Abstract
Objective
To evaluate pharmacokinetic (PK) parameters and oxygen saturation as markers of abuse potential after administration of buprenorphine buccal film (BBF) and immediate-release (IR) oxycodone.
Methods
This was a secondary analysis of data from a phase I randomized controlled trial. A total of 19 healthy subjects who self-identified as recreational opioid users were enrolled, with 15 completing the study. Subjects were administered 300, 600, and 900 µg BBF; 30 and 60 mg orally-administered oxycodone; and placebo. For PK analysis, blood samples were collected before dosing and at 0.5, 1, 2, 3, 4, and 6 h postdose. Respiratory drive/ventilatory response to hypercapnia and oxygen saturation were evaluated before dosing and up to 8 h after administration of test drugs.
Results
Median time to maximum concentration (Tmax) was 2.17 h for 900 µg BBF and 1.17 h for 60 mg oxycodone and was similar across all doses for each drug. Mean maximum concentration (Cmax) was 1.06 ng/mL for 900 µg BBF and 132 ng/mL for 60 mg oxycodone. The abuse quotient, defined as Cmax/Tmax, was substantially higher for oxycodone compared to BBF. Respiratory depression (maximum decrease in minute ventilation) was similar for all 3 doses of BBF, consistent with a potential ceiling effect. In addition, respiratory depression occurred sooner with oxycodone vs BBF, and a greater mean decrease in oxygen saturation was observed for oxycodone 30- and 60-mg doses, compared with BBF.
Conclusion
These results indicate that BBF may have a decreased risk of abuse and respiratory depression compared with the full µ-opioid receptor agonist oxycodone.
Trial Registration
ClinicalTrials.gov identifier, NCT03996694.
Keywords: Abuse quotient, Buprenorphine, Oxygen saturation, Pharmacokinetics
Key Summary Points
| Why carry out this study? |
| Overdose of opioid analgesics, with associated respiratory depression and risk of death, can occur either during medical use or from drug abuse; there is a need to develop safer opioids with a lower potential for abuse. |
| Pharmacokinetic (PK) properties, such as high maximum plasma concentration (Cmax) and short time to reach Cmax (Tmax), are likely related to the abuse potential/drug liking of opioid analgesics. |
| What did the study ask? |
| Buprenorphine, a partial µ-opioid receptor agonist, was evaluated in comparison with oxycodone, a full µ-opioid receptor agonist, for PK properties and respiratory depression effects. |
| What was learned from the study? |
| Buprenorphine buccal film (BBF) compared to immediate-release oxycodone was associated with increased Tmax, lower Cmax, and slower respiratory depression in human subjects administered therapeutic doses of each drug. |
| These results suggest that BBF may have a decreased risk for abuse compared to oxycodone. |
Introduction
A major concern of the opioid epidemic is the occurrence of overdose by respiratory depression, either from abuse or medical use of opioids [1, 2]. Opioids induce respiratory depression (decreased minute ventilation) that is measured by increased end-tidal carbon dioxide or elevated arterial carbon dioxide. In addition, reduced minute ventilation by opioids may lead to decreased oxygen saturation, which can be assessed by a variety of instruments, including pulse oximetry and smart wearable devices in the field [3, 4]. Oxygen saturation below 90% may be considered an indication of respiratory depression, although some patients can recover after oxygen saturation of 80% or lower, depending on patient characteristics [5–7]. In contrast to full µ-opioid receptor agonists that show dose-dependent effects on respiratory depression, buprenorphine is a partial µ-opioid receptor agonist analgesic [8], with evidence of a ceiling effect on respiratory depression [9, 10]. Buprenorphine is classified as a Schedule III drug because it has a lower abuse potential than full µ-opioid receptor agonists, which are Schedule II drugs [10, 11]. As a caveat, subcutaneously administered buprenorphine (as high as 2 mg) led to euphoria in nondependent subjects, although euphoria is more common with opioids administered intravenously [12, 13].
