Abstract
Background
Observational data during the pandemic have demonstrated mixed associations between frailty and mortality.
Aim
To examine associations between frailty and short‐term mortality in patients hospitalised with coronavirus disease 2019 (COVID‐19).
Methods
In this systematic review and meta‐analysis, we searched PubMed, Embase and the COVID‐19 living systematic review from 1 December 2019 to 15 July 2021. Studies reporting mortality and frailty scores in hospitalised patients with COVID‐19 (age ≥18 years) were included. Data on patient demographics, short‐term mortality (in hospital or within 30 days), intensive care unit (ICU) admission and need for invasive mechanical ventilation (IMV) were extracted. The quality of studies was assessed using the Newcastle−Ottawa Scale.
Results
Twenty‐five studies reporting 34 628 patients were included. Overall, 26.2% (n = 9061) died. Patients who died were older (76.7 ± 9.6 vs 69.2 ± 13.4), more likely male (risk ratio (RR) = 1.08; 95% confidence interval (CI): 1.06–1.11) and had more comorbidities. Fifty‐eight percent of patients were frail. Adjusting for age, there was no difference in short‐term mortality between frail and non‐frail patients (RR = 1.04; 95% CI: 0.84–1.28). The non‐frail patients were commonly admitted to ICU (27.2% (4256/15639) vs 29.1% (3567/12274); P = 0.011) and had a higher mortality risk (RR = 1.63; 95% CI: 1.30–2.03) than frail patients. Among patients receiving IMV, there was no difference in mortality between frail and non‐frail (RR = 1.62; 95% CI 0.93–2.77).
Conclusion
This systematic review did not demonstrate an independent association between frailty status and short‐term mortality in patients with COVID‐19. Patients with frailty were less commonly admitted to ICU and non‐frail patients were more likely to receive IMV and had higher mortality risk. This finding may be related to allocation decisions for patients with frailty amidst the pandemic.
Keywords: COVID‐19, frailty, hospital‐related mortality, systematic review, meta‐analysis, older people
Introduction
Coronavirus disease 2019 (COVID‐19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The clinical spectrum ranges widely from asymptomatic to severe respiratory failure, multi‐organ failure and death. 1 , 2 Older age, male sex, obesity and pre‐existing health conditions such as diabetes and hypertension have all been identified as risk factors for poor outcomes. 3 , 4 , 5 There is some evidence for a disproportionate effect on older people with frailty. 6 High degree of frailty and cumulative comorbidities have been associated with higher mortality rates in patients with COVID‐19. 7 It may be that patients with frailty have a poor immune response to SARS‐CoV‐2, leading to higher short‐term mortality, slower recovery and further functional decline in patients. 8
With healthcare resources worldwide overstretched and scarce intensive care resources, frailty is being used in clinical decision‐making for patients with COVID‐19 in some settings. Early evidence on the impact of frailty demonstrated mixed results with some studies demonstrating an association of frailty and mortality, 9 , 10 , 11 while others did not. 12 , 13 A few systematic reviews have demonstrated a prognostic effect of frailty in patients with COVID‐19. 14 , 15 , 16 Many observational studies have been published recently in patients with COVID‐19 comparing patient characteristics and outcomes among survivors and non‐survivors. 7 , 9 , 10 , 11 , 13 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 Several studies used frailty as one of the predictors of mortality. No studies have pooled and analysed the results examining the association between frailty and mortality, adjusting for important confounders such as age. Therefore, we aimed to evaluate the association of frailty and age with all‐cause short‐term mortality and intensive care unit (ICU) pertinent outcomes, such as ICU admission and the need for invasive mechanical ventilation (IMV), in patients hospitalised with COVID‐19.
Methods
The protocol was registered with PROSPERO (CRD42021233599). The study was conducted in adherence with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses Statement. 37
Eligibility criteria
We included studies reporting on consecutive adult hospitalised patients with COVID‐19 with a documented frailty assessment (regardless of the frailty measure used) reporting on survivors and non‐survivors. The studies were excluded if frailty assessment was not reported.
Frailty
People who are susceptible to poorer outcomes, beyond the risk explained by their age or comorbidities, is defined as frailty. There are two accepted paradigms of frailty: phenotypic construct, 38 and deficit accumulation model. 39 The phenotype construct is based on a cluster of signs and symptoms such as self‐reported exhaustion, slowed performance (by walking speed), weakness (by grip strength), unintentional weight loss (4.5 kg in the past year) and low physical activity. 38 In contrast, the deficit accumulation model is quantified based on the number rather than the nature of health problems, 39 , 40 along with biochemical and physiological impairments. An overlap exists between the two constructs, 38 , 39 their sum contributing to a risk state. 41
Frailty tools
Frailty was measured by four tools in the included studies: the clinical frailty scale (CFS), 39 the hospital frailty risk scale (HFRS), 42 the frailty index (FI) 43 and the Frail Non‐Disabled survey (FIND). 44
Search strategy, information sources, study selection and data extraction
Two authors (ZL, SA) independently searched the publicly available COVID‐19 living systematic review, 45 which is updated daily to provide a dynamic database of research papers related to COVID‐19 that are indexed by PubMed, EMBASE, MedRxiv and BioRxiv. This has been validated in previously published COVID‐19‐related research. 46 The last was conducted on 16 July 2021. Studies were extracted between 1 December 2019 and 15 July 2021, using the search terms ‘frail’ and ‘frailty’ within the title and the abstract. These terms were combined with the Boolean operator ‘OR’. Pre‐print and non‐English articles were included. The bibliography of each study was analysed to identify studies that may have been missed during the literature search. Although we mainly focussed on older frail patients, we included all adult patients aged ≥18 years as some younger people can be frail. 47 In the case of overlapping patient data across two or more studies in our primary meta‐analysis, we included the larger study. Data were collected independently by two reviewers (HB, SA) using a prespecified data extraction form; any conflicts were resolved by consensus or by a third reviewer (AS). Corresponding authors were contacted for additional information where data were incomplete. Data collection covered study characteristics (study design, study period, sample size and country where the study was conducted), patient demographics, frailty status, frailty tools used, need for IMV, in hospital mortality and hospital length of stay (LOS). These were independently extracted, tabulated and verified by the two reviewers (HB, SA).
