Abstract
Introduction
In a recent randomized placebo-controlled trial, a single intra-articular injection of a high and low molecular weight hyaluronic acid formulation (HA–HL) was shown to be effective in providing a clinically meaningful reduction in pain and functional limitation up to 24 weeks in subjects with painful knee osteoarthritis (OA). The objective of this post hoc analyses is to assess the cost-effectiveness of HA–HL compared with placebo using individual patient data from this clinical trial in a Swiss health care perspective.
Methods
A total of 692 patients fulfilling the inclusion criteria were randomly allocated to HA–HL or placebo groups. Each patient received one intra-articular injection of HA–HL or placebo at baseline and was then followed-up for a total duration of 24 weeks with five follow-up visits (i.e., after weeks 1, 6, 12, 18, and 24). The EQ-5D-5L five-point verbal Likert scale was used to calculate the health utility and the related quality-adjusted life-years (QALYs) using the area-under-the-curve (AUC) method. For the costs, the price of HA-HL in Switzerland was used. The primary threshold for the incremental cost/effectiveness ratio (ICER) below which HA-HL was considered as cost-effective was 91,540 Swiss francs (CHF) per QALY (i.e., US $100,000).
Results
No significant difference between the baseline characteristics of the HA–HL group and the placebo group was observed. With a mean ICER of 27,212 CHF per QALY (95% CI 20,135–34,289), HA–HL was considered as cost-effective compared to placebo. Sensitivity analyses (e.g., using lower or upper limit prices or using other threshold values) gave similar results, i.e., ICERs far below the threshold values of cost-effectiveness.
Conclusions
These results confirm the role of HA–HL as a cost-effective therapeutic option in the management of OA. However, more studies taking into account the utilization of other health care resources are needed.
Keywords: Osteoarthritis, Hyaluronic acid, Heath economic evaluation
Key Summary Points
| Why was this study carried out? |
| Intra-articular injection of hyaluronic acid is proposed as a treatment option for the management of osteoarthritis. |
| In a world with limited healthcare resources, the allocation of the limited financial resources to cost-effective treatments is crucial. |
| The objective of this study was to assess the cost-effectiveness of an intra-articular solution of high and low molecular weight hyaluronic acid compared to the one of placebo from a Swiss health care perspective. |
| What do we learn from this study? |
| With a mean incremental cost/effectiveness ratio of 27,212 CHF per QALY (95% CI 20,135–34,289), the intra-articular solution of high and low molecular weight hyaluronic acid can be considered cost-effective compared to placebo. |
| This treatment could then be considered as an efficient therapeutic modality, confirming its place in the management of osteoarthritis. |
Introduction
Osteoarthritis (OA), the most common form of arthritis of the knee, causes pain, joint stiffness and functional limitation, decreases the quality of life, and increases the mortality risk of many patients worldwide [1, 2]. The current treatment of OA should include a combination of pharmacological and non-pharmacological therapies as recommended in the update of the guidelines of the Osteoarthritis Research Society International (OARSI) [3] and the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) [4]. Even if there are some differences between these two guidelines, they both also recommend the intra-articular injection of hyaluronic acid (HA) as a treatment option. In a recent systematic review of clinical practice guidelines for the prevention, diagnosis, and treatment of knee OA, it was noted that out of the 27 guidelines identified, 20 were in favor of the use of HA [5].
Recently, a phase 3, randomized, double-blind, placebo-controlled study was conducted to evaluate the clinical efficacy and safety of an intra-articular solution of high and low molecular weight HA (HA–HL) in the treatment of pain in symptomatic knee OA [6]. This study showed that a single injection of HA–HL is more effective than placebo (PL) in improving pain [i.e., assessed by the Visual Analogue Scale (VAS)], with the onset of important effects observable as soon as week 1 post-administration and lasting up to week 24. Similar findings were recorded for all secondary outcome variables. Interestingly, the difference in incidence of adverse events between groups was not statistically significant, suggesting that HA–HL is safe and well tolerated.
