Abstract
Background
Vascular disease burden after lower extremity revascularization (LER) comprises more than the first event, more vascular beds than the local arteries, and more than one clinical event type.
Objectives
Assess total arterial and venous thrombotic burden after LER for symptomatic peripheral artery disease (PAD) and effect of low‐dose anticoagulation added to low‐dose antiplatelet therapy.
Patients/Methods
VOYAGER PAD randomized 6564 symptomatic PAD patients undergoing LER to rivaroxaban 2.5 mg twice‐daily or placebo on aspirin background. Marginal proportional‐hazards models used to generate treatment hazard ratios and associated 95% CIs for first and total events; non‐thrombotic deaths treated as competing terminal events. Incidence rates calculated as number of events per 100 patient‐years follow‐up.
Results
Over 2.5 years (median), first and total thrombotic event rates: 7.1 and 10.3 events/100 patient‐years, respectively, in placebo group. Two‐thirds (925/1372) of total thrombotic events (arterial 95%, venous 5%) were nonfatal first events. Nearly one‐third of patients with first event had a second arterial or venous thrombotic event. Rivaroxaban plus aspirin reduced first and total arterial and venous thrombotic events to 5.4 and 7.9 events/100 patient‐years, respectively, a reduction in total thrombotic events over aspirin of 23% (HR: 0.77, 95%CI: 0.67–0.89, p = .0005), preventing 6.1 total arterial and venous thrombotic events at 3 years.
Conclusions
Assessing total arterial and venous thrombotic events, not just first events, provides more complete information about disease burden and absolute on‐treatment impact. Following LER, judicious modulation of more than one coagulation pathway can provide broader benefit than intensifying inhibition of one hemostatic system component.
Keywords: anticoagulants, atherosclerosis, peripheral arterial disease, rivaroxaban, thrombosis, venous thromboembolism
Essentials.
Arterial and venous events occur frequently in symptomatic peripheral artery disease patients after revascularization.
More than one hemostatic pathway is involved in atherosclerotic vessel thrombotic events.
Rivaroxaban added to aspirin reduces first and subsequent arterial and venous thrombotic events.
Total event analysis enhances understanding of treatment impact on full burden of thrombotic risk.
1. INTRODUCTION
The full burden of chronic vascular disease such as atherosclerosis encompasses more than one vascular bed, includes more than just the first event, involves more than just local arterial thrombi, and comprises clinical events that occur more than once. These events are indicators of disease progression, are associated with patient disability morbidity and mortality, and add to the substantial humanistic and economic encumbrance of the disease. 1
Important pathobiological differences exist in vascular disease patients with lower extremity PAD compared to coronary artery disease (CAD). Luminal thrombosis leads to symptoms both in CAD and PAD, but in contrast to acute coronary syndrome (ACS) where the underlying pathology is acute thrombus formation after atherosclerotic plaque rupture, the causes of acute limb ischemia (ALI) in patients with lower extremity PAD include in situ thrombosis, emboli from heart and proximal vessels, and graft occlusion. 2 , 3 Descriptive pathology studies in PAD demonstrate heterogeneity with luminal compromise in distal arteries more frequently due to thrombotic occlusion, often in the absence of significant atherosclerosis, while in proximal arteries stenosis is predominantly secondary to atherosclerotic plaques. 4 , 5
Peripheral artery disease is prevalent and its incidence is increasing in the setting of an aging population and risk factors such as obesity and diabetes. 6 , 7 Population‐based studies estimate that there are three times as many asymptomatic patients with lower extremity PAD as symptomatic patients. 7 Patients with PAD are at very high risk for thrombotic events, especially those with polyvascular disease (symptomatic in more than one vascular territory) 8 and diabetes, 9 therefore antithrombotic therapy is a core element of preventive medical therapy.
Trials investigating antithrombotic treatment strategies in patients with atherosclerosis have described the beneficial effects and risks of those approaches but mainly studied CAD populations with stable disease. 10 , 11 , 12 These trials also focused on first rather than total events and did not include symptomatic PAD patients randomized in the acute revascularization setting. Patients with symptomatic PAD who have undergone lower extremity revascularization (LER) are at particularly high risk for both major adverse cardiovascular events (MACE) and major adverse limb events (MALE). 13 , 14 , 15 Until very recently there was no proven antithrombotic strategy demonstrating efficacy benefit to reduce these events. The VOYAGER PAD (Efficacy and Safety of Rivaroxaban in Reducing the Risk of Major Thrombotic Vascular Events in Subjects With Symptomatic Peripheral Artery Disease Undergoing Peripheral Revascularization Procedures of the Lower Extremities) trial was designed to overcome the knowledge gap in these patients.
In VOYAGER PAD 1 in 5 patients experienced a first major adverse limb or cardiovascular event despite the use of aspirin in all patients, statins in 80%, and clopidogrel in half. 16 An overall picture of the total load of vascular events in patients with PAD after LER and the efficacy of low‐dose rivaroxaban on total events has been published. 17 In that analysis, 72% of the total vascular events were not clearly thrombotic in nature, e.g. heart failure death, and dominated by repeat revascularization procedures, many of which were not thrombotically driven.
The risk profile for total thrombotic events and the effect of Factor Xa inhibition added to antiplatelet therapy in symptomatic PAD patients after LER have not been fully described. In this pre‐specified analysis, we examine total arterial and venous thrombotic events in VOYAGER PAD in order to obtain a more comprehensive approach to CV outcome assessment and to capture the full picture and benefit of the therapy tested. We delineate the burden of primarily thrombotic events (arterial and venous) in patients with symptomatic PAD after revascularization and evaluate the efficacy of low dose Factor Xa inhibition plus low dose antiplatelet therapy for the prevention of first and total arterial and venous thrombotic events. We hypothesized that there is ongoing and additional thrombotic risk subsequent to the first event and that rivaroxaban in combination with aspirin would reduce both first and succeeding arterial and venous events.
2. METHODS
2.1. Data source and study conduct
Data for this analysis were drawn from the VOYAGER PAD trial, the design and results of which have been previously published. 16 , 18 This double‐blind trial performed in 34 countries at 542 sites randomized in a 1:1 ratio 6564 symptomatic PAD patients who underwent lower extremity revascularization, either for claudication or for critical limb ischemia, to rivaroxaban 2.5 mg twice‐daily or matching placebo on a background of aspirin 100 mg daily. Concomitant clopidogrel use was allowed for up to 6 months per the investigator's discretion. All received standard of care medications and patients were followed for a median of 28 months. The trial protocol was designed and overseen by Colorado Prevention Center (CPC) Clinical Research (an academic research organization affiliated with the University of Colorado), the academic executive committee, and the sponsors, Bayer and Janssen Pharmaceuticals. CPC Clinical Research holds the clinical database and independently performed all analyses for this publication. VOYAGER PAD was registered at www.clinicaltrials.gov (NCT02504216) and performed in accordance with good clinical practice and local regulatory requirements. All patients provided written informed consent, institutional review boards/ethics committees at participating institutions approved the protocols, and consent could be withdrawn at any time.
