Abstract
Introduction
Guselkumab, a novel interleukin-23p19 subunit monoclonal antibody, has been shown to effectively improve the diverse manifestations of active psoriatic arthritis (PsA) in two phase 3 trials (DISCOVER-1, DISCOVER-2). Serum concentrations of extracellular matrix (ECM) biomarkers at baseline and following treatment with guselkumab were evaluated in patients with active PsA, and the relationship of these biomarkers with baseline PsA characteristics and clinical response to guselkumab treatment was explored.
Methods
Serum samples were collected at weeks 0, 4, 24, and 52 from a selected subset (N = 260) of the 739 biologic-naïve patients with PsA treated with guselkumab 100 mg every 4 or 8 weeks or placebo in DISCOVER-2. Demographically matched healthy controls (N = 76) were used for comparison. The samples were analyzed for ECM biomarkers associated with collagen degradation (C1M, C2M, C3M, C4M, C6M, C10C) and collagen formation (PRO-C1, PRO-C2, PRO-C3, PRO-C4, PRO-C6).
Results
Baseline concentrations of collagen degradation biomarkers C1M, C3M, C4M, and C6M and collagen formation biomarkers PRO-C3 and PRO-C6 were significantly higher (i.e., ≥ 1.25-fold and false discovery rate adjusted p < 0.05) in PsA patients than in healthy controls. Serum C1M, C3M, C4M, and C6M levels declined from baseline in guselkumab-treated patients in both dosing regimens. In addition, guselkumab-treated ACR20 responders (≥ 20% improvement in American College of Rhematology response criteria) had significantly lower C1M levels than ACR20 nonresponders.
Conclusion
These data demonstrate that serum collagen biomarkers are elevated in patients with PsA compared with healthy controls and that treatment with guselkumab decreases levels of C1M, C3M, C4M, and C6M. Importantly, C1M serves as a biomarker that associates with improvement of joint signs and symptoms.
Trial Registration
ClinicalTrials.gov identifier: NCT03158285.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-022-00444-x.
Keywords: Biomarkers, Collagen turnover, Extracellular matrix, Guselkumab, Psoriatic arthritis
Key Summary Points
| Why carry out this study? |
| The identification of biomarkers that are associated with psoriatic arthritis (PsA) disease and could potentially serve as predictors of treatment response in patients with PsA is an unmet need. |
| What did the study ask? |
| The effect of guselkumab treatment on serum concentrations of biomarkers associated with collagen degradation and collagen formation was evaluated in patients with active PsA. |
| What was learned from the study? |
| At baseline, serum concentrations of collagen degradation biomarkers (C1M, C3M, C4M, and C6M) and collagen formation biomarkers (PRO-C3 and PRO-C6) were significantly higher in patients with PsA than in healthy controls. |
| In the guselkumab every-4-weeks and every-8-weeks groups, serum levels of C1M, C3M, C4M and C6M decreased as early as week 4 and were lower in guselkumab-treated patients compared with placebo patients at week 24. |
| Among guselkumab-treated patients, ACR20 responders had significantly lower C1M levels at week 24 than did ACR20 nonresponders. |
Introduction
Psoriatic arthritis (PsA) is a progressive, joint-degenerative inflammatory disease characterized by swelling, pain, fatigue, and stiffness in the joints [1, 2]. Joints affected by PsA often show evidence of cartilage destruction, bone erosions, and synovitis, as well as increased new bone growth as evidenced by active osteoblasts and hypertrophic chondrocytes [1, 3]. Several therapies are available for PsA with differing mechanisms of action, and treatment guidelines have focused on individualized treatment to address as many relevant disease domains as possible [2, 4, 5]. Conventional and biologic disease-modifying antirheumatic drug (DMARD) options for PsA include methotrexate and inhibitors of tumor necrosis factor, interleukin-17A (IL-17A), IL-12/23p40, IL-23p19, phosphodiesterase 4, T cell activation, and the Janus kinase pathway [1, 2, 6].
Guselkumab, a monoclonal antibody targeting the IL-23p19 subunit, demonstrated efficacy compared with placebo in reducing skin and musculoskeletal signs and symptoms in patients with active PsA in two phase 3 trials, DISCOVER-1 and DISCOVER-2 [7, 8]. In DISCOVER-2, guselkumab 100 mg every 4 weeks (Q4W) or every 8 weeks (Q8W) improved joint symptoms, physical function, skin symptoms of psoriasis, and overall health and quality of life in biologic-naïve patients with active disease [7, 8]. In addition, evidence of inhibition of structural damage was also demonstrated for the guselkumab 100 mg Q4W dose regimen at week 24. At 1 year, radiographic progression from baseline was similar between the 100 mg Q4W and Q8W dose regimens [9].
Biomarkers can be used to elucidate normal and pathological processes and to measure biological response to a therapeutic intervention [10]. In PsA, there is a clear unmet medical need for biomarkers that can characterize patients and identify which therapy may provide the most benefit for a particular patient [10, 11]. Tissue turnover is shifted in the affected joints of patients with PsA, resulting in the release of neo-epitopic fragments from the extracellular matrix (ECM). Collagen proteins are the predominant structural element of the ECM, with the most abundant collagens of the joint tissue being types I, II, III, IV, VI, or X collagen. Markers quantifying the breakdown and formation peptides of these collagens may be useful as biomarkers of PsA [3, 12–16]. Type I collagen is the most abundant collagen in the body as it is the main structural protein of bone. The remodeling of type I collagen has been assessed by biomarkers for decades, as exemplified by the C-terminal telopeptides of type I collagen (CTX-I), a specific bone resorption marker [17]. Type II collagen is the main collagen found in the articular cartilage [18]. Types III, VI, and IV collagens are found in the interstial matrix, the intermediate tissue layer, and the basement membrane, respectively, the latter being found in the synovial tissue, skin and lungs (as examples) [14, 19]. Type X collagen is produced by hypertrophic chondrocytes as tissues become calcified [3]. Markers that reflect tissue turnover may be useful in evaluating the tissue-associated disease activity.