The cost of OA is substantial. Very recently, using an administrative claims database, Bedenbaugh et al. showed in a US perspective that all-cause costs were significantly higher for knee OA patients compared to matched controls [7]. The authors noted that the difference was attributable to increased medical and treatment costs and the comorbidity treatment burden. In a world with limited resources and healthcare budgets, their allocation to cost-effective treatments is very important, and in that perspective, economic evaluations are playing an increasingly important role in pricing and reimbursement decisions. Indeed, regulatory authorities take into account, at least partly, pharmaco-economic evaluations in guiding their decisions. The aim of this study was to explore the cost-effectiveness of HA-HL compared to PL in the treatment of knee OA. For this purpose, the 24-week time horizon of the recent clinical trial and a Swiss health care perspective were used.
Methods
The Initial Study
This post hoc pharmaco-economic analysis used the data of a multi-center phase 3, randomized, double-blind, PL-controlled clinical trial, previously published (ClinicalTrials.gov identifier NCT03200288) [6]. The aim of the trial was to evaluate the efficacy and safety of an intra-articular solution of high and low molecular weight hyaluronic acid (i.e., HA–HL) in the treatment of pain in knee OA patients. Eligible participants were female and male subjects ≥ 40–80 years of age with primary knee OA according to American College of Rheumatology (ACR) criteria, with Kellgren and Lawrence (K–L) radiographic evidence of OA of grade 2–3. Pain intensity in the target knee measured by a 100-mm VAS was required to be ≥ 40 mm VAS (and ≤ 20 mm in the contralateral knee). The study was conducted in Belgium, Germany, Hungary, Italy, and Poland). The first subject was enrolled on June 29, 2017, and the last subject completed on October 30, 2018. The investigational product (HA–HL, brand names Sinovial® HL and Intragel®, manufactured by IBSA Institut Biochimique SA) was made of 32 mg of high and 32 mg of low molecular weight, non-chemically modified, HA sodium salt per 2 ml of buffered physiological saline solution. In this clinical trial, the HA–HL was administered as a single intra-articular injection, and the impact on pain was primarily assessed by the VAS and compared with PL. Secondary outcomes included the changes, from baseline to five follow-up time points, in the global status assessed by the patients using the EQ-5D-5L questionnaire (EuroQol 5-Dimension Questionnaire, five-level version) [8].
After a wash-out period to avoid any interference of an eventual prior use of analgesics and non-steroidal anti-inflammatory drugs (NSAIDs), 692 patients meeting the inclusion criteria were randomly allocated to HA–HL (n = 347) or PL (n = 345). Each patient received one intra-articular injection of HA–HL or PL at baseline (T0), and was then followed-up for a total duration of 24 weeks. Five follow-up visits were scheduled for the assessment of the outcomes after the baseline visit: after weeks 1, 6, 12, 18, and 24. Only acetaminophen was allowed as rescue analgesic during the trial.
For the purpose of the current pharmaco-economic analysis, data from the intent-to-treat (ITT) population of the clinical trial was considered, which consisted of all randomized patients who received an injection of either HA–HL or PL. No method to replace missing values was used. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
The Effect
The EQ-5D-5L five-point verbal Likert scale (mobility problems, self-care problems, usual activities problems, pain/discomfort problems, and anxiety/depression problems) [8] outcome variables were used. Indeed, the EQ-5D-5L data for each patient can be transformed into health utility (HU) values. There are currently nine countries for which utility value sets for the EQ-5D-5L are available (Denmark, France, Germany, Japan, the Netherlands, Spain, United Kingdom, United States, and Zimbabwe). The utility value sets for the EQ-5D-5L were developed based on the societal preferences of the population of each of these countries; they enable the use of the EQ-5D-5L instrument in the context of economic evaluations [9–11]. First, the EQ-5D-5L values for each patient in the HA–HL and the placebo groups were transformed into utility values (EQ-5D-5L index values) using the data from five major European countries for which utility value sets are currently available (Denmark, France, Germany, the Netherlands, and United Kingdom). Secondly, the mean HU values at each time point (for all patients) were generated using the HU values for the five countries considered in this analysis, which are the mean HU values calculated based on the absolute HU values for each country. Subsequently, the HU changes from baseline values, using data from each country as well as the mean values for these countries, were computed. This was the difference between the absolute HU value at a defined time point (e.g., week 1, week 6, etc.) and the absolute HU value at baseline.