Quality assessment and risk of bias in individual studies
The quality of studies was assessed using the Newcastle−Ottawa Scale (NOS) tool 48 by two independent reviewers (HB, SA) using the same set of decision rules. Any discrepancies were resolved by a third author (AS). Publication bias was examined using the symmetry of funnel plots and Egger regression test. 49 To account for the heterogeneity, sensitivity analysis was performed based on study quality for all outcomes.
Definitions
Short‐term mortality was defined as all‐cause in hospital mortality or death within 30 days of hospitalisation. 6
Study outcomes
The primary aim was to examine associations of frailty status and short‐term mortality. The primary outcome was to evaluate the pooled mortality among hospitalised frail and non‐frail patients with COVID‐19. In addition, secondary outcomes included mortality among patients who required ICU admission or ventilatory supports.
Post hoc analyses
Outcomes were compared between the type of frailty measure (CFS vs others). A further post hoc analysis to evaluate the primary outcome based on studies that used CFS as a frailty measure. For this meta‐analysis, we stratified patients as CFS 1–3, CFS‐4, CFS‐5, CFS‐6 and CFS 7–9.
Data collection and analysis
Statistical analyses were performed using the statistical software package Stata‐Version 16 (StataCorp., College Station, TX, USA). Mean (standard deviation (SD)) was used for numerical data and proportion for categorical data. Where median (interquartile range) was reported, the mean (SD) was derived using an estimation formula. 50 Age stratification was performed based on the mean age of the individual study population. Five studies 9 , 30 , 32 , 34 , 36 that reported on longer‐term outcomes were censored at 30 days to reflect the short‐term mortality. We reported standardised mean difference (MD) with 95% confidence intervals (CI) for physiological parameters and event rates using a random‐effects model to account for both within‐study and between‐study variances. 51 The results were presented in Forest plots as a log risk ratio (RR). For convenience, we also reported the anti‐log RR by calculating the RR using the = EXP(value) function in Microsoft Excel (MS Office 365). Heterogeneity was tested using the χ 2 test on Cochran Q statistic, which was calculated using H and I 2 indices. The I 2 index estimates the percentage of total variation across studies that were based on true between‐study differences rather than on chance. Conventionally, I 2 values of 0–25% indicate low heterogeneity, 26–75% indicate moderate heterogeneity and 76–100% indicate substantial heterogeneity. 52 For the post hoc analysis, we used CFS 1–3 as the control group and compared these patients against those with CFS scores of 4, 5, 6 and 7–9 to assess their respective RR of short‐term mortality. A P‐value <0.05 was considered statistically significant.
Results
A total of 914 studies was extracted from the living systematic review. Eighty‐seven full‐text articles were assessed for eligibility. Twenty‐five studies 7 , 9 , 10 , 11 , 13 , 17 , 18 , 19 , 20 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 across 19 countries (Belgium, Brazil, Cyprus, Egypt, France, Greece, Iraq, Italy, Libya, The Netherlands, Poland, Saudi Arabia, Spain, Sudan, Sweden, Switzerland, Turkey, UK and the USA) reporting on 34 628 patients with COVID‐19 with frailty assessments, from the early phase of the pandemic, were included in the qualitative and quantitative analysis. Study population sizes were variable, ranging between 23 and 18 234 patients (Supporting Information Fig. S1). Most of the studies were from the UK (n = 14). 7 , 9 , 10 , 11 , 13 , 18 , 19 , 23 , 24 , 26 , 30 , 33 , 35 , 36 All studies reported findings from acute care hospitals, one study specifically on transplant patients 22 and another study from a COVID‐19‐specific hospital. 27 Five studies 20 , 24 , 27 , 30 , 31 provided additional data to enable further analysis. Based on the NOS, four studies were of good quality, 30 , 33 , 34 , 35 12 studies were of fair quality 9 , 11 , 18 , 19 , 21 , 26 , 27 , 29 , 31 , 32 , 36 and the remaining nine were of poor quality. 7 , 10 , 13 , 17 , 20 , 22 , 24 , 25 , 28 The CFS was the most common frailty measure. Most studies included all consecutive patients with no specified exclusion criteria. One study randomly selected patients from a list of all patients with confirmed COVID‐19 who were discharged from the hospital during the period. 29 One study excluded nosocomial COVID‐19 cases. 30 Only one large study reported on missing data and those who were still alive in the hospital at the end of the study period. 31 Table 1 illustrates the characteristics and descriptions of the included studies.
Table 1.
