Abstract
Introduction
Postherpetic neuralgia (PHN) is a painful condition that persists for 1 month or more after herpes zoster rash has healed. Radiofrequency thermocoagulation (RF-TC) provides analgesia by destroying the dorsal root ganglion and blocking the pain upload pathway; nonetheless, the concomitant neurological-related side effects and recurrence remain a concern.
Methods
In this study, 228 patients with PHN in the thoracic segment treated with RF-TC of the dorsal root ganglion of the spinal nerve were included, and were followed up regularly after surgery. The numerical rating scale (NRS) scores, time to recurrence, and intraoperative and postoperative adverse events were recorded and analyzed. The Kaplan–Meier method was used to plot survival curves and calculate the cumulative effective rate and recurrence rate. Cox regression analyses were performed to identify factors associated with postoperative recurrence. Predictive models were built to assess the value of applications.
Results
The NRS scores decreased in all postoperative periods compared with preoperative ones. At 10-year-follow-up, recurrence was observed in 34.6% (79/228) of patients that underwent PHN. The main postoperative complications were numbness and reduced abdominal muscle strength, which gradually decreased with time, while the abdominal muscle strength gradually recovered. No other adverse events occurred. Interval-censored multivariable Cox regression analysis demonstrated that disease course, complications, pain grade, and type of RF electrode were associated with a significantly higher risk of relapse. The main intraoperative adverse effect was a transient increase in pain during RF-TC.
Conclusion
CT-guided RF-TC of the dorsal root ganglion of the spinal nerve for PHN is a relatively safe and effective surgical option. Disease course, type of RF electrode, complications, and pain grade are risk factors for postoperative recurrence and can assist in clinical decision-making before the RF-CT procedure.
Keywords: Postherpetic neuralgia, Radiofrequency thermocoagulation, Efficacy, Safety, Recurrence factors, Predictive model
Key Summary Points
| Postherpetic neuralgia (PHN) is the most common and severe complication of herpes zoster. The existing treatments are difficult to cure. |
| Radiofrequency thermocoagulation (RF-TC) provides analgesia by destroying the dorsal root ganglion and blocking the pain upload pathway; nonetheless, the concomitant neurological-related side effects and recurrence remain a concern. |
| With long-term follow-up, we observed that mean postprocedural NRS scores were lower than those at the preprocedural stage. No other complications were found except for numbness and abdominal bulging. Cox regression analysis demonstrated that disease course, complications, pain grade, and type of RF electrode were associated with a significantly higher risk of relapse. |
| In our opinion, CT-guided RF-TC of the dorsal root ganglion for PHN is a relatively safe and effective surgical option. If patients are willing to accept pain relief at the cost of numbness, RF-TC of the dorsal root ganglion should be considered. |
Introduction
Herpes zoster (HZ) is caused by reactivation of varicella-zoster virus (VZV), which is latent and persists in the dorsal root ganglion (DRG) and cranial ganglia after the initial infection [1]. When the patient's immune function decreases, the latent VZV may start replicating again, and the reactivation of the virus leads to inflammation and necrosis in one or more affected ganglia[2]. The global annual incidence of HZ reportedly ranges from 3 to 5 per 1000 people, and its incidence increases dramatically after the age of 50 [3]. Current evidence suggests that 5–30% of patients with herpes zoster develop postherpetic neuralgia (PHN) [4].
PHN is pain that persists for 1 month or more after the herpes zoster rash has healed, and is the most common complication of herpes zoster [5]. Similar to herpes zoster, PHN is age-dependent. PHN occurs predominantly in the chest and abdomen and can account for 50–70% of cases [6]. The main pain features of PHN are persistent, spontaneous or paroxysmal burning pain and abnormal sensory hypersensitivity pain, with the majority of patients with PHN experiencing moderate pain or above. Persistent herpes zoster-related pain is the most common complication of PHN, with nearly half of patients experiencing pain lasting longer than 1 year, while some patients have pain lasting more than 10 years [7]. Long-term pain leads to sleep and daily life disorders, seriously affecting patients' quality of life [8].
Currently, the main treatments for PHN include pharmacological (NSAIDs, gabapentin, pregabalin, opioids, etc.) and interventional (subcutaneous botulinum injections, nerve blocks, nerve radiofrequency, and nerve stimulation) methods [9–11]. These treatment strategies are often not always effective, and with side effects and risks, with up to 50% of patients with PHN exhibiting recurrent episodes that are difficult to manage [12]. Spinal nerve DRG radiofrequency is considered an effective intervention for the treatment of PHN, given its low invasiveness, good accuracy, reproducible operation, low recurrence rate, high effectiveness, and safety and reliability [13, 14]. Although pulsed radiofrequency (PRF) has been shown to yield definite analgesic efficacy in treating herpes zoster-associated pain, some patients have insignificant pain relief and a high short-term recurrence rate after treatment, and often require additional pain relief treatment [15–17]. Radiofrequency thermocoagulation (RF-TC) enables the destruction of the DRG with significant pain relief and high efficiency [18, 19]. Therefore, for patients with PHN, RF-TC may become a effective percutaneous minimally invasive surgical modality to relieve the pain, reduce the recurrence rate, improve sleep, and enhance the quality of life.
In this study, we retrospectively analyzed a large cohort of patients with thoracic PHN who underwent RF-TC and were followed up for a long period. The main objective of this study was to investigate the treatment effects of RF-TC, including preoperative and postoperative pain scores, cumulative efficiency, recurrence rates, and adverse events. In addition, we explored factors influencing postoperative recurrence following RF-TC using the Cox proportional risk model, predicted the probability of recurrence using nomogram plots in R language, and plotted calibration curves to evaluate the consistency of the Cox analytical model.