2.2. Study population
VOYAGER PAD enrolled symptomatic PAD patients ≥50 years old and with an abnormal ankle‐brachial index (ABI) ≤ 0.80 or toe‐brachial index (TBI) ≤ 0.60 (in those without a prior history of LER) and imaging evidence of PAD distal to the external iliac artery. Eligible patients had successfully undergone LER for claudication or critical limb ischemia via either a surgical or an endovascular (including hybrid endovascular plus surgical) approach within the previous 10 days. Key exclusion criteria included a planned course of dual antiplatelet therapy greater than 6 months, clinical indication for therapeutic anticoagulation, including treatment or long‐term prevention of VTE, recent acute limb ischemia (ALI) or ACS, increased risk of bleeding, significantly impaired baseline renal function (eGFR < 15 ml/min/1.73 m2), and documented prior intracranial hemorrhage, stroke, or transient ischemic attack.
2.3. Outcomes
The primary efficacy outcome for VOYAGER PAD was a composite endpoint of time to first occurrence of ALI, major amputation of vascular etiology, myocardial infarction, ischemic stroke, or cardiovascular death. For our analysis we constructed within the VOYAGER PAD ITT dataset an efficacy outcome of total (i.e., first and subsequent) arterial and venous thrombotic events, which is a composite of ALI, major amputation of vascular etiology, myocardial infarction, ischemic stroke, and symptomatic VTE. An independent Central Events Committee blinded to treatment assignment adjudicated all deaths and potential ischemic cardiac, cerebrovascular and vascular limb events, including the first and all subsequent thrombotic events, except for symptomatic VTE. VTE was a prespecified, prospectively ascertained secondary endpoint, which was reported on a specific case report form by investigators blinded to treatment assignment.
2.4. Statistical analysis
Categorical variables are reported as count (percentage), and continuous variables as median (quartile 1‐ quartile 3). Comparisons of baseline characteristics grouped by no, one, or multiple arterial or venous thrombotic events during follow‐up were by Wilcoxon rank‐sum tests for continuous variables and chi‐square or Fisher's exact tests (where possible) for categorical variables.
A multivariable Cox regression model of baseline demographic and clinical variables as predictors of total thrombotic events was estimated by stepwise selection; a p‐value of <.10 was applied for model entry or exit. Candidate variables are listed in Table S1.
First and total events were analyzed by a marginal proportional‐hazards models where non‐thrombotic deaths were treated as competing terminal events. 19 Treatment effects on first and total events were summarized by hazard ratios (HRs) with associated Wald 95% CIs and log‐rank p‐values. For total events, the robust sandwich variance estimate for the estimated standard error of the log hazard ratio was applied to account for the dependence of event times within individual patients. 20 Cumulative incidence functions for first events and mean cumulative functions for total events were used to estimate cumulative incidences with corresponding 95% CIs through 3 years of follow‐up by treatment group in the presence of competing non‐thrombotic deaths. Incidence rates were calculated as number of events per 100 patient‐years of follow‐up.
All outcomes and components were prespecified and analyzed according to the intention‐to‐treat principle. 16 , 18 p‐values < .05, two‐tailed, were considered statistically significant, with no adjustment for multiple testing. Analyses were performed in SAS 9.4 and S+ 8.2 and GraphPAD Prism 9.2.0.
3. RESULTS
3.1. Study population
Among 6564 randomized patients, 929 (14.2%) suffered a total of 1372 arterial and venous thrombotic events over a median of 2.5 (2.0–3.0) years of follow‐up (Table 1). Arterial events comprised about 95% (1299 of 1372) while the 73 venous events made up just over 5%.
TABLE 1.
Categories of total arterial and venous thrombotic events
| Event |
Placebo N = 3278 |
Rivaroxaban N = 3286 |
Total N = 6564 |
|---|---|---|---|
| Total thrombotic events | 772 | 600 | 1372 |
| Arterial events | 725 (93.9) | 574 (95.7) | 1299 (94.7) |
| Acute limb ischemia | 306 (42.2) | 202 (35.2) | 508 (39.1) |
| Major amputation for vascular causes | 133 (18.3) | 117 (20.4) | 250 (19.2) |
| Non‐fatal myocardial infarction | 170 (23.4) | 152 (26.5) | 322 (24.8) |
| Non‐fatal ischemic stroke | 86 (11.9) | 75 (13.1) | 161 (12.4) |
| Fatal myocardial infarction or stroke | 30 (4.1) | 28 (4.9) | 58 (4.5) |
| Venous events | 47 (6.1) | 26 (4.4) | 73 (5.3) |
| Non‐fatal venous thromboembolic event* | 41 (87.2) | 25 (96.1) | 66 (90.4) |
| Fatal pulmonary embolism or other fatal thromboembolic event | 6 (12.8) | 1 (3.8) | 7 (9.6) |
*Investigator‐reported; not subject to adjudication by independent committee.
Numbers in parentheses are % of total thrombotic events.
Fatal thrombotic events, n = 65: 1st event, n = 4; 2nd event, n = 46; 3rd or subsequent event, n = 15.
3.2. Categories of total arterial and venous thrombotic events
The categories of total arterial and venous thrombotic events are shown in Table 1. The thrombotic events were observed in a variety of vascular beds. The most frequent outcomes, accounting for 55% (758/1372) of all events, were in the lower extremity arteries: acute limb ischemia and major amputation of a vascular cause. Coronary artery events (non‐fatal MI) occurred in 23% (322/1372), and nearly 12% (161/1372) were observed in the cerebral vascular tree (non‐fatal ischemic stroke). Over 90% of the venous events were nonfatal VTE. Fatal arterial or venous thrombotic events totaled 65 (4.7%), comprised of 58 arterial and seven venous events. Four of these fatal events were first thrombotic events, 46 were second thrombotic events, and 15 were third or subsequent thrombotic events.
3.3. Characteristics of the population assessed
Patient baseline and procedural characteristics of the study population assessed for total arterial and venous events are summarized in Table 2, which compares patients without an event (n = 5635; 86%), those with one arterial or venous event (n = 645; 10%), and those with multiple arterial or venous events (n = 284; 4%). One or more arterial thrombotic events occurred in 882 patients, 67 had one or more venous event, and 20 had one or more of both (not shown). Patients with one or more thrombotic events were more likely to have CAD, diabetes, or impaired renal function. Patients with characteristics associated with more severe PAD such as history of prior revascularization or amputation, lower ABI, having a surgical qualifying revascularization as opposed to endovascular, and longer target lesions and more complex anatomy were more likely to experience arterial or venous thrombotic events.
TABLE 2.