Pharmacokinetic (PK) properties of opioid formulations can affect both abuse and respiratory depression. Individuals who abuse opioids typically prefer a high maximum observed plasma concentration (Cmax) and short peak time to attain Cmax (Tmax) for the greatest, fastest onset of euphoria [14]. High plasma concentrations also increase risk of respiratory depression [15]. For oxycodone, reported Cmax has ranged from 34.1 ng/mL (immediate-release [IR] 15 mg/650 mg oxycodone/acetaminophen) to 66.2 ng/mL (IR 30 mg/1300 mg oxycodone/acetaminophen), and Tmax ranged from 0.4 h (IR 15 mg/650 mg oxycodone/acetaminophen) to 8.2 h (IR 30 mg/1300 mg oxycodone/acetaminophen) [16]. For buprenorphine, reported Cmax has ranged from 0.2 (for 75 μg buprenorphine buccal film [BBF]) to 6.4 ng/mL (for 16 mg sublingually administered buprenorphine), and Tmax ranged from 0.75 h (16 mg sublingually administered buprenorphine) to 3 h (75 μg BBF) [17, 18].
Abuse quotient is an objective, quantitative measure to compare abuse potential across opioid formulations of the same molecule and dose that is based on PK parameters: Cmax/Tmax. Higher values suggest greater risk for abuse of the drug when using the abuse quotient measure. Abuse quotient values of 5.5–8.6 have been reported in the literature for intact (i.e., not manipulated by crushing or other methods) morphine and oxycodone formulations [11, 19–21].
This analysis of PK and oxygen saturation was conducted to examine secondary outcomes from a previously published study (ClinicalTrials.gov NCT03996694) [22]. The primary outcome of the initial study was the maximum decrease in minute ventilation (Emax) after administration of BBF (BELBUCA®) 300 µg, 600 µg, and 900 µg and IR oxycodone 30 mg and 60 mg by measurement of the ventilatory response to hypercapnia (VRH). Results showed that oxycodone decreased respiratory drive in a dose-dependent manner vs placebo, and BBF did not significantly decrease respiratory drive at any dose tested [22]. This report summarizes the exploratory PK and abuse quotient endpoints and the secondary oxygen saturation endpoint as indications of abuse potential and respiratory depression after administration of BBF and IR oxycodone in this phase I study.
Methods
This was an analysis of secondary outcomes from a previously published phase I study. Detailed methods were previously described [22].
Ethics
The authors have received approval from an institutional review board (Midlands Independent Review Board, Lenexa, KS, USA). This study was conducted in accordance with the principles and requirements of the Declaration of Helsinki and International Council for Harmonization E6 Guidelines for good clinical practice (European Medicines Agency/Committee for Medicinal Products for Human Use).
All subjects were informed verbally and in writing regarding the objectives, procedures, and risks of study participation. The subjects signed the informed consent form that contained information about the objectives of the study, the procedures followed during the study, and the risks and restrictions of the study, with special reference to possible side effects of the medication and potential interactions. This study is registered on ClinicalTrials.gov (NCT03996694).
Study Design
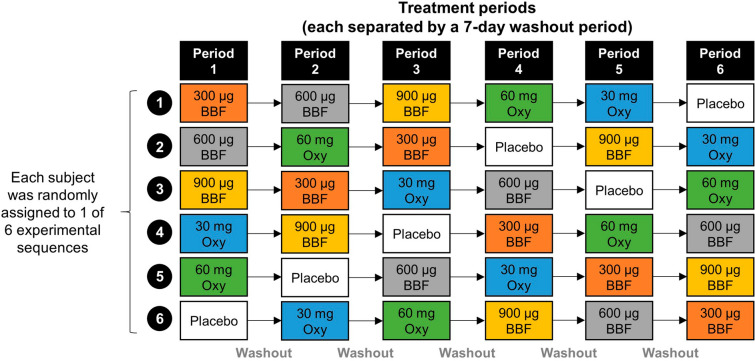
Participants were healthy individuals who self-identified as recreational opioid users and were not dependent on opioids, as confirmed with a naloxone challenge test. This study utilized a double-blind, double-dummy, 6-treatment, 6-period, placebo-controlled, randomized crossover design (Fig. 1). Each treatment was separated by a 7-day washout period to avoid any potential carryover effects. Study treatments were placebo; BBF 300 µg, 600 µg, and 900 µg; and IR oxycodone 30 mg and 60 mg [22]. The doses for BBF and oxycodone were selected on the basis of estimates of equianalgesic dosing. The first patient was screened on July 8, 2019. The clinical study protocol was reviewed and approved by an institutional review board.
Fig. 1.
Study design. BBF indicates buprenorphine buccal film; oxy, oxycodone. Reproduced from Webster et al. [22], under the CC BY-NC 4.0 Open Access License
Assessments
During the treatment phase, blood samples were collected before dosing and at 0.5, 1, 2, 3, 4, and 6 h after dosing for PK analysis. Buprenorphine and oxycodone were analyzed in plasma samples using liquid chromatography with tandem mass spectrometric detection according to validated analytical methods. PK parameters were evaluated using noncompartmental methods, and descriptive summary statistics were calculated for Cmax, Tmax, and abuse quotient (Cmax/Tmax).
