | Iwaku A | 2014 | USA | CCA | 52 | III–IV | 70 | 59.62% | Non-surgery | 4 | OS | NLR ≥ 4 |
| 47 | Dumitrascu T | 2013 | Romania | ECCA | 90 | I–IV | 58 | No | Surgery | 68 | OS/DFS | NLR ≥ 3.3 |
| 48 | Gomez D | 2008 | UK | ICCA | 27 | I–IV | 57 | 31.00% | Surgery | 23 | OS/DFS | NLR ≥ 5 |
Mix*, including 326 surgical and 538 non-surgery cases, CCA cholangiocarcinoma, DFS disease-free surviva, ECCA extrahepatic cholangiocarcinoma, ICCA intrahepatic cholangiocarcinoma, NLR neutrophile-to-lymphocyte ratio, NR not reported, OS overall survival, Ref reference.
The study quality was evaluated between six and nine points on the Newcastle–Ottawa quality assessment scale indicating that the methodology of the investigations was of generally good quality (Table 2).
Table 2.
Qualities of cohort studies are evaluated by modified Newcastle–Ottawa scale.
| Ref | Author | Selection | Comparability | Outcomes | Quality score |
|---|---|---|---|---|---|
| 17 | Zhao JP | ★★★★ | ★★ | ★★ | 9 |
| 18 | Ma B | ★★★ | ★★ | ★★ | 8 |
| 19 | Zhang ZY | ★★★★ | ★★ | ★★ | 9 |
| 20 | Tsilimigras DI | ★★★★ | ★★ | ★★ | 9 |
| 21 | Ohira M | ★★★★ | ★★ | ★★ | 9 |
| 22 | Ji F | ★★★★ | ★★ | ★★ | 9 |
| 23 | Huh G | ★★★★ | ★★ | ★★ | 9 |
| 24 | Filippi L | ★★★★ | ★★ | ★★ | 9 |
| 25 | Zhang Y | ★★★★ | ★★ | ★★ | 9 |
| 26 | Wu YH | ★★★ | ★★ | ★★ | 8 |
| 27 | Sellers CM | ★★★★ | ★★ | ★★ | 9 |
| 28 | Lin J | ★★★★ | ★★ | ★★ | 9 |
| 29 | Hu HJ | ★★★ | ★★ | ★★ | 8 |
| 30 | Hoshimoto S | ★★★ | ★★ | ★★ | 8 |
| 31 | Buettner S | ★★★★ | ★★ | ★ | 8 |
| 32 | Yoh T | ★★★ | ★★ | ★ | 6 |
| 33 | Omichi K | ★★★★ | ★★ | ★★ | 9 |
| 34 | Nam K | ★★★★ | ★★ | ★ | 8 |
| 35 | Kitano Y | ★★★★ | ★★ | ★★ | 9 |
| 36 | Cho H | ★★★★ | ★★ | ★ | 8 |
| 37 | Okuno M | ★★★★ | ★★ | ★★ | 9 |
| 38 | Okuno M | ★★★★ | ★★ | ★★ | 9 |
| 39 | Lin GH | ★★★★ | ★★ | ★ | 8 |
| 40 | Lee BS | ★★★★ | ★★ | ★★ | 9 |
| 41 | Ha H | ★★★★ | ★★ | ★★ | 9 |
| 42 | Beal EW | ★★★★ | ★★ | ★★ | 9 |
| 43 | Chen Q | ★★★★ | ★★ | ★★ | 9 |
| 44 | Chen Q | ★★★★ | ★★ | ★ | 8 |
| 45 | McNamara MG | ★★★★ | ★★ | ★★ | 9 |
| 46 | Iwaku A | ★★★★ | ★★ | ★★ | 9 |
| 47 | Dumitrascu T | ★★★★ | ★★ | ★ | 8 |
| 48 | Gomez D | ★★★★ | ★★ | ★ | 8 |
The quality of the included studies was assessed under six items of Hayden et al. All included translational studies reporting oncological outcome were evaluated in accordance with the Newcastle–Ottawa scale. The maximum score of the scale is nine points with studies being categorized as low (0–3 points), moderate (4–6 points) and high quality (7–9 points), respectively.
Correlation between NLR and OS in CCA
Among the 32 publications related to OS, 19 original papers reported that NLR was an independent predictor for impaired OS18,20,23–27,32–34,38–40.
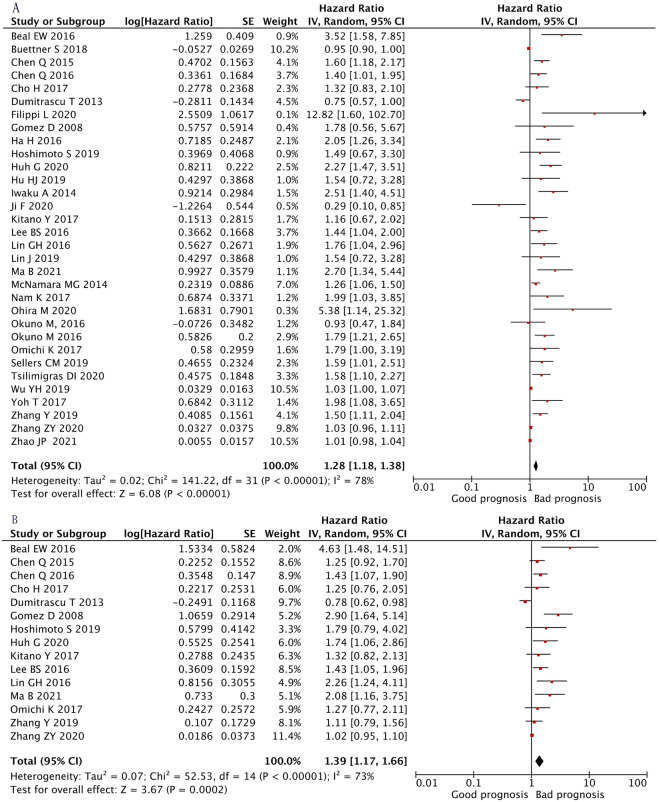
41–46, while 12 studies observed no association17,19,21,28–31,35–37,47,48 and one study identified NLR as prognostic for a longer OS22. The combined analysis of all 32 publications displayed that high NLR values indicated an impaired OS (HR 1.28, 95% CI 1.18–1.38, p < 0.01) with high heterogeneity (I2 = 78%, p < 0.01, Fig. 2A).