Baseline and procedural characteristics of participants by type of thrombotic event
| Characteristics at Randomization |
(A) No Event (n = 5635; 86%) |
(B) One Arterial or Venous (n = 645; 10%) |
(C) Multiple Arterial or Venous Event (n = 284; 4%) |
p‐value | |
|---|---|---|---|---|---|
| (A) vs. (B) + (C) | (B) vs. (C) | ||||
| Baseline characteristics | |||||
| Coronary artery disease, % | 30.6 | 38.9 | 33.1 | <.0001 | n.s. |
| Diabetes mellitus, % | 38.9 | 47.3 | 46.1 | <.0001 | n.s. |
| eGFR<60 ml/min/1.73 m2 | 19.7 | 23.7 | 22.2 | .01 | n.s. |
| BMI, kg/m² |
26.0 (23.3–29.1) |
26.0 (23.0–29.1) |
25.7 (23.2–28.3) |
n.s. | n.s. |
| History of Cancer, % | 4.9 | 7.1 | 4.6 | n.s. | n.s. |
| Medications | |||||
| Statin, % | 79.8 | 83.3 | 75.7 | n.s. | .009 |
| Clopidogrel, % | 51.1 | 47.6 | 45.1 | .02 | n.s. |
| PAD & Procedural characteristics | |||||
| Prior peripheral artery disease history | |||||
| History of claudication, % | 95.7 | 94.4 | 93.3 | .03 | n.s. |
| History of revascularization, % | 34.4 | 42.2 | 43.3 | <.0001 | n.s. |
| History of amputation, % | 5.3 | 9.3 | 10.6 | <.0001 | n.s. |
| Ankle Brachial Index, Median (IQR) | 0.56 (0.43–0.67) | 0.53 (0.40–0.65) | 0.51 (0.38–0.63) | <.0001 | n.s. |
| Type of revascularization | |||||
| Surgical, % | 32.1 | 39.1 | 43.3 | <.0001 | n.s. |
| Endovascular or hybrid, % | 67.9 | 60.9 | 56.7 | ||
| Days from procedure to randomization, median (IQR) | 5 (2–7) | 5 (3–7) | 5 (3–8) | <.0001 | n.s. |
| Target lesion length | n.s. | ||||
| Short (<5 cm), % | 23.4 | 18.8 | 15.5 | <.0001 | |
| Intermediate (5 to <15 cm), % | 40.7 | 35.5 | 32.0 | ||
| Long (≥15 cm), % | 32.7 | 42.8 | 47.5 | <.0001 | n.s. |
| Atherectomy, % | 4.8 | 4.8 | 2.8 | n.s. | n.s. |
3.4. Baseline characteristics associated with thrombotic risk
Baseline characteristics and PAD and procedural characteristics independently associated with arterial and venous thrombotic risk as determined by multivariate modeling are shown in Figure 1. The strongest factors associated with thrombotic risk were related to greater severity of limb disease or anatomy: prior surgical revascularization, target lesion ≥15 cm, prior amputation, and CLI as the indication for index revascularization. The patient factors such as diabetes or presence of polyvascular disease (i.e., concomitant CAD) were next strongest. After multivariable modeling, treatment with rivaroxaban was associated with a lowered risk for total thrombotic events (HR: 0.79, 95% CI: 0.69, 0.90; p = .0003; Figure 1). Importantly, when accounting for all baseline characteristics associated with risk, the efficacy of rivaroxaban was not modified by the use of clopidogrel at randomization: without clopidogrel HR: 0.78, 95% CI: 0.66, 0.94; with clopidogrel HR: 0.79, 95% CI 0.66, 0.96; p‐interaction = 0.93 (not shown).
FIGURE 1.
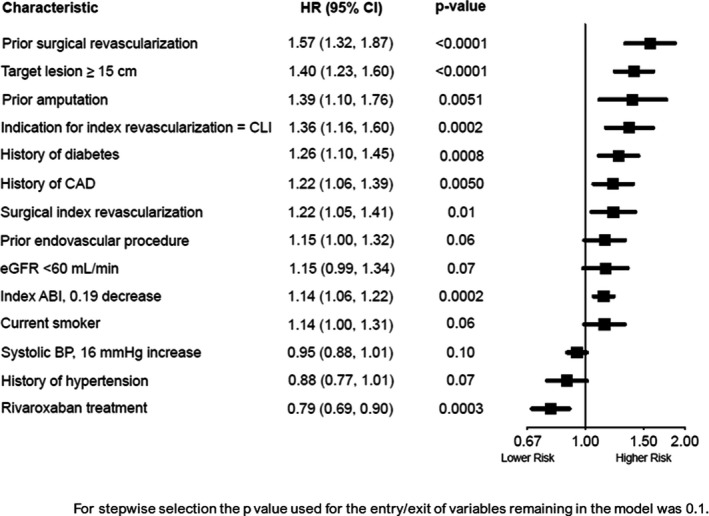
Independent determinants of total thrombotic events. Baseline patient and procedural characteristics associated with arterial and venous thrombotic risk after multivariate modeling are shown. Candidate variables shown in Figure S1. ABI, ankle‐brachial index; BP, blood pressure; CAD, coronary artery disease; CI, confidence interval; CLI, chronic limb ischemia; eGFR, estimated glomerular filtration rate; HR, hazard ratio
3.5. First, second, and total arterial and venous thrombotic events in placebo group
Of the 929 patients with first thrombotic events (407 and 522 patients in the rivaroxaban and placebo groups, respectively), four were fatal (two in each treatment group; Table S2). The rate of first arterial and venous thrombotic events per 100 patient‐years is shown in Figure 2. Over the median duration of follow‐up of 28 months, the rate of first and total events in the Placebo arm were 7.1 and 10.3 per 100 patient‐years, respectively, over the median duration of follow‐up.
FIGURE 2.
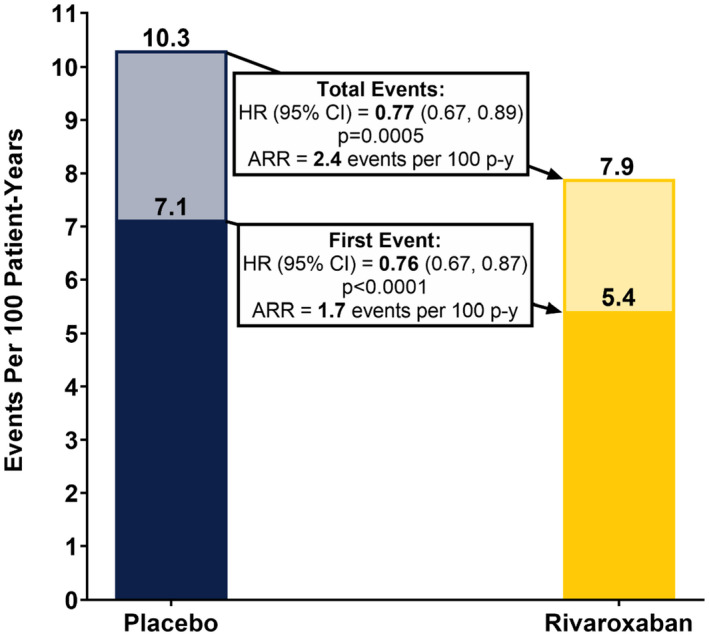
First and total arterial and venous thrombotic events, per 100 patient‐years. The first and total arterial and venous thrombotic events per 100 patient years in the Placebo and Rivaroxaban groups, respectively, are provided. ARR, absolute risk reduction; CI, confidence interval; HR, hazard ratio; p, p‐value; p‐y, patient‐yearsNote: arterial and venous events include acute limb ischemia, major amputation of vascular cause, MI, ischemic stroke, and symptomatic VTE (deep vein thrombsis and pulmonary embolism).
