Abstract
Extracellular vesicles (EVs) are often elevated in obesity and may modulate disease risk. Although acute exercise reduces fasting EVs in adults with obesity, no data exist on insulin-mediated EV responses. This study evaluated the effects of exercise on EV responses to insulin in relation to vascular function. Ten (5M/5F) sedentary adults with obesity (34.3 ± 3.7 kg/m2) completed an evening control and acute exercise condition (70% VO2max to expend 400 kcals). Following an overnight fast, participants underwent a 2-hr euglycemic-hyperinsulinemic clamp (90 mg/dl; 40 mU/m2/min) to determine metabolic insulin sensitivity (M-value), phenotypes of medium- to large-sized EVs, and aortic waveform measures. Endothelial (CD105+, CD41-/CD31+), leukocyte (CD45+), platelet (CD41+, CD41+/31+), and tetraspanin-derived EVs (TX+), as well as platelet endothelial cell adhesion molecule (CD31+) were determined before and after the clamp using high resolution spectral flow cytometry. Although exercise did not alter fasting hemodynamics, it lowered augmentation index (AIx75, P=0.024) and increased the M-value (P=0.042). Further, exercise decreased all fasting EVs (P<0.01) and decreased insulin-stimulated TX+ (P=0.060), CD31+ (P=0.060), and CD41-/31+ (P=0.045) compared to rest. Interestingly, greater insulin-stimulated decreases in CD41-/31+ were associated with reduced AIx75 during the clamp (r=0.62, P=0.059), while insulin-stimulated decreases in CD41+ (r=−0.68, P=0.031), CD41+/31+ (r=−0.69, P=0.262), TX+ (r=−0.66, P=0.037), and CD31+ (r=−0.69, P=0.028) correlated with M-Value following exercise. Thus, acute exercise may decreases fasting and insulin-stimulated medium- to large-size EVs in conjunction with improved M-value and AIx75. More research is needed to understand effects of exercise on EVs in the regulation of glucose homeostasis and vascular function.
Keywords: aerobic exercise, insulin sensitivity, extracellular vesicles, arterial stiffness
Graphical Abstract

A single bout of exercise raises metabolic insulin sensitivity and reduces aortic waveform hemodynamics in relation to extracellular vesicles (EVs).
INTRODUCTION
Excess adiposity is associated with an increased risk of developing type 2 diabetes (T2D) and cardiovascular disease (CVD) (Wilson et al., 2002; Piché et al., 2020). While traditional factors, such as decreased insulin sensitivity and hypertension, are often considered key components of CVD development, these collectively predict only 40–50% of risk (Ridker et al., 2007). Recently, extracellular vesicles (EVs) have emerged as a novel mean of cell-to-cell communication and may contribute to T2D (Xiao et al., 2019) and CVD development (Chong et al., 2019). Indeed, EVs contain lipids, proteins, and genetic information (Zaborowski et al., 2015) that likely modify the phenotype and function of target cells (Tetta et al., 2013; Vechetti et al., 2021). Interestingly, EV cargo is dependent on cellular origin as well as physiological conditions at the time of EV packaging and secretion (Hutcheson & Aikawa, 2018). In fact, states of obesity and insulin resistance are associated with total EV concentrations (Kobayashi et al., 2018; Amosse et al., 2018), which may contribute to elevated systolic and diastolic blood pressure (Eichner et al., 2018b; Kobayashi et al., 2018). Furthermore, recent reviews have highlighted the potential role of EVs in hypertension and vascular dysfunction (La Salvia et al., 2020a; Liu et al., 2021).
Exercise, with or without weight loss, reduces cardiometabolic disease risk, partially via increasing insulin sensitivity and decreasing blood pressure (Bird & Hawley, 2017; Nystoriak & Bhatnagar, 2018). Indeed, acute aerobic exercise increases metabolic (Black et al., 2005; Newsom et al., 2013) and vascular insulin sensitivity (Heiston et al., 2021b) as well as decreases augmentation index, a measure of aortic waveforms and/or surrogacy for arterial stiffness (Hanssen et al., 2015). Although the biological mechanisms by which exercise confers these benefits are still being elucidated, EVs may play a mechanistic role. In fact, one bout of moderate-intensity exercise (60% VO2peak) decreases fasting platelet-derived EV concentrations ~24 hr later in adults with and without obesity (Rigamonti et al., 2020). Similarly, 12 d of high-intensity aerobic exercise lowers fasting CD105+, an endothelial-derived EV (Eichner et al., 2020). However, data regarding exercise effects on post-prandial EVs are limited (Jenkins et al., 2011; Eichner et al., 2018a), despite the important role of post-prandial metabolism on CVD risk (Tushuizen et al., 2005) and all-cause mortality (Cavalot et al., 2011).
Recent work by our group (Eichner et al., 2019b), using a comparable EV detection technique as done in this study, demonstrated relationships between changes in both insulin and augmentation index and EV concentrations during an oral glucose tolerance test (OGTT) in adults at risk for type 2 diabetes. It is difficult though to delineate whether decreases in EVs were driven by insulin concentrations or altered glucose levels observed during the OGTT. In addition, we recently demonstrated that one bout of exercise increases insulin-stimulated brachial diameter and microvascular blood flow (Heiston et al., 2021b). Whether a single bout of exercise affects EVs in a corresponding fashion to aortic waveform responses is unknown. Thus, the purpose of the current study was to examine the effect of a single bout of exercise on fasting and insulin-stimulated EVs. We secondarily sought to determine if changes in insulin-mediated EVs associated with improved metabolic insulin sensitivity and/or aortic waveform in adults with obesity.
METHODS
Participants.
A subgroup of ten individuals (5M/5F; 49.5 ± 7.7 yr; 34.3 ± 3.7 kg/m2) who were part of a larger study designed to understand the effects of acute exercise on vascular insulin sensitivity (Heiston et al., 2021b) underwent additional testing for EV classification. Individuals had no history of cardiovascular, renal, or hepatic disease, were non-smoking, physically inactive (<60 min/wk), and not on medications that affect glucose metabolism (e.g., biguanides, insulin, etc.). All individuals provided written and verbal informed consent as approved by the University of Virginia Institutional Review Board (IRB-HSR: #19060). This study conformed to the standards set by the Declaration of Helsinki.
Metabolic Control.
Resting metabolic rate was determined after an overnight fast using indirect calorimetry. The last 5 min of steady state data were averaged and multiplied by an activity factor of 1.2 to estimate daily caloric needs. A day worth of food and snacks were provided before both testing conditions and individuals were instructed to consume only the supplied food and water, as well as abstain from caffeine, medication, and alcohol 24 hr prior to testing.
Acute Exercise and Control.
Prior to each condition, participants completed an incremental peak oxygen consumption test (VO2peak) on a treadmill using indirect calorimetry (Carefusion, Vmax CART, Yorba Linda, CA, USA) to determine the appropriate exercise prescription. Briefly, individuals completed a control and acute exercise condition in a counterbalanced order between 1600–1830 hours, with at least 1 week between tests. Females with a self-reported regular menstruation cycle completed the conditions ~1 month apart in the follicular phase. The exercise condition consisted of a treadmill walking protocol at moderate intensity (70% VO2peak) to expend 400 kcals, as measured via indirect calorimetry, while the control condition involved resting in a seated position for a similar duration. Thirty minutes following both conditions, participants were provided a standardized dinner (~25% of total daily energy expenditure).
Euglycemic Hyperinsulinemic Clamp.
Participants arrived at the Clinical Research Unit ~16 hr following both conditions. A constant infusion (40 uU/m2/min) of insulin (Humulin R U-100; Eli Lilly and company, Indianapolis, IN, USA) and a variable rate of glucose (20% dextrose; McKesson Corporation, Irving, TX, USA) were infused (PHD 22/200 Syringe Pumps, Harvard Apparatus, Holliston, MA, USA) over 120 min. Glucose was measured every 5 min using the glucose-oxidase method (YSI Instruments 2300, Yellow Springs, OH, USA) to maintain a plasma glucose of 90 mg/dl. Metabolic insulin sensitivity was determined using the mean glucose metabolized infusion rate (M-Value) over the last 30 min of the clamp. Insulin was measured at 0, 90, 105, and 120 min of the clamp and stored at −80°C until later analysis via enzyme-linked immunosorbent assay (ALPCO, Salem, NH, USA). Aortic waveform and EVs were also measured at fasting and 120 min of the clamp.
Pulse Waveform Analysis.