In Switzerland, the pricing of pharmaceuticals to be reimbursed by compulsory health insurances/compulsory basic health insurance plans is based on two kinds of cross comparisons, a comparison with the prices of other pharmaceuticals to treat the same disease and a comparison of the prices of the product to be reimbursed in nine European reference countries, including Denmark, France, Germany, the Netherlands, and the UK. Accordingly, the mean of the HU values of the before-mentioned countries was used in the present study.
The Cost
The ex-factory price of HA–HL in Switzerland is CHF 174.48 excluding VAT. HA–HL is not reimbursed by Swiss compulsory health insurances/compulsory basic health insurance plans. Therefore, its public price is subject to market forces (supply and demand). In Switzerland, for these kinds of products, a profit margin of up to 80% may commonly be applied and accepted. However, because the price of a HA treatment is perceived as relatively high—consider that the patients have to pay it themselves—many physicians and pharmacists only add a margin as low as around 40% to the ex-factory price. Therefore, we used the following public prices of HA–HL in the pharmaco-economic analysis regarding the Swiss market:
public price (“midpoint”): ex-factory price + 40% profit margin + 7.7% VAT = CHF 263.08
lower limit of public price for sensitivity analysis: ex-factory price + 20% profit margin + 7.7% VAT = CHF 225.50
upper limit of public price for sensitivity analysis: ex-factory price + 80% profit margin + 7.7% VAT = CHF 338.25
Statistical Analysis
Normality of each of the data was checked using the Shapiro–Wilk normality test, and by generating QQ plots as well as by plotting histograms with normal distribution curves. The absolute HU values, at baseline and at each follow-up time point (after weeks 1, 6, 12, 18, and 24) were then compared between the HA–HL and the placebo groups, using the one-way ANOVA test. HU changes from baseline values were also compared between HA–HL and PL using HU changes from baseline values for each country as well as the mean changes from baseline values of all countries. These comparisons were made using the one-way ANOVA test when the two variables (i.e., HU change from baseline, in HL-01 or placebo groups) followed a normal distribution. Otherwise, the non-parametric Kruskal–Wallis rank test was used. All these analyses were performed using STATA software, version 14.2 (StataCorp LLC).
Cost-Effectiveness Analysis
The HU estimates were used to calculate the quality-adjusted life-years (QALYs) using the area-under-the-curve (AUC) method, that is, the weighted average of time spent in the study and HU value. The AUC was calculated for the following time periods: 0–1, 1–6, 6–12, 12–18, and 18–24 weeks. Then, the global AUC was considered as the sum AUC for those time periods. The cost-effectiveness analysis assessed the additional resources used for an improvement in the QALYs associated with HA–HL compared to PL. The results were summarized as an incremental cost/effectiveness ratio (ICER) that is a measure of the additional cost per QALY gained. ICER was calculated as the cost of HA–HL divided by the difference between the QALY change in the HA–HL and PL groups.
To get insight into the uncertainty around the ICERs, a bootstrap simulation was conducted using 1000 replications. A bootstrap simulation is a non-parametric method in which a sample of equal size as the original sample is selected, many times at random with replacement. This adds information concerning the distribution of the ICER estimate.
Within the cost-effectiveness framework, the ceiling ratio is important. Indeed, the probability for a new treatment to be cost-effective varies, depending on how much society is willing to pay per gain in effectiveness. Consequently, cost-effectiveness acceptability curves (CEAC) were also calculated.
Limit of the ICER
There is no specific threshold value for ICERs below which an intervention is considered as cost-effective. Indeed, this threshold ranges greatly from country to country and depends on methods and assumptions used. The World Health Organization proposes to use one to three times the gross domestic product (GDP) per capita, but without any robust methodological justification. However, according to a recent systematic review of the literature, it was noted that the commonly referred to value of US $100,000 per QALY may potentially be more scientifically relevant [12]. Consequently, in our analyses, we considered the following thresholds:
Primary threshold: 91,540 Swiss franc (CHF) per QALY (corresponding to the US $100,000).
- Secondary thresholds:
- 79,423 CHF per QALY (corresponding to the 2020 GDP per capita of Switzerland of 86,601.6 CHF https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=CH).
- 254,307 CHF per QALY (three times the GDP per capita).