Summary characteristics and descriptions for the included studies that investigated frailty and COVID‐19‐related mortality
| Author, country | Setting | Study type | Study period (DD/MM/YY) | Sample size, proportion male (%) | Age, mean (SD) (years) | Proportion Caucasian (%) | Frailty measure; proportion frail (%) | COVID‐19 diagnosis | Comments | NOS grading |
|---|---|---|---|---|---|---|---|---|---|---|
| Aliberti, 1 Brazil | COVID‐19 special hospital | Retrospective cohort study | 30/03/20 to 7/07/20 | 1830 (57) | 66 (11) | N/R | CFS†; 25 | RT‐PCR | Although patients were followed up at 6 months, only 30‐day follow up was included in this study | 7 (fair) |
| Apea, 2 UK | Acute hospitals (5 in UK) | Prospective Cohort study | 1/01/20 to 13/05/20 | 1996 (60.6) | 63.4 (18.3) | 35.2 | HFRS; 47.9 | RT‐PCR | The primary outcome was 30‐day mortality from time of first hospital admission with COVID‐19 diagnosis | 8 (good) |
| Aw, 3 UK | Acute hospital | Cohort study | 8/03/20 to 30/04/20 | 677 (61) | 62.2 (17.4) | 35 | CFS; 71.3 | RT‐PCR |
The follow‐up period was the time between admission and death, discharge or 28 days Censored at 28 days from hospitalisation |
6 (fair) |
| Baker, 4 UK | Acute hospital | Retrospective cohort study | 8/01/20 to 12/04/20 | 316 (55) | 72.7 (17.1) | 96 | CFS; N/R | RT‐PCR | Censored at 28 days from hospitalisation | 6 (poor) |
| Bellelli, 5 Italy | General hospital | Cohort study | 27/02/20 to 7/04/20 | 105 (68.6) | N/R | N/R | FI; N/R | RT‐PCR | Follow up at 48 days | 6 (poor) |
| Brill, 6 UK | Acute hospital | Retrospective cohort study | 9/03/20 to 6/04/20 | 410 (35) | 81.1 (8.1) | 60 | CFS; N/R | RT‐PCR | Censored at 28 days from hospitalisation | 6 (fair) |
| Chinnadurai, 7 UK | Acute hospital | Cohort study | 23/03/20 to 30/04/20 | 215 (62) | 72.0 (16.4) | 87 | CFS; 51.2 | RT‐PCR | Censored at 14 days from hospitalisation | 7 (fair) |
| Davis, 8 UK | Acute hospital | Retrospective cohort study | 18/03/20 to 20/04/20 | 222 (33) | 82 (range 56–99) | N/R | CFS; 75 | RT‐PCR | Reported 30‐day mortality post hospitalisation | 6 (poor) |
| De Smet, 9 Belgium | General hospital | Retrospective cohort study | 12/03/20 to 30/04/20 | 81 (41) | 70.3 (20.1) | N/R | CFS; 79.5 | RT‐PCR | − | 6 (poor) |
| Dres, 10 France, Switzerland Belgium | ICU | Prospective cohort study | 25/02/20 to 04/05/20 | 1199 (73) | 74.7 (4.4) | N/R | CFS; 9 | RT‐PCR |
Follow up at 28 days Mortality was 60% at 90 days |
8 (good) |
| Fagard, 11 Belgium | Acute hospital | Retrospective cohort study | 16/03/20 to 16/05/20 | 105 (52.4) | 81.7 (8.3) | N/R | CFS; 59 | RT‐PCR | In hospital mortality | 7 (fair) |
| Hendra, 12 UK | Acute hospital with four satellite dialysis units | Retrospective cohort study | 11/03/20 to 10/05/20 | 148 (56.8) | 64.1 (14.6) | 32.4 | CFS | RT‐PCR | Follow up censored on 26 May 2020 | 8 (good) |
| Hewitt, 13 Italy/UK | Acute hospital (UK 10, Italy 1) | Cohort study | 27/02/20 to 30/04/20 | 1564 (58) | 76.0 (5.2) | N/R | CFS; 35 | RT‐PCR/clinical | Patients still in hospital at follow‐up point were censored for the time‐to‐mortality analysis. Censored at 28 days from hospitalisation | 7 (fair) |
| Hoek, 14 Netherlands | Acute hospital | Cohort study | 27/02/20 to 30/04/20 | 23 (78) | 60.7 (15.0) | 61 | CFS; ~22 | RT‐PCR | Reported on in hospital mortality | 4 (poor) |
| Knights, 15 UK | General hospital | Retrospective cohort study | 01/03/20 to 31/03/20 | 108 (61) | 69.3 (16.3) | 76 | CFS; N/R | RT‐PCR | In hospital deaths included patients discharged for palliative care either at home or a local palliative care inpatient unit | 7 (fair) |
| Koduri, 16 UK | Acute hospital | Retrospective cohort study | 20/02/20 to 07/05/20 | 500 (60) | 87.6 | CFS; 42.9 | RT‐PCR | − | 6 (poor) | |