The SphygmoCor XCEL system (AtCor Medical, Itasca, IL, USA) was used to characterize peripheral and central hemodynamics as well as the aortic waveform, as described previously (Heiston et al., 2021a). Three readings occurred over 10 min and were averaged to determine heart rate (HR), peripheral systolic (pSBP) and diastolic blood pressure (pDBP), central systolic (cSP), diastolic (cDP), and augmentation pressure (AP), peripheral (pPP) and central pulse pressure (cPP), as well as augmentation index corrected to a HR of 75 bpm (AIx75). Central forward pressure (Pf), backward pressure (Pb), and reflection magnitude (RM) were also characterized via deconvolution analysis. Pulse pressure amplification (PPA) was calculated as a ratio (pPP/cPP) (Wykretowicz et al., 2012).
EV Preparation.
Blood was collected in citrate vacutainers and underwent differential centrifugation at 5000 g for 15 min (Sorvall RC 5B Plus Centrifuge: Rotor SS-34 Fixed Angle Rotor) (Eichner et al., 2018b). The generated platelet poor plasma was transferred to 1.5 ml microcentrifuge tubes (Axygen) and frozen (−80°C) until further analysis. After thawing, 0.2 mL of plasma was used to enrich EVs by centrifugation at 17,000 g for 10 min at room temperature (centrifuge: 524/5424 R-Rotor FA-45-24-11, Eppendorf).
EV Characterization.
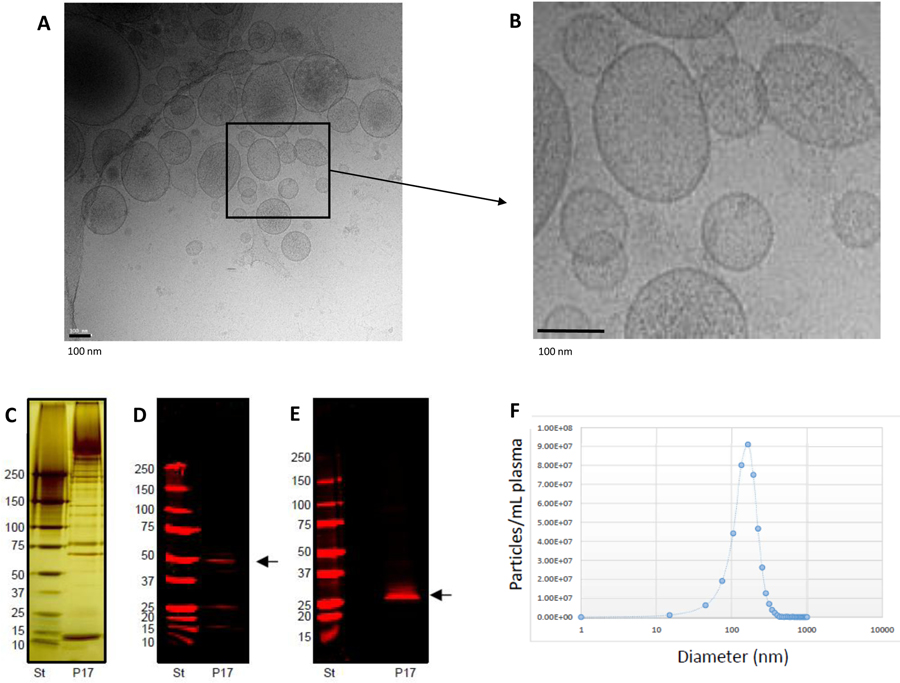
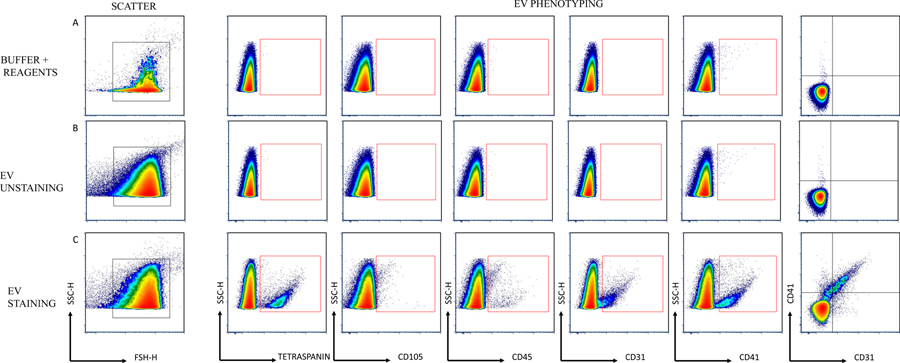
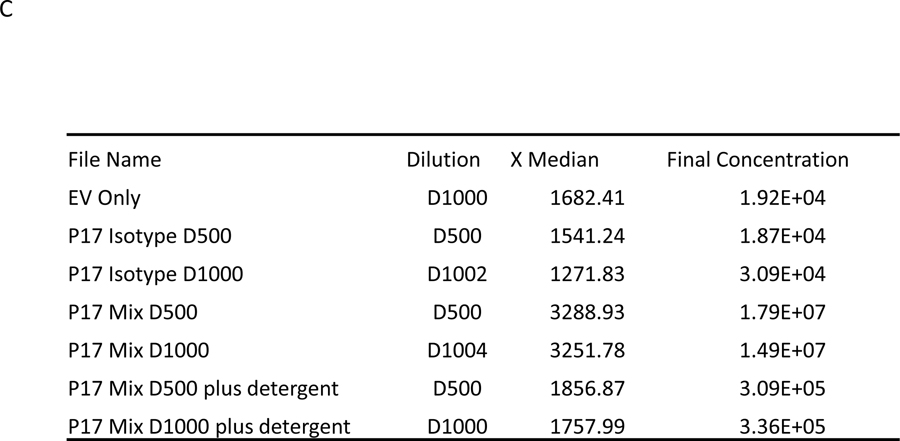
Basic EV characterization of the enriched EV samples was performed using cryo-electron microscopy for EV morphology analysis, nanoparticle tracking analysis for EV count, and Western blotting for detection of EV-related proteins (as shown in Figure 1) and described previously (Eichner et al., 2019b; La Salvia et al., 2020b) following EV guidelines (Théry et al., 2018). EV-targeted phenotyping using Full Spectrum Profiling (FSP™) (Aurora from Cytek®) was performed to detect, count, and size total particle count, endothelial- (CD105+, CD41−/CD31+), leukocyte- (CD45+), platelet- (CD41+), and tetraspanin-derived EVs (TX+, CD9/CD81/CD63 cocktail), as well as platelet endothelial cell adhesion molecule- (CD31+) derived EVs. Before flow acquisition, each sample was diluted in different volumes (D:500; D:1000; D:2000) according to MISEV 2018 guidelines (Minimal information for studies of EVs) to verify single particle definition.30 (See also Table 4 providing the “MiFlowCyt EV checklist”, which is recommended by the EV Flow cytometry working group of ISEV31). Several controls were used for flow cytometry (Figure 2) including testing Antibody cocktail in on the buffer solution, EVs without labeling, EVs as single stained and cocktail, isotype control for each marker, and dilution and detergent experiments, all of which are shown in Figure 1 and summarized in Figure 5.
Figure 1.
This figure summarizes the basic characterization of extracellular vesicles enriched for analysis in this study from platelet poor plasma with differential centrifugation. Figure 1A provides cryo-electron microscopy images in low (A) and high resolution (B). EVs of various sizes and densities are present. The higher magnification shows also the bi-lipid layers of EVs studied here. Figure C to E summarize results of Western blotting analysis of enriched EV pellets with differential centrifugation. The Figure C shows a silver-stain of protein content of the EV pellet (here enriched with differential centrifugation (P17 pellet)) and a standard (ST). EV antigens such as TSG101 (Figure D) and CD9 (Figure E) are present in this P17 pellet. Figure F shows an examples of a particle detection with Nanosight tracking analysis (NTA) using “Zetaview by Particle metrix”. The mean size of EVs in this example is 174.2nm (median 161.8nm and mode 171.8nm) and the majority of EVs is less than 200nm in size. For all other samples size and phenotype of single EVs are detected with flow cytometry.
Table 4.