Respiratory drive was evaluated by VRH as previously published [22]. The equipment was calibrated with known CO2 concentrations prior to each assessment. During VRH, peripheral capillary oxygen saturation was measured by pulse oximetry from at least 15 min before dosing until at least 8 h after dose. Pearson correlations for Tmax and Cmax with Emax minute ventilation and change in pupil size from baseline were analyzed. P values were calculated by 2-tailed tests with a null hypothesis of r = 0.
Results
In total, 19 participants enrolled and were included in the safety population, 15 completed the study (completer population), and 16 completed at least 2 treatments (partial completer population). Among the 4 subjects who did not complete the study, 1 was lost to follow-up, another subject withdrew, 1 had a positive urine drug screen finding, and 1 subject experienced an adverse event of idioventricular rhythm several hours after receiving a 600-µg dose of BBF and was discontinued at that point. This adverse event was judged by the investigator as likely related to the test drug. Most patients enrolled were men (94.7%), White (73.7%), and not of Hispanic or Latino ethnicity (73.7%); the mean age was 33.1 years (Table 1).
Table 1.
Summary of demographics
| Category | Randomized/safety (N = 19) | Completer (N = 15) | Partial completer (N = 16) |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 1 (5.3) | 1 (6.7) | 1 (6.3) |
| Male | 18 (94.7) | 14 (93.3) | 15 (93.8) |
| Race, n (%) | |||
| White | 14 (73.7) | 12 (80.0) | 13 (81.3) |
| Black or African American | 1 (5.3) | 1 (6.7) | 1 (6.3) |
| Asian | 1 (5.3) | 1 (6.7) | 1 (6.3) |
| American Indian or Alaska Native | 3 (15.8) | 1 (6.7) | 1 (6.3) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 5 (26.3) | 3 (20.0) | 3 (18.8) |
| Not Hispanic or Latino | 14 (73.7) | 12 (80.0) | 13 (81.3) |
| Age (y)a | 33.1 (4.5) | 32.9 (4.4) | 32.8 (4.3) |
| Weight (kg)a | 78.6 (15.8) | 80.6 (16.7) | 79.3 (16.9) |
| Height (cm)a | 177.1 (8.4) | 177.4 (9.3) | 177.0 (9.1) |
| BMI (kg/m2)a | 24.9 (3.7) | 25.4 (3.8) | 25.1 (3.9) |
Reproduced from Webster et al. [22], under the CC BY-NC 4.0 Open Access License
BMI body mass index
aValues, mean (SD)
PK
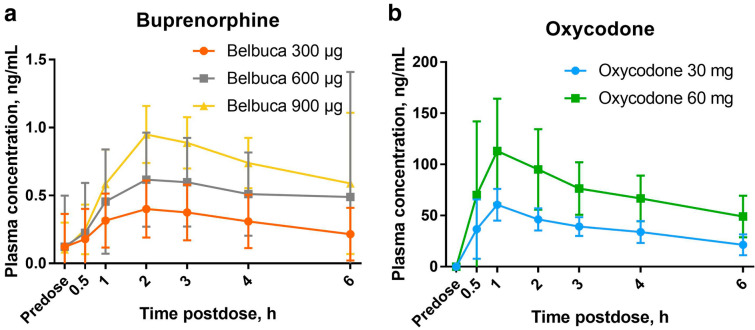
Plasma drug concentration increased with increasing dose, as expected, with most separation between doses occurring at 2 h postdose for buprenorphine and 1 h postdose for oxycodone (Fig. 2). Tmax occurred earlier, and Cmax and the abuse quotient were higher with oxycodone from the IR product than with buprenorphine from BBF (Table 2). Tmax was similar across doses, while Cmax and the abuse quotient increased with higher doses.
Fig. 2.
Mean (± SD) plasma concentration of a buprenorphine and b oxycodone over time, (N = 19)
Table 2.