Methods
Subjects and Ethics
This study was performed in accordance with the Good Clinical Practice guidelines and the principles of the Declaration of Helsinki of 1964 and its subsequent revisions. The study was approved by the Ethics Committee of Jiaxing University Hospital (LS2021-KY-375). A retrospective analysis of 228 patients with PHN in the thoracic segment who underwent RF-TC of the DRG in the Affiliated Hospital of Jiaxing University from January 2012 to August 2021 was performed. All data are derived from the clinical database of our hospital, and the specific researchers conduct regular follow-up of patients and improved the database, with the last follow-up date being October 31, 2021. In addition, the follow-up was conducted as part of the original collection of data, and not part of this current study.
Inclusion Criteria and Exclusion Criteria
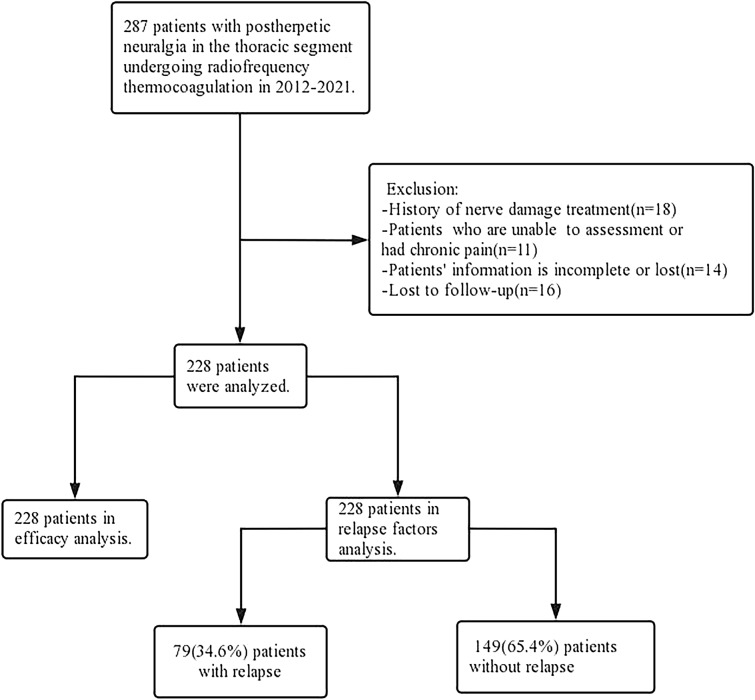
The inclusion criteria were as follows: (1) preoperative numerical rating scale score (NRSs) ≥ 5; (2) lesions located in the T2-T12 innervation area; (3) diagnostic criteria for PHN were met[5]; and (4) patients who failed conventional conservative pharmacological treatment or neuromodulation. The exclusion criteria consisted of: (1) previous treatment related to nerve destruction; (2) patients with psychiatric or psychological disorders who were unable or found it difficult to continue clinical assessment; (3) a combination of other types of acute and chronic pain; and (4) incomplete or missing patient information. The detailed process of patient inclusion is shown in Fig. 1.
Fig. 1.
Flowchart depicting the detailed process of patient enrollment
Surgical Operation
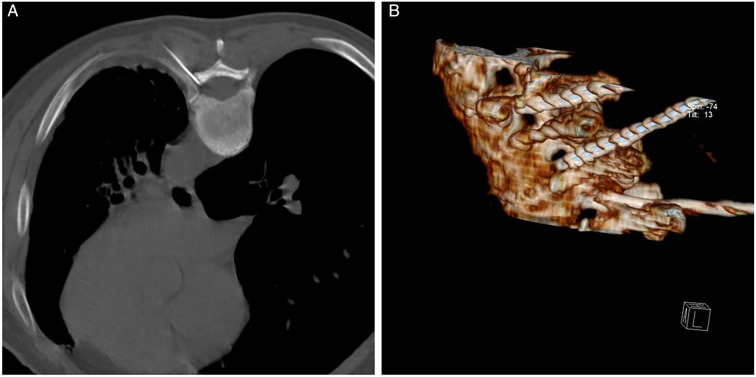
Patients were placed in the prone position with soft pillows under the chest (or abdomen). Each patient received oxygen through a nasal cannula, and vital signs, such as blood pressure, heart rate, and oxygen saturation, were continuously monitored. The target nerve and the body surface location of the puncture site were positioned according to the site of their lesion and the nerve segment involved. The skin was then disinfected with iodophor, and local infiltration anesthesia was performed with 1% lidocaine. A 22G puncture needle was inserted into the upper 1/3 of the corresponding intervertebral foramen under CT guidance (Fig. 2A), and the tip of the puncture needle was reconfirmed to be located in the intervertebral foramen under CT 3D reconstruction (Fig. 2B). The needle core was then withdrawn, and a matching electrode was inserted along the trocar. The tip position was repeatedly adjusted until tissue resistance around the test electrode tip ranged from 250 to 550 Ω.
Fig. 2.
The radiofrequency puncture needle guided by CT is punctured to the rear of 1/3 on the intervertebral foramen (A); the puncture needle located in the intervertebral foramen under the three-dimensional reconstruction of CT (B)
Sensory and motor tests were performed after confirming the absence of blood or gas. Parameters for sensory testing were set at a voltage of 0.5 V and a frequency of 100 Hz; sensory testing was considered positive if it caused soreness, numbness, or tingling in the skin tissue of the corresponding segment. The motor test was performed using a low-frequency current, with parameters set to a voltage of 0.5 V and a frequency of 2 Hz; the motor test was considered positive if the corresponding segment showed trunk muscle fiber fibrillation and pulsation. When the needle was confirmed to be close to the target nerve, local anesthesia was administered, using approximately 1 mL of 2% lidocaine, followed by the RF procedure. The temperature was set to 95 °C for 120 s, and RF-TC was conducted for 2 cycles [20]. According to the lesion site, we usually selected three related segments for RF-TC. After RF-TC was completed and the needle was removed, a sterile patch was applied. All patients were observed in the treatment room for 20 min to ensure stable vital signs and no significant discomfort before being sent back to the ward.