Degradation metabolites of these collagens are elevated in other diseases with musculoskeletal involvement, such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), and systemic sclerosis (SSc). In RA, serum C1M and C4M (markers for degradation of type I and type IV collagen, respectively) are associated with bone and synovium turnover, respectively [20]. Additionally, C1M and C3M, markers for degradation of type III collagen, are associated with inflammatory interstitial matrix tissue degradation in RA and inflammatory status in osteoarthritis [21]. C6M, a marker for degradation of type VI collagen, is elevated in AS as well as in SSc [22, 23].
The collagen formation biomarkers PRO-C1 (type I collagen), PRO-C3 (type III collagen), and PRO-C6 (type VI collagen) are used to assess the release of propeptides as the newly formed collagens are integrated into the tissue after being produced by activated fibroblasts. PRO-C1 (N-terminal propeptide of type I procollagen [PINP]) has been shown to be a biomarker of bone formation [24]. PRO-C3 and PRO-C6 more closely associate with fibrogenesis [25]. PRO-C4 (type IV collagen formation biomarker) associates with basement membrane turnover [25]. PRO-C2, C2M, and C10C (biomarker of cathepsin-degraded type X collagen) were originally used to assess articular cartilage turnover and chondrocyte differentiation induced by growth factors [25–27]. These growth factors are involved in dedifferentiation of joint cells in PsA and, thus, the biomarkers may reflect ongoing damage to the cartilage.
Given the need for predictive and pharmacodynamic/response biomarkers for PsA treatment and increased collagen turnover in PsA [12, 13], serum samples collected from a subset of individuals with PsA who participated in DISCOVER-2 were compared to those from healthy controls. We examined the relationship between serum concentrations of tissue-derived ECM product biomarkers with PsA disease phenotypes and with guselkumab treatment response.
Methods
DISCOVER-2 Participants and Study Design
DISCOVER-2 was a double-blind, randomized, placebo-controlled, phase 3 study of guselkumab in adults with active PsA [8]. Details of the DISCOVER-2 study design and patient populations have been reported elsewhere [8, 9]. Briefly, participants had active PsA, with at least five tender and five swollen joints, and a C-reactive protein (CRP) concentration of at least 0.6 mg/dL despite standard therapies, and were naïve to biologic therapies. Patients were randomized (1:1:1) to receive subcutaneous injections of guselkumab 100 mg Q4W; guselkumab 100 mg at weeks 0 and 4, then Q8W; or placebo. At week 24, patients in the placebo group crossed over to receive guselkumab Q4W.
Biomarker Study Participants
Of the 739 treated patients in DISCOVER-2, these exploratory analyses were limited to a subset of 260 participants (Q4W, n = 82; Q8W, n = 92; placebo, n = 86). Serum collagen biomarkers were evaluated for patients for whom serum samples were available at baseline and at least one post-baseline visit, with priority given to those who had samples available at weeks 0, 4, 24, and 52. Patients were further selected by baseline demographic and disease characteristics to ensure that the biomarker subset was reflective of the total study population (Table 1).
Table 1.
Baseline demographic and disease characteristics of biomarker subset of DISCOVER-2 patients
| Baseline demographic and disease characteristics | DISCOVER-2 biomarker subset | DISCOVER-2 study cohorta | |||
|---|---|---|---|---|---|
| Guselkumab 100 mg | Placebo | Total | |||
| Q4W | Q8W | ||||
| Patients, N | 82 | 92 | 86 | 260 | 739 |
| Demographics | |||||
| Age (years) | 45.1 (10.8) | 45.1 (11.6) | 46.1 (10.9) | 45.4 (11.1) | 45.7 (11.7) |
| Male | 51 (62.2%) | 49 (53.3%) | 42 (48.8%) | 142 (54.6%) | 388 (52.5%) |
| White | 81 (98.8%) | 87 (94.6%) | 85 (98.8%) | 253 (97.3%) | 724 (98.0%) |
| Weight (kg) | 87.0 (18.5) | 82.3 (18.5) | 80.6 (18.4) | 83.2 (18.6) | 84.3 (19.5) |
| Disease characteristics | |||||
| PsA disease duration (years) | 6.2 (5.9) | 5.5 (5.1) | 7.0 (5.9) | 6.2 (5.6) | 5.5 (5.7) |
| DAS28-CRP | 5.3 (1.0) | 5.2 (1.0) | 5.5 (0.9) | 5.3 (1.0) | 5.2 (1.0) |
| Swollen joint count (0–66) | 14.5 (9.1) | 12.1 (7.8) | 13.1 (7.0) | 13.2 (8.0) | 12.3 (7.2) |
| Tender joint count (0–68) | 24.5 (14.4) | 20.0 (12.5) | 22.5 (13.1) | 22.3 (13.4) | 21.3 (12.9) |
| Psoriasis Area Severity Index (0–72) | 11.5 (11.6) | 8.9 (10.9) | 9.9 (11.4) | 10.1 (11.3) | 9.9 (11.1)b |
| Body surface area affected by psoriasis, % | 18.5 (19.3) | 17.4 (21.3) | 17.6 (20.9) | 17.8 (20.5) | 17.4 (20.4) |
| Concomitant medications | |||||
| MTX | 48 (58.5%) | 56 (60.9%) | 55 (64.0%) | 159 (61.1%) | 443 (59.9%) |
| Oral corticosteroids | 11 (13.4%) | 21 (22.8%) | 16 (18.6%) | 48 (18.5%) | 145 (19.6%) |
| NSAIDs | 66 (80.5%) | 62 (67.4%) | 58 (67.4%) | 186 (71.5%) | 504 (68.2%) |
Data are reported as the mean with the standard deviation (SD) in parenthesis, or as n with the percentage (%) in parentheses, unless otherwise noted
DAS28-CRP Disease Activity Score in 28 Joints using C-reactive protein, MTX methotrexate, NSAID nonsteroidal anti-inflammatory drug, PsA psoriatic arthritis, Q4W every 4 weeks, Q8W every 8 weeks