Pharmacokinetic parameters
| Median Tmax (min, max), h | Mean Cmax (SD) | Mean (SD) abuse quotient | |
|---|---|---|---|
| Buprenorphine | |||
| 300 µg | 2.15 (2.13, 3.20) | 0.413 (0.215) | 0.17 (0.09) |
| 600 µg | 3.13 (1.12, 6.00) | 0.796 (0.853) | 0.30 (0.20) |
| 900 µg | 2.17 (2.13, 6.00) | 1.06 (0.414) | 0.41 (0.11) |
| Oxycodone | |||
| 30 mg | 1.15 (0.63, 3.15) | 65.8 (19.1) | 67.4 (39.2) |
| 60 mg | 1.17 (0.67, 6.0) | 132 (46.2) | 110 (75.3) |
Correlations
Pearson’s correlation between Tmax and maximum change from baseline in pupil size was moderate (r = 0.31; p = 0.03) for BBF, with no appreciable correlation (r = 0.09; p = 0.64) for oxycodone. For BBF, the maximum decrease in pupil size occurred from 2 to 3 h postdose. For oxycodone, the maximum decrease in pupil size occurred from 0.5 to 2 h postdose. Pearson’s correlation between Tmax and Emax was moderate (r = 0.36; p = 0.01) for BBF and very small (r = 0.16; p = 0.38) for oxycodone. For BBF, mean Emax was observed at 3 h postdose for 300- and 600-µg doses and at 4 h for 900 µg, whereas for oxycodone, mean Emax values occurred at 1 and 2 h postdose for 30- and 60-mg doses, respectively [22]. No compelling correlations were observed between Cmax and either Emax or change in pupil size from baseline.
Oxygen Saturation
With oxycodone, oxygen saturation decreased in an apparent dose-dependent manner in the first hour after dosing, after which the dose-dependent effect was less pronounced (Fig. 3). The lowest mean oxygen saturation with oxycodone was 95%, 1.5 h after dosing with oxycodone 60 mg. BBF resulted in change to oxygen saturation similar to that observed with placebo (Fig. 3). Only one subject had a clinically significant decrease in oxygen saturation (to 86%, approximately 1.5 h after dosing with oxycodone 60 mg).
Fig. 3.
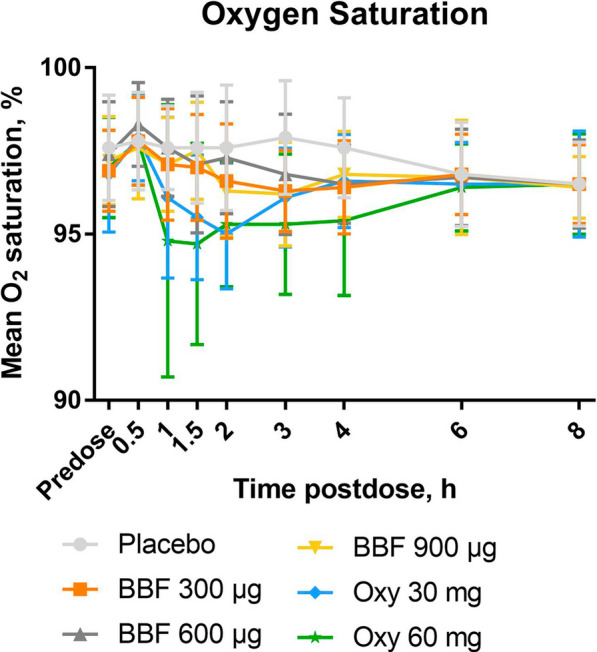
Mean (± SD) oxygen saturation in response to BBF and oxycodone administration, (N = 15). BBF indicates buprenorphine buccal film; Oxy oxycodone. Consort flowchart and checklist: not applicable because the primary results of the study were already published; this report focuses on a secondary analysis
Discussion
Previous studies have shown that faster Tmax and higher Cmax in opioids indicate a higher risk for abuse [14]. Although it can be challenging to interpret the clinical implications of pharmacokinetic differences across molecules, IR oxycodone illustrated a faster Tmax and a higher Cmax compared with BBF at estimated equianalgesic doses. These results were expected, given the difference in onset of action for each drug. The abuse quotient was substantially higher for both oxycodone doses compared with all 3 BBF doses. The abuse quotient may not be directly comparable across different molecules, but it is intuitive that the slower increase in plasma concentration with BBF may make it less appealing for abuse than an IR oxycodone formulation. Tmax was significantly moderately correlated with maximum change from baseline in pupil size as well as Emax for BBF, which suggests that later Tmax among subjects receiving this drug may be related to improved oxygen saturation and less drug liking. Moreover, previous studies have demonstrated a relationship between drug liking and pupil diameter [23, 24], and delayed miosis seen with BBF is consistent with the lower abuse quotient compared to IR oxycodone observed in this study. In the current study, oxygen saturation showed an initial dose-dependent decrease up to 1 h with oxycodone, while levels for BBF remained similar to those for placebo. Taken together, these results suggest that BBF was well tolerated with regard to respiratory function and may have a lower potential for abuse relative to oxycodone.