Figure 2.
Forest plot of the correlation between NLR and survival in CCA. High NLR values indicated a worse OS (A) (HR 1.28, 95% CI 1.18–1.38, p < 0.01) with high heterogeneity (Tau2 = 0.02, Chi2 = 141.22 p < 0.01, I2 = 78%) and a higher NLR level was associated with worse DFS (B) (HR 1.39, 95% CI 1.17–1.66, p < 0.01) with high heterogeneity (Tau2 = 0.07, Chi2 = 52.53 p < 0.01, I2 = 73%).
Correlation between NLR and DFS in CCA
Among the 15 publications which reported DFS, 7 cohort studies showed that NLR was an independent predictor of reduced DFS in patients with CCA18,23,39,40,42,43,48, whereas 7 publications observed no significant relationship between NLR19,25,30,33,35,36,44 and DFS and one study showed NLR was a prognostic for a longer DFS47. The pooled analysis of all studies showed that a higher NLR level was associated with impaired DFS (HR 1.39, 95% CI 1.17–1.66, p < 0.01) with high heterogeneity (I2 = 73%, p = 0.02, Fig. 2B).
Subgroup analyses of correlation between NLR and survival in CCA
Significant heterogeneity was detected in the HRs of OS for NLR (I2 = 78%, p < 0.01, Fig. 2A) and of DFS for NLR (I2 = 73%, p < 0.01, Fig. 2B). Thus, causes of the heterogeneity were investigated by subgroup analyses focusing on NLR cut-off values, treatment (surgical vs. non-surgical), cancer type, geographical region, age, size and sample.
For OS, NLR was prognostic in each defined subgroup of treatment type (surgical: p < 0.01; non-surgical: p < 0.01), cut-off value (> 3: p < 0.01; ≤ 3: p < 0.01), geographical region (western: p = 0.02; eastern: p < 0.01), sample size (n ≥ 200: p < 0.01; n < 200: p < 0.01) and age (≥ 60: p < 0.01; < 60: p = 0.01; Table 3, Supplementary Fig. S1) However, in the stratified analysis for cancer type, the pooled analysis of studies exclusively reporting on ECCA showed no significant effect of NLR on OS (p = 0.38), while statistical significance was obtained for ICCA (p < 0.01) and CCA (p < 0.01; Table 3).
Table 3.
Summary of the subgroup analyses of the correlation between NLR and overall survival in CCA patients.
| Subgroup | Number of studies | HR [95%CI] | P value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | p | ||||
| Cancer type | |||||
| CCA* | 4 | 1.60 [1.20–2.12] | < 0.01 | 61% | 0.05 |
| ICCA | 20 | 1.21 [1.11–1.31] | < 0.01 | 78% | < 0.01 |
| ECCA | 8 | 1.20 [0.79–1.82] | 0.38 | 75% | < 0.01 |
| Treatment | |||||
| Surgery | 23 | 1.14 [1.06–1.23] | < 0.01 | 73% | < 0.01 |
| Non-surgery | 9 | 1.71 [1.39–2.10] | < 0.01 | 49% | 0.05 |
| Cut-off value | |||||
| NLR > 3 | 15 | 1.40 [1.23–1.60] | < 0.01 | 85% | < 0.01 |
| NLR ≤ 3 | 17 | 1.25 [1.10–1.42] | < 0.01 | 67% | < 0.01 |
| Region | |||||
| Eastern | 21 | 1.28 [1.17–1.40] | < 0.01 | 75% | < 0.01 |
| Western | 11 | 1.36 [1.05–1.76] | 0.02 | 83% | < 0.01 |
| Sample size | |||||
| ≥ 200 | 14 | 1.29 [1.15–1.45] | < 0.01 | 80% | < 0.01 |
| < 200 | 18 | 1.39 [1.19–1.61] | < 0.01 | 77% | < 0.01 |
| Age** | |||||
| ≥ 60 | 15 | 1.72 [1.44–2.05] | < 0.01 | 42% | 0.04 |
| < 60 | 17 | 1.09 [1.02–1.18] | 0.01 | 40% | < 0.01 |
*Includes both ICCA and ECCA. **Mean/median age of the study cohort. ECCA extrahepatic cholangiocarcinoma, HR hazard ratio, ICCA intrahepatic cholangiocarcinoma, NLR neutrophil-to-lymphocyte ratio.