The absolute number of events in patients who had a second arterial or venous thrombotic event are presented in Figure 3. Of the 925 patients with a first nonfatal event, 284 (30.7%) suffered a second event, with 94.4% being arterial and 5.6% venous.
FIGURE 3.
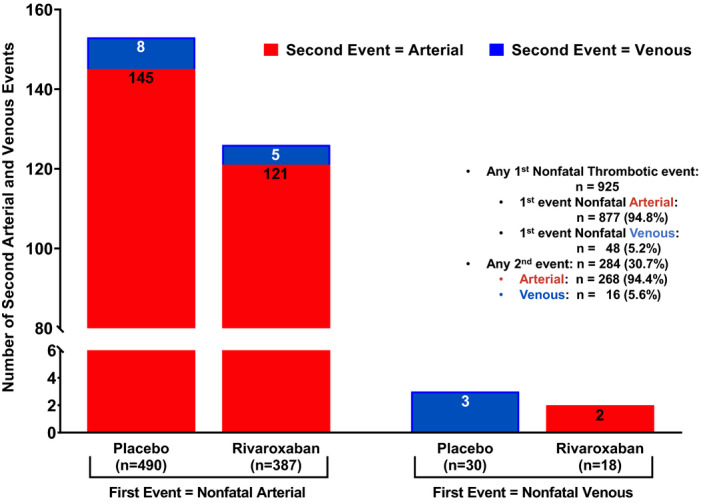
Second Arterial and Venous Thrombotic Events by Type of First Nonfatal Event. The number of second thrombotic events by type of first nonfatal event, categorized by arterial and venous and by treatment group, are presentedNote: Of the 929 patients with first events, all but 4 were nonfatal. Arterial and venous events include acute limb ischemia, major amputation of vascular cause, MI, ischemic stroke, and symptomatic VTE (deep vein thrombsis and pulmonary embolism).
Figure 4 depicts the distribution of the first, second, third and subsequent, and total arterial and venous thrombotic events, arrayed into their component outcome events of ALI, major amputation of vascular etiology, myocardial infarction, ischemic stroke, symptomatic VTE, and fatal events. Subsequent thrombotic events followed a distribution pattern similar to that seen with first events.
FIGURE 4.
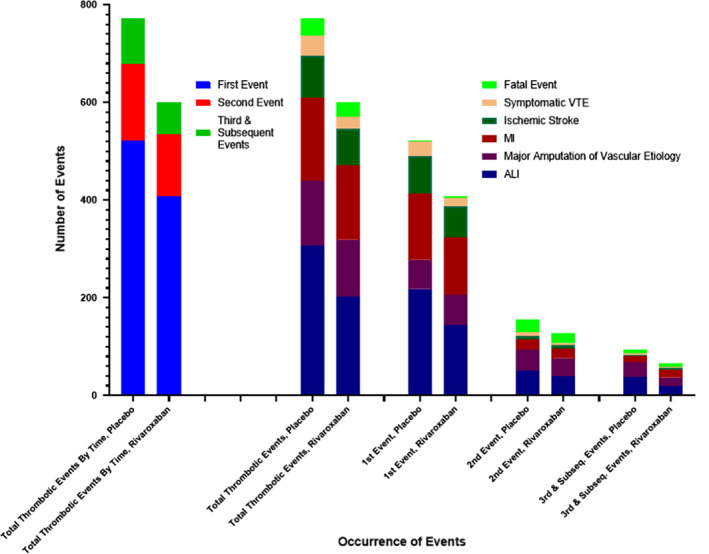
Distribution of total (first and subsequent) arterial and venous thrombotic events. The distribution of first, second, and third and subsequent arterial and venous thrombotic events within the placebo and rivaroxaban‐treated groups is shown, along with the occurrence of the nonfatal outcome components and fatal events
Cumulative incidence (time to event) function and mean cumulative function curves for first and total arterial and venous thrombotic events are illustrated in Figure 5. The cumulative incidence of the composite outcome (i.e., acute limb ischemia, major amputation of vascular cause, MI, ischemic stroke, and symptomatic VTE) for patients randomized to placebo for first and total arterial and venous thrombotic events was 19.0 (95% CI: 17.3, 21.0) and 26.2 (95% CI: 24.2, 28.4) per 100 patients at 3 years, respectively.
FIGURE 5.
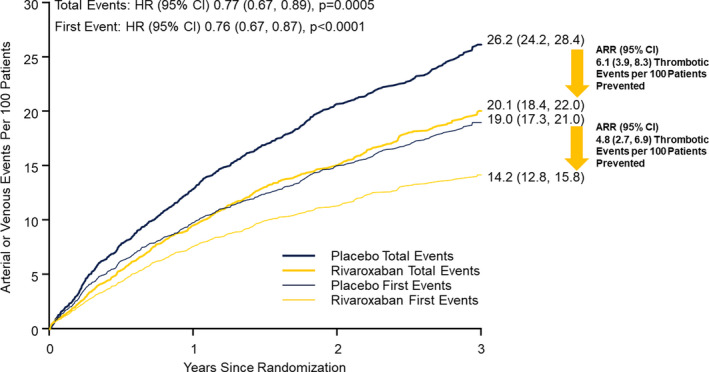
Effect of rivaroxaban on arterial and venous thrombotic events. Shown are the cumulative incidence curves for the composite first and total arterial and venous thrombotic events, defined as acute limb ischemia, major amputation of vascular etiology, myocardial infarction, ischemic stroke, or symptomatic VTE, in patients randomized to placebo versus rivaroxaban. The treatment hazard ratios (HRs) for first and total events and associated 95% confidence intervals (CIs) are provided, along with the estimated rates at 3 years and associated 95% CIs, and the absolute risk reductions (ARR) in terms of the number of thrombotic events per 100 patients prevented with associated 95% CIs. p, p‐value
3.6. Effect of rivaroxaban on arterial and venous thrombotic events
Of the 1372 total thrombotic events, 600 were in the rivaroxaban group and 772 in the placebo group (Table 1). Across subcategories of arterial and venous thrombotic events, the rivaroxaban‐treated group had consistently fewer events of each type.
In the rivaroxaban arm, the rate of first events was 5.4 per 100 patient‐years, and for total events was 7.9 per 100 patient‐years (Figure 2). Rivaroxaban reduced the first arterial and venous thrombotic event rate by 24%, for an absolute risk reduction (ARR) of 1.7 events per 100 patient‐years and reduced the total arterial and venous thrombotic event rate by 23%, for an ARR of 2.4 events per 100 patient‐years.
The cumulative incidence of first and total arterial and venous thrombotic events for rivaroxaban‐allocated patients was 14.2 (95% CI: 12.8, 15.8) and 20.1 (95% CI: 18.4, 22.0) events per 100 patients, respectively, at 3 years (Figure 5). With the addition of rivaroxaban, an estimated 4.8 (95% CI: 2.7, 6.9) first thrombotic events and 6.1 (95% CI: 3.9, 8.3) total thrombotic events per 100 patients would be prevented through 3 years.
