Abstract
The expansion of Multi-Gene Panel Testing (MGPT) has led to increased detection of variants of uncertain significance (VUS) among individuals with personal or family history of cancer. However, having a VUS result can impact on emotional and psychological wellbeing and cause challenges for non-geneticist healthcare providers. The purpose of this mixed methods systematic review was to examine what is currently known about the experiences of individuals with a VUS on genetic testing for inherited cancer susceptibility. The initial search was conducted in June 2020 using PUBMED, CINAHL, Web of Science, and PsychInfo according to the Joanna Briggs methodology for systematic reviews. A total of 18 studies met the inclusion criteria. Studies included in this review identified a range of emotional reactions to a VUS result, a general lack of understanding of a VUS result and its implications, frustration with a lack of healthcare provider knowledge, and a need for clear communication with healthcare providers. This review identified critical gaps in current knowledge to guide genetic counseling praxis, specifically in the knowledge of communication patterns and methods of improving communication with healthcare providers and family members and preferred risk management strategies. This will help to improve the counseling process and the management of care during and after genetic testing.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12687-022-00600-4.
Keywords: Variant of uncertain significance (VUS), Hereditary cancer, Genetic testing, Literature review
Introduction
Hereditary cancer syndromes caused by inherited mutations (pathogenic variants) in certain genes increase the risk of developing specific tumor types (Rahner and Steinke 2008). There are known pathogenic variants in dozens of genes associated with increased cancer susceptibility (Reid and Pal. 2020). Hereditary breast and ovarian cancer (HBOC), Lynch syndrome, adenomatous polyposis syndrome (APC), and Li-Fraumeni syndrome (LFS) are some of the most commonly known hereditary cancer syndromes. Genetic testing for known hereditary cancer syndrome helps estimate the chance of developing cancer in a lifetime (Milanese and Wang 2019). Individuals may be considered eligible for cancer risk assessment if they have a personal and/or family history of cancer or other clinical indication consistent with increased cancer risk (Rahner and Steinke 2008; Reid and Pal. 2020; Reid et al. 2022). These features vary by type of cancer and specific hereditary syndrome (Rahner and Steinke 2008).
Regardless of the genetic test result, genetic testing benefits both affected and unaffected individuals. A positive genetic test means that all first-degree relatives have a 50% chance of carrying the same variant and should be tested. Identifying pathogenic variants allows these individuals to make informed decisions and reduce their risk of cancer through tailored cancer screening approaches and risk reduction interventions. A negative test result may allow individuals to avoid aggressive cancer screenings and unnecessary interventions (Reid and Pal. 2020). However, a variant of uncertain significance (VUS) test result may bring more uncertainty, as it cannot be determined to increase cancer risk or be normal human variation. Therefore, a VUS result does not help to clarify individual risk and is not recommended for use in making health care decisions (Federici and Soddu 2020). The NCCN guideline recommends cancer screenings as appropriate to the family history of those with a VUS result (NCCN 2020; NCCN 2021). However, it is not always clear to healthcare providers without training in genetics how to manage these cases. Occasionally a VUS is over-managed, leading to inappropriate surgeries, or under-managed, leading to missed opportunities for screening based on family history (Donohue et al. 2021). This confusion among healthcare professionals can lead to increased stress and poorer health outcomes for individuals with a VUS result (Hamilton et al. 2019; Richter et al. 2013).
Over the past decade, multi-gene panel testing for hereditary cancer risk has expanded to include more than 60 genes (Reid and Pal. 2020; Rosenthal et al. 2017). Although it is difficult to estimate exact odds due to different reporting methods among commercial laboratories, the chance of a VUS result increases with each gene included in the test panel. It is reported that a BRCA1 and BRCA2 analysis has a 1–3% chance of a VUS result; for a 25 gene panel test, the chance increases to 30% or more (Idos et al. 2019; NCCN 2020; Rosenthal et al. 2017). An analysis showed that 28.7% of variants found were classified as a VUS (Rosenthal et al. 2017). This considerable possibility of having a VUS adds to the complexity of counseling for multi-gene testing (Reid et al. 2022). Despite this, many studies have focused on individuals with an identified pathogenic variant to improve informed decisions, cascade testing, and cancer screening. Few studies have focused on the psychosocial effects of a VUS result and the experiences of these individuals (Brédart et al. 2019; Esteban et al. 2018; Hamilton et al. 2019). Even though most reclassified VUS are categorized as benign, the individual still experiences uncertainty about its pathogenicity, which may have negative psychological effects. There might also be confusion for an individual to be told that this VUS itself does not incur a higher risk of cancer, yet they are at elevated risk due to personal and family history. Therefore, while an increasing proportion of individuals undergo genetic testing and rapidly growing multi-gene testing leads to more VUS detection (Idos et al. 2019), we need to understand how these individuals are affected by a VUS result and how they can be best supported. This will ultimately reduce the confusion and miscommunication, which leads to poorer patient experiences and outcomes.