| Kokosz‐Bargiel, 17 Poland | Acute hospital and ICU | Retrospective cohort study | 10/03/20 to 10/06/20 | 67 (32 ICU) (69) | 62.4 (10.4) | N/R | CFS; 55 | RT‐PCR | − | 5 (poor) |
| Kundi, 18 Turkey | All acute hospitals in Turkey | Retrospective cohort study | 11/03/20 to 22/06/20 | 18 234 (46.6) | 74.1 (7.4) | N/R | HFRS; 67.4 | RT‐PCR | In hospital all‐cause mortality | 7 (fair) |
| Maguire, 19 UK | General hospital | Retrospective cohort study | 17/03/20 to 01/05/20 | 224 (55) | Most >70 | 93.3 | CFS; 46 | RT‐PCR/clinical | Censored at 30 days from hospitalisation | 7 (fair) |
| Marengoni, 20 Italy | COVID‐19 special hospital | Retrospective cohort study | 08/03/20 to 14/04/20 | 165 (61) | 69.3 (14.5) | N/R | CFS; 15.2 | RT‐PCR/clinical | To death or discharge. Maximum 40 days | 7 (fair) |
| Osuafor, 21 UK | Acute hospital | Retrospective cohort study | 01/03/20 to 15/05/20 | 214 (55.1) | 80.7 (8.9) | 83.2 | CFS; 66.4 | RT‐PCR | Follow up at 45 days | 7 (fair) |
| Owen, 22 UK | Acute hospital | Retrospective observational study | 23/01/20 to 13/03/20 | 301 (56) | 68.7 (15.6) | N/R | CFS; 43.8 | RT‐PCR/clinical |
The primary outcome was time to death (all‐cause mortality). Deaths occurring outside the hospital were captured daily Censored at 30 days of hospitalisation |
6 (poor) |
| Steinmeyer, 23 France | Acute hospital | Retrospective cohort study | 13/03/20 to 04/05/20 | 94 (45) | 85.5 (7.5) | N/R |
FIND 76.6 dependent 10.6 frail |
RT‐PCR | Patients were followed up from hospital admission to hospital discharge or death | 5 (poor) |
| Tehrani, 24 Sweden | Acute hospital | Retrospective cohort study | 05/03/20 to 28/04/20 | 255 (59) | 66.0 (17.0) | N/R | CFS; 50 | RT‐PCR | Follow up at 60 days | 7 (fair) |
| Welch, 25 UK, USA, Italy Libya, Egypt, Iraq, Saudi Arabia, Spain, Greece, Sudan, Turkey, Cyprus | 55 acute hospitals | Cohort study | 01/02/20 to 31/05/20 | 5711 (55.1) | 71.7 (18.8) | N/R | CFS; 42.8 | RT‐PCR | Censored at 30 days from hospitalisation | 8 (good) |
Only five patients had a CFS score of 9.
CFS, clinical frailty score; FI, frailty index; FIND, frail non‐disabled survey; HFRS, hospital risk frailty score; ICU, intensive care unit; NOS, Newcastle−Ottawa Quality Assessment Score; N/R, not reported; RT‐PCR, reverse transcription−polymerase chain reaction.
NOS study quality.
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.
Survivor versus non‐survivor demographics
Overall mortality and demographic predictors
Table 2 summarises the study features and the characteristics of patients with COVID‐19, comparing survivors and non‐survivors. The pooled mortality was 26.2% (range 13.3–56%). The mean (SD) age was 73.0 (±11.5) years; the patients who died were older (76.7 ± 9.6 vs 69.2 ± 13.4; MD = 7.4 years; 95% CI 4.0–10.8; P < 0.001; I 2 = 99.2%) and mortality increased with age (Fig. S2). Over half the patients were male (52%; 17 768/34 141; range 33% to 78%; 22 studies 7 , 9 , 10 , 11 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ). Male patients, compared with female patients, had higher short‐term mortality risk (RR = 1.08; 95% CI 1.06–1.11; Fig. S3). Although the heterogeneity was minimal (I 2 = 23%), the Egger regression test suggested publication bias (P = 0.038). The sensitivity analysis adjusting for study quality demonstrated consistently higher mortality in male patients in higher quality studies. There was no difference in mortality by ethnicity (Caucasian vs Others: 52% vs 48%; n = 6056; 10 studies 9 , 10 , 18 , 19 , 23 , 24 , 26 , 32 , 33 , 35 ). Patients with acute kidney injury (11 studies 7 , 10 , 11 , 18 , 19 , 20 , 25 , 26 , 28 , 29 , 30 ; 48.1% vs 24.1%) and delirium (six studies 7 , 20 , 24 , 26 , 28 , 30 ; 26.8% vs 16.6%) were more likely to die. The treatment limitation documentation was only reported in five studies 10 , 17 , 20 , 23 , 29 (34%; 286/840), most (84.1%) with treatment limitations died.
Table 2.