ap values were calculated for comparisons of the total biomarker cohort vs. the overall DISCOVER-2 study cohort using two-tailed Fisher's exact test for categorical variables and the two-tailed t test for continuous variables. All p values were > 0.05 with the exception of the mean DAS28-CRP score, which had a p value of 0.049
bN = 738
Healthy Controls
Serum samples from healthy controls (N = 76) were selected from an internal biobank at Nordic Bioscience (Herlev, Denmark). The biobank consists of samples acquired from commercial sources, including from BioIVT (West Sussex, UK) and Discovery Life Sciences (San Luis Obispo, CA, USA), and samples were collected according to the European Union General Data Protection Regulation and U.S. ethical regulations and privacy act. These samples were matched for age and gender to the DISCOVER-2 PsA biomarker subset (n = 260). The median age of the healthy control population was 45 (range 20–69) years, and there were 38 (50%) males.
Biomarker Analyses
Blood samples were collected from the subset of DISCOVER-2 participants (N = 260) for biomarker analyses at weeks 0 (pre-treatment, referred to here as baseline), 4, 24, and 52. The samples were collected in standard serum separation tubes, prepared into aliquots, frozen at less than or equal to − 20 °C, and then shipped frozen on dry ice to Covance Central Laboratory Services (Indianapolis, IN, USA) for storage prior to biomarker measurement.
For this study, serum protein concentrations of 11 serum biomarkers of ECM collagen formation and degradation were measured at Nordic Bioscience following methods previously described [12, 13]. The biomarkers assayed were collagen formation biomarkers PRO-C1, PRO-C2, PRO-C3, PRO-C4, and PRO-C6 and collagen degradation biomarkers C1M, C2M, C3M, C4M, C6M, and C10C [22, 23, 27]. All biomarkers were measured by competitive immunoassays using manual enzyme-linked immunosorbent assays (ELISAs) or the Immunodiagnostic Systems robotic platform (IDS-i10; Immunodiagnostic Systems (IDS), Bolden, Tyne & Wear, UK). Samples were rerun if the duplicate coefficient of variation was > 15.0%. Intra- and inter-assay variation was < 15.0%. All runs included the same three quality control samples, which were to be within 20% range of the set target value.
Statistical Methods
Biomarker data were log2-transformed, and Spearman rank correlation or general linear models (GLMs) were used for analyses. For correlations, statistical significance was defined as GLM-adjusted p < 0.05. The strength of the correlation was defined using Spearman rank correlation coefficient (rs) as weak (|rs| < 0.25), modest (0.60 > |rs| ≥ 0.25), or strong (|rs| ≥ 0.60). Significance of associations of demographic and clinical variables across treatment groups was tested by analysis of variance F tests and Chi-square tests for continuous and categorical variables, respectively. For comparison of biomarkers between patients with PsA and healthy controls, between guselkumab-treated and placebo-treated PsA patients, and between PsA clinical responders and non-responders, GLM was used and significance was defined by false discovery rate adjusted (adj) p < 0.05 and fold difference of ≥ 1.25. Clinical response was defined as achieving ≥ 20% improvement in American College of Rhematology response criteria (ACR20 response), the primary endpoint of DISCOVER-2, ACR50 response (≥ 50% improvement in ACR criteria), and ≥ 75% improvement in the Psoriasis Area and Severity Index (PASI75 response) at week 24. For response analyses, guselkumab arms were combined to increase the power of the analyses.
Compliance with Ethics Guidelines
This study (ClinicalTrials.gov identifier: NCT03158285) was conducted according to the Declaration of Helsinki of 1964 and its later amendments and the International Committee on Harmonisation good clinical practices. Protocols were approved by ethics committees at each site (Sterling IRB approval number for US sites: 5910C), and all patients provided written informed consent.
Results
Baseline Characteristics and Efficacy of DISCOVER-2 Biomarker Subset
The baseline characteristics of the biomarker subset of DISCOVER-2 PsA patients were assessed (Table 1). As with the overall DISCOVER-2 population [8], demographics, baseline clinical characteristics, and baseline collagen biomarker levels were generally balanced across treatment arms within the subset (Table 1; Electronic Supplementary Material [ESM] Table 1). In addition, baseline demographic and disease characteristics were similar between the biomarker subset and the overall DISCOVER-2 study population (Table 1) [8].
Similar to the reported findings from the full clinical cohort in DISCOVER-2 [8], the biomarker subset had significantly higher rates of clinical response in guselkumab-treated patients compared to placebo-treated patients (measured at week 24 by ACR20 [67% in the Q4W group, 59% in the Q8W group, and 26% in the placebo group; adj p < 0.0001] and by PASI75 [78% in the Q4W and Q8W groups, and 24% in the placebo group; adj p < 0.0001]) (ESM Table 2).