MIFlowCyt-EV check list.
| Framework Criteria | What to report | Please complete each criterion | |
|---|---|---|---|
| 1.1 Preanalytical variables conforming to MISEV guidelines. | Preanalytical variables relating to EV sample including source, collection, isolation, storage, and any others relevant and available in the performed study. | Plasma EV sample were enriched by centrifugation | |
| 1.2 Experimental design according to MIFlowCyt guidelines. | EV-FC manuscripts should provide a brief description of the experimental aim, keywords, and variables for the performed FC experiment(s) using MIFlowCyt checklist criteria: 1.1, 1.2, and 1.3, respectively. Template found at www.evflowcytometry.org. | Detectable EV recovery was measured using flow cytometry | |
| 2.1 Sample staining details | State any steps relating to the staining of samples. Along with the method used for staining, provide relevant reagent descriptions as listed in MIFlowCyt guidelines (Section 2.4 Fluorescence Reagent(s) Descriptions). | Reported in the manuscript | 0.25 μL of anti CD9 conjugated with FITC (BD, clone HI9a Cat number 312104), anti CD63 conjugated with FITC (BD, clone H5C6 Cat number 353006), anti CD81 conjugated with FITC (BD, clone 5A6 Cat number 349504))); 2 μL anti CD45 conjugated with PE-Dazzle 594 (BD, clone HI30 Cat number 304052); 1 μL of anti CD105 conjugated with PE (BD, clone 43A3 Cat number 323206), 1 μL of anti CD31 conjugated with AF647 (BD, clone WM59 Cat number 303112) and 1 μL of anti CD41 conjugated with Pacific Blue(BD, Clone HIP8 Cat number 303714). All the antibodies were purchased from Biolegend including isotype controls diluted and processed likewise as follow. The antibody cocktails were diluted to 20 μL with HEPES Buffer (10 mM HEPES pH7.4 and 0.15 M NaCl) filtered through a 0.1 μm syringe filter (Minisart PES syringe filter code 16553K, Sartorious) and centrifuge for 1 hour at 21.130g (max speed of the centrifuge: 524/5424 R-Rotor FA-45-24-11, Eppendorf). EV pellets P17 were resolubilized in 30 μL of HEPES buffer and stained with 20 μL of centrifuge antibody mix over night at RT and in dark condition. |
| 2.2 Sample washing details | State any steps relating to the washing of samples. | Not applicable | |
| 2.3 Sample dilution details | All methods and steps relating to sample dilution. | Reported in the manuscript, see Figure 5. | sample was diluted with filtered HEPES buffer in different volume (D:500; D1000; D:2000) |
| 3.1 Buffer alone controls. | State whether a buffer-only control was analyzed at the same settings and during the same experiment as the samples of interest. If utilized it is recommended that all samples be recorded for a consistent set period of time e.g. 5 minutes, rather than stopping analysis at a set recorded event count e.g. 100,000 events. This allows comparisons of total particle counts between controls and samples. | HEPES buffer control was included | |
| 3.2 Buffer with reagent controls. | State whether a buffer with reagent control was analyzed at the same settings, same concentrations, and during the same experiment as the samples of interest. If used state what the results were. | HEPES buffer plus antibodies mix was included. Number of events recorded was negligible and subtracted from the sample reading | |
| 3.3 Unstained controls. | State whether unstained control samples were analyzed at the same settings and during the same experiment as stained samples. If used, state what the results were, preferably in standard units. | HEPES buffer control was included | |
| 3.4 Isotype controls. | The use of isotype controls is applicable to immunofluorescence labelling only. State whether isotype controls were analyzed at the same settings and during the same experiment as stained samples. If utilized, state which antibody they are matched to, the concentration used, and what the results were (Section 4.2, 4.3, 4.4). Due to conjugation differences between manufacturers if should be stated if the isotype controls are from the same manufacturer as the matched antibodies. | Isotype controls were included. They were purchased from Biolegend and used at the same working concentration. Mouse IgG1, κ-FITC clone MOPC-21 cat number 981802; Mouse IgG1, κ-Pacific Blue clone MOPC-21 cat number 981812; Mouse IgG1, κ-AF647 clone MOPC-21 cat number 400130; Mouse IgG1, κ-PE Dazzle 594 clone MOPC-21 cat number 981814 and Mouse IgG1, κ-PE clone MOPC-21 cat number 400112 | |
| 3.5 Single-stained controls. | State whether single-stained controls were included. If used state whether the single-stained controls were recorded using the same settings, dilutions, and during the same experiment as stained samples and state what the results were, preferably in standard units (Section 4.2, 4.3, 4.4). | Single stain controls were included and used as reference controls for the unmixing. | |
| 3.6 Procedural controls. | State whether procedural controls were included. If used, state the procedure and if the procedural controls were acquired at the same settings and during the same experiment as stained samples. | The same healthy plasma sample control was used and process according to the protocol in each experiment to check inter day variability. | |
| 3.7 Serial dilutions. | State whether serial dilutions were performed on samples and note the dilution range and manner of testing. The fluorescence and/or scatter signal intensity would ideally be reported in standard units (see Section 4.3, 4.4) but arbitrary units can also be used. This data is best reported by plotting the recorded number events/concentration over a set period of time at different sample dilution. The median fluorescence intensity at each of the dilutions should also ideally be plotted on the same or a separate plot. | Samples were run at a serial dilution to establish the concentration that produced a single EV detection and this happened when abort rate was less than the 10 percent of the count rate. This parameter was used to dilute each sample to avoid swarming. See Figure 5. | |
| 3.8. Detergent treated EV-samples | State whether samples were detergent treated to assess lability. If utilized, state what detergent was used, the end concentration of the detergent, and what the results were of the lysis. | Ripa buffer was used to lyse the EV. The concentration dropped to less than 30 percent after incubation for 30 minutes at room temperature at the same dilution. See Figure 5. | |
| 4.1 Trigger Channel(s) and Threshold(s). | The trigger channel(s) and threshold(s) used for event detection. Preferably, the fluorescence calibration (Section 4.3) and/or scatter calibration (Section 4.4) should be used in order to report the trigger channel(s) and threshold(s) in standardized units. | EV analyses were carried out using a 405 SSC trigger and the threshold set at 500 arbitrary unit | |
| 4.2 Flow Rate / Volumetric quantification. | State if the flow rate was quantified/validated and if so, report the result and how they were obtained. | Samples were run at the lowest flow rate setting. Acquisition volume was measured using the internal flow rate sensor of the cytometer. | |
| 4.3 Fluorescence Calibration. | State whether fluorescence calibration was implemented, and if so, report the materials and methods used, catalogue numbers, lot numbers, and supplied reference units for the standards. Fluorescence parameters may be reported in standardized units of MESF, ERF, or ABC beads. The type of regression used, and the resulting scatter plot of arbitrary data vs standard data for the reference particles should be supplied. | MESF beads for FITC (Bangs Laboratory Catalogue Number 555A, Lot number 12690), PE (Bangs Laboratory Catalogue Number827A, Lot number 12845) and AF647 (Bangs Laboratory Catalogue Number 647A, Lot number 12929) were used. | |
| 4.4 Light Scatter Calibration. | State whether and how light scatter calibration was implemented. Light scatter parameters may be reported in standardized units of nm2, along with information required to reproduce the model. | Light scatter calibration was not performed due to no Light scatter parameters being used. | |
| 5.1 EV diameter/surface area/volume approximation. | State whether and how EV diameter, surface area, and/or volume has been calculated using FC measurements. | Not applicable | |
| 5.2 EV refractive index approximation. | State whether the EV refractive index has been approximated and how this was done. | Not applicable | |
| 5.3 EV epitope number approximation. | State whether EV epitope number has been approximated, and if so, how it was approximated. | Not applicable | |
| 6.1 Completion of MIFlowCyt checklist. | Complete MIFlowCyt checklist criteria 1 to 4 using the MIFlowCyt guidelines. Template found at www.evflowcytometry.org. | Complete | |
| 6.2 Calibrated channel detection range | If fluorescence or scatter calibration has been carried out, authors should state whether the upper and lower limits of a calibrated detection channel were calculated in standardized units. This can be done by converting the arbitrary unit scale to a calibrated scaled, as discussed in Section 4.3 and 4.4, and providing the highest unit on this scale and the lowest detectable unit above the unstained population. The lowest unit at which a population is deemed ‘positive’ can be determined a variety of ways, including reporting the 99th percentile measurement unit of the unstained population for fluorescence. The chosen method for determining at what unit an event was deemed positive should be clearly outlined. | Done | |
| 6.3 EV number/concentration. | State whether EV number/concentration has been reported. If calculated, it is preferable to report EV number/concentration in a standardized manner, stating the number/concentration between a set detection range. | Use of the volumetric methods. | |
| 6.4 EV brightness. | When applicable, state the method by which the brightness of EVs is reported in standardized units of scatter and/or fluorescence. | Not applicable | |
| 7.1. Sharing of data to a public repository. | Provide a link to the experimental data in a public data repository. | Uploaded in flowrepository.com | Repository ID: FR-FCM-Z4PZ |
Figure 2.
Manual gating strategy. Scatter was based on FSC-H/SSC-H properties. Arrows are used to visualize x and y across plots. Letters A, B, and C are used to call attention to the gating strategy. A) Buffer plus Reagents. B) EV unstaining. C) EV staining. EV phenotyping was performed for the main EV subsets.