For DFS, NLR was prognostic in each defined subgroup of treatment type (surgical: p < 0.01; non-surgical: p < 0.01), cut-off value (> 3: p = 0.03; ≤ 3: p < 0.01), sample size (n ≥ 200: p < 0.01; n < 200: p < 0.01) and age (≥ 60: p < 0.01; < 60: p = 0.01; Table 4, Supplementary Fig. S1). Similar to OS, the stratified analysis for cancer type, showed no significant effect of NLR on DFS in ECCA (p = 0.67) while again statistical significance was obtained for ICCA (p < 0.01) and CCA (p = 0.02; Table 4, Supplementary Fig. S2). Also, no prognostic effect of NLR on DFS was obtained for western patients (p = 0.16) in this sub analysis (Table 4, Supplementary Fig. S2).
Table 4.
Summary of the subgroup analyses of the correlation between NLR and DFS in CCA patients.
| Subgroup | Number of studies | HR [95%CI] | P value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | p | ||||
| Cancer type | |||||
| CCA* | 1 | 1.43 [1.05–1.96] | 0.02 | - | - |
| ICCA | 10 | 1.44 [1.17–1.77] | < 0.01 | 73% | < 0.01 |
| ECCA | 3 | 1.11 [0.68–1.83] | 0.67 | 70% | 0.03 |
| Treatment | |||||
| Surgery | 11 | 1.40 [1.13–1.74] | < 0.01 | 78% | < 0.01 |
| Non-surgery | 4 | 1.42 [1.15–2.75] | < 0.01 | 0% | 0.78 |
| Cut-off value | |||||
| NLR > 3 | 6 | 1.56 [1.04–2.34] | 0.03 | 84% | < 0.01 |
| NLR ≤ 3 | 9 | 1.33 [1.10–1.62] | < 0.01 | 59% | 0.01 |
| Region | |||||
| Eastern | 11 | 1.36 [1.15–1.61] | < 0.01 | 62% | < 0.01 |
| Western | 4 | 1.72 [0.81–3.63] | 0.16 | 88% | < 0.01 |
| Sample size | |||||
| ≥ 200 | 6 | 1.34 [1.13–1.59] | < 0.01 | 21% | 0.28 |
| < 200 | 9 | 1.44 [1.11–1.88] | < 0.01 | 79% | < 0.01 |
| Age** | |||||
| ≥ 60 | 4 | 1.70 [1.23–2.34] | < 0.01 | 24% | 0.26 |
| < 60 | 11 | 1.30 [1.08–1.57] | 0.01 | 74% | < 0.01 |
*Includes both ICCA and ECCA. **Mean/median age of the study cohort. ECCA extrahepatic cholangiocarcinoma, HR hazard ratio, ICCA intrahepatic cholangiocarcinoma, NLR neutrophil-to-lymphocyte ratio.
Sensitivity analyses of correlation between NLR and prognosis of CCA patients
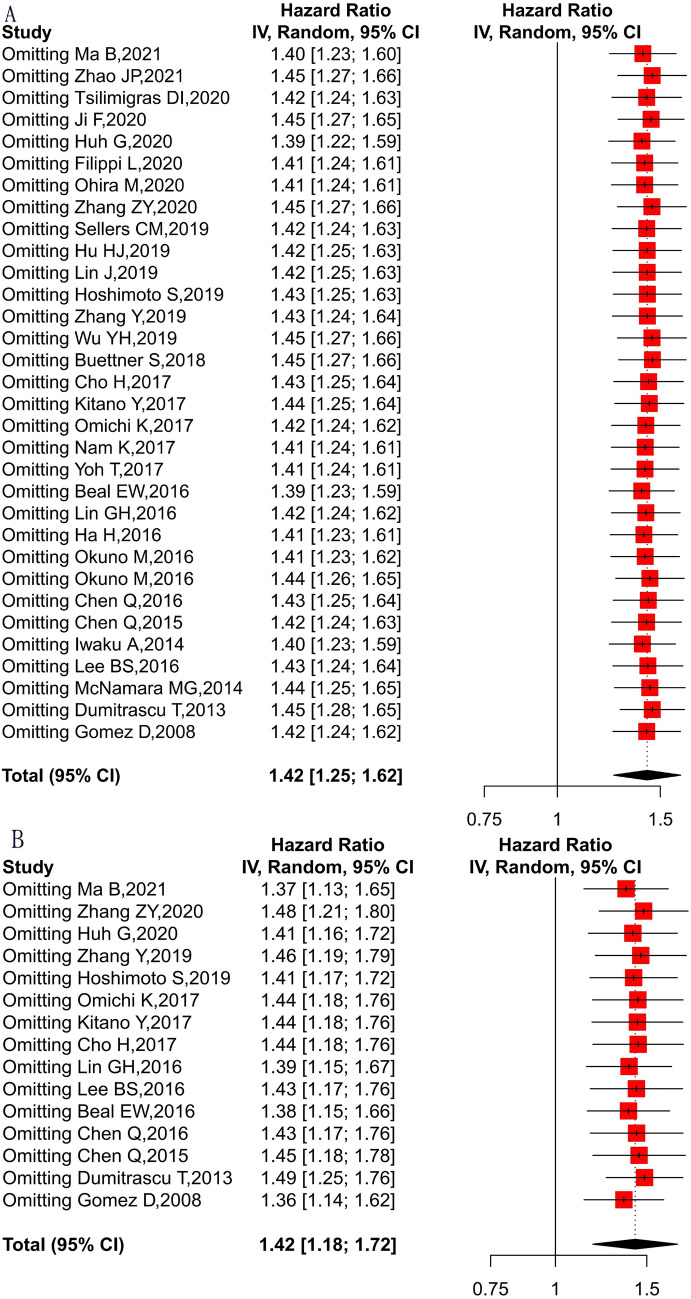
To determine the prognostic robustness of NLR, a random effects model in sensitivity analyses was adopted, deleting each study in each turn. As shown in Fig. 3, the results of the pooled HRs changed in each analysis, but high NLR still displayed an unfavorable effect on OS and DFS. These results indicate that the association between NLR and survival in CCA is certainly robust.