Collagen Biomarkers at Baseline in PsA Patients Versus Healthy Controls
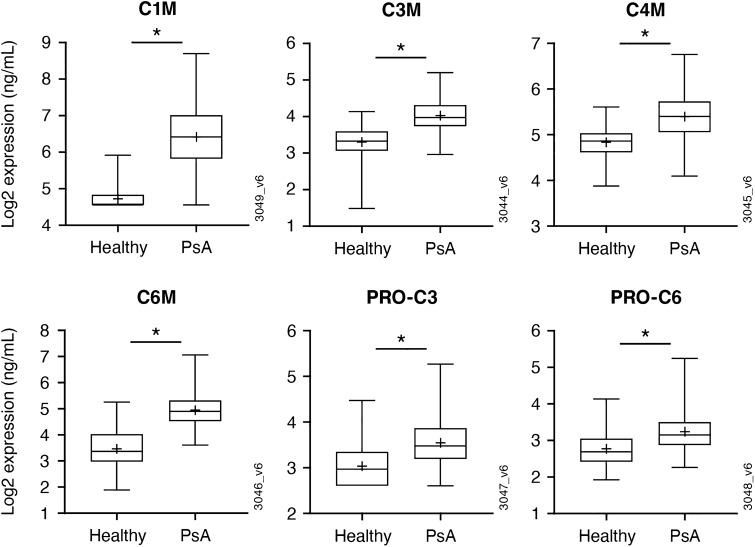
Differences in the serum concentrations of collagen biomarkers in patients with PsA versus healthy controls were assessed (Fig. 1; ESM Fig. 1). At baseline, concentrations of collagen degradation biomarkers C1M, C3M, C4M, and C6M and collagen formation biomarkers PRO-C3 and PRO-C6 were significantly higher (i.e., ≥ 1.25-fold and adj p < 0.05) in patients with PsA compared with matched healthy controls. C10C and PRO-C1 also trended toward upregulation in PsA patients compared with healthy controls, but the difference did not reach statistical significance (1.17-fold; ESM Fig. 1). Serum C2M, PRO-C2, and PRO-C4 concentrations at week 0 did not differ between PsA patients and healthy controls (ESM Fig. 1).
Fig. 1.
Upregulation of collagen degradation and formation biomarkers in the serum of PsA patients (PsA biomarker subset: N = 260) compared to healthy controls (healthy control subset: N = 76). Healthy controls were demographically matched (age and sex) to the PsA biomarker subset. Values are shown as the median (mean is shown as a (plus [+] sign). Boxes represent the interquartile range, while whiskers represent the minimum and maximum values. The asterisk indicates significance defined by a fold change ≥ 1.25 and adjusted (adj) p < 0.05. C1M, C3M, C4M, C6M collagen degradation biomarkers, PRO-C1, PRO-C3 collagen formation biomarkers, PsA psoriatic arthritis
Collagen Biomarkers and Baseline Clinical Features in PsA Patients
Collagen biomarkers were assessed for their association with baseline clinical features in patients with PsA, including PASI score, baseline body surface area (BSA) with psoriasis, CRP, and joint manifestations (Table 2). Significant and modest correlations were seen between baseline levels of collagen degradation biomarkers C1M, C3M, C4M, and C6M and collagen formation biomarker PRO-C4 and baseline PASI score (rs = 0.25–0.32; p < 0.05). Significant and modest correlations were also seen between C3M, C4M, and C6M and baseline BSA with psoriasis involvement (rs = 0.27–0.30; p < 0.05). Regarding PsA joint manifestations, baseline levels of C1M, C3M, C4M, and C6M were positively correlated with baseline Disease Activity Score in 28 Joints using CRP (DAS28-CRP) (rs = 0.29–0.35; p < 0.05) and baseline CRP levels (rs = 0.65–0.74; p < 0.05). C10C was inversely correlated with baseline CRP (rs = − 0.27; p < 0.05). Correlations between baseline levels of select collagen degradation biomarkers and baseline swollen joint and tender joint counts and enthesitis and dactylitis scores were weak (all rs < 0.25), but statistically significant (p < 0.05). Other than baseline PRO-C4 levels modestly correlating with PASI score (rs = 0.28; p < 0.05), correlations between collagen formation biomarkers and baseline clinical features of PsA were generally weak or not statistically significant.
Table 2.