4. DISCUSSION
This paper provides the first long‐term evaluation of the total thrombotic burden in the arterial and venous vascular bed in symptomatic PAD patients following LER. This analysis revealed several noteworthy findings regarding the occurrence and importance of considering the total number of arterial and venous thrombotic events during the clinical course of patients with symptomatic PAD after LER and the efficacy benefit rivaroxaban provided. First, the thrombotic burden and occurrence of events in symptomatic PAD after LER is high, with 14% of patients developing at least one arterial or venous thrombotic event over a median of 2.5 years, of which two‐thirds were first nonfatal events. Nearly one‐third of patients with a first event developed a second arterial or venous thrombotic event. Considering total events, over 25 arterial or venous thrombotic events per 100 patients with symptomatic PAD could be expected to occur by 3 years (26.2 events at 3 years, Figure 5). Second, after multivariable modeling, the determinants most strongly associated with thrombotic risk in this population were related to limb disease severity and anatomy, which given the predominance of lower extremity arterial thrombotic events might be expected. Thrombosis after prior LER is often related to the type of revascularization, the nature of the conduit, and the distal target. However, patient clinical characteristics such as diabetes and history of CAD were also significant. Third, the arterial and venous thrombotic events were observed in a variety of vascular beds with the preponderance being arterial, as anticipated in an atherosclerotic and post‐revascularization population, but despite receiving guideline recommended therapy (statins, aspirin, clopidogrel). Fourth, rivaroxaban reduced the thrombotic risk, and its effect was independent of P2Y12 inhibition and statins and was robust both for first and subsequent events. Rivaroxaban 2.5 mg twice‐daily in combination with aspirin reduced the total arterial and venous thrombotic events over aspirin by 23% (Figure 2) and is estimated to prevent 6.1 total arterial and venous thrombotic events at 3 years (Figure 5). Because fatal events were usually subsequent rather than first events, initiation of this treatment soon after LER or even after a non‐fatal thrombotic event provides the opportunity to minimize future mortal events.
Many large phase 3 trials use primary and secondary outcomes composites comprised of multiple component events to account for both nonfatal and fatal consequences of chronic disease. Utilizing composite outcomes in time‐to‐event trials can offer higher event rates and permit smaller sample sizes or shorter follow‐up or both. 21 However, concentrating on time‐to‐first event means outcomes occurring subsequently are disregarded in the primary analysis, which ignores potentially pertinent information and limits understanding of the natural course of diseases characterized by progression and recurrent events. 22 , 23 , 24 For example, approximately 40%–50% of heart failure events and cardiovascular deaths that occur in heart failure trials may be omitted from analyses that only use each patient's first event. 23 , 24 Including outcomes that occur beyond what is provided by time‐to‐first event analyses more fully captures the cumulative clinical consequences to the patient.
After revascularization, patients with peripheral artery disease of the lower extremities are at elevated risk both for MACE and for MALE, such as severe limb ischemia and major amputation. 25 , 26 , 27 These thrombotic events are not rare and therapeutic direction needs to turn toward prevention rather than to treatment once they occur. The VOYAGER PAD trial confirmed the high rate of MACE and MALE after revascularization with single agent antiplatelet therapy, and the addition of rivaroxaban reducing this risk by approximately 15%: Kaplan–Meier estimates of the incidence of the primary efficacy outcome (composite of ALI, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes) at 3 years were 17.3% for rivaroxaban‐ and 19.9% for placebo‐treated patients (HR: 0.85, 95% CI: 0.76–0.96; p = .009), 16 further substantiating the benefit of combining low dose anticoagulation with platelet inhibition. The benefit was apparent early and consistent over time with a number needed to treat of 39. As anticipated, adding rivaroxaban to aspirin did lead to more bleeding, with a number needed to harm of 125, but no excess in the irreversible harm events of intracranial hemorrhage or fatal bleeding. If 10 000 patients were treated for 1 year with rivaroxaban, 181 first severe cardiovascular and limb events would be prevented at a cost of 29 TIMI (Thrombolysis In Myocardial Infarction) major bleeding events, translating to an approximately 6:1 benefit risk ratio. 16 , 28 Clopidogrel is regularly used as a short‐term adjunct to aspirin after endovascular revascularization, but in this clinical situation there is minimal primary evidence to underpin administering DAPT (dual antiplatelet therapy). 29 In the VOYAGER PAD trial, rivaroxaban combined with aspirin diminished the risk of MACE and MALE with prompt benefit for reducing ALI regardless of clopidogrel use. 28 , 30 , 31 Potentiation of effect by combined antithrombotic action from inhibition of platelet function and coagulation is hypothesized but other possible effects of factor Xa inhibition and COX‐1 blockade facilitated through less well understood pathways must also be considered. 32
During the course of their disease, patients with symptomatic PAD after LER can have more than one thrombotic complication over time, more than one type of thrombosis (arterial or venous), and thrombosis at different sites (e.g., stroke, MI, PE, DVT). A focus on the first event, however, can steer the clinical decision‐making process toward how to prevent that one type of thrombotic complication and hinder consideration of broader therapeutic options that may address future associated thrombotic complications at other locations of the vascular tree, and which could potentially work in tandem or even synergistically. Given our penchant in clinical practice to cling to traditionally understood mechanistic concepts of athero‐ and venous thrombosis, it can be tempting to think of treatment of arterial disease as primarily, if not exclusively, addressed by antiplatelet therapy alone (to treat “platelet‐rich” clots) and venous disease treated only with anticoagulants (for “fibrin‐rich” clots). However, evidence is accumulating to the contrary. Recurrent thrombotic events may still occur in approximately 1 in 10 patients in the first year following an ACS event, despite treatment with aspirin plus potent P2Y12 blockade. 32 Dual antiplatelet therapy is not consistently superior to single antiplatelet therapy in reducing MACE and MALE in patients with peripheral artery disease. 33 , 34 This suggests that mechanisms beyond platelet function may be driving thrombus occurrence. 32 , 35 , 36 Recent developments in our understanding of the contribution of thrombin generation to arterial thrombosis and the role of platelets in venous thrombosis give credence to re‐thinking the current therapeutic paradigm. 37
Rivaroxaban at low dose still delivers considerable inhibition of ex vivo thrombin generation. 38 The initial clinical evidence to suggest the benefit of a low factor Xa inhibitor dose on top of antiplatelet therapy came from the ATLAS ACS studies. 32 Among patients with stabilized ACS treated with ASA alone and ASA plus a thienopyridine, the lowest doses of rivaroxaban (2.5 and 5 mg twice‐daily) were associated with a trend toward a reduced risk of death, MI, or stroke (hazard ratio [HR], 0.54; p = .08 and HR: 0.55; p = .09, respectively) in comparison with placebo. The COMPASS trial studied the 2.5 mg twice‐daily dose of rivaroxaban on top of ASA in patients with stable atherosclerotic vascular disease (chronic CAD and/or PAD). The results of the primary analysis showed a 24% reduction in MACE in comparison with ASA alone (HR, 0.76; 95% CI: 0.66–0.86) 12 and in those COMPASS patients with symptomatic lower extremity PAD a 30% reduction in MACE plus MALE (HR, 0.70; 95% CI: 0.53–0.93), further reinforcing the idea of enhancement of antithrombotic effects by combining blockade of cyclooxygenase‐1 (COX‐1) with a low dose of a factor Xa inhibitor in patients with stable atherosclerotic vascular disease, specifically in those with polyvascular disease. 39
Patients with atherosclerosis, which primarily involves specific regions of the arterial tree, 40 are at heightened risk for subsequent venous thromboembolism, 41 , 42 , 43 while patients with VTE have a higher risk of experiencing myocardial infarction, ischemic stroke or both. 31 , 41 , 44 , 45 A recent meta‐analysis of cohort studies confirmed that the risk of arterial events is significantly higher in patients with VTE than the general population without VTE, and in patients with unprovoked VTE compared to patients with provoked VTE. 46 Avoiding DVT is pertinent because the development of significant venous outflow obstruction after a lower extremity DVT in a PAD patient may lead to worsened symptoms of intermittent claudication due to loss of venous return (termed “venous” claudication). 47
While the connection between arterial and venous thrombotic events is not clearly elucidated, the association is likely underpinned by shared pathobiology which includes endothelial dysfunction, inflammation, and the balance of thrombin generation and platelet activation. 48 The hemostatic system, comprising four integrated components, the coagulation system, endothelium and regulatory proteins, platelets, and fibrinolysis, exerts an assortment of actions on the vasculature. 49 Strategies targeting more than one of these pathways may provide broad benefit by more comprehensively reducing thrombotic events across the spectrum of vascular territories in atherosclerosis.