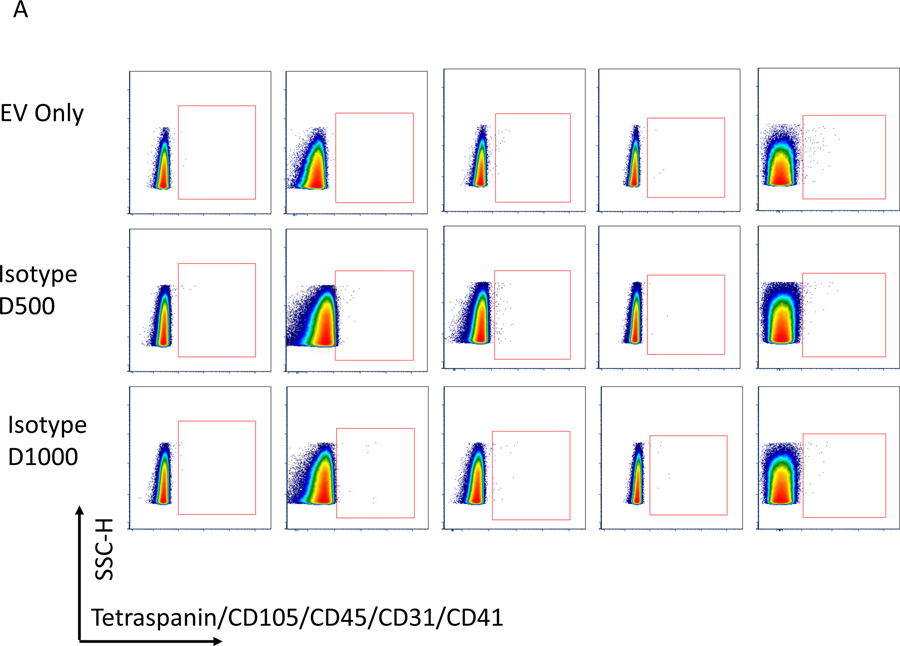
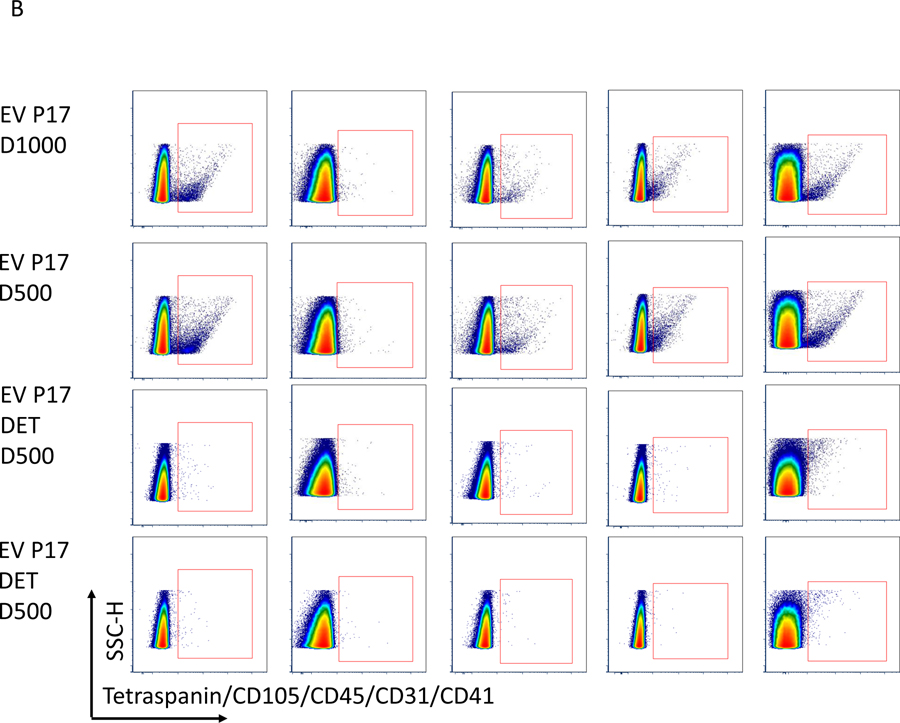
Figure 5.
Before flow acquisition each sample is diluted with filtered HEPES buffer in different volumes to rule out effects of co-incidence (“swarming”). In this figure, we show in Figure A and B flow blots of examples for one Isotype control and an EV samples without flourescent labeling (EV Only), Isotype control at dilution 1:500 (D500) and 1:1000 (D1000), EVs with antibody mix (EV P17) in 2 dilutions (EV P17 D500 and EV P17 D1000) and EVs with antibodies also treated with detergent (EV P17 DET D500 and EV P17 DET D500). C summarizes the actual counts for each plot and demonstrates that there is no change in the Median particle count between different dilutions. However, there is a significant reduction in counts with use of detergents.
Antibody titer was performed for each antibody and the following amount was optimized in the cocktail: 0.25 μL of anti CD9 conjugated with FITC (clone HI9a Cat number 312104, BD Biosciences), anti CD63 conjugated with FITC (clone H5C6 Cat number 353006, BD Biosciences), anti CD81 conjugated with FITC (clone 5A6 Cat number 349504, BD Biosciences); 2 μL anti CD45 conjugated with PE-Dazzle 594 (clone HI30 Cat number 304052, BD Biosciences); 1 μL of anti CD105 conjugated with PE (clone 43A3 Cat number 323206, BD Biosciences), 1 μL of anti CD31 conjugated with AF647 (clone WM59 Cat number 303112, BD Biosciences) and 1 μL of anti CD41 conjugated with Pacific Blue (Clone HIP8 Cat number 303714, BD Biosciences). The antibody cocktails were diluted to 20 μL with HEPES Buffer (10 mM HEPES pH7.4 and 0.15 M NaCl), filtered through a 0.1 μm syringe filter (Minisart PES syringe filter code 16553K, Sartorious), and centrifuged for 1-hr at 21,130g (max speed of the centrifuge: 524/5424 R-Rotor FA-45-24-11, Eppendorf). EV pellets P17 were resolubilized in 30 μL of HEPES buffer and stained with 20 μL of centrifuge antibody mix overnight at RT and in a dark condition. All the antibodies were purchased from Biolegend, including isotype controls. FCS-EXPRESS 7-De Novo Software was used for EV analysis. EV counts were described per microliter plasma of the starting volume.
Statistical Analysis.
Data were analyzed using the Statistical Package for Social Sciences (SPSS Software v. 27) and the statistical software R (v. 4.0.2). Normality was assessed using Shapiro-Wilk and non-normally distributed data were log-transformed. After log transformation, data met criteria to utilize parametric tests. Paired t-tests were used to evaluate fasting measurements between exercise and control conditions. Repeated measures analysis of variance (ANOVA) was also used to assess the insulin-stimulated interactions (i.e. condition × time). Cohen’s d and partial η2 were used to calculate effect size, when appropriate, while Pearson correlations were performed to determine outcome relationsips. Statistical significance was accepted as P≤0.05. Non-normally distributed data are presented in text/tables as medians (ranges) and in figures as medians with 95% confidence intervals. Normally distributed data are mean (SD).
RESULTS
Acute Exercise Protocol:
All individuals (n=10) completed the moderate-intensity exercise session (VO2peak: 69.1 [4.1%]). Further, participants expended 398 (7.2) kcals over 54.2 (16.4) min.
Euglycemic Hyperinsulinemic Clamp.
Individuals had similar fasting and 120 min levels of glucose (P=0.163, d=0.48 and P=0.338, d=0.32) and insulin (P=0.750, d=0.10 and P=0.151, d=0.50) following both conditions. Likewise, the average glucose (P=0.169, d=0.473) and insulin levels (P=0.411, d=0.27) between 90–120 min of the clamp was comparable. However, a single session of exercise increased the M-Value (P=0.042, d=0.80, Table 1) compared to control.
Table 1.
Euglycemic-Hyperinsulinemic Clamp Data.
| Control | Exercise | P-Value | |
|---|---|---|---|
|
| |||
| Weight (kg) | 102 (17.6) | 102 (17.3) | 0.490 |
| Glucose (mg/dl) | |||
| Fasting | 96.8 (8.0) | 94.1 (6.8) | 0.163 |
| 120 min | 90.4 (4.5) | 88.2 (5.0) | 0.338 |
| Average (90–120 min) | 90.2 (2.3) | 88.7 (2.6) | 0.169 |
| Insulin (uU/ml) | |||
| Fasting | 9.14 (4.9) | 9.59 (6.9) | 0.750 |
| 120 min | 63.5 (14.9) | 77.8 (35.5) | 0.151 |
| Average (90–120 min) | 66.1 (13.0) | 70.0 (14.4) | 0.411 |
| Insulin Sensitivity | |||
| M-Value (mg/kg/min)^ | 2.49 (1.19, 6.07) | 3.18 (1.13, 8.52) | 0.042 |
Data are complete (n=10) for all variables.
Non-normally distributed data are presented as median (min, max). All other data are mean (SD). Average glucose metabolized between 90–120 min (M-Value).
Pulse Waveform Analysis.