Figure 3.
Sensitivity analyses of correlation between NLR and prognosis of CCA patients. Adopting a random effects model in sensitivity analyses, deleting each study in each turn, to further determine the robustness of the prognostic role of NLR. High NLR still displayed an unfavorable effect on OS(A) and DFS(B).
Contribution of studies to the heterogeneity and pooled outcome
Baujat plots were used to detect studies which overly contributed to the heterogeneity in this meta-analysis. Here, Huh et al. contributed heavily to the overall heterogeneity in this meta-analysis, while Zhao et al. had a significant influence on the overall result and Buettner S et al. influenced both the estimated heterogeneity and the pooled effect (Supplementary Fig. S3A). Similarly, for DFS the study of Ma et al. contributed to the overall heterogeneity of this meta-analysis and Zhang et al. had the greatest impact on the pooled effect (Supplementary Fig. S3B).
Publication bias
No bias influencing the HRs could be detected as the results from a funnel plot analysis displayed no asymmetry (Supplementary Fig. S4).
Discussion
Recently, a number of studies have investigated the interaction between inflammation and cancer8,49. NLR, as an inflammatory index, has been shown to be associated with various clinical endpoints including long-term prognosis, disease recurrence and response to treatment50. Previous meta-analyses demonstrated that high NLR values are linked to poor oncological survival in hepatocellular51, breast52, esophageal53, colorectal54, lung55, pancreatic cancer56. In contrast, a meta-analysis by Templeton et al. identified significant differences in pooled effect estimates when stratifying studies by cancer type and metastatic versus non-metastatic disease, suggesting the prognostic potential of the NLR may not be equal among all patient and cancer subgroups8. Considering the effects in other tumor entities, the main research question of the present meta-analysis was whether NLR has also prognostic value in cholangiocarcinoma patients (CCA). To investigate this, 32 studies with a total of 8572 patients were assessed. Results show that high NLR is associated with significantly poor OS and DFS with notable hazard ratios.
Notably, the subgroup analysis revealed that the unfavorable effect of NLR is independent from sample size, age, NLR cut-off value, different treatment types including palliative or curative therapy and geographical region. Although, we observed no statistical significance in DFS for Western patients, the number of included studies from Western countries was limited (n = 4) and displayed a high level of heterogeneity (I2 = 88%, p < 0.01, Table 4, Fig. S2). The observation that the prognostic ability of NLR is independent from the applied oncological treatment is of particular interest since the oncological outcome of curative treatment is significantly different to the palliative setting in CCA. While 5-year survival rates higher than 50% have been demonstrated in selected subgroups of CCA patients undergoing curative-intent liver resection, the median OS in the palliative setting remains discouraging (< 12 months) due to lack of effective systemic therapy57–59. Given this observation, one might speculate that NLR could be closely associated with the individual tumor biology and therefore might be a suitable prognostic biomarker irrespective of the applied treatment across the oncological stages. Interestingly, Zheng et al. carried out a comparable meta-analysis for hepatocellular carcinoma and also noticed that high NLR is an independent risk factor for DFS and OS in HCC patients in a palliative and curative setting51.
Further, the subgroup analysis marginally failed to detect a statistically significant association between oncological outcomes and NLR in patients with ECCA (for OS: HR 1.20, 95% CI 0.79–1.82, p = 0.38; for DFS: HR 1.11, 95% CI 0.68–1.83, p = 0.07). However, the number of studies on ECCA was limited (nOS = 6, nDFS = 3). Furthermore, these reports included the distal as well as perihilar subtype of CCA, resulting in a significant heterogeneity (I2 = 60% and 70%, respectively) among the analyzed publications. In addition, ECCA is usually characterized by recurrent cholangitis with following septic complications interfering with long-term survival5. As the data suggests a primary association of NLR with OS and DFS, it seems plausible that the prognostic value of NLR might be mitigated in the scenario of ECCA as septic events might also result in deaths which are pre see not cancer-related.
Chronic inflammation is believed to play a notable role in 15% of cancer cases globally and it is accepted that a systemic inflammatory activation is an important player in carcinogenesis and progression60. However, the underlying mechanisms explaining how NLR influences oncological outcomes are yet to be explored7. In the clinical setting, systemic inflammation is primarily reflected and quantified by changes in blood parameters and can be determined by the counts of various cell components of the peripheral blood (neutrophils, lymphocytes, monocytes and platelets) using standard thresholds61. Previous studies have already shown that the association between neutrophils and circulating tumor cells (CTCs) drives cell cycle progression to confer proliferative advantage of CTC clusters, leading to faster metastasis development and enhanced metastatic potential of CTC clusters62.
Local and systemic inflammation is often involved in the initial carcinogenesis, cell proliferation, angiogenesis, and metastasis or progression of malignant tumors6. Quail et al. have linked neutrophilia to tumor-derived granulocyte colony-stimulating factor (GCSF) which at the same time accelerates tumor development63. Other studies depicted that neutrophils itself promote the survival and proliferation of malignant cells by secreting pro-inflammation mediators, such as tumor necrosis factor alpha (TNFa), interleukin (IL) 1, IL 6 and vascular endothelial growth factor64. Furthermore, a meta-analysis on NLR in solid tumors in general also demonstrated that high NLR is associated with poor survival in many malignancies, showing a particularly pronounced effect in metastatic advanced disease8. Park et al. also found that an elevated NLR is associated with a poor lymphocyte-mediated cytotoxicity against tumor cells characterized by a lower density of tumor-infiltrating lymphocytes (CD3+ and CD8+ T cells) in individuals with colorectal cancer65.