Our analysis has limitations. First, our observations are limited to the subpopulation of symptomatic PAD patients who had successfully undergone LER within the previous 10 days. However, similar benefit in the reduction of vascular risk was observed in the analysis of the 6391 patients with stable established PAD in the COMPASS trial. 50 , 51 Second, VTE was a secondary endpoint in VOYAGER PAD and was investigator‐reported, not adjudicated; however, VTE was prospectively ascertained, and this analysis was prespecified. Third, adjusted models accounted for known baseline characteristics but post‐randomization variables were not included, so residual confounding may exist. Fourth, whether study medication was continuously taken throughout or discontinued at some point in the trial is an issue with all therapeutic clinical trials and in practice. Discontinuation of the study drug before a patient's first event can impact both time‐to‐first and recurrent‐event analyses, whereas those that occur after a first event would affect only a recurrent‐event analysis. 22 Adherence can wane after a patient has multiple events. However, our analysis showed a consistent, statistically significant reduction in first and total events (HR: 0.77 and 0.76, respectively), and as we took a conservative approach by performing the analysis in the ITT population, which may present an attenuated treatment effect as the result, it is suggested that a greater absolute benefit in total thrombotic events may actually exist.
In this atherosclerotic patient population at heightened thrombotic risk, VOYAGER PAD provides evidence for a broad and persistent benefit of dual pathway inhibition, combining low‐dose anticoagulation with low‐dose antiplatelet therapy, over antiplatelet therapy alone, to affect both thrombin generation and platelet activation and lead to a reduction in first and subsequent arterial and venous thrombotic events. Future clinical trials should continue this focus on a holistic, that is, comprehensive CV outcome assessment, including total arterial and venous thrombotic events, to capture the full spectrum of clinical benefit. Improving the therapy for our patients with vascular disease requires consideration of the hemostatic system in its entirety and an appreciation of how it may interact locally in various vascular territories and disease states. Judicious modulation of the hemostatic system through more than one pathway appears to provide more benefit than simply ramping up the intensity of inhibition of only one of its integrated components.
CONFLICT OF INTEREST
Drs. Berkowitz, Nehler, Hsia, Capell, Hess, Hsia, and Bonaca report that their University of Colorado salary is partially supported through funds to the University from the Colorado Prevention Center, a non‐profit academic research organization affiliated with the University of Colorado, that receives research grant/consulting funding from: Abbott, Agios, Alexion Pharma, Alnylam, Amgen, Angionetics, ARCA Biopharma, Array, AstraZeneca, Atentiv, Audentes, Bayer, Better Therapeutics, Brigham and Women's Hospital, Bristol‐Myers Squibb, Cardiol Therapeutics, CellResearch, Cook Medical, Cook, CSL Behring, Eidos Therapeutics, EP Trading Co, Esperion Therapeutics, Everly Health, Faraday, Fortress Biotech, HDL Therapeutics, Heartflow, Hummingbird Bioscience, Insmed, Janssen, Kowa Research, Lexicon, Merck, MedPace, Medtronic, Moderna, Novate Medical, NovoNordisk, Pfizer, PhaseBio, PPD Development, Prairie Education and Research, Prothena Biosciences, Regeneron, Regio Biosciences, Sanifit Therapeutics, Sanofi, Smith and Nephew, Stealth BioTherapeutics, University of Colorado, University of Pittsburgh, Worldwide Clinical Trials, Wraser, Yale Cardiovascular Research Group.
Dr. Bauersachs reports consultation and speaker´s honoraria from Aspen, Bayer, Bristol Myers Squibb and Pfizer.
Dr. Szarek reports grant support from Resverlogix, Baxter, and Janssen; Personal fees from CiVi and Esperion; Grant support, personal fees, and non‐financial support from Sanofi; and grant support and non‐financial support from Regeneron.
Dr. Debus reports grants and personal fees from Bayer AG, grants from Cook LTD, grants from Terumo Aortic, during the conduct of the study.
Dr. Patel reports receiving Advisory Board/Consultant Fees: Bayer, Janssen, Heartflow, Novartis, Phillips and Research Grants from Bayer, Janssen, and Heartflow.
Dr. Anand discloses receiving lecture fees from Bayer and Janssen.
Dr. Leeper reports having has received consulting fees from Janssen within the last 2 years.
-
Dr. Brasil reports the following:
-
o
Employee at:
-
▪
Assistant Professor of Medicine, Faculdade de Ciências Médicas de Minas Gerais FCMMG/FELUMA School of Medicine. Principal Investigator in the Centro de Investigacao Clinica (CIC) at Hospital Universitario Ciencias Medicas (HUCM). Belo Horizonte ‐ MG, Brazil.
-
▪
Assistant Professor of Medicine, Faculdade de Ciências da Saúde (FCS), Departamento de Medicina (DME), Universidade Federal de Lavras (UFLA) School of Medicine. Lavras ‐ MG, Brazil.
-
▪
-
o
His institution Faculdade de Ciencias Medicas de Minas Gerais FCMMG/FELUMA School of Medicine received payments to conduct Voyager‐PAD from the sponsor (Bayer). DPB served as the NLI for Brazil during Voyager‐PAD.
-
o
Institutional grants from BAYER during the conduct of the Voyager PAD study.
-
o
Personal fees outside the submitted work from:
-
▪
LIBBS (Brazil) and SERVIER (Brazil) to write scientific educational materials and speak in meetings organized by those pharmaceutical companies.