Acute exercise did not alter fasting measurements of HR (P=0.384, d=0.31), pSBP (P=0.200, d=0.44), pDBP (P= 0.453, d=0.25), pPP (P=0.151, d=0.50), cSP (P=0.162, d=0.48), cDP (P= 0.494, d=0.23), AP (P=0.094, d=0.59), AIx75 (P=0.251, d=0.39), Pf (P=0.155, d=0.49), RM (P=0.179, d=0.46), or PPA (P=0.161, d=0.48). Although not statistically significant, exercise tended to reduce fasting cPP (P=0.064, d=0.69) and Pb (P=0.064, d=0.67) compared to control. Insulin infusion decreased AP (P=0.02, η2=0.47) and AIx75 (P=0.041, η2=0.39) following both conditions, with greater decreases in AIx75 following exercise (interaction: P=0.024, η2=0.45). Although PPA tended to decrease in response to insulin following exercise (P=0.067, η2=0.33), Pf (P=0.064, η2=0.33) and cPP (P=0.046, η2=0.37) tended to increase following exercise compared to a small decrease during control (Table 2).
Table 2.
Effect of exercise and insulin on peripheral and central hemodynamics.
| Control | Exercise | P-Value | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 min | Δ | 0 min | Δ | C | T | C*T | |
|
| |||||||
| HR (bpm) | 65.0 (9.4) | 10.0 (19.7) | 67.9 (9.5) | −3.1 (11.3) | 0.943 | 0.729 | 0.227 |
| Peripheral | |||||||
| pSBP (mmHg) | 133 (15.9) | −0.8 (11.0) | 127 (10.0) | 6.8 (9.0) | 0.429 | 0.263 | 0.101 |
| pDBP (mmHg) | 83.6 (11.9) | −0.7 (7.4) | 81.3 (10.2) | 1.6 (5.2) | 0.505 | 0.744 | 0.466 |
| pPP (mmHg) | 49.4 (7.2) | −0.1 (6.7) | 45.5 (4.7) | 5.2 (5.9) | 0.530 | 0.170 | 0.032 |
| Central | |||||||
| cSP (mmHg) | 123 (13.5) | −3.5 (12.5) | 116 (10.5) | 5.2 (10.4) | 0.328 | 0.739 | 0.136 |
| cDP (mmHg) | 84.4 (11.8) | −6.6 (25.5) | 82.3 (10.4) | −3.2 (18.2) | 0.852 | 0.486 | 0.375 |
| cPP (mmHg) | 38.3 (4.9) | −3.8 (5.2) | 33.8 (5.8) | 8.4 (16.0) | 0.590 | 0.420 | 0.046 |
| AP (mmHg)^ | 11 (7, 16) | −3.5 (−8, 4) | 8.5 (3, 18) | −1.0 (−7, 4) | 0.106 | 0.020 | 0.576 |
| AIx75 (%)^ | 23 (15, 31) | −3.5 (−14, 8) | 25 (5, 37) | −7.5 (−23, 13) | 0.061 | 0.041 | 0.024 |
| Wave Reflection | |||||||
| Pb (mmHg) | 16.4 (2.2) | −2.0 (3.6) | 13.9 (3.0) | 1.1 (3.8) | 0.179 | 0.615 | 0.077 |
| Pf (mmHg)^ | 27.5 (19, 60) | −0.5 (−34, 2) | 23.5 (21, 33) | 2.5 (−4, 6) | 0.309 | 0.670 | 0.064 |
| RM (%) | 62.0 (9.9) | −5.4 (9.3) | 57.4 (13.3) | 1.0 (11.6) | 0.440 | 0.373 | 0.204 |
| PPA | 1.29 (0.8) | 0.15 (0.2) | 1.36 (0.2) | −0.08 (0.3) | 0.518 | 0.600 | 0.067 |
Data are complete (n=10) for all variables.
Non-normally distributed data are presented as median (min, max). All other data are mean (SD). Augmentation index (AIx75); Augmentation pressure (AP); Backward pressure (Pb); Forward pressure (Pf); Heart rate (HR); Peripheral and central diastolic blood pressure (pDBP and cDP); Peripheral and central pulse pressure (pPP and cPP); Peripheral and central systolic blood pressure (pSBP and cSP); Pulse pressure amplification (PPA); Reflection magnitude (RM); Δ = 120–0 min.
EVs.
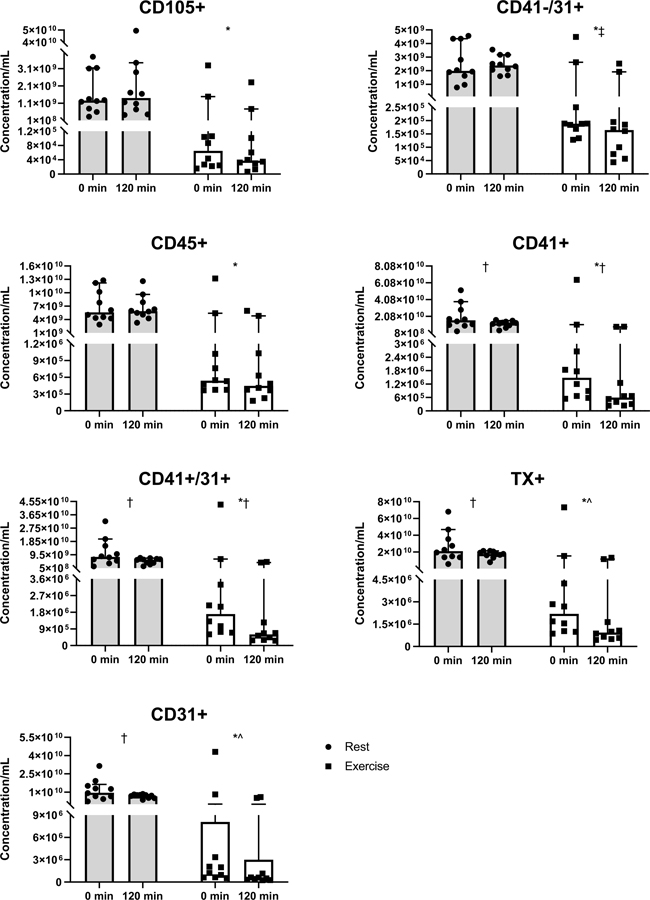
Acute exercise decreased all fasting EVs, including CD105+ (P=0.001, d=1.59), CD41-/31+ (P=0.001, d=1.65), CD45+ (P<0.001, d=1.70), CD41+ (P<0.001, d=1.68), CD41+/31+(P=0.001, d=1.65), TX+ (P<0.001, d=1.69), and CD31+ (P=0.001, d=1.64) compared to control (Figure 3). Insulin infusion decreased CD41+ (P=0.010, η2=0.52), CD41+/31+ (P=0.006, η2=0.58), TX+ (P=0.010, η2=0.54), and CD31+ (P=0.007, η2=0.58) following both conditions. However, insulin tended to reduce TX+ (interaction: P=0.060, η2=0.34), and CD31+ (interaction: P=0.060, η2=0.34) more following exercise compared to control. Although insulin somewhat increased CD41−/31+ following control, exercise resulted in a significant decrease (interaction: P=0.045, η2=0.37, Figure 3).
Figure 3.
The impact of control (circles) and exercise (squares) on EV concentrations. *Paired t-test between fasting control and exercise (P≤0.001); †ANOVA main effect of time (P≤0.01); ANOVA interaction ^(P=0.060), ‡(P=0.045). Data are presented as medians with 95% confidence intervals
Correlations.