The purpose of this mixed methods systematic review was to explore the experiences of individuals receiving a variant of uncertain significance (VUS) on genetic testing for hereditary cancer susceptibility. The research question was, “what is the current evidence on the experiences of individuals with a VUS genetic test result for hereditary cancer susceptibility”. For this review, “experience” is defined broadly, including the effect of genetic test results on biological, psychological, or social wellbeing of individuals.
Methods
Inclusion and exclusion criteria
Studies were deemed eligible if they (a) were original research published in the English language, (b) included individuals with a VUS result in their sample, (c) focused on an adult sample of any gender, and (d) included individuals tested for any type of hereditary cancer syndromes with or without a personal history of cancer. Studies that examined non-VUS genetic test results or non-cancer genes were also included if either of these two areas were explored in addition to individuals with VUS results in the gene associated with cancer risks. The articles with any type of genetic testing such as single gene, small panels, or multi-gene panels were also included. Exclusion criteria were review articles, dissertations, or topics not consistent with the review’s aims. This mixed methods systematic review considered the quantitative and qualitative studies, and data related to the review aim was clearly extracted.
Search strategy
We conducted this Mixed Methods systematic review with an integrated approach according to the recommendations outlined by the Joanna Briggs Institute (Lizarondo et al. 2020). The search was conducted in June 2020 using the PubMed, CINAHL, Web of Science, and PsychInfo databases. We used search terms to capture all possible articles to map current evidence on experiences of individuals with a VUS result for hereditary cancer. The exact search used for PUBMED was ((hereditary cancer risk OR hereditary cancer syndrome) AND (experience OR need OR belief OR attitude OR reaction OR perception OR perspective OR consequence OR view)) AND (VUS OR variants of uncertain significance). Similar combinations were used for CINAHL, Web of Science, and PsychInfo. Preliminary searches to select databases and search terms were conducted with the consultation of the health sciences librarian at a research-intensive public university. There were no limitations on the publication dates of the articles searched.
Study selection
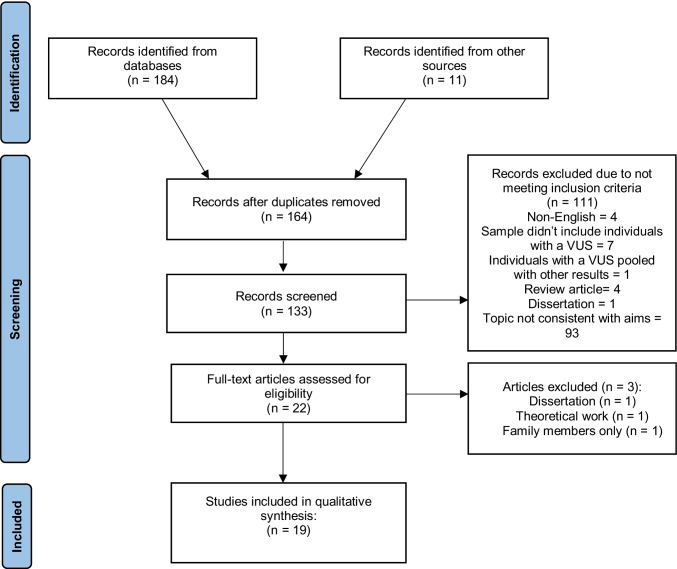
We used the RefWorks reference manager to manage citations from multiple databases. All titles and abstracts identified in the search process were imported into RefWorks. Duplicates were identified and removed using the automated feature in RefWorks. The remaining articles were imported into Rayyan QCRI (https://rayyan.qcri.org). A manual check for duplicates was then performed. An initial title scan was conducted and followed by a scan of the abstracts. Items published only as abstracts were excluded from the review. The full text of any articles identified as relevant after the abstract scan was obtained. Full texts of included articles were retrieved and reviewed by the researchers using the inclusion and exclusion criteria, resulting in a sample of 12 articles. The reference lists of the full text of all articles identified seven articles, which were also assessed against the inclusion criteria, resulting in a final sample of 19 articles. The search process results are depicted in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram (Fig. 1).
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram
Assessment of methodological quality
All articles selected for inclusion were research articles. We used The Joanna Briggs Institute (JBI) Critical Appraisal Tools to assess the methodological quality of each study and to determine the extent to which studies had addressed potential bias (Lizarondo et al. 2020). Papers were scored independently by two researchers, and the total scores were then converted to percentages to facilitate direct comparisons. Any disagreements in scoring were resolved through discussion. Since there is no specific cutoff point to exclude articles, we decided that a cutoff point of 70% was appropriate to exclude poor-quality papers. The range of scores for the included papers was 75–100%; therefore, all were included.