Results
Table 1 shows the baseline characteristics of the study population including radiographic OA severity (K–L grade), pain intensity in the target knee (VAS pain), and algofunctional status (Lequesne index). No significant difference between the baseline characteristics of the HA–HL group and the PL group was observed. More particularly, no significant difference was noted for the mean HU between the two groups.
Table 1.
Summary of baseline (T0) characteristics of the study population
| N | n | HA–HL | n | Placebo | p value | |
|---|---|---|---|---|---|---|
| HU T0, mean of all countries, mean ± SD | 692 | 347 | 0.63 ± 0.16 | 345 | 0.61 ± 0.16 | 0.112 |
| Age, mean ± SD | 347 | 63.68 ± 8.74 | 345 | 63.77 ± 8.12 | 0.884 | |
| Sex, female (%) | 692 | 232 (66, 86) | 230 (66, 67) | 0.957 | ||
| BMI, median (IQR) | 692 | 28.96 (26.04–30.86) | 29.38 (26.99–30.82) | 0.316 | ||
| K–L grade, n (%) | 692 | 347 | 345 | 0.989 | ||
| 2 | 205 (59.08) | 204 (59.13) | ||||
| 3 | 142 (40.92) | 141 (40.87) | ||||
| VAS pain, mean ± SD | 692 | 347 | 63.53 ± 13.13 | 345 | 64.89 ± 13.87 | 0.187 |
| Lequesne index, mean ± SD | 691 | 347 | 11.40 ± 3.58 | 344 | 11.60 ± 3.58 | 0.451 |
IQR interquartile range (P25, P75). All p values are based either on the one-way ANOVA test or the non-parametric Kruskal–Wallis rank test for quantitative variables, and on the Pearson’s Chi2 test for qualitative variables (sex and K–L grade)
The mean absolute HU changes from baseline in the HA–HL and PL groups at each evaluation are presented in Table 2. A significant difference between groups was noted after 6 weeks of follow-up, but not at the other time points.
Table 2.
Summary of HU changes from baseline, using the mean index values for all countries
| n | HA–HL | n | Placebo | p value† | |
|---|---|---|---|---|---|
| HU change—mean index, mean ± SD | |||||
| Δ T1–T0 | 347 | 0.10 ± 0.14 | 345 | 0.08 ± 0.14 | 0.072 |
| Δ T6–T0 | 342 | 0.12 ± 0.17 | 343 | 0.09 ± 0.17 | 0.048 |
| Δ T12–T0 | 341 | 0.14 ± 0.17 | 336 | 0.11 ± 0.17 | 0.058 |
| Δ T18–T0 | 339 | 0.15 ± 0.17 | 330 | 0.12 ± 0.17 | 0.055 |
| Δ T24–T0 | 336 | 0.14 ± 0.17 | 327 | 0.12 ± 0.17 | 0.365 |
†p value comparing the HA–HL to the placebo group using the one-way ANOVA test
Using the mean HU value of Denmark, France, Germany, the Netherlands and UK assessed during the whole study period, incremental QALYs were calculated for the HA–HL and PL groups and are shown in Table 3. Using the base case scenario for the cost of HA–HL, the calculated ICER was 27,860 CHF/QALY gained, far below the threshold values of cost-effectiveness. The bootstrap method gave similar results with an ICER of 27,212 CHF/QALY (95% CI 20,135–34,289 CHF/QALY). The use of the lower and upper limit prices gave ICERs of 23,888 and 35,815 CHF/QALY, respectively.
Table 3.
Incremental cost-effectiveness ratio assessment
| Incremental QALYs per patient in the HA-HL group | 0.0580 |
| Incremental QALYs per patient in the PL group | 0.0486 |
| Incremental cost of PL per patient (in CHF) | 0 |
| Incremental cost of HA-HL per patient (in CHF) | |
| Base-case scenario | 263.08 |
| Lower limit | 225.50 |
| Upper limit | 338.25 |
| ICER (CHF/QALY) | |
| Base-case scenario | |
| Standard method | 27,860 |
| Bootstrapping | 27,212 (95% CI 20,135–34,289) |
| Lower limit | 23,888 |
| Upper limit | 35,815 |
QALYs quality-adjusted life-years, PL placebo, HA–HL high and low molecular weight hyaluronic acid, ICER incremental cost/effectiveness ratio, CHF Swiss franc
The results of the bootstrap simulation also showed that in 99% of the cost-effectiveness pairs, the HA–HL treatment was cost-effective compared to the one with PL, whereas in 1% of the pairs, HA–HL was inferior. According to the cost-effectiveness acceptability curve (Table 4), given a maximum acceptable ceiling ratio between 80,000 and 90,000 CHF per QALY gained, the probability that HA–HL is cost-effective compared to PL is 95% (Fig. 1).