Escape from immune surveillance is considered to be a key characteristic of tumorigenesis and cancer progression. Novel treatment modalities, e.g. immune checkpoint inhibitors (ICIs), tumor vaccines and chimeric antigen receptor (CAR) T-cells are currently under investigation and suggested to have high potential to improve treatment66. However, in contrast to other solid tumors, the response rates to immunotherapy have not shown satisfying results which may be attributed to the spatial heterogeneity in CCA per se. In fact, there is a lack of reliable prognostic biomarkers and risk-assessment tools which would be suitable to predict the future response to these therapies. This is also considered a main obstacle in the use of immunotherapies in CCA patients. Katayama et al. studied NLR in 81 patients diagnosed with non-small cell lung cancer (NSCLC) who received atezolizumab as monotherapy and observed that patients with high NLR at baseline exhibited shorter progression-free survival and OS compared to those with a low NLR67. Li et al. reported that patients receiving ICIs for metastatic disease with NLR < 5 showed significantly longer OS68. In addition, Ota et al. studied the data of 98 patients who received nivolumab and found that poor prognostic factors of OS were pretreatment NLR of > 3 and NLR difference of > 2 over 60 days before and after receiving nivolumab. Those individuals with NLR difference > 2 displayed a longer median OS69. Hence, NLR holds promise to predict treatment response to ICIs in CCA as well.
The pure prognostic value of NLR was frequently investigated in other tumor entities. Yang et al. conducted a meta-analysis based 1804 pancreatic cancer patients and showed that high NLR was linked to reduced OS in individuals treated by chemotherapy or surgical resection. Furthermore, a high NLR was associated to tumor metastasis, poor tumor differentiation, poor performance status and elevated carbohydrate antigen 19–956. Moreover, NLR indicated reduced OS and DFS in breast cancer patients, with its prognostic value being retained across different clinicopathologic parameters such disease stage and subtypes70. In patients with esophageal cancer, a higher pretreatment NLR was linked to shorter survival as well as deeper tumor invasion and the presence of lymph node metastases53. Surprisingly, ethnicity had also an impact on certain studies. For example, Gu et al. discovered that NLR has consistent prognostic value in metastatic castration-resistant prostate cancer patients and predicts poor PFS/RFS in Asian, but not in Caucasian individuals71. In colon cancer patients undergoing a variety of treatments such as resection of the primary tumor, palliative chemotherapy and resection or ablation for liver metastases, higher preoperative NLR was prognostic for a lower survival rate72. Given these reports in other solid tumors, our results were in line with previous results and support a general role NLR as prognostic in solid malignancies.
As all meta-analyses with limited available studies, the analysis has certainly limitations due to the lack of high-level evidence:
The study comprised a variety of methodologies and, most importantly, different NLR cutoff levels.
Further, the included studies were retrospective in nature and therefore have an inherent potential of selection bias. As several studies did not explicitly report HRs and CIs, these variables were extrapolated from the Kaplan–Meier curves in some papers27,28,36,38,46,48.
A detailed investigation of the association between NLR and tumor clinicopathological characteristics was not feasible as the published data were unfortunately not detailed enough. This also accounts for the different types of cholangiocarcinoma as some studies include both ICCA and ECCA in a unified analysis as well as the distinct molecular subtypes of CCA.
Future research should therefore focus on the role of NLR in different subtypes and the identification of a uniform NLR cut-off to facilitate the implantation into clinical management of patients. Despite these obvious limitations, the present meta-analysis has also inherent strengths:
Representative data set especially for patients undergoing surgical treatment.
Detailed sub-analysis for study sample size, age, NLR cut-off value, geographical region, tumor subtypes and different types.
The inclusion of a sensitivity analysis indicating that no single study is responsible for the overall significant effect of NLR on OS and DFS.
Conclusions
Considering the aforementioned limitations and the limited available sample size, this study indicates a notable likelihood of NLR to be prognostic for reduced OS and DFS. Elevated NLR before treatment might therefore serve as biomarker for reduced oncological outcome (OS and DFS) in CCA patients. As patients undergoing surgery for CCA display high rates of perioperative morbidity and mortality, preoperative patient selection is fundamental to balance surgical risk with oncological benefits. Here, NLR provides additional information for treatment selection and risk stratification.
Supplementary Information
Abbreviations
- BTC
Biliary tree cancers
- CAR
Chimeric antigen receptor
- CCA
Cholangiocarcinoma
- CI
Confidence interval
- CRP
C-reactive protein
- CTCs
Circulating tumor cells
- DCCA
Distal cholangiocarcinoma
- DFS
Disease-free survival
- ECCA
Extrahepatic cholangiocarcinoma
- GCSF
Granulocyte colony-stimulating factor
- GPS
Glasgow prognostic score
- HR
Hazard ratio
- ICC
Intrahepatic cholangiocarcinoma
- ICIs
Immune checkpoint inhibitors
- GCSF
Granulocyte colony-stimulating factor
- NLR
Neutrophil-to-lymphocyte ratio
- NR
No reported
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PCCA
Perihilar cholangiocarcinoma
- PROSPERO
Prospective register of systematic reviews
- Ref
Reference
Author contributions
Conceptualization, D.L. and J.B.; methodology, D.L., U.P.N., and J.B.; formal analysis, D.L., Z.C., L.R.H., T.F.U., and J.B.; investigation, D.L., E.D., M.d.D., S.A.L., and J.B.; resources, U.P.N.; data curation, Z.C., S.A.L., and J.B.; writing—original draft preparation, D.L., L.R.H., E.D., M.d.D., and J.B.; writing—review and editing, Z.C., S.A.L., T.F.U., and U.P.N.; supervision, U.P.N.; project administration, UPN. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by DFG (German Research Foundation)—Project-ID 403224013—SFB 1382.