Correlations between collagen biomarkers and baseline clinical features of PsA (rs values)
| Collagen biomarkers | PSO BSA | PASI | DAS28-CRP | SJC66 | TJC68 | Baseline CRP | Enthesitis Score | Dactylitis Score |
|---|---|---|---|---|---|---|---|---|
| C1M | 0.24 | 0.25 | 0.30 | 0.13 | 0.09 | 0.74 | 0.10 | 0.11 |
| C2M | − 0.02 | − 0.04 | 0.07 | 0.04 | 0.04 | − 0.05 | 0.01 | 0.03 |
| C3M | 0.28 | 0.31 | 0.29 | 0.18 | 0.08 | 0.65 | 0.10 | 0.15 |
| C4M | 0.30 | 0.32 | 0.34 | 0.17 | 0.12 | 0.73 | 0.13 | 0.14 |
| C6M | 0.27 | 0.27 | 0.35 | 0.22 | 0.16 | 0.68 | 0.14 | 0.15 |
| C10C | − 0.08 | − 0.09 | 0.01 | 0.03 | 0.01 | − 0.27 | − 0.02 | − 0.05 |
| PRO-C1 | − 0.05 | 0.01 | − 0.01 | 0.08 | − 0.01 | 0.02 | − 0.07 | 0.07 |
| PRO-C2 | 0.14 | 0.13 | − 0.02 | − 0.03 | − 0.04 | − 0.12 | − 0.04 | 0.02 |
| PRO-C3 | 0.03 | 0.05 | 0.09 | 0.05 | 0.01 | 0.11 | 0.01 | 0.04 |
| PRO-C4 | 0.23 | 0.28 | 0.07 | 0.08 | 0.02 | 0.15 | 0.05 | 0.13 |
| PRO-C6 | 0.15 | 0.15 | 0.04 | 0.04 | − 0.06 | 0.14 | − 0.03 | 0.01 |
N = 260
Values presented in italics are statistically significant (GLM adusted [adj] p < 0.05). Values presented in italics with underlining are statistically significant (GLM adj p < 0.05) for modest (0.60 >|rs|≥ 0.25) or strong (|rs|≥ 0.60) correlation
BSA Body surface area, GLM general linear model, PASI Psoriasis Area and Severity Index, PSO psoriasis, rs Spearman rank correlation coefficient, SJC66 swollen joint count (0–66), TJC68 tender joint count (0–68), Enthesitis Score Leeds Enthesitis Index 0–6, Dactylitis Score Dactylitis Severity Score 0–60
Effect of Guselkumab on Collagen Biomarkers
The effect of guselkumab on collagen biomarkers was measured at weeks 4, 24, and 52 (Fig. 2). Beginning at week 4, serum C1M, C3M, C4M, and C6M levels declined from baseline in guselkumab-treated patients in both dosing regimens, with further decreases from baseline levels noted at week 24. At week 24, serum C1M, C3M, C4M, and C6M levels were lower in both guselkumab groups versus placebo (C1M: Q4W adj p < 0.05, Q8W adj p = 0.05; C3M and C4M: adj p < 0.05 for each dose arm; C6M: Q4W adj p = 0.066, Q8W adj p < 0.05) (Fig. 2; ESM Table 3). Trends were consistent for both guselkumab dosing regimens, although patients receiving guselkumab 100 mg Q4W exhibited a trend for greater decline in C1M. Reduced serum C1M, C3M, C4M, and C6M levels were maintained at week 52. Following crossover to guselkumab Q4W at week 24, patients randomized to placebo also demonstrated a reduction in C1M, C2M, C3M, and C6M levels by week 52 (adj p < 0.0001) (ESM Table 3), thus confirming the pharmacodynamic effect of IL-23p19 inhibition on these collagen biomarkers.
Fig. 2.
Collagen degradation biomarkers (C1M, C3M, C4M, and C6M) significantly decrease with GUS vs. PBO at week 24. GUS treatment Q4W or Q8W significantly reduced collagen degradation biomarkers C1M, C3M, C3M, and C6M compared to PBO at 24 weeks. Asterisk indicates significant difference vs. PBO at adj pGUSvsPBO < 0.05. Dagger indicates signficant difference vs. placebo at pGUSvsPBO < 0.07. Error bars represent 1 standard error. GUS Guselkumab, Q4W, Q8W every 4 weeks, every 8 weeks, PBO placebo
No significant changes in other biomarkers, including collagen formation biomarkers PRO-C1, PRO-C2, PRO-C3, PRO-C4, and PRO-C6 and degradation biomarkers C2M and C10C, were observed following guselkumab or placebo treatment (data not shown).
Relationships Between Collagen Biomarker Levels and Clinical Outcomes
Levels of C1M at baseline, week 4, and week 24 were analyzed by ACR20 response at week 24, the primary endpoint for the DISCOVER-2 study (Fig. 3). Expression of collagen biomarkers at baseline and at week 4 was similar between week 24 ACR20 responders and nonresponders across treatment groups. In the combined guselkumab-treated Q4W and Q8W group at week 24, ACR20 responders had significantly lower C1M levels than did ACR20 nonresponders (− 1.26 fold; adj p = 0.0065). Similar results were observed for ACR50 responders versus ACR20 nonresponders (data not shown).
Fig. 3.
Significantly lower C1M expression in ACR20 responders vs. nonresponders at baseline and weeks 4 and 24. Response was defined by ACR20 (≥ 20% improvement according to the American College of Rheumatology criteria) at week 24. Values are shown as the median (mean is shown as a (plus [+] sign). Boxes represent the interquartile range, while whiskers represent the minimum and maximum values. Asterisk indicates signficant difference at p = 0.0065 between responder and nonresponder defined by fold change ≥ 1.25 and adj p < 0.05
No significant differences in biomarker levels between PASI75 responders and nonresponders were seen at any timepoint (including baseline; data not shown), indicating a lack of association between these biomarker changes and PsA skin response.
Discussion
This work demonstrates that collagen biomarkers are detectable and elevated in the serum of patients with PsA compared with healthy controls [12, 28] and that guselkumab treatment reduces the levels of specific collagen biomarker proteins.
The collagen biomarkers assessed in the current study are released from the ECM of connective tissues. In healthy tissues, there is a delicate balance between the formation and degradation of ECM components, such as collagens. The imbalance that occurs in inflammatory and fibro-proliferative diseases results in increased degradation and/or formation, leading to loss of tissue, altered tissue composition, or thickening and stiffening of the tissue [25]. To our knowledge, there are limited reports on how the tissue balance is altered in PsA compared to the homeostasis maintained between formation and degradation in healthy individuals. Therefore, in this study, biomarkers of both collagen formation (fibrogenesis) and collagen degradation (fibrolysis) were evaluated to gain greater insight into the shift in tissue balance in patients with PsA.