-
▪
LIBBS (Brazil) to function as member of consulting boards in hypertension, hyperlipidemias, diabetes, and peripheral artery disease.
-
▪
VIATRIS (Brazil) and BIOLAB (Brazil) to function as speaker in scientific meetings.
-
▪
SERVIER (Brazil) to serve as a scientific consultant, deliver interview, and function as member of a consulting board in hypertension.
-
▪
-
o
Sponsored in transport and/or hotel accommodations to attend national or international scientific congresses by SERVIER.
-
o
Dr. Brasil does not hold any patents, whether planned, pending or issued, broadly relevant to this work.
-
o
Dr. Lajos Mátyás notes no conflicts of interest to declare.
Dr. Diaz reports Bayer support via a grant for a trial in Argentina.
Dr. Brodmann reports no conflicts of interest to declare.
Dr. Muehlhofer reports being employed a Bayer AG employee.
Dr. Haskell, being employed by Janssen Pharmaceuticals and owning stock in Johnson & Johnson.
AUTHOR CONTRIBUTIONS
Scott D. Berkowitz: wrote the first draft, revised the subsequent drafts, made substantial contributions to concept and design, analysis and interpretation of data, critical writing and revising the intellectual content, and gave final approval of the version to be published. Rupert M. Bauersachs, Mark R. Nehler, E. Sebastian Debus, Manesh Patel, Sonia S. Anand, Warren H. Capell, Eva Muehlhofer and Lloyd Haskell: substantial contribution to concept and design, analysis and/or interpretation of data, critical writing or revising the intellectual content, and final approval of the version to be published. Michael Szarek and Marc Bonaca: substantial contribution to concept and design, analysis and/or interpretation of data, critical writing (first and subsequent drafts) and revising the intellectual content, and final approval of the version to be published. Connie N. Hess, Lajos Mátyás, Rafael Diaz and Marianne Brodmann: analysis and/or interpretation of data, revising the intellectual content, and final approval of the version to be published. Judy Hsia, Nicholas J. Leeper and David P. Brasil: analysis and interpretation of data, revision of the intellectual content, and gave final approval of the version to be published.
Supporting information
Table S1‐S2
Berkowitz SD, Bauersachs RM, Szarek M, et al. Prevention of arterial and venous thrombotic events in symptomatic peripheral arterial disease patients after lower extremity revascularization in the VOYAGER PAD trial: Dual anticoagulant/antiplatelet regimen vs antiplatelet therapy alone. J Thromb Haemost. 2022;20:1193–1205. doi: 10.1111/jth.15673
Manuscript handled by: Jean Connors
Final decision: Jean Connors, 10 February 2022
Funding information
Bayer AG, Berlin, Germany, and Janssen Research & Development LLC, Raritan, NJ, USA.
REFERENCES
- 1. Bauersachs R, Zeymer U, Briere JB, Marre C, Bowrin K, Huelsebeck M. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther. 2019;2019:8295054. doi: 10.1155/2019/8295054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonaca MP, Scirica BM, Creager MA, et al. Vorapaxar in patients with peripheral artery disease: results from TRA2{degrees}P‐TIMI 50. Circulation. 2013;127:1522‐1529. [DOI] [PubMed] [Google Scholar]
- 3. Creager MA, Kaufman JA, Conte MS. Acute limb ischemia. N Engl J Med. 2012;366:2198‐2206. [DOI] [PubMed] [Google Scholar]
- 4. Narula N, Olin JW, Narula N. Pathologic disparities between peripheral artery disease and coronary artery disease. Arterioscler Thromb Vasc Biol. 2020;40:1982‐1989. doi: 10.1161/ATVBAHA.119.312864 [DOI] [PubMed] [Google Scholar]
- 5. Narula N, Dannenberg AJ, Olin JW, et al. Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol. 2018;72:2152‐2163. [DOI] [PubMed] [Google Scholar]
- 6. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329‐1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 7. Conte MS, Pomposelli FB, Clair DG, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication. J Vasc Surg. 2015;61:2S–41S. e1. doi: 10.1016/j.jvs.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 8. Suarez C, Zeymer U, Limbourg T, et al. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc Med. 2010;15:259‐265. doi: 10.1177/1358863X10373299 [DOI] [PubMed] [Google Scholar]
- 9. Low Wang CC, Blomster JI, Heizer G, et al. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: The EUCLID Trial. J Am Coll Cardiol. 2018;72:3274‐3284. doi: 10.1016/j.jacc.2018.09.078 [DOI] [PubMed] [Google Scholar]
- 10. Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404‐1413. doi: 10.1056/NEJMoa1200933 [DOI] [PubMed] [Google Scholar]
- 11. Bonaca MP, Storey RF, Theroux P, et al. Efficacy and safety of ticagrelor over time in patients with prior MI in PEGASUS‐TIMI 54. J Am Coll Cardiol. 2017;70:1368‐1375. doi: 10.1016/j.jacc.2017.07.768 [DOI] [PubMed] [Google Scholar]
- 12. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319‐1330. doi: 10.1056/nejmoa1709118 [DOI] [PubMed] [Google Scholar]
- 13. Sigvant B, Kragsterman B, Falkenberg M, et al. Contemporary cardiovascular risk and secondary preventive drug treatment patterns in peripheral artery disease patients undergoing revascularization. J Vasc Surg. 2016;64(1009–17):1009–1017. e3. doi: 10.1016/j.jvs.2016.03.429 [DOI] [PubMed] [Google Scholar]
- 14. Jones WS, Baumgartner I, Hiatt WR, et al. Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135:241‐250. doi: 10.1161/CIRCULATIONAHA.116.025880 [DOI] [PubMed] [Google Scholar]
- 15. Hess CN, Wang TY, Weleski FUJ, et al. Long‐term outcomes and associations with major adverse limb events after peripheral artery revascularization. J Am Coll Cardiol. 2020;75:498‐508. doi: 10.1016/j.jacc.2019.11.050 [DOI] [PubMed] [Google Scholar]
- 16. Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382:1994‐2004. doi: 10.1056/NEJMoa2000052 [DOI] [PubMed] [Google Scholar]
- 17. Bauersachs RM, Szarek M, Brodmann M, et al. Total Ischemic event reduction with rivaroxaban after peripheral arterial revascularization in the VOYAGER PAD trial. J Am Coll Cardiol. 2021;78:317‐326. doi: 10.1016/j.jacc.2021.05.003 [DOI] [PubMed] [Google Scholar]
- 18. Capell WH, Bonaca MP, Nehler MR, et al. Rationale and design for the Vascular Outcomes study of ASA along with rivaroxaban in endovascular or surgical limb revascularization for peripheral artery disease (VOYAGER PAD). Am Heart J. 2018;199:83‐91. doi: 10.1016/j.ahj.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 19. Andersen PK, Angst J, Ravn H. Modeling marginal features in studies of recurrent events in the presence of a terminal event. Lifetime Data Anal. 2019;25:681‐695. doi: 10.1007/s10985-019-09462-4 [DOI] [PubMed] [Google Scholar]