Data extraction
Data were extracted from the eligible articles using a table developed by the author based on the key information suggested by Joanna Briggs Institute (Lizarondo et al. 2020). In addition to the authors of the studies, the year of publication, and the country of origin, the extracted data included study aims, genetic test results for the sample, sample size and characteristics, assessment and measures, methods, results, and interpretation or recommendations (Table 1).
Table 1.
Descriptive characteristics of studies included in the integrative review
| N | % | ||
|---|---|---|---|
| Country | USA | 11 | 63.61.11 |
| USA and Canada | 1 | 5.26 | |
| Canada | 1 | 5.26 | |
| France, Germany, and Spain | 1 | 5.26 | |
| France | 1 | 5.26 | |
| Spain | 1 | 5.26 | |
| The Netherlands | 2 | 5.26 | |
| Singapore | 1 | 5.26 | |
| Study design | Quantitative | 12 | 57.89 |
| Qualitative | 5 | 27.78 | |
| Mixed | 2 | 10.53 | |
| Sample | Women only | 13 | 63.16 |
| Men only | 1 | 5.26 | |
| Any gender | 5 | 27.78 | |
| Outcome measurements | Recall/ understanding of the test result | 11 | 52.63 |
| Communication with healthcare providers | 8 | 44.44 | |
| Family communication on the rest result | 4 | 22.22 | |
| Emotional/psychological effects of test result | 10 | 52.63 | |
| Perception of cancer risks | 6 | 27.78 | |
| Screening and risk-reducing surgery decision making | 7 | 36.84 | |
| Type of genetic test | BRCA 1/2 genes | 10 | 47.37 |
| Lynch or single gene | 2 | 10.53 | |
| Multigene panel | 7 | 38.89 | |
| Results of sample | VUS only | 6 | 38.33 |
| PV or VUS | 4 | 21.05 | |
| Negative or VUS | 2 | 10.53 | |
| PV or VUS or Negative | 7 | 31.58 | |
| Publication date | 2000–2004 | 2 | 5.26 |
| 2005–2009 | 3 | 15.79 | |
| 2010–2014 | 3 | 15.79 | |
| 2014–2020 | 11 | 61.11 |
Data synthesis and integration
This review followed a convergent integrated approach according to the JBI methodology for mixed-method systematic review (Lizarondo et al. 2020). This involves assembling the qualitative data with the quantitative data. The assembled data are categorized and pooled together based on similarity in meaning to produce a set of integrated findings.
Results
Description of the studies included in the review
Table 1 shows the descriptive characteristics of the 19 studies included in this review. Of the studies, 11 were conducted in the USA. A quantitative study design was used in 12 studies, and other studies used either a qualitative (n = 5) or a mixed-methods study design (n = 2). In most studies, the sample consisted of only women (n = 12) studies and no study included nonbinary or intersex as a gender category. Although the individuals tested were the focus of 17 studies, one study included individuals and families, and one study included individuals and clinicians. The participants in eight studies had undergone multi-gene panel testing (Conley et al. 2019; Esteban et al. 2018; Garcia 2014; Giri et al. 2018; Li et al. 2018; Makhnoon et al. 2019a, b; Reuter et al. 2019; Vos et al. 2008). A theoretical framework was used in only five studies, such as Mishel’s Theory of Uncertainty in Illness (Reuter et al. 2019; Solomon et al. 2017) and Han’s Taxonomy of Uncertainty (Makhnoon et al. 2019a, b).
Of the studies included in this review, six included individuals only with VUS results (Cypowyj et al. 2008; Makhnoon et al. 2019a, b; Reuter et al. 2019; Solomon et al. 2017; Vos et al. 2008). Individuals with a VUS result were compared with those who had a negative test result in two studies (Chern et al. 2019; Culver and Brinkerhoff 2013), and with those who had a positive test result in four studies (Garcia 2014; Giri et al. 2018; Hamilton et al. 2019; Miron et al. 2000). In seven studies, individuals with different genetic test results, including a VUS result, positive, or negative result, were compared (Brédart et al. 2019; Conley et al. 2019; Elsayegh et al. 2018; Esteban et al. 2018; Li et al. 2018; Richter et al. 2013; Van Dijk et al. 2004).
The outcomes measured in the studies were condensed to five main domains, including (1) understanding, knowledge, and recall of the test result; (2) communication; (3) emotional and psychological effects of the result; (4) cancer risk perception; and (5) screening and risk reduction strategies used. These domains were the most commonly examined in the articles and aligned best with the aim of this review.
Understanding, knowledge, or recall
Most studies examined factual recall or perceived understanding of the genetic test result. Factual recall, which is described as accurate recall of the test result, was measured in five studies (Giri et al. 2018; Hamilton et al. 2019; Richter et al. 2013; Solomon et al. 2017; Vos et al. 2008). Perceived understanding or knowledge of results were examined in four studies (Cypowyj et al. 2008; Makhnoon et al. 2019a, b; Reuter et al. 2019).