Efficacy Evaluation
The numerical rating scale (NRS) was used to evaluate the patients' pain level by drawing a 10-cm horizontal line on the paper, with 0 at one end of the horizontal line indicating no pain and 10 at the other end indicating intolerable pain; the middle part indicated different degrees of pain. Patients were instructed to draw a mark on the horizontal line to indicate the degree of pain. The numerical rating scale scores (NRSs) criteria were as follows: 0, no pain symptoms; 1–3, mild pain, not affecting daily activities; 4–6, moderate pain, affecting daily rest during episodes; 7–10, severe pain, necessitating bed rest during episodes. The clinical database manager of our hospital arranged for specialist researchers to follow-up the discharged patients regularly (once every 3 months). The NRSs of patients before surgery and at the last postoperative follow-up, duration of adverse events were observed and recorded. Postoperative pain relief of ≥ 50% preoperatively was defined as effective. Postoperative recurrence of pain at the primary site and NRSs ≥ preoperative values were defined as recurrence. The follow-up was ended w hen the patient relapsed.
Patients were divided into ten time periods according to the duration of postoperative follow-up: T1, within 1 year after surgery; T2, between 1 and 2 years after surgery; T3, between 2 and 3 years after surgery; T4, between 3 and 4 years after surgery; T5, between 4 and 5 years after surgery, T6, between 5 and 6 years after surgery; T7, between 6 and 7 years after surgery; T8, between 7 and 8 years after surgery; T9, between 8 and 9 years after surgery; and T10, between 9 and 10 years after surgery. The annual cumulative effictive rate, cumulative relapse rate, and median time to relapse within 10 years after RF-TC was calculated in years.
Analysis of Recurrence Factors
Clinical characteristics were collected for ten variables, including patient age, gender, body mass index (BMI), lesion segment, lesion side, disease course, type of RF electrode, complications, treatment history, and pain grade, and all variables were adjusted as categorical variables. The primary endpoint was defined as the time from surgery to recurrence or death or last follow-up. A positive outcome was defined as recurrence, and the rest were considered a negative outcome. The time to recurrence was recorded for all recurrent patients.
Statistical Analysis
SPSS 23.0 (IBM, Chicago, USA) was used to analyze the data. The Shapiro–Wilk test was used to determine whether the measured data had a normal distribution. Normally distributed data were expressed as mean ± standard deviation, the counting data were expressed by the number of cases (%), and non-parametric data were expressed as median (interquartil). Paired t tests were used for pre-treatment and post-treatment comparisons, and repeated data were analyzed using repeated-measures ANOVA. A P value < 0.05 was statistically significant.
Survival curves were plotted using the Kaplan–Meier method, log-rank tests were performed to compare the non-recurrence rate between patient subgroups, and cumulative efficiency and cumulative recurrence rates were calculated. After adjusting for related confounders, the interval-censored multivariable Cox regression analyses were performed to identify independent factors associated with postoperative recurrence. To minimize the inclusion of significant independent variables in the regression model, the adjusted significance level was set to 0.20. The screened independent variables with significant differences were included in the interval-truncated multivariate Cox regression to remove covariance between independent variables, then we adjusted for all confounders in the model. Risk ratios (HR) and 95% confidence intervals (CI) were calculated for each variable, and differences were considered statistically significant at P < 0.05. Time-independent receiver operating characteristic (ROC) curves were used to assess the overall variance of the models, and AUC was calculated to predict the value of the models. An AUC greater than 0.7 suggested good prediction ability of the model. Based on the results of the multivariate analysis, nomogram plots were plotted using R software packages (v.3.6) to predict the probability of recurrence, and calibration curves were plotted to evaluate the consistency of the models. The R packages used included survival, survminer, rms, foreign, rmda, survROC, and riskRegression.
Results
Baseline Information
According to the inclusion and exclusion criteria, 228 patients diagnosed with PHN from 2012 to 2021 were included in the analysis. The mean follow-up time was 29.77 ± 26.55 months. The recurrence rate was 34.6% (79/228). The basic statistics of the numerical and categorical variables are shown in Table 1. It is worth mentioning that the baseline data do not include the recurrence rate and cumulative response rate.
Table 1.
Baseline characteristics (preoperative) of the numerical variables [mean ± SD or n (%) or median (IQR)]
| Variable | Negative (n = 149) | Positive (n = 79) |
|---|---|---|
| Age, years | 70.88 ± 9.17 | 71.92 ± 8.64 |
| BMI, kg/m2 | 23.16 ± 2.87 | 22.84 ± 2.52 |
| Course of disease, months | 3 (2–8) | 8 (3–36) |
| NRSs | 6 (5–6) | 6 (6–7) |
| Gender | ||
| Female | 63 (42.3) | 39 (49.4) |
| Male | 86 (57.7) | 40 (50.6) |
| Lesion side | ||
| Left | 69 (46.3) | 34 (43.0) |
| Right | 80 (53.7) | 45 (57.0) |
| Type of RF electrode | ||
| Monopole | 125 (83.9) | 78 (98.7) |
| Bipolar | 24 (16.1) | 1 (1.3) |
| Lesion segment | ||
| Chest and back | 104 (69.8) | 59 (74.7) |
| Waist and abdomen | 45 (30.2) | 20 (25.3) |
| Complications | ||
| Yes | 88 (59.1) | 60 (75.9) |
| No | 61 (40.9) | 19 (24.1) |
| Treatment history | ||
| Medication | 115 (77.2) | 69 (87.3) |
| Neuromodulation | 34 (22.8) | 10 (12.7) |
BMI Body mass index, NRSs numerical rating scale scores
Efficacy Analysis
Immediate postoperative pain was significantly relieved in all patients. As shown in Table 2, we compared the NRSs of patients with PHN before and after RF-TC treatment from T1 to T10. Excepting T10, the NRSs in other periods significantly decreased after surgery (P < 0.05). After surgery, patients with the cumulative effective rates, the cumulative relapse rates, and the median relapse time are shown in Table 3. The main intraoperative adverse effect was increased pain during RF-TC, significantly relieved after low-dose sedation or analgesia. The main postoperative complications were skin numbness and abdominal muscle weakness in the area of radiofrequency innervation. Skin numbness was mild and gradually decreased or even disappeared. All patients recovered abdominal muscle strength within 1 year. No other adverse events occurred (Table 4).