Patient demographics among survivors and non‐survivors
| Overall, % (95% CI) (n/N) | Survivors, % (95% CI) (n/N) | Non‐survivors, % (95% CI) (n/N) | |
|---|---|---|---|
| Total patients with documented frailty† | 34 628 | 25 567 (73.8%) | 9061 (26.2%) |
| Female, % (n) [22 studies] | 48 (47.4–48.5%) (16 373/34 141) | 80.2 (79.6–80.9%) (13 139/16 373) | 19.8 (19.1–20.4%) (3234/16 373) |
| Age, mean (SD) (years) [20 studies] | 73.0 (±11.5) | 69.2 (±13.4) | 76.7 (±9.6) |
| Patient residence prior to hospitalisation, % (n) [13 studies] | |||
| Nursing home resident | 15.5 (14.8–16.3%) (1369/8832) | 12.2 (11.4–13.0%) (735/6027) | 22.7 (21.2–24.3%) (634/2795) |
| Own home | 63.0 (62.0–64.0%) (5564/8832) | 66.2 (65.0–67.4%) (3992/6027) | 56.2 (54.4–58.1%) (1572/2795) |
| Residential care/other‡ | 15.0 (14.3–15.8%) (1325/8832) | 14.9 (14.0–15.9%) (899/6027) | 20.3 (18.9–21.8%) (568/2795) |
| Ethnicity, % (n) [9 studies] | |||
| Caucasian | 59.3 (58.0–60.5%) (3612/6094) | 64.5 (62.9–66.0%) (2288/3549) | 52.0 (50.1–54.0%) (1324/2545) |
| Other | 40.7 (39.5–42.0%) (2482/6094) | 35.5 (34.0–37.1%) (1261/3549) | 48.0 (46.0–49.9%) (1221/2545) |
| Frailty data, % (n) [20 studies] | |||
| Total non‐frail† | 42.1 (41.5–42.6%) (13 751/32 687) | 80.6 (80.0–81.3%) (11 089/13 751) | 19.4 (18.7–20.0%) (2662/13 751) |
| Total frail† | 57.9 (57.4–58.5%) (18 936/32 687) | 69.4 (68.7–70.0%) (13 137/18 936) | 30.6 (30.0–31.3%) (5799/18 936) |
| Comorbidities, % (n) | |||
| Charlson comorbidity index <2 [4 studies] | 45.5 (42.2–48.9%) (388/852) | 56.6 (52.4–60.7%) (305/539) | 26.5 (21.9–31.6%) (83/313) |
| Charlson comorbidity index >2 [4 studies] | 54.5 (51.1–57.8%) (464/852) | 43.4 (39.3–47.6%) (234/539) | 73.5 (68.4–78.1%) (230/313) |
| Acute kidney injury [11 studies] | 31.1 (30.1–32.0%) (2837/9134) | 24.1 (23.0–25.1%) (1560/6483) | 48.2 (46.3–50.1%) (1277/2651) |
| Delirium [6 studies] | 17.0 (16.2–17.8%) (1472/8662) | 15.7 (14.8–16.7%) (870/5526) | 19.2 (17.8–20.6%) (602/3136) |
| Hospital‐specific data | |||
| Hospital LOS, mean (SD) (days) [14 studies] | 9.8 (±8.4) | 11.0 (±9.4) | 9.9 (±7.6) |
| Goals of care documentation, % (n) [5 studies] | 34.0 (30.9–37.3%) (286/840) | 13.3 (10.7–16.2%) (79/594) | 84.1 (79.2–88.3%) (207/246) |
| ICU‐specific data, % (n) | |||
| ICU admission [19 studies] | 26.0 (25.5–26.5%) (8317/32 028) | 47.3 (46.2–48.4%) (3932/8317) | 52.7 (51.6–53.8%) (4385/8317) |
| Non‐frail [11 studies] | 29.1 (28.3–29.9%) (3567/12 274)§ | 56.2 (54.5–57.8%) (2004/3567)§ | 43.8 (42.2–45.5%) (1563/3567)§ |
| Frail [11 studies] | 27.2 (26.5–27.9%) (4256/15 639)§ | 39.7 (38.2–41.2%) (1690/4256)§ | 60.3 (58.8–61.8%) (2566/4256)§ |
| Invasive mechanical ventilation [14 studies] | 76.9 (76.0–77.9%) (5850/7602) | 35.3 (34.1–36.5%) (2066/5850) | 64.7 (63.5–65.9%) (3784/5850) |
| Non‐frail [7 studies] | 75.5 (73.6–77.4%) (1499/1985) | 39.4¶ (36.1–42.7%) (326/828) | 56.3¶ (52.9–59.6%) (466/828) |
| Frail [7 studies] | 68.8 (67.3–70.2%) (2790/4057) | 29.0†† (24.6–34.3%) (98/335) | 71.0†† (66.0–75.7%) (238/335) |
Comparison between frail and non‐frail requiring ICU admission also P‐value of <0.0001.
Based on three studies that had granular data. P‐value 0.024 for both survivors and non‐survivors when compared between frail and non‐frail requiring mechanical ventilation.
Frailty measure:17 studies CFS; one study each from FI, FIND and HFRS.
Other and missing data.
Binomial 95% confidence interval (CI) (alpha 0.05).
CFS, clinical frailty scale; HFRS, hospital frailty risk scale; ICU, intensive care unit; LOS, length of stay; SD, standard deviation.
Comorbidities
Table 3 summarises the comorbidities comparing survivors and non‐survivors. Mortality was higher among patients with dementia, 7 , 10 , 17 , 20 , 21 , 23 , 26 , 27 , 28 , 29 , 30 , 33 (RR = 1.39; 95% CI 1.22–1.58), chronic kidney disease 10 , 17 , 19 , 20 , 21 , 22 , 25 , 26 , 29 , 31 , 33 , 34 , 35 (RR = 1.23; 95% CI 1.12–1.35), heart failure 7 , 13 , 19 , 22 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 35 , 53 (RR = 1.22; 95% CI 1.08–1.36), diabetes mellitus 10 , 11 , 17 , 18 , 19 , 20 , 21 , 24 , 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 (RR = 1.11; 95% CI 1.05–1.07), hypertension 7 , 10 , 11 , 17 , 18 , 19 , 20 , 21 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 31 , 32 , 33 , 34 , 35 (RR = 1.13; 95% CI 1.07–1.19) and cerebrovascular accident 17 , 19 , 20 , 23 , 24 , 25 , 29 , 31 , 33 (RR = 1.28; 95% CI 1.07–1.39). Chronic respiratory disease and obesity (body mass index ≥30 kg/m2) was not associated with mortality. Patients who died were more likely to have acute kidney injury (11 studies 7 , 10 , 11 , 18 , 19 , 20 , 25 , 26 , 28 , 29 , 30 ; 48.1% vs 24.1%; P < 0.0001) and delirium (six studies 7 , 20 , 24 , 26 , 28 , 30 ; 26.8% vs 16.6%; P < 0.0001).
Table 3.