Some studies reported the misinterpretation of a VUS as a PV result (Solomon et al. 2017; Vos et al. 2008; Giri et al. 2018). In Giri et al. (2018), 79.2% of individuals who stated that they understood their results incorrectly reported that they carried a PV showing a misunderstanding of the VUS test result, and found that having a VUS result was associated with a lack of understanding. The studies comparing individuals with VUS and other test results generally reported a higher rate of incorrect recall (36% and 33%) (Cypowyj et al. 2008; Richter et al. 2013). However, some studies reported low incorrect recall rates (14.2–21.4%) for a VUS result (Hamilton et al. 2019; Reuter et al. 2019).
Misunderstanding of the clinical recommendations after having a VUS result was also reported. Individuals with a VUS had the lowest levels of comprehension of clinical recommendations (Van Dijk et al. 2004) such as cancer risk management (Makhnoon et al. 2019a). Esteban et al. (2018) noted that patients with a VUS or moderate penetrance PV reported they understood their cancer risk management recommendations less frequently than those with a high-penetrance PV. Another study reported that conflicting recommendations from physicians and genetic counselors were source of confusion for patients (Mahknoon 2019).
Communication
Communication with healthcare providers was a critical component of the experience. Nine studies examined the communication process between individuals with a VUS result and their healthcare providers. Mahknoon et al. (2019a; 2019b) found that participants with a VUS result were frustrated with their providers and highlighted the importance of pretest preparation for a possible VUS on understanding of the result. Hamilton et al. (2019) found that those with a VUS result were less satisfied with the provider’s knowledge implying the lack of recommendations received from their provider. Conley et al. (2019) found that the only factor affecting the test result disclosure in the family was whether a provider encouraged them to tell their families. Another study reported that most physicians would incorrectly refer the family members of a patient with a VUS for genetic testing (Richter et al. 2013).
Different counseling styles (Culver and Brinkerhoff 2013) and disclosure methods influenced the risk perception of those with a VUS result, with those receiving their result via telephone or telehealth having a greater misunderstanding of test results (Giri et al. 2018). Esteban et al. (2018) noted that most participants preferred to be informed about all results, including a VUS, instead of only being informed of pathogenic results in high-penetrance genes. Assisting in decision-making about medical care (Culver and Brinkerhoff 2013) and screening based on personal or family history of cancer (Richter et al. 2013) were reported as the most helpful component of counseling.
Communication with family members was also an important component of the experience, and five studies examined aspects of family communication on the genetic test result and its implications for family members (Brédart et al. 2019; Cypowyj et al. 2008; Hamilton et al. 2019; Li et al. 2018; Solomon et al. 2017). Studies reported that most participants had communicated the test results to their family members (Cypowyj et al. 2008; Hamilton et al. 2019; Solomon et al. 2017). Cypowj et al. also reported that 76% had communicated the test result, mostly due to a misunderstanding that their family members required genetic testing or cancer screening because of the VUS result.
Various factors influencing the decision or motivations of the participant to communicate the VUS test results with their family members were reported in the studies. Studies reported that family members’ gender (Conley et al. 2019), belief that family members need to be tested or increased cancer surveillance (Cypowyj et al. 2008), and thinking of that family members would not understand the implication of the test results (Li et al. 2018) effect family risk communication. In one study, although some participants were cautious about sharing the result due to confusion about a VUS and a desire not to cause a false alarm, test result communication was related to a feeling of closeness or duty to the family member, and whether they felt the family member would understand the information (Li et al. 2018).
Emotional and psychological effects of having a VUS result
Emotional responses and psychological effects describe a key aspect of the experience with a VUS. Most studies (n = 10) examined emotional or psychological effects of having a VUS result, including worry, anxiety, and depression. Culver and Brinkerhoff (2013) found that the VUS group reported a significant change in concerning thoughts, with 92% reporting a decrease after receiving the test result. Richter et al. (2013) found that individuals with a VUS result had intermediate worry, which was significantly different than those with a PV and similar to those with a negative result. Esteban et al. (2018) found similar uncertainty level, but higher levels of distress among individuals with a VUS than individuals with a negative result. Bredart et al. (2019) found that participants with a VUS had more significant decreases in psychologic distress related to family and social issues such as hereditary predisposition and risk communication after receiving their genetic test result.
Emotional reactions to having a VUS result were varied in the studies. Solomon et al. (2017) reported varied reactions from relief to shock experienced by participants after having their VUS result. They also found that some considered the result as a threat and an opportunity and mobilizing or planning for cancer screening was the most common coping mechanism. Some studies also reported relief or indifference (Makhnoon et al. 2019a, 2019b) or not thinking about the test result (Reuter et al. 2019) among participants with a VUS result.