At baseline, serum concentrations of collagen degradation biomarkers C1M, C3M, C4M, and C6M and collagen formation biomarkers PRO-C3 and PRO-C6 were significantly higher in patients with PsA compared with matched healthy controls, which was indicative of the cartilage turnover experienced by patients with PsA that often results in swollen and tender joints [28]. The collagen degradation markers C1M, C3M, C4M, and C6M were positively correlated with measures of baseline skin disease, DAS28-CRP, and general inflammation (CRP) in this study. In addition, weak, but statistically significant correlations were observed between these biomarkers and baseline swollen joint count. C3M and C4M have been implicated in synovitis, or inflammation of the joint synovial membrane, which is associated with symptoms of pain, swelling, and stiffness [29, 30], and type VI collagen plays a role in regulating synovial joint physiology [19]. Guselkumab treatment resulted in a decrease in collagen degradation biomarkers C1M, C3M, C4M, and C6M, suggesting that guselkumab may suppress inflammation-driven tissue degradation. On the other hand, collagen formation biomarkers PRO-C3 and PRO-C6 were not modulated in response to guselkumab. These biomarkers have been reported to be associated with the activation of dermal and lung fibroblasts and to be elevated in patients with active scleroderma [22, 31], suggesting that the biomarkers may be associated with skin remodeling; as such, they are interesting in the context of PsA and psoriasis. This raises two questions that cannot be answered in the current study. Are the elevated levels of the collagen formation biomarkers associated with increased fibrosis of, for example, the skin in PsA, or are these biomarkers a reflection of the repair mechanism that is initiated to counteract the increased inflammation-driven tissue degradation reflected by the collagen degradation biomarkers?
C2M, a degradation product of type II collagen, is a biomarker of cartilage destruction [32]. Interestingly, in this set of PsA patients, we did not detect an increase in either C2M or PRO-C2 in comparison to healthy controls, potentially indicating a lack of cartilage involvement in this cohort.
This work identified C1M as an important biomarker for PsA as C1M levels were found to be elevated in the serum of the patients with PsA. C1M has been reported to be elevated in RA and associated with radiographic progression [33]. In contrast to the bone resorption marker C-terminal telopeptide of type 1 collagen, C1M is highly correlated with CRP and derived through the action of matrix metalloproteinases, indicating that C1M is a direct marker of inflammation-driven bone degradation [34]. Thus, the increased levels observed in patients with PsA may reflect the relevance of this protein in the joint damage associated with PsA. Guselkumab treatment significantly reduced serum C1M levels at 24 weeks. For context, the log2 reduction of − 0.6 shown in Fig. 2 translates to a reduction of approximately 42% in C1M from baseline. In patients with RA, this level of reduction was correlated with improvement in joint space narrowing scores [35].
Importantly, this work has shown that C1M also serves as a biomarker that associates with joint response. Patients achieving ACR20 or ACR50 response at week 24 showed lower C1M levels than ACR20 nonresponders, further emphasizing the association of this biomarker with clinical disease activity. Specifically, diminution of inflammation-driven bone degradation—as reflected by lower C1M levels (Fig. 3)—may lead to improvements in ACR components (e.g., joint swelling and tenderness), as reflected by achievement of ACR response. ACR20 and ACR50 responders in the placebo group also had numerically lower C1M levels at week 24 compared with ACR20 nonresponders, suggesting that C1M may be associated with disease activity rather than the pharmacodynamic effect of guselkumab. No differences in serum protein levels were observed in patients who achieved PASI75 compared with those who did not realize this degree of skin response, indicating that collagen biomarker changes may not be associated with skin clearance.
There are several limitations to this work, which will require further exploration. First, this study evaluated collagen biomarker levels in the serum, and additional work is needed to understand, more directly, the collagen turnover in affected tissues. Also, while we showed that biomarkers may serve as a peripheral measure of underlying joint inflammation and damage, additional work is needed to further explore the relationship of these biomarkers to PsA. In addition, data on other characteristics that may influence these biomarker levels, such as factors related to diet, environment, or genetics, were not collected for either the DISCOVER-2 study population or the healthy controls. The understanding of collagen turnover biomarkers, particularly in relation to rheumatic diseases, is an evolving field, and will require additional, large cohort studies to further delineate the role of these biomarkers.
Conclusions
In summary, results of this investigation indicate that serum collagen biomarkers C1M, C3M, C4M, C6M, PRO-C3, and PRO-C6 are elevated in patients with PsA compared with healthy controls. We also observed significant correlations between serum C1M, C3M, C4M, and C6M levels and PASI scores, DAS28-CRP scores, and CRP level at baseline. Levels of C1M, C3M, C4M, and C6M significantly decreased with guselkumab treatment at week 24, and lower C1M levels at week 24 were associated with achievement of ACR response at week 24, suggesting that collagen degradation biomarkers, individually or in combination, might be useful in evaluating changes in disease activity after treatment. The need for surrogate blood-based biomarkers of the joint to provide a more granular understanding of disease progression or improvement to support PsA patient care and clinical trials of new therapies is clear, and the results of this study suggest that serum collagen degradation and formation biomarkers have potential as PsA biomarkers. This work provides insight into the potential utility of ECM biomarkers in PsA patients and evidence that guselkumab may decrease collagen turnover.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients in DISCOVER-2 for their participation in the study.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Janssen Research & Development, LLC.