Comorbidities among survivors and non‐survivors, along with risk ratio (including log‐transformed)
| Comorbidities | No. studies | Mortality for patients with each comorbidity, % (n/N) | Mortality for patients without each comorbidity, % (n/N) | Log of risk ratio (95% CI) | Risk ratio (95% CI) | I 2 |
|---|---|---|---|---|---|---|
| Dementia | 12 | 44.8 (657/1466) | 28.6 (2496/8735) | 0.33 (0.20, 0.46) | 1.39 (1.22, 1.58) | 70.7% |
| Chronic kidney disease | 13 | 39.2 (1041/2658) | 20.6 (4358/21 131) | 0.21 (0.11, 0.30) | 1.23 (1.12, 1.35) | 55.9% |
| Smoking | 6 | 35.6 (580/1628) | 32.6 (1186/3635) | 0.07 (0.02, 0.12) | 1.07 (1.02, 1.13) | 6.4% |
| Heart failure | 8 | 32.6 (956/2931) | 16.4 (2685/16 398) | 0.28 (0.11, 0.46) | 1.22 (1.08, 1.36) | 72.7% |
| Cardiovascular disease | 19 | 29.7 (3627/12 214) | 21.5 (4323/20 153) | 0.25 (0.18, 0.33) | 1.28 (1.07, 1.54) | 89.2% |
| Cerebrovascular accident | 9 | 29.4 (1172/3990) | 20.2 (3714/18 409) | 0.25 (0.07, 0.43) | 1.28 (1.20, 1.39) | 83.3% |
| Hypertension | 20 | 24.3 (4733/19 461) | 22.2 (1635/7358) | 0.12 (0.07, 0.17) | 1.13 (1.07, 1.19) | 76.4% |
| Diabetes mellitus | 21 | 27.6 (3054/11 084) | 24.2 (5197/21 461) | 0.10 (0.05, 0.14) | 1.11 (1.05, 1.15) | 62.5% |
| Chronic respiratory disease † | 20 | 24.6 (2347/9528) | 21.5 (3888/18 121) | 0.04 (0.03, 0.07) | 1.02 (0.97, 1.07) | 64.3% |
| Obesity | 9 | 26.7 (556/2079) | 31.9 (2402/7528) | 0.06 (−0.12, 0.012) | 0.94 (0.89, 1.01) | 49.2% |
Respiratory diseases include a composite of asthma, chronic obstructive pulmonary disease and pulmonary fibrosis. Bold values are statistically significant.
Primary outcome for short‐term mortality based on frailty status
Association of frailty with mortality adjusting for covariates
Of patients with COVID‐19, 57.9% were classified as frail (18 936/32 687). Eight studies reported mortality over time using hazard ratios, 9 , 11 , 13 , 19 , 27 , 30 , 32 , 34 six studies report mortality risk as odds ratio 17 , 20 , 21 , 24 , 27 , 31 whilst another seven studies 7 , 10 , 18 , 22 , 23 , 25 , 36 using other descriptions all demonstrated an association between increased mortality risk with increasing levels of frailty (Table S1). Four studies 26 , 28 , 33 , 35 reported no association between frailty and mortality. Despite the higher univariate pooled mortality amongst patients with frailty (30.6% vs 19.4%) when compared with non‐frail patients, there was no independent increased risk of dying (RR = 1.27; 95% CI 0.97–1.42) when compared with non‐frail patients when adjusting for age and other covariates (Fig. 1). Although there was high heterogeneity (I 2 = 98.9%), Egger test suggested no publication bias (P = 0.32). The sensitivity analysis adjusting for study quality showed patients with frailty were more likely to die if the studies were of fair quality (RR = 1.43; 95% CI: 1.30–1.58), but no difference in good or poor qualities studies (Fig. S4).
Figure 1.
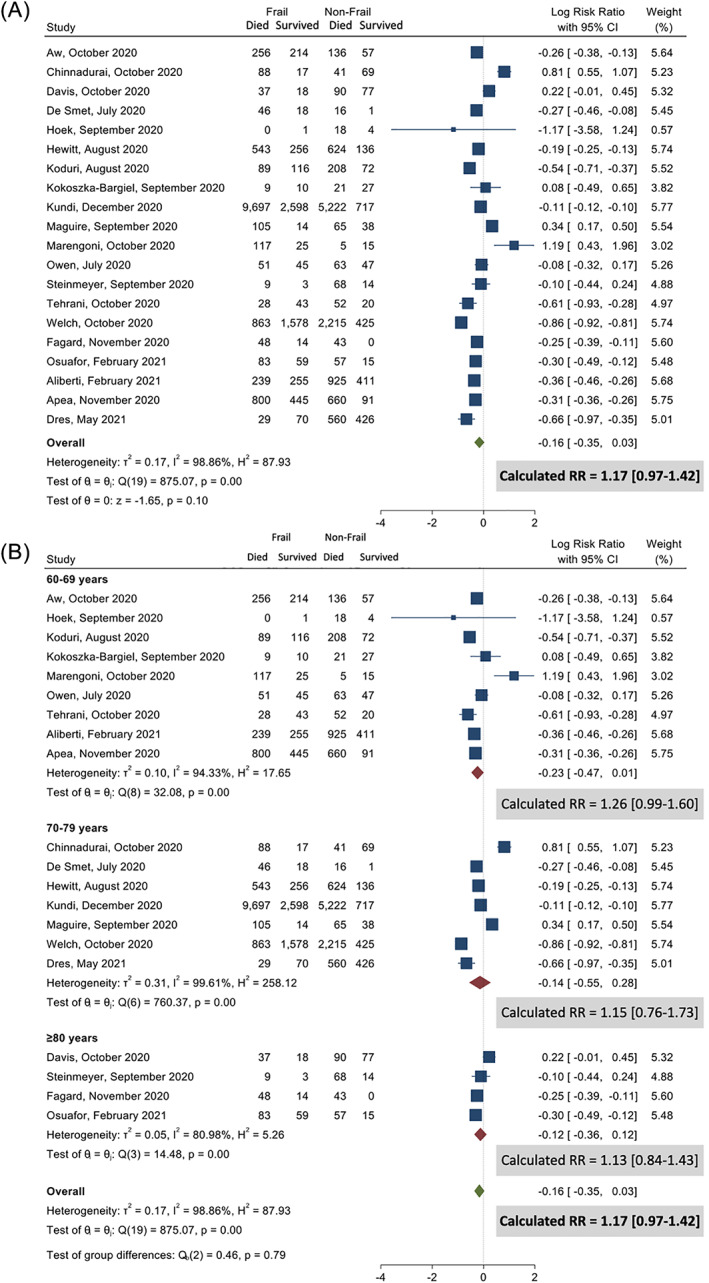
Non‐survivors among frail and non‐frail patients. (A) All studies and (B) age stratified.