Risk perception for developing cancer among individuals with VUS result
Risk perception also played a key role in the experience of a VUS, and six studies examined risk perception specifically in those with a VUS (Culver and Brinkerhoff 2013; Hamilton et al. 2019; Makhnoon et al. 2019a, b; Van Dijk et al. 2004; Vos et al. 2008). Mahknoon (2019) found uncertainty and unclear interpretations regarding risks among individuals with a VUS result. Miron et al. (2000) reported significant differences between self-estimated risks and calculated risks of those with a VUS result. In Hamilton et al. (2019) study, participants with VUS results and those with a PV had similar cancer risk perception. In Culver and Brinkerhoff (2013), 15% of those with a VUS considered themself high risk, while it was 10% of those with a negative result. Two studies reported that individuals with a VUS result had decreases in their perceived risk after genetic testing (Van Dijk et al. 2004; Vos et al. 2008).
Discussion
There is generally a lack of understanding among individuals with a VUS result, including misinterpretation of the test result and incorrect recall (Cypowyj et al. 2008; Hamilton et al. 2019; Reuter et al. 2019; Richter et al. 2013; Solomon et al. 2017; Vos et al. 2008). This was more common among those with a VUS result when compared to those with a PV or negative result (Esteban et al. 2018; Giri et al. 2018). The incorrect recall of the result could be a misinterpretation that anything besides a negative is pathogenic, or an interpretation that every variant is pathogenic. It was especially concerning when participants stated they could recall the result when their recall was incorrect. Considering the counselor and counseling related factors affecting individuals' understanding, these findings show the need for improvement in the counseling process for those with a VUS result. As reported in the articles, preparation for a possible VUS result during the pre-testing counseling (Makhnoon et al. 2019a, b), providing a written copy of the test result along with an appropriate counseling session (Giri et al. 2018), and assisting these individuals in deciding on risk management (Culver and Brinkerhoff 2013) were areas to focus on to improve patient outcomes. At the time of the posttest counseling, the emotional state of individuals should also be considered, as anxiety and distress are linked to forgetting medical information or the perception that the information is unimportant (Kessels 2003).
Participants expressed frustration with a lack of provider knowledge regarding their test results and occasionally felt they were not getting the appropriate medical care. Individuals with a VUS result reported feeling “brushed off” by their healthcare provider. There were also some results indicating that healthcare providers, especially non-genetic providers, made incorrect recommendations regarding screening for individuals with a VUS or their family members (Richter et al. 2013), which is consistent with other literature showing poor genetics knowledge among providers (Edwards et al. 2011; Ha et al. 2018; Nair et al. 2017). However, it is unclear if they were getting the appropriate care, as risk calculation data based on family history were not included in any studies. Even if they were being correctly managed, the stated frustrations show miscommunication between provider and patient and decreased satisfaction associated with unclear communication from healthcare providers (Culver and Brinkerhoff 2013). This is important to address because poor communication with the healthcare provider disclosing the test results may negatively influence the sharing of VUS results with family members (Makhnoon et al. 2019a, b). Since result disclosure by telephone or telehealth was found to be associated with higher levels of misunderstanding (Giri et al. 2018), it is important to understand what differences exist between these methods and in person disclosure, especially given the emerging data on the necessity for telehealth visits.
As genetic information or a test result information was described as confusing, unhelpful, or even potentially harmful (Makhnoon et al. 2019a, b), clear communication indicating the implications of a VUS for family members should be part of posttest counseling. Even if genetic testing is not recommended for family members, this is still helpful knowledge to share that effect the appropriate utilization of testing and cancer risk management services. Clinicians should include a discussion of family communication within their counseling visits, including identification of which relatives to speak to and strategies to do so. The sharing of medical information among family members can be encouraged through projects such as the CDC “My Family Health Portrait” (CDC 2020a, 2020b).
Individuals experienced a number of emotional reactions to receiving a VUS (Reuter et al. 2019), including relief, distress (Culver and Brinkerhoff 2013), confusion (Cypowyj et al. 2008; Richter et al. 2013), and frustration (Makhnoon et al. 2019a, b). Distress appeared to be less among individuals with a VUS than those with a PV result (Brédart et al. 2019; Culver and Brinkerhoff 2013). In general, having a VUS appeared not to be a significant life event and was not associated with intrusive thoughts. There was a preference among some for a definitive result, even if it were a PV. In other studies, negative emotions have been linked to uncertainty (Tsai et al. 2020). Accounting for these variations can potentially improve satisfaction and decrease frustrations with perceived failures of the healthcare system.
The differentiation between recalling actual test results and personal interpretation of the test result had consequences on risk perception (Vos et al. 2008). The combination of poor recall and misinterpretation of test results is likely the underlying cause of confusion regarding screening recommendations for individuals with a VUS. Although no studies examined screening uptake based on family history, it is important to address to improve the appropriate utilization of cancer risk management strategies, including cancer screening based on the family history (NCCN Guidelines Panel 2020). This is also important to address in clinical practice, as under-screening can result in the identification of cancers at a later stage, and over-screening can lead to excessive procedures and increased costs.