Table 2.
The NRS score of the patient at the preoperative and postoperative in each time period (mean ± SD)
| Time | n | Preoperative | Postoperative | p value |
|---|---|---|---|---|
| T1 | 42 | 5.90 ± 0.69 | 2.86 ± 2.08 | < 0.001 |
| T2 | 41 | 6.15 ± 0.79 | 3.49 ± 2.55 | < 0.001 |
| T3 | 39 | 5.85 ± 0.67 | 3.36 ± 2.42 | < 0.001 |
| T4 | 29 | 5.69 ± 0.71 | 3.28 ± 1.93 | < 0.001 |
| T5 | 21 | 5.95 ± 0.67 | 3.67 ± 2.31 | < 0.001 |
| T6 | 23 | 5.91 ± 0.67 | 3.30 ± 2.06 | < 0.001 |
| T7 | 10 | 6.00 ± 0.94 | 3.50 ± 2.95 | 0.008 |
| T8 | 11 | 6.00 ± 0.89 | 4.18 ± 2.44 | 0.008 |
| T9 | 8 | 5.88 ± 0.64 | 3.75 ± 2.96 | 0.049 |
| T10 | 4 | 6.00 ± 0.82 | 4.25 ± 2.63 | 0.188 |
T1 within 1 year after surgery, T2 between 1 and 2 years after surgery, T3 between 2 and 3 years after surgery, T4 between 3 and 4 years after surgery, T5 between 4 and 5 years after surgery, T6 between 5 and 6 years after surgery, T7 between 6 and 7 years after surgery, T8 between 7 and 8 years after surgery, T9 between 8 and 9 years after surgery, T10 between 9 and 10 years after surgery
Table 3.
Cumulative effictive rate, cumulative relapse rate, and median time to relapse after radiofrequency thermocoagulation
| Time | Cumulative effective rate (%) | Cumulative relapse rate (%) | Median time to relapse (months) |
|---|---|---|---|
| Within 1 year | 75.6 | 22.2 | 5 |
| Within 2 years | 63.3 | 32.6 | 13 |
| Within 3 years | 55.8 | 36.6 | 19 |
| Within 4 years | 44.6 | 42.0 | 28 |
| Within 5 years | 42.1 | 44.6 | 29 |
| Within 6 years | 37.6 | 47.9 | 33 |
| Within 7 years | 37.6 | 47.9 | 36 |
| Within 8 years | 37.6 | 47.9 | 37 |
| Within 9 years | 37.6 | 47.9 | 38 |
| Within 10 years | 37.6 | 47.9 | 38 |
Table 4.
Adverse events regarding surgery in all patients at each time point [n (%)]
| Adverse events | t0 | t1 | t2 | t3 | t4 | t5 |
|---|---|---|---|---|---|---|
| Total number | 228 | 228 | 228 | 228 | 186 | 146 |
| Intraoperative | ||||||
| The pain intensified | 164 (71.9) | |||||
| Bleeding | 7 (3.1) | |||||
| Nerve injury | 0 | |||||
| Postoperative | ||||||
| Abdominal muscle weakness | 42 (18.4) | 15 (6.6) | 0 | 0 | 0 | |
| Numbness | 203 (89.0) | 190 (83.3) | 111 (54.1) | 44 (23.7) | 15 (10.3) | |
t0 intraoperative, t1 lasting 1 day after surgery, t2 lasting 1 month after surgery, t3 lasting 6 months after surgery, t4 lasting 1 year after surgery, t5 lasting 2 years after surgery
Kaplan–Meier Survival Analysis
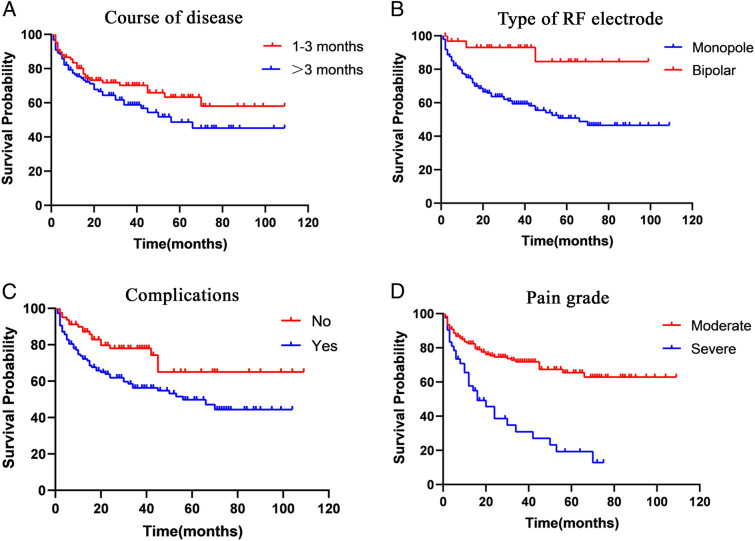
Kaplan–Meier survival analysis was performed according to patients' 10 relational variables. The results showed that the disease course was significantly associated with recurrence (Fig. 3A). Recurrence was significantly lower in patients treated with bipolar radiofrequency needles than monopolar (Fig. 3B). Patients with complications exhibited a higher postoperative recurrence rate than patients without underlying preoperative complications (Fig. 3C). There was also a significant difference in the time to recurrence between different pain grades, while patients with severe preoperative pain experienced a higher recurrence rate than those with moderate pain (Fig. 3D).