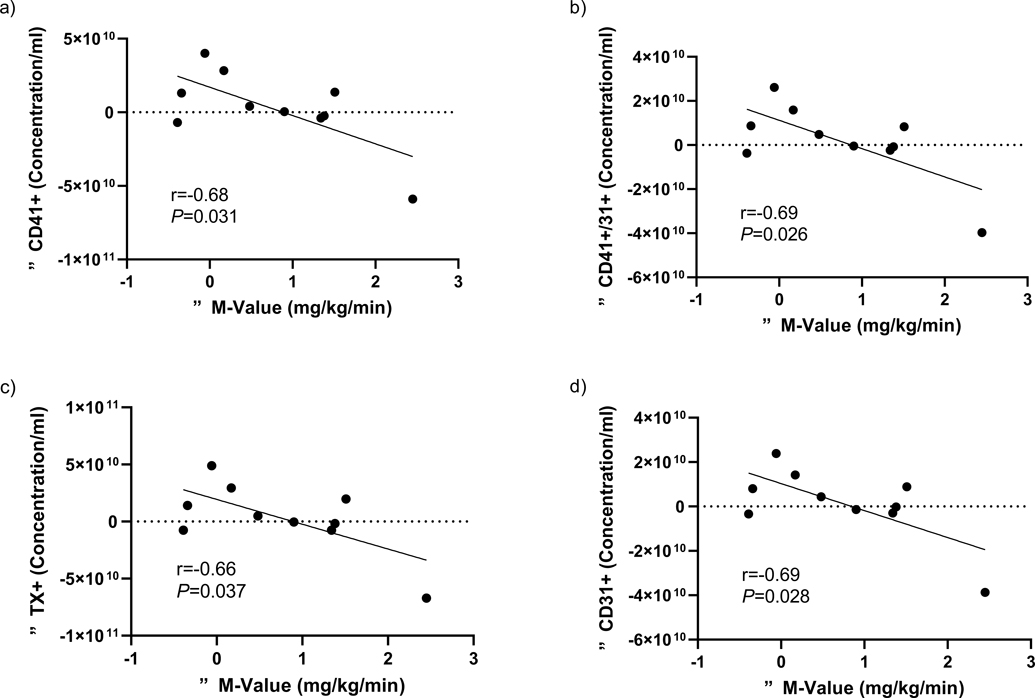
Decreased fasting EVs following exercise did not correlate with any changes in fasting hemodynamic or aortic waveform measures (data not shown). However, insulin-stimulated decreases in CD41−/31+ (Δ = (Exercise: 120 min – 0 min) – (Rest: 120 min – 0 min)) were associated with insulin-stimulated decreases in AIx75 (r=0.62, P=0.059) and increases in DP (r=−0.65, P=0.043), while decreases in CD105+ were related to decreased HR (r=0.83, P=0.003; Table 3). Interestingly, insulin-stimulated decreases in CD41+ (r=−0.68, P=0.031), CD41+/31+ (r=−0.69, P=0.026), TX+ (r=−0.66, P=0.037), and CD31+ (r=−0.69, P=0.028) correlated with increased M-Value following exercise (Figure 4).
Table 3.
Correlations between changes in EVs with peripheral and central hemodynamics.
| Variable | ΔCD105+ | ΔCD41−/31+ | ΔCD45+ | ΔCD41+ | ΔCD41+/31+ | ΔTX+ | ΔCD31+ |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Δ HR | 0.83 * | 0.43 | −0.29 | −0.21 | −0.17 | −0.16 | −0.16 |
| Δ pSBP | −0.14 | −0.42 | −0.21 | −0.24 | −0.23 | −0.28 | −0.24 |
| Δ pDBP | −0.14 | −0.31 | 0.16 | −0.12 | −0.10 | −0.15 | −0.13 |
| Δ pPP | −0.32 | −0.37 | −0.29 | −0.46 | −0.47 | −0.41 | −0.44 |
| Δ cSP | −0.12 | −0.27 | −0.05 | −0.28 | −0.18 | −0.26 | −0.27 |
| Δ cDP | −0.10 | −0.65 * | −0.60 | −0.53 | −0.53 | −0.55 | −0.56 |
| Δ cPP | −0.22 | −0.53 | −0.62 | −0.73 * | −0.68 * | −0.74 * | −0.71 * |
| Δ AP | 0.36 | −0.04 | 0.11 | 0.09 | 0.10 | 0.04 | 0.11 |
| Δ AIx75 | 0.46 | 0.62 ^ | 0.38 | 0.03 | 0.09 | 0.05 | 0.12 |
| Δ Pb | −0.04 | −0.14 | −0.10 | −0.11 | −0.08 | −0.14 | −0.90 |
| Δ Pf | 0.20 | 0.26 | 0.32 | 0.39 | 0.30 | 0.34 | 0.26 |
| Δ RM | −0.11 | −0.54 | −0.50 | −0.37 | −0.37 | 0.42 | −0.38 |
| Δ PPA | 0.03 | 0.32 | 0.35 | 0.51 | 0.42 | 0.54 | 0.49 |
Note: Δ = (Ex: 120 min – 0 min) – (Rest: 120 min – 0 min).
P<0.05
=0.059. Augmentation index (AIx75); Augmentation pressure (AP); Backward pressure (Pb); Forward pressure (Pf); Heart rate (HR); Peripheral and central diastolic blood pressure (pDBP and cDP); Peripheral and central pulse pressure (pPP and cPP); Peripheral and central systolic blood pressure (pSBP and cSP); Pulse pressure amplification (PPA); Reflection magnitude (RM).
Figure 4.
Associations between the changes in metabolic insulin sensitivity (ΔM-value) and a) CD31+, b) CD41+/31+, c) TX+, and d) CD31+.
DISCUSSION
The major finding of this study is that a single bout of exercise not only decreases fasting EVs, but also enhances the effect of insulin to lower EV concentrations. This may be of clinical relevance since both obesity (Amosse et al., 2018) and T2D (Freeman et al., 2018) have been characterized by elevated EV levels compared with healthy counterparts. While aerobic exercise often increases EV concentrations during the immediate post-exercise period (Frühbeis et al., 2015; Whitham et al., 2018; Neuberger et al., 2021), the decrease in fasting EVs observed herein corroborates recent work by Rigamonti et al. (2020), in which acute exercise decreased total EVs ~24 hr later in adults with obesity. Of note, these investigators detected EVs with NTA and single EV analysis with flow cytometry, which was used within our study. This highlights that medium- to large-sized EVs likely respond to exercise in a time-dependent manner and this observation overall may help understand the mixed results in EVs among prior exercise work in the literature. In either case, this study shows for the first time that exercise enhances the effect of insulin to lower EVs. This finding expands on previous work from our group studying the post-prandial EV responses utilizing an oral glucose challenge (Eichner et al., 2019b) as well as others after a high fat meal (Tushuizen et al., 2007; Jenkins et al., 2011). These prior data were unable to determine direct effects of insulin as glucose and incretin concentrations were also altered. Indeed, the post-prandial response is often characterized by elevated circulating insulin levels. Interestingly, we previously showed that elevations in blood insulin in response to a 75 g oral glucose load related to elevated CD31+ EVs (Eichner et al., 2019b). Although this work is consistent with prior in vitro data showing that hyperinsulinemic conditions increase EV secretion and decrease insulin signaling (Liem et al., 2017; Freeman et al., 2018), it is possible that the elevated insulin levels we previously saw following the glucose load related to ambient insulin resistance per se rather than representing a direct effect of insulin on EVs. Thus, more research is warranted to understand how different insulin concentrations impact EVs.
We are only aware of one study regarding the effects of exercise on post-prandial EVs, which noted that ~50 min of aerobic exercise blunted the increase in CD31+ EVs following a high fat meal compared to control (Jenkins et al., 2011). The exact mechanism by which exercise attenuated the EV response to the high fat meal remains unclear, however, insulin sensitivity was not considered. Herein, we directly tested the effect of insulin infusion on EVs and observed that insulin-stimulated decreases in CD41+ concentrations were associated with increased metabolic insulin sensitivity following exercise. This indicates that reductions in platelet-derived EVs following insulin infusion may contribute to improved metabolic insulin sensitivity following exercise. This is consistent with animal work showing that the transfer of EVs from insulin resistant rodents caused glucose intolerance and insulin resistance in healthy mice (Ying et al., 2017). How exercise enhances the effect of insulin to lower EV levels though is beyond the scope of the study. We speculate nonetheless that it may be a direct effect of insulin on EV protein signaling. Evidence supporting this assertion comes from data in individuals with high levels of insulin resistance, whereby insulin signaling proteins in EVs, such as AKT and IRS-1 detected by ELISA technique of lysed EVs, are reduced (Freeman et al., 2018). However, it is important to note that ELISA techniques are bulk analyzed and not necessarily EV specific and Freeman et al. (2018) did not utilize a secondary method to corroborate these findings. Nonetheless, this possibly highlights that EVs not only have the capacity to directly respond to insulin, but that EVs may in turn have functional changes in response to insulin that mediates insulin action in other tissues (e.g. liver, skeletal muscle, etc.). Work in mice support this given that exosomes released by muscle are modified by high-intensity exercise and promote increases in whole-body insulin sensitivity via changes in the liver via a FoXO-1 mechanism (Castaño et al., 2020). We did not utilize isotope tracers in the current study to differentiate liver versus skeletal muscle metabolism, but it should be noted that exercise did not lower fasting glucose or insulin concentrations in this study. Since the liver primarily regulates fasting glucose homeostasis, it is possible that skeletal muscle was primarily responsible for gains in insulin sensitivity. In either case, addition work is needed to understand how exercise affects specific EV function to promote insulin sensitivity.