Limitations
Limitations of this study include the small sample size (N = 16 partial completer population) and that differences in drug release of BBF and IR oxycodone in this study affect their PK profiles and the abuse quotient. In addition, comparing abuse quotient of different molecules may not represent a difference in abuse potential because of differences in opioid properties and potency across molecules. This was a single-dose study, and results may not be applicable to patients receiving long-term therapy. Moreover, mechanisms of respiratory depression (and death from overdose) are complicated by polypharmacy, in which drug interactions or additive effects may occur. This was illustrated in an animal study where a combination of buprenorphine and benzodiazepine was administered and led to early-onset sedation and respiratory depression in rats [25].
Conclusions
Respiratory depression is the leading cause of death from opioids [26]. Opioids slow breathing and reduce tidal volume, which decreases oxygen saturation in the blood [2, 3]. Abuse increases the risk of taking doses of opioids that can cause respiratory depression. Results from this study suggest that BBF leads to decreased risk of abuse and respiratory depression compared with full µ-opioid receptor agonists such as oxycodone, which has important safety implications.
Acknowledgements
Funding Source
The journal’s Rapid Service Fee and study and editorial support for the manuscript were funded by BioDelivery Sciences International, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to manuscript revision, read, and approved the submitted version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
LW Principal Investigator.
JC Participated in the analysis and interpretation of the data.
TS Participated in the analysis and interpretation of the data.
Medical Writing, Editorial, and Other Assistance
Technical and editorial support for this manuscript was provided by MedLogix Communications, LLC, and funded by BioDelivery Sciences International, Inc.
Prior Presentation
Posters presented as encores at the American Society of Pain Management Nursing, September 18–October 2, 2021, virtually. PAINWeek conference, September 6–10, 2021, Las Vegas, NV, USA. American Association of Physicists Medicine Conference, July 25–29, 2021, virtually. North American Neuromodulation Society Conference, July 15–17, 2021, virtually.
Disclosures
LW has received consultation, advisory board, and travel fees from BioDelivery Sciences International, Inc.; advisory board and travel fees from Ensysce Biosciences, Neurana Pharmaceuticals, and Salix Pharmaceuticals; consultation fees from Arbor Pharmaceuticals; and travel fees from Elysium Health. JC declares no conflicts of interest. TS is an employee of BioDelivery Sciences International, Inc.
Compliance with Ethics Guidelines
The authors have received approval from an institutional review board (Midlands Independent Review Board, Lenexa, KS, USA). This study was conducted in accordance with the principles and requirements of the Declaration of Helsinki and International Council for Harmonization E6 Guidelines for good clinical practice (European Medicines Agency/Committee for Medicinal Products for Human Use).
Data Availability
The datasets generated during and/or analyzed during the current study are available at https://clinicaltrials.gov/ct2/show/NCT03996694.