There is a lack of gender, racial, and ethnic diversity in the samples of the included studies, which is consistent with the current landscape of research in this area. Of the studies included in this review, 12 studies were focused exclusively on women (Brédart et al. 2019; Conley et al. 2019; Culver and Brinkerhoff 2013; Cypowyj et al. 2008; Elsayegh et al. 2018; Garcia 2014; Ha et al. 2018; Li et al. 2018; Makhnoon et al. 2019a, b; Miron et al. 2000; Richter et al. 2013; Vos et al. 2008) and no studies operationalized gender outside of a nonbinary variable. Only one study reported that the group with a VUS was more racially diverse, which is likely due to lower testing rates in non-white, non-European populations (Chern et al. 2019). A lack of diversity not only limits the ability to generalize study findings but also slows the progress in the application of precision medicine in the clinical setting (Bentley et al. 2017; Huang 2019; Tan et al. 2016).
Limitations
The mixed methods systematic review method used has some limitations. The search criteria and strategy were restricted to genetic testing for hereditary cancers; therefore, findings cannot be generalized to those with a VUS in genes associated with other conditions. Due to our aim to include literature focusing on cancer genetics, the applicability of this review to other settings and specialties is limited. Including articles published only in English may have excluded studies from non-English speaking countries with different counseling practices or screening processes.
Recommendations
This review established that individuals with a VUS result have a lower level of recall and understanding of their test results. Clinicians providing VUS results should support the patient’s knowledge and implication of having a VUS result for patients and family members. Research should focus on communication between individuals with a VUS result and healthcare providers, including genetic counselors and primary health care providers, to improve patients’ understanding of a VUS result. Communication between family members regarding genetic testing, specifically VUS results, also needs further investigation to describe the motivations and factors that impact the successful sharing of a VUS result.
Although the limited studies in this review showed that individuals with a VUS result had better emotional outcomes compared with those with a PV, continued research into the factors impacting long-term emotional or psychological outcomes in this group would improve the counseling process. Particular emphasis should be placed on the effective use of telehealth services for pre and post-test counseling. Additionally, further research is needed regarding provider use of family history in making risk management recommendations, as no studies reported the use of family history in addition to the genetic test result. Finally, the patterns of cancer screening in individuals with a VUS should be further examined in order to understand the uptake of the provider recommendations and identify missed opportunities to reduce risks.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Danielle Gould and Memnun Seven. The first draft of the manuscript was written by Danielle Gould, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Code availability
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent for publication
All authors consent to the publication of this manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bentley AR, Callier S, Rotimi CN. Diversity and inclusion in genomic research: why the uneven progress? J Community Genet. 2017;8(4):255–266. doi: 10.1007/s12687-017-0316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brédart A, Kop J-L, Dick J, Cano A, De Pauw A, Anota A, Brunet J, Devilee P, Stoppa-Lyonnet D, Schmutzler R, Dolbeault S. Psychosocial problems in women attending French, German and Spanish genetics clinics before and after targeted or multigene testing results: an observational prospective study. BMJ Open. 2019;9(9):e029926. doi: 10.1136/bmjopen-2019-029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2020a). Genetic Testing. https://www.cdc.gov/genomics/gtesting/genetic_testing.htm. Accessed 14 Jun 2022
- CDC (2020b). Knowing is Not Enough—Act on Your Family Health History | CDC. https://www.cdc.gov/genomics/famhistory/knowing_not_enough.htm. Accessed 14 Jun 2022