A single session of exercise did not alter fasting heart rate, peripheral or central blood pressure, or wave reflection measures. This was not surprising though as some (Eichner et al., 2019a; Heiston et al., 2021a) but not all short-term exercise studies (Way et al., 2020), with or without caloric restriction, show little impact on fasting hemodynamic measures in adults with obesity. In contrast, we show here that both augmentation pressure and AIx75 decreased in response to insulin following both conditions, mirroring recent work in which insulin reduced AP and AIx75 in individuals with metabolic syndrome (Dotson et al., 2021). Despite showing that exercise enhanced the reduction of AIx75 in response to insulin, exercise tended to increase central pulse and forward pressure as well as decrease pulse pressure amplification. This is somewhat surprising because Pf is associated with elevations in AP and AIx. Since HR did not differ between rest and exercise, we interpret this to suggest that that AIx75 reduction was not via changes in left ventricular ejection fraction (Pf) or autonomic innervation (HR). Rather, we previously observed increases in conduit artery diameter along with microcirculatory blood flow in response to insulin following acute exercise in adults with obesity (Heiston et al., 2021b). In turn, a greater need to deliver nutrients and oxygen may have promoted compensatory changes in cPP and Pf to provide adequate blood pressure to raise blood flow from the heart to periphery following exercise. Therefore, the reduction in AIx75 to insulin following exercise may have occurred via a nitric oxide (NO)-mediated mechanism to support endothelial function (Wilkinson et al., 2002). Interestingly, endothelial-derived EVs have been noted to impair vasorelaxation by inhibiting NO production through decreases in endothelial NO synthase (eNOS) phosphorylation and activity (Densmore et al., 2006). Although size was not evaluated in this study, it is likely that only larger EVs were detected with flowcytometric tools available at the time of publication. In vitro work has highlighted that high levels of laminar shear stress, similar to what is observed during aerobic exercise, also decreases endothelial-derived microparticles (larger EVs) in accordance with increased NO (Kim et al., 2015). While neither NO nor eNOS were measured herein, the reduction of larger EVs in responses to exercise may have increased NO bioavailability, resulting in greater insulin-stimulated AIx75 reductions. Our work thus supports the findings by Kim et al. (2015) as we observed that medium- to large-sized endothelial-derived EVs (CD105+ and CD41−/31+) were reduced following exercise in response to insulin, but not in the control condition. Further, it is worth noting that CD41+ was correlated with metabolic insulin sensitivity and paralleled the decline in AIx75. This suggests that CD41+ may play a direct role in insulin sensitivity compared with other EVs measured in the current study. CD41+ is a platelet- rather than endothelial-derived EV. Platelet-derived EVs have been previously described as a biomarker to monitor CVD risk and tend to be higher in individuals with hypertension and diabetes (Oggero et al., 2019). Additional work is warranted to understand how insulin impacts EVs in relation to AIx75, as it could have clinical ramifications for improving blood flow and/or arterial stiffness (Jurgielewicz et al., 2020).
The current study does have limitations that warrant discussion. Total concentration but not EV size was measured. However, our flow cytometry method can detect and count medium- to large-sized EVs down to 200 nm and characterize specific surface proteins of each individual EV, which can provide information regarding the pathophysiology of underling disease processes (Erdbrügger et al., 2014). Although our methodology not include smaller EVs, such as exosomes, not one instrument has the capability to characterize a specific single EV phenotype, which covers the whole range of very small (e.g. 40 nm exosomes to large microparticles up to 1000 nm). NTA (without use of a laser) is often used to characterize EV size range, but it detects particles including EVs, but also debris and protein complexed. We employed single EV detection with flow cytometry in order to define specific EV phenotypes. This offers more specific EV analysis to open up new mechanistic insight into the pathophysiology of T2D and CVD as the markers used herein are derived from cells/organs releasing the EVs during stress and stimulus (here insulin and exercise). In addition, the current study did not involve EV cargo analyses nor their direct vascular effects, which may have been altered in response to exercise and/or insulin. Another consideration is that while significant changes were detected in EV concentrations, it is likely that the sample size was inadequate to address differences in hemodynamic measures. Further, we were unable to test potential effects of sex or metabolic phenotypes (e.g. obesity with or without metabolic syndrome), which may modify the effects of insulin and/or exercise and reduce the generalizability of these results. Exercise was also of moderate to high intensity and on the treadmill. It is possible that other exercise intensities and/or modalities could modify the EV response observed herein. Although a strength of the current study is that individuals were provided a standardized diet, it is important to note that the 400 kcals expended during exercise were not refed. Thus, it is possible that the results herein were driven, in part, by energy deficit rather than the exercise per se. We also did not measure EVs across time of the clamp to gain temporal understandings or study EV responses to different doses of insulin. Finally, the associations amongst EVs and metabolic insulin sensitivity are only correlative/associative and do not represent causation.
CONCLUSION
A single bout of exercise lowers fasting and insulin-stimulated medium- to larger-sized EVs in adults with obesity. In addition, acute exercise decreases insulin-stimulated AIx75 and increases metabolic insulin sensitivity, which are related to decreases in insulin-stimulated EVs. Collectively, this work highlights that exercise may confer CVD risk reduction through an EV-insulin interaction. Further work is needed to understand how exercise affects EVs, including the smaller fraction, during fasting and insulin-stimulated states to maximize our understanding of their implications in obesity-related disease prevention, treatment, and/or management.
Supplementary Material
KEY POINTS.
What is already known that led to the present work?
Extracellular vesicles (EVs) are increased in states of obesity and may play a role in altered insulin sensitivity and blood pressure.
Aerobic exercise decreases fasting EV concentrations the following day in adults with obesity.
What does this paper add to existing knowledge?
This study directly tested the effects of insulin on EVs and how a single bout of exercise impacts these responses.
Together, these data highlight the positive effects of a single bout of exercise on fasting and insulin-stimulated EVs, with the latter relating to increased insulin sensitivity and decreased augmentation index.
What is the importance of the results for body function in health and/or disease?
These results support future research identifying EV as a mechanistic factor in glucose regulation and vascular function as well as clinical use of exercise to reduce cardiovascular disease risk.
Acknowledgements
We thank the participants who partook in this study and the staff of the Exercise Physiology Core Lab, Clinical Research Unit, and Applied Metabolism and Physiology Lab at the University of Virginia for assistance with data acquisition.
Funding
ACSM Doctoral Study Research Grant (EMH), NIH R01-HL130296 (SKM), UVA LaunchPad (SKM, UE)
FIRST AUTHOR PROFILE

Emily Heiston received her PhD from the University of Virginia (mentor: Steven Malin, PhD) where she studied the effects of exercise on vascular and metabolic insulin sensitivity in adults with obesity. Emily is currently a postdoctoral fellow at Virginia Commonwealth University (mentor: W. Gregory Hundley, MD) where she is researching the effects of cancer therapies on cardiometabolic health and body composition. Specifically, Emily is interested in identifying mechanisms by which exercise and/or pharmaceutical agents, can reduce the cardiovascular risk associated with cancer treatments.
Footnotes
Competing Interests
The authors declare no competing interests.
ADDITIONAL INFORMATION
Clinical Trial Registration: NCT03894527
Data Availability
These data are not publicly available. However, the corresponding author (SKM) can provide further information on the data upon reasonable request.