Data availability
Available upon request. JB and UPN had full access to the data and act both as guarantor for the data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-16727-w.
References
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat. Rev. Gastroenterol. Hepatol. 2016;13(5):261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 3.Lurje G, Bednarsch J, Czigany Z, Lurje I, Schlebusch IK, Boecker J, et al. The prognostic role of lymphovascular invasion and lymph node metastasis in perihilar and intrahepatic cholangiocarcinoma. Eur. J. Surg. Oncol. 2019;45(8):1468–1478. doi: 10.1016/j.ejso.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Bednarsch J, Czigany Z, Heij LR, Liu D, den Dulk M, Wiltberger G, et al. Compelling long-term results for liver resection in early cholangiocarcinoma. J Clin Med. 2021;10(13):2939. doi: 10.3390/jcm10132959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17(9):557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D, Czigany Z, Heij LR, Bouwense SAW, van Dam R, Lang SA, et al. The value of platelet-to-lymphocyte ratio as a prognostic marker in cholangiocarcinoma: A systematic review and meta-analysis. Cancers. 2022;14(2):438. doi: 10.3390/cancers14020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014;106(6):124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 9.McMillan DC. The systemic inflammation-based Glasgow prognostic score: A decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Amygdalos I, Bednarsch J, Meister FA, Erren D, Mantas A, Strnad P, et al. Clinical value and limitations of the preoperative C-reactive-protein-to-albumin ratio in predicting post-operative morbidity and mortality after deceased-donor liver transplantation: A retrospective single-centre study. Transpl. Int. 2021;34(8):1468–1480. doi: 10.1111/tri.13957. [DOI] [PubMed] [Google Scholar]
- 11.Hakeem AR. Does the extent of lymphadenectomy, number of lymph nodes, positive lymph node ratio and neutrophil–lymphocyte ratio impact surgical outcome of perihilar cholangiocarcinoma? Springer; 2014. [DOI] [PubMed] [Google Scholar]
- 12.Hamed MO, Roberts KJ, Smith AM, Stiff GM. Elevated pre-operative neutrophil to lymphocyte ratio predicts disease free survival following pancreatic resection for periampullary carcinomas. Pancreatology. 2013;13(5):534–538. doi: 10.1016/j.pan.2013.07.283. [DOI] [PubMed] [Google Scholar]
- 13.Tan DW, Fu Y, Su Q, Guan MJ, Kong P, Wang SQ, et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: A meta-analysis. Sci. Rep. 2016;6:33789. doi: 10.1038/srep33789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012;10:1–10. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GSB, Oconnell D, Peterson J, Welch V, Losos M. The Newcastleottawa Scale (NOS) for Assessing the Quality if Non-randomized Studies in Meta-Analyses. University of Ottawa; 2022. [Google Scholar]
- 17.Zhao JP, Chen Y, Wang JJ, Wang J, Wang Y, Chai SS, et al. Preoperative risk grade predicts the long-term prognosis of intrahepatic cholangiocarcinoma: A retrospective cohort analysis. Bmc Surg. 2021;21(1):10. doi: 10.1186/s12893-020-00954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma B, Meng H, Shen A, Ma Y, Zhao D, Liu G, et al. Prognostic value of inflammatory and tumour markers in small-duct subtype intrahepatic cholangiocarcinoma after curative-intent resection. Gastroenterol. Res. Pract. 2021;2021:6616062. doi: 10.1155/2021/6616062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZY, Zhou YF, Hu K, Huang Y. Investigating effects of preoperative inflammatory biomarkers on predicting survival outcomes of intrahepatic cholangiocarcinoma after curative resection. World J. Surg. Oncol. 2020;18(1):2053. doi: 10.1186/s12957-020-02053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsilimigras DI, Moris D, Mehta R, Paredes AZ, Sahara K, Guglielmi A, et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: An international multi-institutional analysis. HPB. 2020;22(12):1667–1674. doi: 10.1016/j.hpb.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Ohira M, Yoshizumi T, Yugawa K, Kosai-Fujimoto Y, Inokuchi S, Motomura T, et al. Association of inflammatory biomarkers with long-term outcomes after curative surgery for mass-forming intrahepatic cholangiocarcinoma. Surg. Today. 2020;50(4):379–388. doi: 10.1007/s00595-019-01905-7. [DOI] [PubMed] [Google Scholar]
- 22.Ji F, Kang Q, Wang L, Liu L, Ke Y, Zhu Y, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio with distal cholangiocarcinoma patients. Medicine. 2020;99(43):e22827. doi: 10.1097/MD.0000000000022827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh G, Ryu JK, Chun JW, Kim JS, Park N, Cho IR, et al. High platelet-to-lymphocyte ratio is associated with poor prognosis in patients with unresectable intrahepatic cholangiocarcinoma receiving gemcitabine plus cisplatin. BMC Cancer. 2020;20(1):7390. doi: 10.1186/s12885-020-07390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippi L, Di Costanzo GG, Tortora R, Pelle G, Saltarelli A, Marino Marsilia G, et al. Prognostic value of neutrophil-to-lymphocyte ratio and its correlation with fluorine-18-fluorodeoxyglucose metabolic parameters in intrahepatic cholangiocarcinoma submitted to 90Y-radioembolization. Nucl. Med. Commun. 2020;41(1):78–86. doi: 10.1097/MNM.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Shi SM, Yang H, Yang LX, Wang Z, Li XD, et al. Systemic inflammation score predicts survival in patients with intrahepatic cholangiocarcinoma undergoing curative resection. J. Cancer. 