- Chern J-Y, Lee SS, Frey MK, Lee J, Blank SV. The influence of BRCA variants of unknown significance on cancer risk management decision-making. J Gynecol Oncol. 2019;30(4):e60. doi: 10.3802/jgo.2019.30.e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley CC, Ketcher D, Reblin M, Kasting ML, Cragun D, Kim J, Ashing KT, Knott CL, Hughes-Halbert C, Pal T, Vadaparampil ST. The big reveal: family disclosure patterns of BRCA genetic test results among young Black women with invasive breast cancer. J Genet Couns. 2019;2020(29):410–422. doi: 10.1002/jgc4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver J, Brinkerhoff C. Variants of uncertain significance in BRCA testing: evaluation of surgical decisions, risk perception, and cancer distress. Clin Genet. 2013;2013(84):464–472. doi: 10.1111/cge.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypowyj C, Eisinger F, Huiart L, Sobol H, Morin M, Julian-Reynier C. Subjective interpretation of inconclusive BRCA1/2 cancer genetic test results and transmission of information to the relatives. Psychooncology. 2008;18:209–215. doi: 10.1002/pon.1407. [DOI] [PubMed] [Google Scholar]
- Donohue KE, Gooch C, Katz A, Wakelee J, Slavotinek A, Korf BR. Pitfalls and challenges in genetic test interpretation: an exploration of genetic professionals experience with interpretation of results. Clin Genet. 2021;99(5):638–649. doi: 10.1111/cge.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards QT, Maradiegue A, Seibert D, Jasperson K. Pre- and postassessment of nurse practitioners’ knowledge of hereditary colorectal cancer: nurse practitioners’ knowledge of hereditary colorectal cancer. J Am Acad Nurse Pract. 2011;23(7):361–369. doi: 10.1111/j.1745-7599.2011.00625.x. [DOI] [PubMed] [Google Scholar]
- Elsayegh N, Webster RD, Gutierrez Barrera AM, Lin H, Kuerer HM, Litton JK, Bedrosian I, Arun BK. Contralateral prophylactic mastectomy rate and predictive factors among patients with breast cancer who underwent multigene panel testing for hereditary cancer. Cancer Med. 2018;7(6):2718–2726. doi: 10.1002/cam4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban I, Vilaró M, Adrover E, Angulo A, Carrasco E, Gadea N, Sánchez A, Ocaña T, Llort G, Jover R, Cubiella J, Servitja S, Herráiz M, Cid L, Martínez S, Oruezábal-Moreno MJ, Garau I, Khorrami S, Herreros-de-Tejada A, Balmaña J. Psychological impact of multigene cancer panel testing in patients with a clinical suspicion of hereditary cancer across Spain. Psychooncology. 2018;27(6):1530–1537. doi: 10.1002/pon.4686. [DOI] [PubMed] [Google Scholar]
- Federici G, Soddu S. Variants of uncertain significance in the era of high-throughput genome sequencing: a lesson from breast and ovary cancers. J Exp Clin Cancer Res. 2020;39(1):46. doi: 10.1186/s13046-020-01554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C. Comparison of risk management strategies between women testing positive for a BRCA variant of unknown significance and women with known BRCA deleterious mutations. Genet Med. 2014;16(12):7. doi: 10.1038/gim.2014.48. [DOI] [PubMed] [Google Scholar]
- Giri VN, Obeid E, MPhil SEH, Gross L, Bealin L, Hyatt C, Fang CY, Leader A. Understanding of multigene test results among males undergoing germline testing for inherited prostate cancer: implications for genetic counseling. The Prostate. 2018;2013(78):879–888. doi: 10.1002/pros.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha VTD, Frizzo-Barker J, Chow-White P. Adopting clinical genomics: a systematic review of genomic literacy among physicians in cancer care. BMC Med Genomics. 2018;11(1):18. doi: 10.1186/s12920-018-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Long JM, Brandt AC, Brower J, Symecko H, Salo-Mullen EE, Christian SN, Harstad T, Couch FJ, Garber JE, Offit K, Robson ME, Domchek SM. Patients’ medical and psychosocial experiences after detection of a CDH1 variant with multigene panel testing. JCO Precis Oncol. 2019;3:1–14. doi: 10.1200/PO.18.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P (2019). Mistrust and lack of genetic diversity slow gains in precision medicine. NPR.Org. https://www.npr.org/sections/health-shots/2019/07/25/742736466/mistrust-and-lack-of-genetic-diversity-slow-gains-in-precision-medicine. Accessed 14 Jun 2022
- Idos GE, Kurian AW, Ricker C, Sturgeon D, Culver JO, Kingham KE, Koff R, Chun NM, Rowe-Teeter C, Lebensohn AP, Levonian P, Lowstuter K, Partynski K, Hong C, Mills MA, Petrovchich I, Ma CS, Hartman A-R, Allen B, Gruber SB. Multicenter prospective cohort study of the diagnostic yield and patient experience of multiplex gene panel testing for hereditary cancer risk. JCO Precis Oncol. 2019;3:1–12. doi: 10.1200/PO.18.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RPC. Patients’ memory for medical information. J R Soc Med. 2003;96:219–222. doi: 10.1258/jrsm.96.5.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-T, Sun S, Lie D, Met-Domestici M, Courtney E, Menon S, Lim GH, Ngeow J. Factors influencing the decision to share cancer genetic results among family members: an in-depth interview study of women in an Asian setting. Psychooncology. 2018;27(3):998–1004. doi: 10.1002/pon.4627. [DOI] [PubMed] [Google Scholar]