REFERENCES
- Amosse J, Durcin M, Malloci M, Vergori L, Fleury A, Gagnadoux F, Dubois S, Simard G, Boursier J, Hue O, Martinez MC, Andriantsitohaina R & Le Lay S (2018). Phenotyping of circulating extracellular vesicles (EVs) in obesity identifies large EVs as functional conveyors of Macrophage Migration Inhibitory Factor. Mol Metab 18, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird SR & Hawley JA (2017). Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med; DOI: 10.1136/bmjsem-2016-000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SE, Mitchell E, Freedson PS, Chipkin SR & Braun B (2005). Improved insulin action following short-term exercise training: role of energy and carbohydrate balance. J Appl Physiol 99, 2285–2293. [DOI] [PubMed] [Google Scholar]
- Castaño C, Mirasierra M, Vallejo M, Novials A & Párrizas M (2020). Delivery of muscle-derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down-regulation of hepatic FoxO1 in mice. Proc Natl Acad Sci U S A 117, 30335–30343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalot F, Pagliarino A, Valle M, Martino LD, Bonomo K, Massucco P, Anfossi G & Trovati M (2011). Postprandial Blood Glucose Predicts Cardiovascular Events and All-Cause Mortality in Type 2 Diabetes in a 14-Year Follow-Up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 34, 2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, Neupane YR, Pavon MV, Zharkova O, Pastorin G & Wang J-W (2019). Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers. Int J Mol Sci 20, 3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densmore JC, Signorino PR, Ou J, Hatoum OA, Rowe JJ, Shi Y, Kaul S, Jones DW, Sabina RE, Pritchard KA, Guice KS & Oldham KT (2006). Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock 26, 464–471. [DOI] [PubMed] [Google Scholar]
- Dotson BL, Heiston EM, Miller SL & Malin SK (2021). Insulin stimulation reduces aortic wave reflection in adults with metabolic syndrome. American Journal of Physiology-Heart and Circulatory Physiology 320, H2305–H2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner NZM, Erdbrügger U & Malin SK (2018a). Extracellular Vesicles: A Novel Target for Exercise-Mediated Reductions in Type 2 Diabetes and Cardiovascular Disease Risk. J Diabetes Res 2018, 7807245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner NZM, Gaitán JM, Gilbertson NM, Khurshid M, Weltman A & Malin SK (2019a). Postprandial augmentation index is reduced in adults with prediabetes following continuous and interval exercise training. Experimental Physiology 104, 264–271. [DOI] [PubMed] [Google Scholar]
- Eichner NZM, Gilbertson NM, Gaitan JM, Heiston EM, Musante L, LaSalvia S, Weltman A, Erdbrügger U & Malin SK (2018b). Low cardiorespiratory fitness is associated with higher extracellular vesicle counts in obese adults. Physiol Rep; DOI: 10.14814/phy2.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner NZM, Gilbertson NM, Heiston EM, Musante L, Salvia SL, Weltman A, Erdbrugger U & Malin SK (2020). Interval Exercise Lowers Circulating CD105 Extracellular Vesicles in Prediabetes. Med Sci Sports Exerc 52, 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner NZM, Gilbertson NM, Musante L, La Salvia S, Weltman A, Erdbrügger U & Malin SK (2019b). An Oral Glucose Load Decreases Postprandial Extracellular Vesicles in Obese Adults with and without Prediabetes. Nutrients 11, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdbrügger U, Rudy CK, Etter ME, Dryden KA, Yeager M, Klibanov AL & Lannigan J (2014). Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytometry A 85, 756–770. [DOI] [PubMed] [Google Scholar]
- Freeman DW, Hooten NN, Eitan E, Green J, Mode NA, Bodogai M, Zhang Y, Lehrmann E, Zonderman AB, Biragyn A, Egan J, Becker KG, Mattson MP, Ejiogu N & Evans MK (2018). Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 67, 2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis C, Helmig S, Tug S, Simon P & Krämer-Albers E-M (2015). Physical exercise induces rapid release of small extracellular vesicles into the circulation. Journal of Extracellular Vesicles 4, 28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen H, Nussbaumer M, Moor C, Cordes M, Schindler C & Schmidt-Trucksäss A (2015). Acute effects of interval versus continuous endurance training on pulse wave reflection in healthy young men. Atherosclerosis 238, 399–406. [DOI] [PubMed] [Google Scholar]
- Heiston EM, Gilbertson NM, Eichner NZM & Malin SK (2021a). A Low-Calorie Diet with or without Exercise Reduces Postprandial Aortic Waveform in Females with Obesity. Med Sci Sports Exerc 53, 796–803. [DOI] [PubMed] [Google Scholar]
- Heiston EM, Liu Z, Ballantyne A, Kranz S & Malin SK (2021b). A single bout of exercise improves vascular insulin sensitivity in adults with obesity. Obesity; DOI: 10.1002/oby.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson JD & Aikawa E (2018). Extracellular vesicles in cardiovascular homeostasis and disease. Curr Opin Cardiol 33, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NT, Landers RQ, Thakkar SR, Fan X, Brown MD, Prior SJ, Spangenburg EE & Hagberg JM (2011). Prior endurance exercise prevents postprandial lipaemia-induced increases in reactive oxygen species in circulating CD31+ cells. The Journal of Physiology 589, 5539–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgielewicz BJ, Yao Y & Stice SL (2020). Kinetics and Specificity of HEK293T Extracellular Vesicle Uptake using Imaging Flow Cytometry. Nanoscale Research Letters 15, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, Kim B, Lee H, Thakkar S, Babbitt DM, Eguchi S, Brown MD & Park J-Y (2015). Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. American Journal of Physiology-Heart and Circulatory Physiology 309, H425–H433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Eguchi A, Tempaku M, Honda T, Togashi K, Iwasa M, Hasegawa H, Takei Y, Sumida Y & Taguchi O (2018). Circulating extracellular vesicles are associated with lipid and insulin metabolism. Am J Physiol Endocrinol Metab 315, E574–E582. [DOI] [PubMed] [Google Scholar]
- La Salvia S, Gunasekaran PM, Byrd JB & Erdbrügger U (2020a). Extracellular Vesicles in Essential Hypertension: Hidden Messengers. Curr Hypertens Rep 22, 76. [DOI] [PubMed] [Google Scholar]
- La Salvia S, Musante L, Lannigan J, Gigliotti JC, Le TH & Erdbrügger U (2020b). T cell-derived extracellular vesicles are elevated in essential HTN. Am J Physiol Renal Physiol 319, F868–F875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem M, Ang C-S & Mathivanan S (2017). Insulin Mediated Activation of PI3K/Akt Signalling Pathway Modifies the Proteomic Cargo of Extracellular Vesicles. PROTEOMICS 17, 1600371. [DOI] [PubMed] [Google Scholar]
- Liu ZZ, Jose PA, Yang J & Zeng C (2021). Importance of extracellular vesicles in hypertension. Exp Biol Med (Maywood) 246, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger EWI, Hillen B, Mayr K, Simon P, Krämer-Albers E-M & Brahmer A (2021). Kinetics and Topology of DNA Associated with Circulating Extracellular Vesicles Released during Exercise. Genes (Basel); DOI: 10.3390/genes12040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom SA, Everett AC, Hinko A & Horowitz JF (2013). A Single Session of Low-Intensity Exercise Is Sufficient to Enhance Insulin Sensitivity Into the Next Day in Obese Adults. Diabetes Care 36, 2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystoriak MA & Bhatnagar A (2018). Cardiovascular Effects and Benefits of Exercise. Front Cardiovasc Med; DOI: 10.3389/fcvm.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oggero S, Austin-Williams S & Norling LV (2019). The Contrasting Role of Extracellular Vesicles in Vascular Inflammation and Tissue Repair. Front Pharmacol; DOI: 10.3389/fphar.2019.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piché M-E, Tchernof A & Després J-P (2020). Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res 126, 1477–1500. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Rifai N & Cook NR (2007). Development and Validation of Improved Algorithms for the Assessment of Global Cardiovascular Risk in WomenThe Reynolds Risk Score. JAMA 297, 611–619. [DOI] [PubMed] [Google Scholar]
- Rigamonti AE, Bollati V, Pergoli L, Iodice S, De Col A, Tamini S, Cicolini S, Tringali G, De Micheli R, Cella SG & Sartorio A (2020). Effects of an acute bout of exercise on circulating extracellular vesicles: tissue-, sex-, and BMI-related differences. International Journal of Obesity 44, 1108–1118. [DOI] [PubMed] [Google Scholar]
- Tetta C, Ghigo E, Silengo L, Deregibus MC & Camussi G (2013). Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 44, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushuizen ME, Diamant M & Heine RJ (2005). Postprandial dysmetabolism and cardiovascular disease in type 2 diabetes. Postgraduate Medical Journal 81, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ & Diamant M (2007). Elevated Endothelial Microparticles Following Consecutive Meals Are Associated With Vascular Endothelial Dysfunction in Type 2 Diabetes. Diabetes Care 30, 728–730. [DOI] [PubMed] [Google Scholar]
- Vechetti IJ, Valentino T, Mobley CB & McCarthy JJ (2021). The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. The Journal of Physiology 599, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way KL, Lee AS, Twigg SM & Johnson NA (2020). The effect of acute aerobic exercise on central arterial stiffness, wave reflection and hemodynamics in adults with diabetes: A randomized cross-over design. Journal of Sport and Health Science; DOI: 10.1016/j.jshs.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham M et al. (2018). Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab 27, 237–251.e4. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, MacCallum H, Cockcroft JR & Webb DJ (2002). Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol 53, 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PWF, D’Agostino RB, Sullivan L, Parise H & Kannel WB (2002). Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 162, 1867–1872. [DOI] [PubMed] [Google Scholar]
- Wykretowicz A, Rutkowska A, Krauze T, Przymuszala D, Guzik P, Marciniak R & Wysocki H (2012). Pulse pressure amplification in relation to body fatness. Br J Clin Pharmacol 73, 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Zheng L, Zou X, Wang J, Zhong J & Zhong T (2019). Extracellular vesicles in type 2 diabetes mellitus: key roles in pathogenesis, complications, and therapy. J Extracell Vesicles 8, 1625677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P & Olefsky JM (2017). Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 171, 372–384.e12. [DOI] [PubMed] [Google Scholar]
- Zaborowski MP, Balaj L, Breakefield XO & Lai CP (2015). Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 65, 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
These data are not publicly available. However, the corresponding author (SKM) can provide further information on the data upon reasonable request.