References
- 1.Dolinak D. Opioid toxicity. Acad Forensic Pathol. 2017;7(1):19–35. doi: 10.23907/2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747–758. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- 3.Boland J, Boland E, Brooks D. Importance of the correct diagnosis of opioid-induced respiratory depression in adult cancer patients and titration of naloxone. Clin Med (Lond) 2013;13(2):149–151. doi: 10.7861/clinmedicine.13-2-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance. Ann Am Thorac Soc. 2020;17(9):1040–1046. 10.1513/AnnalsATS.202005-418FR. [DOI] [PMC free article] [PubMed]
- 5.Gupta K, Prasad A, Nagappa M, Wong J, Abrahamyan L, Chung FF. Risk factors for opioid-induced respiratory depression and failure to rescue: a review. Curr Opin Anaesthesiol. 2018;31(1):110–119. doi: 10.1097/ACO.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi RS, Papadimos TJ. Life-threatening. Hypoxemia in one-lung ventilation. Anesthesiology. 2011;115(2):438. 10.1097/ALN.0b013e318223bbad (author reply 439–41). [DOI] [PubMed]
- 7.Guler S, Brunner-Agten S, Bartenstein S, et al. Oxygen saturation of 75%, but no symptoms! Respiration. 2016;92(6):420–424. doi: 10.1159/000451030. [DOI] [PubMed] [Google Scholar]
- 8.Khanna IK, Pillarisetti S. Buprenorphine—an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res. 2015;8:859–870. doi: 10.2147/JPR.S85951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94(6):825–834. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 10.Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96(5):627–632. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- 11.Webster LR, Pantaleon C, Iverson M, Smith MD, Kinzler ER, Aigner S. Intranasal pharmacokinetics of morphine ARER, a novel abuse-deterrent formulation: results from a randomized, double-blind, four-way crossover study in nondependent. Opioid-experienced subjects. Pain Res Manag. 2018;2018:7276021. doi: 10.1155/2018/7276021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev. 2011;4(1):28–41. doi: 10.2174/1874473711104010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35(4):501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 14.Katz N, Dart RC, Bailey E, Trudeau J, Osgood E, Paillard F. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37(4):205–217. doi: 10.3109/00952990.2011.569623. [DOI] [PubMed] [Google Scholar]
- 15.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112(1):226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 16.Morton TL, Devarakonda K, Kostenbader K, Montgomery J, Barrett T, Webster L. Correlation of subjective effects with systemic opioid exposure from fixed-dose combinations of oxycodone/acetaminophen in recreational users of prescription drugs. Pain Med. 2016;17(3):539–550. doi: 10.1111/pme.12884. [DOI] [PubMed] [Google Scholar]
- 17.McAleer SD, Mills RJ, Polack T, et al. Pharmacokinetics of high-dose buprenorphine following single administration of sublingual tablet formulations in opioid naive healthy male volunteers under a naltrexone block. Drug Alcohol Depend. 2003;72(1):75–83. 10.1016/s0376-8716(03)00188-1. [DOI] [PubMed]
- 18.BELBUCA [package insert]. BioDelivery Sciences International, Inc; 2019.
- 19.Webster LR, Smith MD, Lawler J, Lindhardt K, Dayno JM. Human abuse potential of an abuse-deterrent (AD), extended-release (ER) morphine product candidate (morphine-ader injection-molded tablets) vs extended-release morphine administered intranasally in nondependent recreational opioid users. Pain Med. 2017;18(9):1695–1705. doi: 10.1093/pm/pnw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MD, Webster LR, Lawler J, Lindhardt K, Dayno JM. Human abuse potential of an abuse-deterrent (AD), extended-release (ER) morphine product candidate (morphine-ADER injection-molded tablets) versus extended-release morphine administered orally in nondependent recreational opioid users. Pain Med. 2017;18(5):898–907. 10.1093/pm/pnw174. [DOI] [PMC free article] [PubMed]
- 21.Webster LR, Kopecky EA, Smith MD, Fleming AB. A randomized, double-blind, double-dummy study to evaluate the intranasal human abuse potential and pharmacokinetics of a novel extended-release abuse-deterrent formulation of oxycodone. Pain Med. 2016;17(6):1112–1130. doi: 10.1093/pm/pnv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster LR, Hansen E, Cater J, Smith T. A phase I placebo-controlled trial comparing the effects of buprenorphine buccal film and oral oxycodone hydrochloride administration on respiratory drive. Adv Ther. 2020;37(11):4685–4696. doi: 10.1007/s12325-020-01481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopecky EA, Fleming AB, Levy-Cooperman N, O'Connor M, Sellers EM. Oral human abuse potential of oxycodone DETERx(®) (Xtampza(®) ER). J Clin Pharmacol. 2017;57(4):500–512. 10.1002/jcph.833. [DOI] [PMC free article] [PubMed]
- 24.Shram MJ, Silverman B, Ehrich E, Sellers EM, Turncliff R. Use of remifentanil in a novel clinical paradigm to characterize onset and duration of opioid blockade by samidorphan, a potent mu-receptor antagonist. J Clin Psychopharmacol. 2015;35(3):242–249. doi: 10.1097/JCP.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vodovar D, Chevillard L, Caillé F, et al. Mechanisms of respiratory depression induced by the combination of buprenorphine and diazepam in rats. Br J Anaesth. 2021. 10.1016/j.bja.2021.10.029. [DOI] [PubMed]
- 26.Bubier JA, He H, Philip VM, et al. Genetic variation regulates opioid-induced respiratory depression in mice. Sci Rep. 2020;10(1):14970. doi: 10.1038/s41598-020-71804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available at https://clinicaltrials.gov/ct2/show/NCT03996694.