Fig. 3.
Kaplan–Meier survival plot. For patients with disease course of 1–3 months versus with more than 3 months (A); for patients with monopolar versus with bipolar needles (B); for patients with complications versus with no complications (C); for patients with moderate versus with severe pain (D)
Cox Proportional Risk Regression Analysis of Recurrence Factors
We performed univariate and multifactorial Cox proportional risk regression analyses for PHN patients treated with RF-TC to identify independent risk factors for postoperative recurrence rates (Table 5). Interval-censored Cox analysis showed a significant correlation between disease course, type of RF electrode, complications, pain grade, and the primary outcome. Disease course (unadjusted HR, 2.389; 95% CI 1.484–3.845 and adjusted HR, 2.086; 95% CI 1.277–3.409), complications (unadjusted HR, 2.010; 95% CI 1.198–3.372 and adjusted HR, 1.740; 95% CI 1.015–2.983), and pain grade (unadjusted HR, 3.188; 95% CI 2.013–5.050 and adjusted HR, 2.724; 95% CI 1.654–4.485) were positively correlated with recurrence. In addition, the unadjusted and adjusted HR (95% CI) with type of RF electrode were 0.200 (0.063–0.633) and 0.258 (0.080–0.831), respectively. During multivariate COX regression analysis, four variables (disease course, type of RF electrode, complications, and pain grade) were identified as independent prognostic factors for recurrence.
Table 5.
Factors associated with relapse after radiofrequency thermocoagulation for patients with postherpetic neuralgia
| Factors | Unadjusted analysis | Adjusted analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p values | HR (95% CI) | p values | |
| Gender | ||||
| Female | 0.724 (0.465–1.126) | 0.151 | 0.741 (0.466–1.176) | 0.203 |
| Male | ||||
| Age, years | ||||
| < 65 | 1.321 (0.753–2.318) | 0.332 | 1.278 (0.712–2.294) | 0.411 |
| ≥ 65 | ||||
| BMI, kg/m2 | ||||
| < 18.5 | Ref | Ref | ||
| 18.5–23.9 | 1.717 (0.517–5.702) | 0.378 | 1.860 (0.526–6.582) | 0.336 |
| ≥ 24 | 1.344 (0.378–0.226) | 0.226 | 1.046 (0.628–1.741) | 0.863 |
| Lesion segment | ||||
| Chest and back | 0.826 (0.497–1.372) | 0.461 | 1.131 (0.658–1.946) | 0.655 |
| Waist and abdomen | ||||
| Lesion side | ||||
| Left | 1.137 (0.727–1.778) | 0.574 | 1.139 (0.717–1.810) | 0.580 |
| Right | ||||
| Course of disease, months | ||||
| 1–3 | 2.389 (1.484–3.845) | < 0.001 | 2.086 (1.277–3.409) | 0.003 |
| > 3 | ||||
| Type of RF electrode | ||||
| Monopole | 0.200 (0.063–0.633) | 0.006 | 0.258 (0.080–0.831) | 0.023 |
| Bipolar | ||||
| Complications | ||||
| No | 2.010 (1.198–3.372) | 0.008 | 1.740 (1.015–2.983) | 0.044 |
| Yes | ||||
| Treatment history | ||||
| Medication | 0.710 (0.365–1.382) | 0.313 | 0.836 (0.422–1.658) | 0.609 |
| Neuromodulation | ||||
| Pain grade | ||||
| Moderate | 3.188 (2.013–5.050) | < 0.001 | 2.724 (1.654–4.485) | < 0.001 |
| Severe | ||||
BMI Body mass index
Predictive Model Evaluation
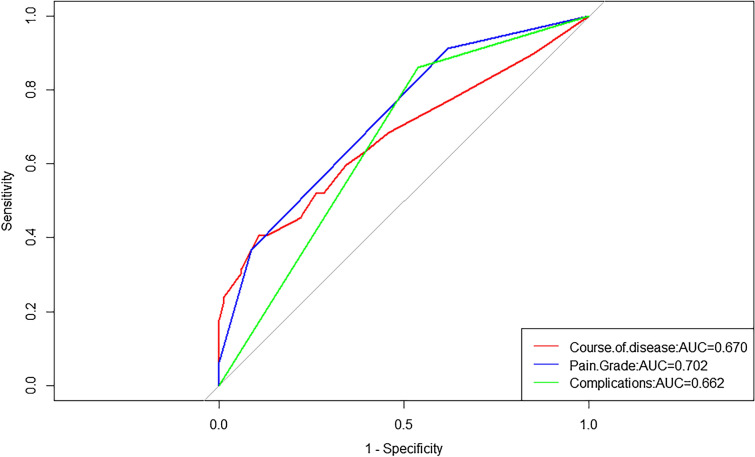
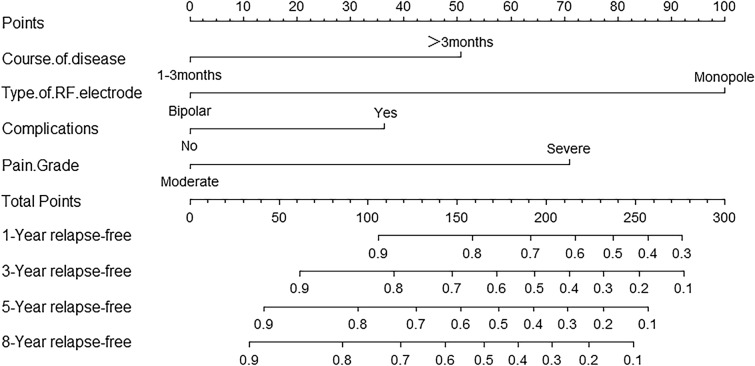
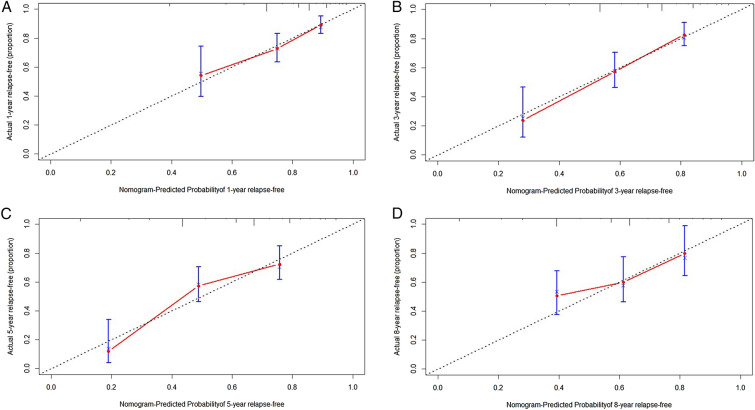
According to the area under the curve (AUC) of a 10-year overall recurrence prediction model, this indicated that the risk model had good sensitivity and specificity in predicting survival risk (Fig. 4). Based on multivariate COX regression analysis coefficients, the recurrence rate of PHN patients after RF was predicted at 1, 3, 5, and 8 years using a nomogram (Fig. 5). Each variable included in the model was assigned a score by locating it to the point scale. Then, the total score obtained by the addition of every risk factor in the vertical line corresponds to the predicted recurrence rate. The calibration curves showed good consistency between the predictions and the actual observations (Fig. 6).