2019;10(2):494–503. doi: 10.7150/jca.26890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu YH, Ren FG, Chai YC, Xue Z, Shen C, Zhang XF, et al. Prognostic value of inflammation-based indexes for intrahepatic cholangiocarcinoma following curative resection. Oncol. Lett. 2019;17(1):165–174. doi: 10.3892/ol.2018.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sellers CM, Uhlig J, Ludwig JM, Stein SM, Kim HS. Inflammatory markers in intrahepatic cholangiocarcinoma: Effects of advanced liver disease. Cancer Med. 2019;8(13):5916–5929. doi: 10.1002/cam4.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Fang T, Zhu M, Xu X, Zhang J, Zheng S, et al. Comparative performance of inflammation-based prognostic scores in patients operated for intrahepatic cholangiocarcinoma. Cancer Manag. Res. 2019;11:9107–9119. doi: 10.2147/CMAR.S198959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu HJ, Jin YW, Zhou RX, Ma WJ, Yang Q, Wang JK, et al. Clinical value of inflammation-based prognostic scores to predict the resectability of hyperbilirubinemia patients with potentially resectable hilar cholangiocarcinoma. J. Gastrointest. Surg. 2019;23(3):510–517. doi: 10.1007/s11605-018-3892-9. [DOI] [PubMed] [Google Scholar]
- 30.Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, Ogata Y. Association of preoperative platelet-to-lymphocyte ratio with poor outcome in patients with distal cholangiocarcinoma. Oncology. 2019;96(6):290–298. doi: 10.1159/000499050. [DOI] [PubMed] [Google Scholar]
- 31.Buettner S, Spolverato G, Kimbrough CW, Alexandrescu S, Marques HP, Lamelas J, et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery. 2018;164(3):411–418. doi: 10.1016/j.surg.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Yoh T, Seo S, Hatano E, Taura K, Fuji H, Ikeno Y, et al. A novel biomarker-based preoperative prognostic grading system for predicting survival after surgery for intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2017;24(5):1351–1357. doi: 10.1245/s10434-016-5708-z. [DOI] [PubMed] [Google Scholar]
- 33.Omichi K, Cloyd JM, Yamashita S, Tzeng CWD, Conrad C, Chun YS, et al. Neutrophil-to-lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162(4):752–765. doi: 10.1016/j.surg.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Nam K, Hwang DW, Shim JH, Song TJ, Lee SS, Seo DW, et al. Novel preoperative nomogram for prediction of futile resection in patients undergoing exploration for potentially resectable intrahepatic cholangiocarcinoma. Sci. Rep. 2017;7:42954. doi: 10.1038/srep42954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitano Y, Yamashita YI, Yamamura K, Arima K, Kaida T, Miyata T, et al. Effects of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios on survival in patients with extrahepatic cholangiocarcinoma. Anticancer Res. 2017;37(6):3229–3237. doi: 10.21873/anticanres.11685. [DOI] [PubMed] [Google Scholar]
- 36.Cho H, Yoo C, Kim KP, Chang HM, Ryoo BY. Prognostic implication of inflammation-based prognostic scores in patients with intrahepatic cholangiocarcinoma (iCCA) treated with first-line Gemcitabine plus Cisplatin (GEMCIS) Ann. Oncol. 2017;28:1–10. doi: 10.1093/annonc/mdx141. [DOI] [Google Scholar]
- 37.Okuno M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, et al. Appraisal of inflammation-based prognostic scores in patients with unresectable perihilar cholangiocarcinoma. J. Hepato-Bil-Pan. Sci. 2016;23(10):636–642. doi: 10.1002/jhbp.386. [DOI] [PubMed] [Google Scholar]
- 38.Okuno M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, et al. Evaluation of inflammation-based prognostic scores in patients undergoing hepatobiliary resection for perihilar cholangiocarcinoma. J. Gastroenterol. 2016;51(2):153–161. doi: 10.1007/s00535-015-1103-y. [DOI] [PubMed] [Google Scholar]
- 39.Lin GH, Liu YC, Li SH, Mao YZ, Wang J, Shuang ZY, et al. Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget. 2016;7(32):50963–50971. doi: 10.18632/oncotarget.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BS, Lee SH, Son JH, Jang DK, Chung KH, Lee YS, et al. Neutrophil-lymphocyte ratio predicts survival in patients with advanced cholangiocarcinoma on chemotherapy. Cancer Immunol Immunother. 2016;65(2):141–150. doi: 10.1007/s00262-015-1780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7(47):76604–76612. doi: 10.18632/oncotarget.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beal EW, Wei L, Ethun CG, Blacks SM, Dillhoff M, Salem A, et al. Elevated NLR in gallbladder cancer and cholangiocarcinoma: Making bad cancers even worse: Results from the US Extrahepatic Biliary Malignancy Consortium. HPB. 2016;18(11):950–957. doi: 10.1016/j.hpb.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q, Yang LX, Li XD, Yin D, Shi SM, Chen EB, et al. The elevated preoperative neutrophil-to-lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumor Biol. 2015;36(7):5283–5289. doi: 10.1007/s13277-015-3188-6. [DOI] [PubMed] [Google Scholar]
- 44.Chen Q, Dai Z, Yin D, Yang LX, Wang Z, Xiao YS, et al. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine. 2015;94(13):e574. doi: 10.1097/MD.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNamara MG, Templeton AJ, Maganti M, Walter T, Horgan AM, McKeever L, et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur. J. Cancer. 2014;50(9):1581–1589. doi: 10.1016/j.ejca.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Iwaku A, Kinoshita A, Onoda H, Fushiya N, Nishino H, Matsushima M, et al. The Glasgow Prognostic Score accurately predicts survival in patients with biliary tract cancer not indicated for surgical resection. Med. Oncol. 2014;31(1):787. doi: 10.1007/s12032-013-0787-1. [DOI] [PubMed] [Google Scholar]