Secondary ICU‐specific outcome comparing among survivors and non‐survivors
ICU admission
Of all patients hospitalised with COVID‐19, 26% were admitted to the ICU (8317/32 028; 19 studies 9 , 10 , 13 , 18 , 19 , 20 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ). More than half the patients admitted to ICU died (52.7%), but the pooled analysis demonstrated that no increased risk of death among patients admitted to the ICU (RR 0.94; 95% CI 0.78–1.15; Fig. 2A). Despite the high heterogeneity (I 2 = 96.3%), Egger test suggested no publication bias (P = 0.95). The sensitivity analysis based on study quality demonstrated similar observations (Fig. S5). We found that patients with frailty were commonly admitted to ICU (27.2%; 4256/15 639). Based on 11 studies, 9 , 13 , 20 , 22 , 24 , 25 , 30 , 31 , 32 , 33 , 34 a higher proportion of non‐frail patients were admitted to the ICU compared with patients with frailty (29.1% (3567/12 274) vs 27.2% (4256/15 639); P = 0.011) and non‐frail patients had higher mortality risk compared with patients with frailty (RR = 1.63; 95% CI: 1.30–2.03; Fig. 2B).
Figure 2.
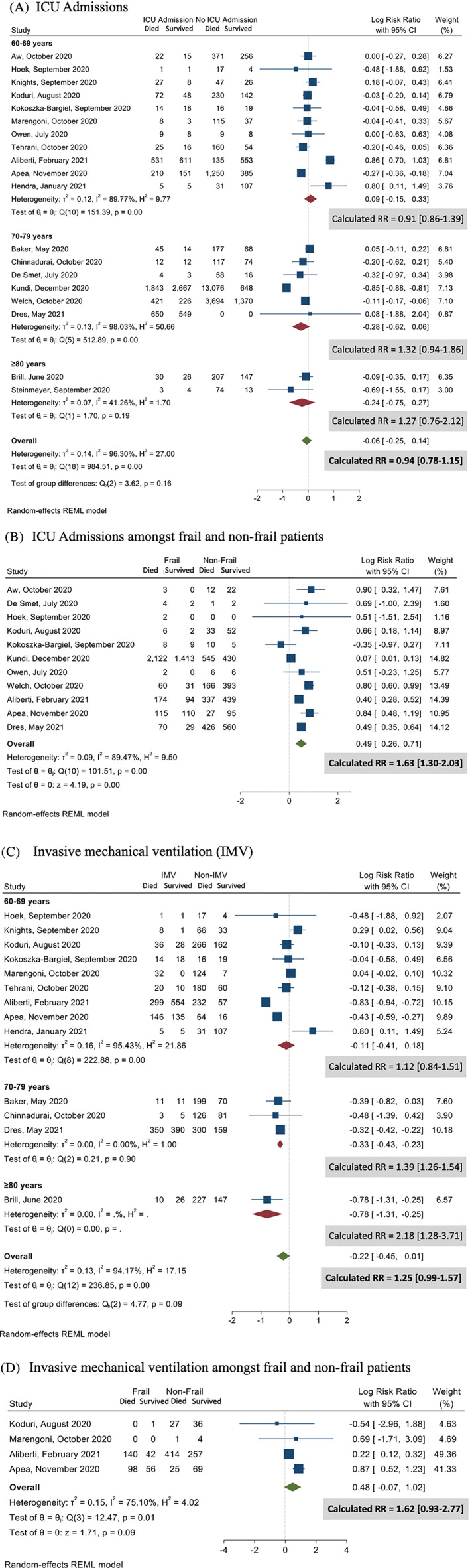
Intensive care unit therapy among survivors and non‐survivors. (A) Age group for patients admitted to intensive care unit (ICU); (B) ICU admissions among frail and non‐frail patients; (C) patients who required invasive mechanical ventilation (IMV); and (D) invasive mechanical ventilation (IMV) among frail and non‐frail patients.