Fig. 4.
Receiver operating characteristic (ROC) curve of a 10-year overall recurrence prediction model in patients with postherpetic neuralgia after radiofrequency thermocoagulation
Fig. 5.
An established nomogram to predict survival based on a Cox model. The total score obtained by the addition of every risk factor in the vertical line corresponds to the predicted recurrence rate
Fig. 6.
Calibration curves of the nomogram for 1-year (A), 3-year (B), 5-year (C), and 8-year (D) relapse-free. Horizontal coordinate the predicted probability, vertical coordinate the actual probability, red line the goodness-of-fit, that is the actual value corresponding to the predicted value, blue line the 95% confidence interval of the relapse-free rate
Discussion
VZV is a human neurotropic alpha-herpesvirus. It is well-established that viral particles enter the terminals of sensory neurons in the peripheral nervous system and then travel retrograde along axons toward the cell body to reach the cerebral nerve, dorsal root nerve, or autonomic ganglion cells, where the genome is deposited into the nucleus to establish lifelong latency [21]. As patients age or their autoimmune function declines, the latent virus is reactivated, and herpes zoster occurs.
PHN is the most common and severe complication of herpes zoster, manifesting as unilateral neuropathic pain in the skin after the rash has healed [22]. In recent years, the incidence and prevalence of herpes zoster and PHN have increased with the aging population. The mechanism of neuropathic pain caused by PHN remains largely unclear. Neuroplasticity is thought to be the basis for developing PHN; the mechanism may be peripheral, and central sensitization and abnormal changes in the excitability of the associated neurons cause a decrease in the pain threshold and amplification of pain signals [23]. PHN is complex in etiology, long-lasting, challenging to cure, and very harmful. Current evidence suggests that RF-TC is a minimally invasive procedure with huge prospects for treating PHN.
Radiofrequency (RF) [24] neurolysis is a technique that harnesses the heat generated to cause tissue and protein destruction, blocking pain transmission to reduce pain. RF neurolysis is classified as a minimally invasive percutaneous procedure for acute pain, chronic pain, and intractable pain. The two types of RF techniques currently used are RF-TC and PRF. RF-TC produces a temperature of 45 °C or higher by outputting a constant high-frequency current, resulting in ablative neurothermal coagulation, where the thermal effect inactivates the sensitive nerve endings caused by the lesion. In contrast, PRF uses short pulses, but does not induce heating to the point of tissue coagulation to produce a transient inhibition of evoked synaptic activity. PRF is thought to modulate only the nerve, and therefore causes little to no nerve damage [25]. Importantly, both RF-TC and PRF can cause distance-dependent tissue destruction under RF electrode needle action, but the effect of RF thermocoagulation is more pronounced. This finding suggests that RF-TC may produce a more durable efficacy.
In this study, 228 patients with PHN in the thoracic segment after RF-TC were followed up for nearly 10 years (range 2–109 months). Patients had an immediate postoperative efficiency of 100% and a 1-year efficiency of 75.6%. The effectiveness rate declined as the postoperative period progressed, but, 10 years after surgery, more than one-third of the patients were still symptom-free. By comparing the NRSs of patients at different periods before and after surgery, it was found that the postoperative NRSs were lower. However, PHN patients will use painkillers both before and after surgery. It is undeniable that the efficacy of RF-TC could be influenced by the concurrent use of other analgesics and therapy. We also took this problem into account when designing the project, and followed up the medication and treatment. However, because the type, dose, and frequency of analgesic drugs taken by each patient before and after operation are different, it is difficult for us to achieve unified data statistics and description. So we had to abandon this indicator. Furthermore, we found that intraoperative transient pain increase caused by high-temperature stimulation during RF-TC was the main intraoperative complication. After administering small doses of sedative and analgesic drugs or at the end of RF, the severe pain subsided. This discomfort caused patient anxiety and fear of a second surgery.
It remains subject to debate whether the destruction of the DRG of the spinal nerve causes motor deficits. The DRG is well-recognized as a critical structure in sensory transduction and modulation. The DRG's anatomical structure and physiological features make it an ideal target for neuromodulation, and may explain the excellent results observed in treating certain chronic neuropathic pain states [26]. Although RF thermal coagulation causes diminished sensation or numbness in the skin it innervates, it does not cause dyskinesia, since its damaged nerves innervate non-dominant movements. The present study found that the main postoperative complication was numbness, which gradually decreased in the number of people and in the degree over time.