- Lizarondo L, Stern C, Carrier J, Godfrey C, Rieger K, Salmond S, Apostolo J, Kirkpatrick P, Loveday H (2020) Chapter 8: mixed methods systematic reviews. In Aromataris E, Munn Z (eds) JBI Manual for Evidence Synthesis JBI. Available from https://synthesismanual.jbi.global; 10.46658/JBIMES-20-09 [DOI] [PubMed]
- Makhnoon S, Garrett LT, Burke W, Bowen DJ, Shirts BH (2019a) Experiences of patients seeking to participate in variant of uncertain significance reclassification research. J Community Genet 10:189–196. 10.1007/s12687-018-0375-3 [DOI] [PMC free article] [PubMed]
- Makhnoon S, Shirts BH, Bowen DJ (2019b) Patients’ perspectives of variants of uncertain significance and strategies for uncertainty management. J Genet Couns 28(2):313–325. 10.1002/jgc4.1075 [DOI] [PubMed]
- Milanese J-S, Wang E. Germline mutations and their clinical applications in cancer. Breast Cancer Manag. 2019;8(1):BMT23. doi: 10.2217/bmt-2019-0005. [DOI] [Google Scholar]
- Miron A, Schildkraut JM, Rimer BK, Winer EP, Sugg Skinner C, Futreal PA, Culler D, Calingaert B, Clark S, Kelly Marcom P, Iglehart JD. Testing for hereditary breast and ovarian cancer in the Southeastern United States. Ann Surg. 2000;231(5):624–634. doi: 10.1097/00000658-200005000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair N, Bellcross C, Haddad L, Martin M, Matthews R, Gabram-Mendola S, Crane B, Meaney-Delman D. Georgia primary care providers’ knowledge of hereditary breast and ovarian cancer syndrome. J Cancer Educ. 2017;32(1):119–124. doi: 10.1007/s13187-015-0950-9. [DOI] [PubMed] [Google Scholar]
- NCCN (2020) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Version 2.2021. NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [DOI] [PubMed]
- NCCN Guidelines Panel (2021) Genetic/Familial High-Risk Assessment: Colorectal. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Accessed 14 Jun 2022
- Rahner N, Steinke V. Hereditary cancer syndromes. Deutsches Ärzteblatt. International. 2008;105(41):706–714. doi: 10.3238/arztebl.2008.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid S, Pal T. Update on multi-gene panel testing and communication of genetic test results. Breast J. 2020;26(8):1513–1519. doi: 10.1111/tbj.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid S, Spalluto LB, Lang K, Weidner A, Pal T. An overview of genetic services delivery for hereditary breast cancer. Breast Cancer Res Treat. 2022;191(3):491–500. doi: 10.1007/s10549-021-06478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter C, Chun N, Pariani M, Hanson‐Kahn A (2019) Understanding variants of uncertain significance in the era of multigene panels: through the eyes of the patient. J Genet Couns 28(4):878–886. 10.1002/jgc4.1130 [DOI] [PubMed]
- Richter S, Haroun I, Graham TC, Eisen A, Kiss A, Warner E. Variants of unknown significance in BRCA testing: impact on risk perception, worry, prevention and counseling. Annals Oncol. 2013;24:viii69–viii74. doi: 10.1093/annonc/mdt312. [DOI] [PubMed] [Google Scholar]
- Rosenthal ET, Bernhisel R, Brown K, Kidd J, Manley S. Clinical testing with a panel of 25 genes associated with increased cancer risk results in a significant increase in clinically significant findings across a broad range of cancer histories. Cancer Genet. 2017;218–219:58–68. doi: 10.1016/j.cancergen.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Solomon I, Harrington E, Hooker G, Erby L, Axilbund J, Hampel H, Semotiuk K, Blanco A, Klein WMP, Giardiello F, Leonard L. Lynch syndrome limbo: patient understanding of variants of uncertain significance. J Genet Couns. 2017;26(4):866–877. doi: 10.1007/s10897-017-0066-y. [DOI] [PubMed] [Google Scholar]
- Tan DSW, Mok TSK, Rebbeck TR. Cancer genomics: diversity and disparity across ethnicity and geography. J Clin Oncol. 2016;34(1):12. doi: 10.1200/JCO.2015.62.0096. [DOI] [PubMed] [Google Scholar]
- Tsai GJ, Chen AT, Garrett LT, Burke W, Bowen DJ, Shirts BH. Exploring relatives’ perceptions of participation, ethics, and communication in a patient-driven study for hereditary cancer variant reclassification. J Genet Couns. 2020;29(5):857–866. doi: 10.1002/jgc4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk S, Van Asperen CJ, Jacobi CE, Vink GR, Tibben A, Breuning MH, Otten W (2004) Variants of uncertain clinical significance as a result of BRCA1/2 testing: impact of an ambiguous breast cancer risk message. Genet Test 8(3). 10.1089/gte.2004.8.235 [DOI] [PubMed]
- Vos J, Otten W, van Asperen C, Jansen A, Menko F, Tibben A. The counsellee’s’ view of an unclassified variant in BRCA1/2: recall, interpretation, and impact on life. Psychooncology. 2008;17(8):822–830. doi: 10.1002/pon.1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Not applicable.