- 20. Lin DY, Wei L‐J. The robust inference for the Cox proportional hazards model. J Am Statis Assoc. 1989;84:1074‐1078. [Google Scholar]
- 21. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289:2554‐2559. doi: 10.1001/jama.289.19.2554 [DOI] [PubMed] [Google Scholar]
- 22. Claggett B, Pocock S, Wei LJ, Pfeffer MA, McMurray JJV, Solomon SD. Comparison of time‐to‐first event and recurrent‐event methods in randomized clinical trials. Circulation. 2018;138:570‐577. doi: 10.1161/CIRCULATIONAHA.117.033065 [DOI] [PubMed] [Google Scholar]
- 23. Rogers JK, Pocock SJ, McMurray JJ, et al. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM‐Preserved. Eur J Heart Fail. 2014;16:33‐40. doi: 10.1002/ejhf.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murphy SA, Cannon CP, Blazing MA, et al. Reduction in total cardiovascular events with ezetimibe/simvastatin post‐acute coronary syndrome: The IMPROVE‐IT trial. J Am Coll Cardiol. 2016;67:353‐361. doi: 10.1016/j.jacc.2015.10.077 [DOI] [PubMed] [Google Scholar]
- 25. Steg PG, Bhatt DL, Wilson PW, et al. One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197‐1206. doi: 10.1001/jama.297.11.1197 [DOI] [PubMed] [Google Scholar]
- 26. Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350‐1357. [DOI] [PubMed] [Google Scholar]
- 27. Gerhard‐Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hess CN, Debus ES, Nehler MR, et al. Reduction in acute limb ischemia with rivaroxaban versus placebo in peripheral artery disease after lower extremity revascularization: insights from VOYAGER PAD. Circulation. 2021;144(23):1831‐1841. doi: 10.1161/CIRCULATIONAHA.121.055146 [DOI] [PubMed] [Google Scholar]
- 29. Hess CN, Norgren L, Ansel GM, et al. A Structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: A TASC (InterSociety Consensus for the Management of Peripheral Artery Disease) Initiative. Circulation. 2017;135:2534‐2555. doi: 10.1161/CIRCULATIONAHA.117.024469 [DOI] [PubMed] [Google Scholar]
- 30. Hiatt WR, Bonaca MP, Patel MR, et al. Rivaroxaban and aspirin in peripheral artery disease lower extremity revascularization: impact of concomitant clopidogrel on efficacy and safety. Circulation. 2020;142:2219‐2230. doi: 10.1161/CIRCULATIONAHA.120.050465 [DOI] [PubMed] [Google Scholar]
- 31. Hess CN, et al. Risk of venous thromboembolism and effect of rivaroxaban in patients with symptomatic peripheral artery disease after lower extremity revascularization, submitted for publication. [DOI] [PMC free article] [PubMed]
- 32. Gurbel PA, Fox KAA, Tantry US, ten Cate H, Weitz JI. Combination antiplatelet and oral anticoagulant therapy in patients with coronary and peripheral artery disease. Circulation. 2019;139:2170‐2185. doi: 10.1161/circulationaha.118.033580 [DOI] [PubMed] [Google Scholar]
- 33. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706‐1717. doi: 10.1056/NEJMoa060989 [DOI] [PubMed] [Google Scholar]
- 34. Cacoub PP, Bhatt DL, Steg PG, Topol EJ, Creager MA, Investigators C. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J. 2009;30:192‐201. doi: 10.1093/eurheartj/ehn534 [DOI] [PubMed] [Google Scholar]
- 35. Belch JJ, Dormandy J, Committee CW, et al. Results of the randomized, placebo‐controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg. 2010;52: 825–33:33 e1–2. doi: 10.1016/j.jvs.2010.04.027 [DOI] [PubMed] [Google Scholar]
- 36. Gurbel PA, Tantry US. Combination antithrombotic therapies. Circulation. 2010;121:569‐583. doi: 10.1161/CIRCULATIONAHA.109.853085 [DOI] [PubMed] [Google Scholar]
- 37. Chan NC, Weitz JI. Antithrombotic agents. Circ Res. 2019;124:426‐436. doi: 10.1161/CIRCRESAHA.118.313155 [DOI] [PubMed] [Google Scholar]
- 38. Borst O, Munzer P, Alnaggar N, et al. Inhibitory mechanisms of very low‐dose rivaroxaban in non‐ST‐elevation myocardial infarction. Blood Adv. 2018;2:715‐730. doi: 10.1182/bloodadvances.2017013573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anand SS, Hiatt W, Dyal L, et al. Low‐dose rivaroxaban and aspirin among patients with peripheral artery disease: a meta‐analysis of the COMPASS and VOYAGER trials. Eur J Prev Cardiol. 2021. doi: 10.1093/eurjpc/zwab128 [DOI] [PubMed] [Google Scholar]
- 40. Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z [DOI] [PubMed] [Google Scholar]
- 41. Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435‐1441. doi: 10.1056/nejmoa022157 [DOI] [PubMed] [Google Scholar]
- 42. Prandoni P. Venous thromboembolism and atherosclerosis: is there a link? J Thromb Haemost. 2007;5(Suppl 1):270‐275. doi: 10.1111/j.1538-7836.2007.02467.x [DOI] [PubMed] [Google Scholar]
- 43. Anand SS. Smoking. Circulation. 2017;135:17‐20. doi: 10.1161/circulationaha.116.025024 [DOI] [PubMed] [Google Scholar]
- 44. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism. Circulation. 2008;117:93‐102. doi: 10.1161/circulationaha.107.709204 [DOI] [PubMed] [Google Scholar]
- 45. Spyropoulos AC, Ageno W, Albers GW, et al. Post‐Discharge Prophylaxis With Rivaroxaban Reduces Fatal and Major Thromboembolic Events in Medically Ill Patients. J Am Coll Cardiol. 2020;75:3140‐3147. doi: 10.1016/j.jacc.2020.04.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noumegni SR, Hoffmann C, Tromeur C, et al. Frequency and incidence of arterial events in patients with venous thromboembolism compared to the general population: A systematic review and meta‐analysis of cohort studies. Thromb Res. 2021;203:172‐185. [DOI] [PubMed] [Google Scholar]
- 47. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long‐term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118‐126. doi: 10.1097/01.sla.0000103067.10695.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prandoni P. Is there a link between venous and arterial thrombosis? A Reappraisal. Intern Emerg Med. 2020;15:33‐36. doi: 10.1007/s11739-019-02238-6 [DOI] [PubMed] [Google Scholar]
- 49. Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746‐1760. doi: 10.1056/NEJMra1011670 [DOI] [PubMed] [Google Scholar]
- 50. Kaplovitch E, Eikelboom JW, Dyal L, et al. Rivaroxaban and aspirin in patients with symptomatic lower extremity peripheral artery disease a subanalysis of the COMPASS randomized clinical trial. JAMA Cardiol. 2021;6:21‐29. doi: 10.1001/jamacardio.2020.4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71:2306‐2315. doi: 10.1016/j.jacc.2018.03.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