In addition, abdominal bulging is another major postoperative complication and a sign of decreased muscle strength, not visible in the chest due to the strong support of the intercostal muscles and ribs, while the abdomen is only supported by the abdominal muscles. It is worth mentioning that these side effects are mild and temporary, and do not cause significant inconvenience to the patients in their daily life. Upon follow-up, patients reported that these adverse effects were acceptable compared to the uncomfortable experience of pain. Therefore, RF-TC for PHN of the thoracic segment is relatively safe and effective, and provides a new treatment option for patients with PHN.
Recurrence is a concerning adverse event for patients and physicians. Currently, the causes and mechanisms of neuralgia recurrence after radiofrequency thermal coagulation of the DRG are poorly understood, and are widely thought to be related to functional recovery after damaged nerve repair [27]. A study confirmed that motor (grip strength) and sensory (touch, pain, and temperature) functions were restored in rats after the spinal nerve was damaged and repaired [28]. None of the existing treatments can lead to the eradication of PHN. Accordingly, we sought to develop a new approach for pain relief and reduction of recurrence rate. Based on previous studies, we included ten covariates for Cox univariate and multifactorial analyses, and found that disease course, type of RF electrode, complications, and pain grade were associated with recurrence.
Age was closely related to herpes zoster-associated pain onset factors, and it has been reported that the risk of PHN increases every 10 years after the age of 50 [29]. Our findings showed that age was not associated with recurrence, which is inconsistent with our initial hypothesis. This finding may be related to the fact that we used 65 years as the threshold for stratifying patients, making the data unevenly distributed. It has been found that patients treated with bipolar RF-TC experience higher treatment efficiency, more significant pain relief, and longer maintenance than monopolar patients after surgery. In the present study, the recurrence rate of bipolar thermocoagulation was lower than that of monopolar, since bipolar RF needle thermocoagulation leads to a more radical destructive effect reaching a greater range of tissues, and therefore has a longer-lasting efficacy [19]. Patients with preoperative comorbidities such as hypertension, diabetes, coronary artery disease, or malignancy are more prone to recurrence than those without comorbidities [30]. This phenomenon may be attributed to patients with complications or comorbidities having a poorer immune function and, therefore, more susceptible to relapse.
Furthermore, similar to our previous findings [20], recurrence was 1.724 times more likely in patients with severe pain (NRSs ≥ 7) than in those with moderate pain (NRSs 4–6). This phenomenon may be attributed to the fact that the corresponding nerve is more severely affected at the peak of viral replication. Even after nerve thermal coagulation, the latent virus may invade the nerve and cause pain again during nerve repair. Moreover, we found that the disease course is associated with recurrence, with recurrence being 1.086 times greater in patients who had a long course of disease (> 3 months) than a short course of disease (1–3 months). A study on long-term follow-up regarding the course of disease and pain grade found that patients with a disease duration of more than 3 months were more likely to have severe pain than within 3 months[31].
Limitations of this study include a lack of randomized controlled trials, and outliers were not excluded. The stratification based on BMI and age was relatively simple, and a more comprehensive stratification approach may lead to more precise results. The present study was retrospective, and the follow-up data may be subject to recall bias. The lack of control, such as the use of propensity score matching, and the relatively small sample made the value of the regression model much weaker than in a larger cohort study. In addition, all the cases were from the same center, and a multicenter cohort needs to be examined in larger trials. Finally, this model lacked external validation.
Conclusions
CT-guided RF-TC of the dorsal root ganglion for PHN is a relatively safe and effective surgical option. Disease course, type of RF electrode, complications, and pain grade are risk factors for postoperative recurrence, and can assist in clinical decision-making before the RF-CT procedure. If patients are willing to accept pain relief at the cost of numbness and the risk of recurrence, RF-TC of the dorsal root ganglion should be considered. Nonetheless, further studies are warranted to determine the exact indications and clinical extension of RF-TC in PHN treatment.
Acknowledgements
The authors would like to thank the undergraduate and graduate students that have worked on the data collection for this study. We also acknowledge the staff and patients of the Affiliated Hospital of Jiaxing University Pain Department who partici-pated in this project.
Funding
This work was supported by Key discipline established by Zhejiang Province and Jiaxing City Jointly-Pain Medicine [2019-ss-ttyx]; Jiaxing Key Laboratory of Neurology and Pain Medicine. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
The following statement that all authors have made significant contributions. Ming Yao, Zhiqiang Zhang, and Ge Luo: program design; Zhiqiang Zhang, Zhangtian Xia, and Ge Luo: collection, analysis, and interpretation of data, and drafting of the article; Ming Yao: critical review of the intellectual content of the article; and administrative, technical, and informational support.
Disclosures
Zhiqiang Zhang, Zhangtian Xia, Ge Luo and Ming Yao have nothing to disclose.