Medical Writing and Editorial Assistance
The authors thank Michelle L. Perate, M.S. of Janssen Scientific Affairs, LLC, and Diane Harrison, M.D. (consultant funded by Janssen Research & Development, LLC) for substantive review. Medical writing support was provided by Amy C. Porter, PhD of Certara Synchrogenix, under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2015;163:461-4) and was funded by Janssen Scientific Affairs, LLC.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Georg Schett, Matthew J. Loza, Arumugam Palanichamy, Oliver FitzGerald, Christopher Ritchlin, Elizabeth C. Hsia, Alexa P. Kollmeier, Xie L. Xu, Frédéric Baribaud, and Kristen Sweet were responsible for the study design. Matthew J. Loza, Anne-Christine Bay-Jensen, Signe Holm Nielsen, Sheng Gao and Kristen Sweet were responsible for data collection and analysis. Georg Schett, Matthew J. Loza, Arumugam Palanichamy, Oliver FitzGerald, Christopher Ritchlin, Anne-Christine Bay-Jensen, Signe Holm Nielsen, Sheng Gao Elizabeth C. Hsia, Alexa P. Kollmeier, Xie L. Xu, Frédéric Baribaud and Kristen Sweet were responsible for interpreting the data. Drafting and critical revisions were carried out by Georg Schett, Matthew J. Loza, Arumugam Palanichamy, Oliver FitzGerald, Christopher Ritchlin, Anne-Chrisitne Bay-Jensen, Signe Holm Nielsen, Sheng Gao, Elizabeth C. Hsia, Alexa P. Kollmeier, Xie L. Xu, Frédéric Baribaud and Kristen Sweet.
Prior Presentation
These data have been presented, in part, at the American College of Rheumatology 2020 Annual Meeting.
Disclosures
Georg Schett has received speaker's honoraria from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Gilead, Janssen, Novartis, and UCB. Christopher Ritchlin has received grant/research support from AbbVie, Amgen, and UCB Pharma and consulting fees from AbbVie, Amgen, AbbVie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB Pharma. Anne-Christine Bay-Jensen and Signe Holm Nielsen are employees of and own stock in Nordic Bioscience. Matthew J. Loza, Arumugam Palanichamy, Sheng Gao, Elizabeth C Hsia, Alexa P Kollmeier, Kristen Sweet, Xie L. Xu, and Frédéric Baribaud are or were employees of Janssen Research & Development, LLC, when this work was performed and own stock in Johnson & Johnson, of which Janssen Research & Development, LLC is a wholly-owned subsidiary. Arumugam Palanichamy is currently an employee of Cerecor, Inc. Frédéric Baribaud is currently an employee of Bristol Myers Squibb.
Compliance with Ethics Guidelines
This study (ClinicalTrials.gov identifier: NCT03158285) was conducted according to the Declaration of Helsinki of 1964 and its later amendments and the International Committee on Harmonisation good clinical practices. Protocols were approved by ethics committees at each site (Sterling IRB approval number for US sites: 5910C), and all patients provided written informed consent.
Data Availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Footnotes
“The original online version of this article was revised:” to correct errors in the result, keyword and table values
Change history
7/2/2022
A Correction to this paper has been published: 10.1007/s40744-022-00470-9
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 2.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gudmann NS, Munk HL, Christensen AF, et al. Chondrocyte activity is increased in psoriatic arthritis and axial spondyloarthritis. Arthritis Res Ther. 2016;18:141. doi: 10.1186/s13075-016-1040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res. 2019;71:2–29. doi: 10.1002/acr.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 6.Noviani M, Feletar M, Nash P, Leung YY. Choosing the right treatment for patients with psoriatic arthritis. Ther Adv Musculoskelet Dis. 2020;12:17. doi: 10.1177/1759720X20962623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–1125. doi: 10.1016/S0140-6736(20)30265-8. [DOI] [PubMed] [Google Scholar]
- 8.Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1126–1136. doi: 10.1016/S0140-6736(20)30263-4. [DOI] [PubMed] [Google Scholar]
- 9.McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through 1 year in biologic-naïve psoriatic arthritis patients. Arthritis Rheumatol. 2021;73:604–616. doi: 10.1002/art.41553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. 2016. https://www.ncbi.nlm.nih.gov/books/NBK338448/. Accessed 29 Nov 2021. [PubMed]
- 11.Jadon DR, Stober C, Pennington SR, FitzGerald O. Applying precision medicine to unmet clinical needs in psoriatic disease. Nat Rev Rheumatol. 2020;16:609–627. doi: 10.1038/s41584-020-00507-9. [DOI] [PubMed] [Google Scholar]
- 12.Gudmann NS, Siebuhr AS, Christensen AF, et al. Type I and III collagen turnover is increased in axial spondyloarthritis and psoriatic arthritis. Associations with disease activity and diagnostic capacity. Clin Exp Rheumatol. 2017;35:653–659. [PubMed] [Google Scholar]
- 13.Sardar S, Kristensen S, Siebuhr AS, et al. Accelerated extracellular matrix remodeling in psoriatic arthritis and value of ECM-related biomarkers. Ann Rheum Dis. 2019;78:867. doi: 10.1136/annrheumdis-2019-215221. [DOI] [PubMed] [Google Scholar]
- 14.Hušáková M, Bay-Jensen AC, Forejtová Š, et al. Metabolites of type I, II, III, and IV collagen may serve as markers of disease activity in axial spondyloarthritis. Sci Rep. 2019;9:11218. doi: 10.1038/s41598-019-47502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holm Nielsen S, Sardar S, Siebuhr AS, et al. Effect of n-3 PUFA on extracellular matrix protein turnover in patients with psoriatic arthritis: a randomized, double-blind, placebo-controlled trial. Rheumatol Int. 2021;41:1065–1077. doi: 10.1007/s00296-021-04861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groen SS, Holm Nielsen S, Magee C, et al. Neo-epitopes of type I collagen can be utilized as translational biomarkers for skin and joint turnover in patients with psoriasis and psoriatic arthritis. Arthritis Rheumatol. 2020;720.