- 47.Dumitrascu T, Chirita D, Ionescu M, Popescu I. Resection for hilar cholangiocarcinoma: Analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J. Gastrointest. Surg. 2013;17(5):913–924. doi: 10.1007/s11605-013-2144-2. [DOI] [PubMed] [Google Scholar]
- 48.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2008;97(6):513–518. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 49.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer: A Glasgow Inflammation Outcome Study. Eur. J. Cancer. 2011;47(17):2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Zheng J, Cai JY, Li H, Zeng KN, He LY, Fu HY, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: A meta-analysis and systematic review. Cell Physiol. Biochem. 2017;44(3):967–981. doi: 10.1159/000485396. [DOI] [PubMed] [Google Scholar]
- 52.Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 2019;8(9):4135–4148. doi: 10.1002/cam4.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. Ann. Surg. Oncol. 2016;23(2):646–654. doi: 10.1245/s10434-015-4869-5. [DOI] [PubMed] [Google Scholar]
- 54.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int. J. Cancer. 2014;134(10):2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 55.Yin Y, Wang J, Wang X, Gu L, Pei H, Kuai S, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics. 2015;70(7):524–530. doi: 10.6061/clinics/2015(07)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2015;21(9):2807–2815. doi: 10.3748/wjg.v21.i9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 58.Bednarsch J, Tan X, Czigany Z, Liu D, Lang SA, Sivakumar S, et al. The presence of small nerve fibers in the tumor microenvironment as predictive biomarker of oncological outcome following partial hepatectomy for intrahepatic cholangiocarcinoma. Cancers. 2021;13(15):3661. doi: 10.3390/cancers13153661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bednarsch J, Kather J, Tan X, Sivakumar S, Cacchi C, Wiltberger G, et al. Nerve fibers in the tumor microenvironment as a novel biomarker for oncological outcome in patients undergoing surgery for perihilar cholangiocarcinoma. Liver Cancer. 2021;10(3):260–274. doi: 10.1159/000515303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulich TR, del Castillo J, Keys M, Granger GA, Ni RX. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha-induced changes in circulating numbers of neutrophils and lymphocytes. J. Immunol. 1987;139(10):3406–3415. [PubMed] [Google Scholar]
- 61.Riesco A. Five-year cancer cure: Relation to total amount of peripheral lymphocytes and neutrophils. Cancer. 1970;25(1):135–140. doi: 10.1002/1097-0142(197001)25:1<135::AID-CNCR2820250120>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 62.Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 63.Schulz M, Salamero-Boix A, Niesel K, Alekseeva T, Sevenich L. Microenvironmental regulation of tumor progression and therapeutic response in brain metastasis. Front. Immunol. 2019;10:1713. doi: 10.3389/fimmu.2019.01713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Invest. 2010;120(4):1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park JH, van Wyk H, Roxburgh CSD, Horgan PG, Edwards J, McMillan DC. Tumour invasiveness, the local and systemic environment and the basis of staging systems in colorectal cancer. Br. J. Cancer. 2017;116(11):1444–1450. doi: 10.1038/bjc.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saeed A, Park R, Al-Jumayli M, Al-Rajabi R, Sun W. Biologics, immunotherapy, and future directions in the treatment of advanced cholangiocarcinoma. Clin. Colorectal Cancer. 2019;18(2):81–90. doi: 10.1016/j.clcc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 67.Katayama Y, Yamada T, Chihara Y, Tanaka S, Tanimura K, Okura N, et al. Significance of inflammatory indexes in atezolizumab monotherapy outcomes in previously treated non-small-cell lung cancer patients. Sci. Rep. 2020;10(1):73. doi: 10.1038/s41598-019-56704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li MJ, Spakowicz D, Burkart J, Patel S, Husain M, He K, et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J. Cancer Res. Clin. 2019;145(10):2541–2546. doi: 10.1007/s00432-019-02982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ota Y, Takahari D, Suzuki T, Osumi H, Nakayama I, Oki A, et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother. Pharmacol. 2020;85(2):265–272. doi: 10.1007/s00280-019-04023-w. [DOI] [PubMed] [Google Scholar]
- 70.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu X, Gao X, Li X, Qi X, Ma M, Qin S, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: Evidence from 16,266 patients. Sci. Rep. 2016;6:22089. doi: 10.1038/srep22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malietzis G, Giacometti M, Kennedy RH, Athanasiou T, Aziz O, Jenkins JT. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: a systematic review and meta-analysis. Ann. Surg. Oncol. 2014;21(12):3938–3946. doi: 10.1245/s10434-014-3815-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon request. JB and UPN had full access to the data and act both as guarantor for the data.