Compliance with Ethics Guidelines
This study was performed in accordance with the Good Clinical Practice guidelines and the principles of the Declaration of Helsinki of 1964 and its subsequent revisions. The study was approved by the Ethics Committee of Jiaxing University Hospital (LS2021-KY-375), and informed consent was obtained from each subject.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Kennedy PGE, Mogensen TH, Cohrs RJ. Recent issues in varicella-zoster virus latency. Viruses. 2021;13(10):e2018. doi: 10.3390/v13102018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrei G, Snoeck R. Advances and perspectives in the management of varicella-zoster virus infections. Molecules. 2021;26(4):e1132. doi: 10.3390/molecules26041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18(1):55–78. doi: 10.1111/ddg.14013. [DOI] [PubMed] [Google Scholar]
- 4.Yu S, Fan B, Yang F, et al. Patient and economic burdens of postherpetic neuralgia in China. Clinicoecon Outcomes Res. 2019;11:539–550. doi: 10.2147/CEOR.S203920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner RN, Nikkels AF, Marinović B, et al. European consensus-based (S2k) guideline on the management of Herpes Zoster-guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 2: treatment. J Eur Acad Dermatol Venereol. 2017;31(1):20–29. doi: 10.1111/jdv.13957. [DOI] [PubMed] [Google Scholar]
- 6.Saguil A, Kane S, Mercado M, Lauters R. Herpes Zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96(10):656–663. [PubMed] [Google Scholar]
- 7.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):1–18. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey M, Prosser L, Rose A, Ortega-Sanchez IR, Harpaz R. Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain. 2020;161(2):361–368. doi: 10.1097/j.pain.0000000000001718. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Wei L, Zeng Q, Lin K, Zhang J. The treatment of topical drugs for postherpetic neuralgia: a network meta-analysis. Pain Physician. 2020;23(6):541–551. [PubMed] [Google Scholar]
- 10.Wen B, Wang Y, Zhang C, Xu W, Fu Z. Efficacy of different interventions for the treatment of postherpetic neuralgia: a Bayesian network meta-analysis. J Int Med Res. 2020;48(12):1–21. doi: 10.1177/0300060520977416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue S, Yang WJ, Cao ZX, Sun T. Comparing the efficacy and safety of short-term spinal cord stimulation and pulsed radiofrequency for zoster-related pain: a systematic review and meta-analysis. Medicine (Baltimore) 2022;101(11):1–14. doi: 10.1097/MD.0000000000029073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngo AL, Urits I, Yilmaz M, et al. Postherpetic neuralgia: current evidence on the topical film-forming spray with bupivacaine hydrochloride and a review of available treatment strategies. Adv Ther. 2020;37(5):2003–2016. doi: 10.1007/s12325-020-01335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(1 Suppl 1):S19–30. doi: 10.1016/j.jpain.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Contreras Lopez WO, Navarro PA, Vargas MD, Alape E, Camacho Lopez PA. Pulsed radiofrequency versus continuous radiofrequency for facet joint low back pain: a systematic review. World Neurosurg. 2019;122:390–396. doi: 10.1016/j.wneu.2018.10.191. [DOI] [PubMed] [Google Scholar]
- 15.Usmani H, Dureja GP, Andleeb R, Tauheed N, Asif N. Conventional radiofrequency thermocoagulation vs pulsed radiofrequency neuromodulation of ganglion impar in chronic perineal pain of nononcological origin. Pain Med. 2018;19(12):2348–2356. doi: 10.1093/pm/pnx244. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Jo D, Kim E. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia. Pain Physician. 2017;20(3):E411–e418. doi: 10.36076/ppj.2017.E418. [DOI] [PubMed] [Google Scholar]
- 17.Peng Z, Guo J, Zhang Y, et al. Development of a model for predicting the effectiveness of pulsed radiofrequency on zoster-associated pain. Pain Ther. 2022;11(1):253–267. doi: 10.1007/s40122-022-00355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sindou M, Mifsud JJ, Boisson D, Goutelle A. Selective posterior rhizotomy in the dorsal root entry zone for treatment of hyperspasticity and pain in the hemiplegic upper limb. Neurosurgery. 1986;18(5):587–595. doi: 10.1227/00006123-198605000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Luo G, He Q, Yao M. Evaluation of the efficacy of unipolar and bipolar spinal dorsal root ganglion radiofrequency thermocoagulation in the treatment of postherpetic neuralgia. Korean J Pain. 2022;35(1):114–123. doi: 10.3344/kjp.2022.35.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo G, Zhang Z, Zhu J, et al. Association between the risk of relapse and the type of surgical procedure for Herpes Zoster-related pain. Pain Physician. 2021;24(8):E1227–1236. [PubMed] [Google Scholar]
- 21.Bader MS. Herpes zoster: diagnostic, therapeutic, and preventive approaches. Postgrad Med. 2013;125(5):78–91. doi: 10.3810/pgm.2013.09.2703. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JI. Clinical practice: Herpes Zoster. N Engl J Med. 2013;369(3):255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devor M. Rethinking the causes of pain in herpes zoster and postherpetic neuralgia: the ectopic pacemaker hypothesis. Pain Rep. 2018;3(6):e702. doi: 10.1097/PR9.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racz GB, Ruiz-Lopez R. Radiofrequency procedures. Pain Pract. 2006;6(1):46–50. doi: 10.1111/j.1533-2500.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 25.Ding Y, Li H, Hong T, et al. Efficacy and safety of computed tomography-guided pulsed radiofrequency modulation of thoracic dorsal root ganglion on Herpes Zoster neuralgia. Neuromodulation. 2019;22(1):108–114. doi: 10.1111/ner.12858. [DOI] [PubMed] [Google Scholar]
- 26.Esposito MF, Malayil R, Hanes M, Deer T. Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med. 2019;20(Suppl 1):S23–s30. doi: 10.1093/pm/pnz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang MC, Chang PT, Tsai MJ, et al. Sensory and motor recovery after repairing transected cervical roots. Surg Neurol. 2007;68(Suppl 1):S17–24. doi: 10.1016/j.surneu.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 28.Eldabe S, Tariq A, Nath S, et al. Best practice in radiofrequency denervation of the lumbar facet joints: a consensus technique. Br J Pain. 2020;14(1):47–56. doi: 10.1177/2049463719840053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon HJ, Anderson J, Damle JS. Evidence for interventional procedures as an adjunct therapy in the treatment of shingles pain. Adv Skin Wound Care. 2012;25(6):276–284. doi: 10.1097/01.ASW.0000415345.22307.f3. [DOI] [PubMed] [Google Scholar]
- 30.Kawai K, Yawn BP. Risk factors for Herpes Zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–1821. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ. 2000;321(7264):794–796. doi: 10.1136/bmj.321.7264.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.