- 17.Nagy EE, Nagy-Finna C, Popoviciu H, Kovacs B. Soluble biomarkers of osteoporosis and osteoarthritis, from pathway mapping to clinical trials: an update. Clin Interv Aging. 2020;15:501–518. doi: 10.2147/CIA.S242288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudmann NS, Wang J, Hoielt S, et al. Cartilage turnover reflected by metabolic processing of type II collagen: a novel marker of anabolic function in chondrocytes. Int J Mol Sci. 2014;15:18789–18803. doi: 10.3390/ijms151018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Sinkeviciute D, He Y, et al. The minor collagens in articular cartilage. Protein Cell. 2017;8:560–572. doi: 10.1007/s13238-017-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thudium CS, Nielsen SH, Sardar S, et al. Bone phenotypes in rheumatology—there is more to bone than just bone. BMC Musculoskelet Disord. 2020;21:789. doi: 10.1186/s12891-020-03804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjelgaard-Petersen CF, Platt A, Braddock M, et al. Translational biomarkers and ex vivo models of joint tissues as a tool for drug development in rheumatoid arthritis. Arthritis Rheumatol. 2018;70:1419–1428. doi: 10.1002/art.40527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhl P, Bay-Jensen AC, Karsdal M, Siebuhr AS, Franchimont N, Chavez J. Serum biomarkers of collagen turnover as potential diagnostic tools in diffuse systemic sclerosis: a cross-sectional study. PLoS ONE. 2018;13:e0207324. doi: 10.1371/journal.pone.0207324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bay-Jensen AC, Leeming DJ, Kleyer A, Veidal SS, Schett G, Karsdal MA. Ankylosing spondylitis is characterized by an increased turnover of several different metalloproteinase-derived collagen species: a cross-sectional study. Rheumatol Int. 2012;32:3565–3572. doi: 10.1007/s00296-011-2237-8. [DOI] [PubMed] [Google Scholar]
- 24.Szulc P. Bone turnover: biology and assessment tools. Best Pract Res Clin Endocrinol Metab. 2018;32:725–738. doi: 10.1016/j.beem.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Karsdal MA, Kraus VB, Shevell D, et al. Profiling and targeting connective tissue remodeling in autoimmunity—a novel paradigm for diagnosing and treating chronic diseases. Autoimmun Rev. 2021;20:102706. doi: 10.1016/j.autrev.2020.102706. [DOI] [PubMed] [Google Scholar]
- 26.Reker D, Siebuhr AS, Thudium CS, et al. Sprifermin (rhFGF18) versus vehicle induces a biphasic process of extracellular matrix remodeling in human knee OA articular cartilage ex vivo. Sci Rep. 2020;10:6011. doi: 10.1038/s41598-020-63216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Manon-Jensen T, Arendt-Nielsen L, et al. Potential diagnostic value of a type X collagen neo-epitope biomarker for knee osteoarthritis. Osteoarthr Cartil. 2019;27:611–620. doi: 10.1016/j.joca.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Jadon DR, Nightingale AL, McHugh NJ, Lindsay MA, Korendowych E, Sengupta R. Serum soluble bone turnover biomarkers in psoriatic arthritis and psoriatic spondyloarthropathy. J Rheumatol. 2015;42:21–30. doi: 10.3899/jrheum.140223. [DOI] [PubMed] [Google Scholar]
- 29.Gudmann NS, Junker P, Juhl P, et al. Type IV collagen metabolism is associated with disease activity, radiographic progression and response to tocilizumab in rheumatoid arthritis. Clin Exp Rheumatol. 2018;36:829–835. [PubMed] [Google Scholar]
- 30.Petersen KK, Siebuhr AS, Graven-Nielsen T, et al. Sensitization and serological biomarkers in knee osteoarthritis patients with different degrees of synovitis. Clin J Pain. 2016;32:841–848. doi: 10.1097/AJP.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 31.Kubo S, Siebuhr AS, Bay-Jensen AC, et al. Correlation between serological biomarkers of extracellular matrix turnover and lung fibrosis and pulmonary artery hypertension in patients with systemic sclerosis. Int J Rheum Dis. 2020;23:532–539. doi: 10.1111/1756-185X.13804. [DOI] [PubMed] [Google Scholar]
- 32.Bay-Jensen AC, Liu Q, Byrjalsen I, et al. Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM–increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin Biochem. 2011;44:423–429. doi: 10.1016/j.clinbiochem.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Drobinski PJ, Bay-Jensen AC, Karsdal MA, Sardar S, Siebuhr AS. Connective tissue remodelling is differently modulated by tocilizumab versus methotrexate monotherapy in patients with early rheumatoid arthritis: the AMBITION study. Arthritis Res Ther. 2021;23:13. doi: 10.1186/s13075-020-02378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bay-Jensen AC, Platt A, Jenkins MA, et al. Tissue metabolite of type I collagen, C1M, and CRP predicts structural progression of rheumatoid arthritis. BMC Rheumatol. 2019;3:3. doi: 10.1186/s41927-019-0052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siebuhr AS, Bay-Jensen AC, Leeming DJ, et al. Serological identification of fast progressors of structural damage with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R86. doi: 10.1186/ar4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.