Table 4.
Probability that HA–HL is cost-effective based on ICER
| Limit on ICER (CHF) | Probability that HA–HL is cost-effective |
|---|---|
| 0 | 0% |
| 10,000 | 0% |
| 20,000 | 38% |
| 30,000 | 72% |
| 40,000 | 84% |
| 50,000 | 89% |
| 60,000 | 92% |
| 70,000 | 94% |
| 80,000 | 95% |
| 90,000 | 95% |
| 100,000 | 96% |
| 110,000 | 96% |
| 120,000 | 96% |
| 130,000 | 96% |
| 140,000 | 97% |
| 150,000 | 97% |
Fig. 1.
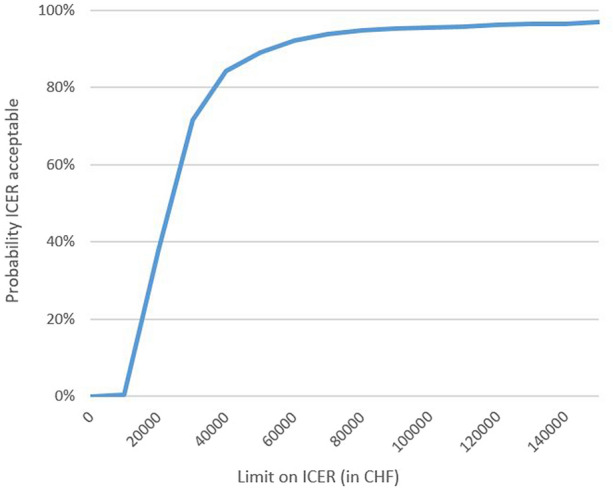
Cost-effectiveness acceptability curve showing the probability that HA–HL is cost-effective compared with placebo over a range of values for the maximum acceptable ceiling ratio
Discussion
In the present study, taking advantage of the individual patient data from a recently published 24-week, multicenter, double-blind randomized placebo controlled trial, we showed that HA–HL is cost-effective compared to placebo and can be considered as a cost-effective option in the management of OA. This study confirms the place of HA–HL as one of the therapeutic modalities of OA management as acknowledged in most of the evidence-based treatment guidelines, consensus statements, and decision algorithms for intra-articular HA in general.
There are, however, many formulations with HA, very heterogeneous in term of molecular weight that could range from 500,000 to more than 5,000,000 Daltons, and so far there is no clear understanding regarding which could be the most appropriate molecular weight for the treatment of OA. In addition, treatment regimens are also quite different and vary from a single injection to courses of 5-weekly injections every 6 months. Therefore, it is important to have specific efficacy and safety data for each of the marketed HA products even though no major differences between most of them have been observed so far in terms of efficacy in meta-analyses [13, 14]. However, in the absence of direct comparisons and due to the methodological differences between the individual clinical trials, the comparison of different HA products is quite complex.
In addition, few studies have compared the cost or the cost-effectiveness of different HA products. Recently, the authors of a real-life retrospective observational study that compared three different formulations of HA with different treatment modalities (weekly for 5 weeks, weekly for 3 weeks or a single injection) came to the conclusion that all three treatments were equally effective [15]. However, the one that had one single injection had the best cost profile, from both a National Italian Health System and a social cost point of view. A pilot study in China comparing 3-weekly intra-articular injections of platelet-rich plasma (PRP) and HA showed that PRP injections were associated with higher costs [16]. The two treatments were also compared in another study with a US cost perspective, and the authors noted that both treatment options could be considered as cost-effective (with cost per QALY below $50,000) [17]. In another study comparing usual care plus high molecular weight HA to usual care only over 52 weeks in 156 subjects, a cost–utility analysis was performed from the societal and health care perspective [18]. The authors noted an ICER of €9100/QALY from a societal perspective and €8700/QALY from a health care perspective, suggesting that the HA added to usual care for is probably cost-effective in the management of knee OA. At last, an interesting study using a national French Health Insurance System perspective concluded that HA did not generate additional costs to the national health insurance [19]. Moreover, for HA, the authors observed an additional gain of QALYs equivalent to half a month after a 6-month follow-up period compared to non-steroidal anti-inflammatory drugs. In any case, it is essential that the efficacy of each HA is clearly supported by strong evidence, e.g., a double-blind RCT, as this is the case with this HA-HL.
In the current study, in the absence of clear recommendations, we used different threshold values for the ICER below which HA-HL could be considered cost-effective. We applied the recommendation of a recent review suggesting that US $100,000 per QALY may be more scientifically relevant [12]. However, we also used the suggested threshold of the WHO (i.e., 1–3 times the GDP per capita). It should also be noted that some countries also use different arbitrary thresholds far below these recommendations (e.g., 20,000 or 30,000 euros/QALY). Our results show that using the average public price of HA–HL in Switzerland, this HA is cost-effective when applying the standard threshold of 91,540 CHF per QALY (corresponding to the common value of US $100,000). Remarkably, all our sensitivity analyses (i.e., with variation in the price of HA–HL and using other thresholds of incremental cost-effectiveness ratio) confirmed the initial analysis highlighting the robustness of our results. At last, the bootstrapping method, with which the confidence interval at 95% can be assessed, also showed that HA–HL is cost-effective whatever primary or secondary cost/QALY threshold values were considered. This highlights the confidence we can have in our results.
However, we should also acknowledge some limitations of our study. First, we only assessed the cost of the HA–HL treatment, omitting a more global perspective taking into account other costs potentially borne by the patients (joining the clinic, management of adverse event, use of other concomitant treatment). However, as the present post hoc study is based on a published RCT where no significant differences were found with respect to adverse events and the concomitant use of rescue medication, the indicated limitation is unlikely to have a real impact. We should, however, acknowledge that no statistically significant difference in the adverse events cannot guarantee that the non-drug health care costs are the same between the two treatment arms. Secondly, as requested by health authorities, we compared HA–HL with placebo and consequently, no comparison is possible with other pharmacological agents. However, more relevant health economics analysis should be carried out in more pragmatic RCTs where interventions are open labeled and applied in a way that is more consistent with the real world. Thirdly, we used data from a clinical trial which, in one way, may not fully reflect patients’ real life experiences, but offers the advantage of providing reliable and accurate data as patients are rigorously followed.
In conclusion, our data show that an intra-articular solution of high and low molecular weight HA is cost-effective compared to placebo in a Swiss health care perspective. The results confirm the role of HA–HL as an important therapeutic option in the management of knee OA.
Acknowledgements
Funding
This study was funded by IBSA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization, OB; methodology, OB, JYR and GH; formal analyses, OB and GH; interpretation of the results, OB, JYR and GH; writing—original draft preparation, OB; writing—review and editing, OB, JYR and GH. All authors have read and agreed to the published version of the manuscript.
Disclosures
Olivier Bruyère and Jean-Yves Reginster have received fees as consultants for IBSA. Germain Honvo has nothing to declare.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The initial trial was approved by the local Ethical Committee of each participating site and the National Regulatory Authorities of Italy, according to the specific national regulation. All patients provided written informed consent. The initial trial was conducted in accordance with the Declaration of Helsinki and its modifications, the rules of the International Conference on Harmonization (ICH) Good Clinical Practices (GCP), and ISO 14155, the European Union Council Directive 93/42/EEC amended by 2007/47/EC, the MEDDEV 2. 12-1 rev. 6 and amendments.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Leyland KM, Gates LS, Sanchez-Santos MT, Nevitt MC, Felson D, Jones G, Jordan JM, Judge A, Prieto-Alhambra D, Yoshimura N, et al. Knee osteoarthritis and time-to all-cause mortality in six community-based cohorts: an international meta-analysis of individual participant-level data. Aging Clin Exp Res. 2021;33:529–545. doi: 10.1007/s40520-020-01762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llopart-Carles N, García-López S, Rejas-Gutierrez J. Disability-adjusted life expectancy lost due to pain severity and usual analgesic treatment among older adults with osteoarthritis in Spain. Aging Clin Exp Res. 2021;33:1285–1295. doi: 10.1007/s40520-020-01630-z. [DOI] [PubMed] [Google Scholar]
- 3.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Bruyère O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, Al-Daghri NM, Herrero-Beaumont G, Martel-Pelletier J, Pelletier JP, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) Semin Arthritis Rheum. 2019;49:337–350. doi: 10.1016/j.semarthrit.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Phillips M, Bhandari M, Grant J, Bedi A, Trojian T, Johnson A, Schemitsch E. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliore A, Blicharski T, Plebanski R, Zegota Z, Gyula G, Rannou F, Reginster JY. Knee osteoarthritis pain management with an innovative high and low molecular weight hyaluronic acid formulation (HA–HL): a randomized clinical trial. Rheumatol Ther. 2021;8:1617–1636. doi: 10.1007/s40744-021-00363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedenbaugh AV, Bonafede M, Marchlewicz EH, Lee V, Tambiah J. Real-world health care resource utilization and costs among US patients with knee osteoarthritis compared with controls. ClinicoEcon Outcomes Res CEOR. 2021;13:421–435. doi: 10.2147/ceor.s302289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res Int J Qual Life Aspects Treat Care Rehabil. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludwig K, Graf von der Schulenburg JM, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36:663–674. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versteegh MM, Vermeulen KM, Evers SM, De Wit GA, Prenger R, Stolk EA. Dutch tariff for the five-level version of EQ-5D. Value Health J Int Soc Pharmacoecon Outcomes Res. 2016;19:343–352. doi: 10.1016/j.jval.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Andrade LF, Ludwig K, Goni JMR, Oppe M, de Pouvourville G. A French value set for the EQ-5D-5L. Pharmacoeconomics. 2020;38:413–425. doi: 10.1007/s40273-019-00876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11:1447828. doi: 10.1080/16549716.2018.1447828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaudart C, Lengelé L, Leclercq V, Geerinck A, Sanchez-Rodriguez D, Bruyère O, Reginster JY. Symptomatic efficacy of pharmacological treatments for knee osteoarthritis: a systematic review and a network meta-analysis with a 6-month time horizon. Drugs. 2020;80:1947–1959. doi: 10.1007/s40265-020-01423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichenbach S, Blank S, Rutjes AW, Shang A, King EA, Dieppe PA, Jüni P, Trelle S. Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Rheum. 2007;57:1410–1418. doi: 10.1002/art.23103. [DOI] [PubMed] [Google Scholar]
- 15.Galluccio F, D'Angela D, Polistena B, Porta F, Barskova T, Tofani L, Spandonaro F, Matucci-Cerinic M. Comparison of three treatment protocols with intra-articular low or intermediate molecular weight hyaluronic acid in early symptomatic knee osteoarthritis. Therap Adv Musculoskelet Dis. 2021 doi: 10.1177/1759720x21994024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Huang Z, Wang S, Di Z, Zhang J, Liu H. Intra-articular injections of platelet-rich plasma vs. hyaluronic acid in patients with knee osteoarthritis: preliminary follow-up results at 6-months. Exp Therap Med. 2021;21:598. doi: 10.3892/etm.2021.10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuelson EM, Ebel JA, Reynolds SB, Arnold RM, Brown DE. The cost-effectiveness of platelet-rich plasma compared with hyaluronic acid injections for the treatment of knee osteoarthritis. Arthrosc J Arthrosc Relat Surg. 2020;36:3072–3078. doi: 10.1016/j.arthro.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Hermans J, et al. Cost-utility analysis of high molecular weight hyaluronic acid for knee osteoarthritis in everyday clinical care in patients at a working age: an economic evaluation of a randomized clinical trial. Arthritis Care Res (Hoboken) 2018;70(1):89–97. doi: 10.1002/acr.23242. [DOI] [PubMed] [Google Scholar]
- 19.Thomas T, Amouroux F, Vincent P. Intra articular hyaluronic acid in the management of knee osteoarthritis: pharmaco-economic study from the perspective of the national health insurance system. PLoS One. 2017;12:e0173683. doi: 10.1371/journal.pone.0173683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