Invasive mechanical ventilation
A majority of patients admitted to ICU required IMV (76.9%; 5850/7602; 14 studies 10 , 18 , 19 , 22 , 23 , 24 , 25 , 27 , 29 , 31 , 32 , 33 , 34 , 36 ). The patients who received IMV were at higher risk of dying if they were older: patients aged between 70 and 79 years (13 studies 10 , 18 , 19 , 22 , 23 , 24 , 25 , 27 , 29 , 32 , 33 , 34 , 35 ; RR = 1.39; 95% CI 1.26–1.54) or ≥80 years (RR = 2.18; 95% CI 1.28–3.71; Fig. 2C). Despite the high heterogeneity (I 2 = 94.2%), there was no publication bias (Egger test P = 0.78). The sensitivity analyses based on the study quality were consistent (Fig. S6). The patients with frailty were less likely to receive IMV (68.8% (2790/4057) vs 75.5% (1499/1985); P = 0.026) and demonstrated no increased mortality risk compared with non‐frail patients (RR = 1.62; 95% CI 0.93–2.77; Fig. 2D).
Post hoc analysis
The CFS was the most common frailty screening tool, used in 21 studies. 7 , 9 , 10 , 11 , 13 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 29 , 30 , 32 , 34 , 35 , 36 The other measures included were FI, 17 HFRS 31 , 33 and FiND. 28 The outcomes were similar comparing CFS and the other frailty screening tools (Fig. S7). When we analysed the studies that used CFS as a frailty screening tool, compared to CFS 1–3 (control group), the CFS scores of 4, 5, 6 and 7–9 had higher RR of short‐term mortality; however, it was not significantly different between CFS 4 and CFS 7–9 (Figs. 3, S8).
Figure 3.
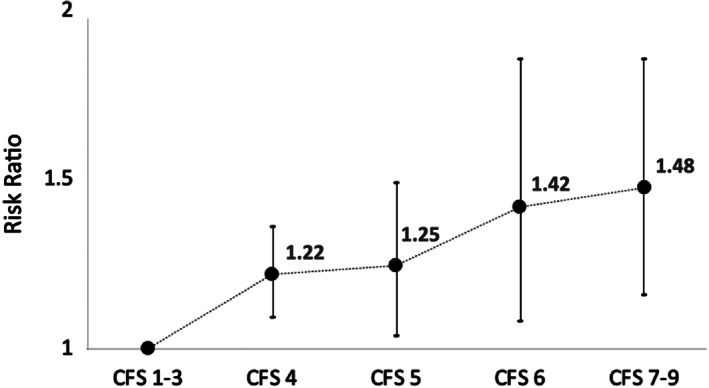
Sensitivity analysis using only clinical frailty scale (CFS): risk associated with increased frailty: CFS 1–3 (reference) with increasing CFS scores.
Discussion
Key findings
This systematic review and meta‐analysis evaluated studies that compared survivors and non‐survivors predominantly among older patients with COVID‐19 who had frailty assessments. We identified five key messages. First, the patients who died were likely to be older, of the male sex, and more likely to have specific comorbidities (dementia, chronic kidney disease, cardiovascular disease, heart failure, diabetes mellitus and previous stroke). Second, there was no increased mortality risk among patients with frailty compared with non‐frail patients, after adjusting for age and other covariates. Third, non‐frail patients were more commonly admitted to ICU and, once in the ICU, had a higher risk of short‐term mortality. Fourth, approximately 75% of patients with frailty were not admitted to ICU, suggesting a more stringent triaging for ICU admission for such patients. Fifth, patients with frailty admitted to ICU were less likely to receive IMV when compared with non‐frail patients, and their short‐term mortality risk was similar to non‐frail patients receiving IMV.
Relation to previous studies
Almost 60% of patients included in our review were identified as frail. The prevalence of frailty in our cohort of patients requiring ICU (57.9%) was comparable with pre‐COVID‐19 pandemic studies of 30–59%. 54 , 55 , 56 , 57 The pooled mortality in patients with frailty (30.6%) was higher than previously reported in hospitalised patients without COVID‐19. 57 , 58 A recent prospective cohort study before the COVID‐19 pandemic identified that frailty on admission was associated with a higher risk of death (15.8%) at 30 days, independent of the pneumonia severity in older adults hospitalised with non‐COVID‐19 pneumonia. 58
The relationship between frailty and ICU admission or IMV is likely to be complex, as ICU admission and IMV for patients with frailty may be preferentially avoided by patients, their families, or clinicians, while increased vulnerability to illness may increase the need for organ support and ICU resource use. 57 Our study observed that more than a quarter of frail older patients were admitted to ICU. A retrospective study of Australian and New Zealand adult ICU patients aged ≥65 years admitted with pneumonia before the COVD‐19 pandemic found that although the patients with frailty were twice as likely to die in the ICU and hospital (12% vs 6%), the adjusted increased risk of death was only observed in those with severe and very severe frailty. 57 Contrastingly, we demonstrated significantly higher mortality rates in those admitted to ICU. The quality of care and patient outcomes may have been compromised in many jurisdictions due to resource constraints and overwhelming caseloads, with several studies demonstrating an association of higher mortality with a higher hospital or regional COVID‐19 caseloads, regardless of whether the patients were frail or not, 59 during the peak of the pandemic. Furthermore, our study identified that non‐frail patients were more commonly admitted to ICU and more likely to die.
A recent study found that patients with frailty were less likely to receive IMV in the ICU and more commonly received non‐invasive ventilatory support. 57 Similarly, we observed that patients with frailty were less likely to receive IMV compared with non‐frail patients. The survival proportions in our review were somewhat lower than a recent systematic review that had a reported case fatality rate of 45% in patients with COVID‐19 who needed IMV. 60 We also observed that there was no mortality risk difference between frail and non‐frail patients who needed IMV. This might suggest the ICU triaging process and being selective in offering potentially life‐saving organ supports, more commonly for patients with frailty, by withholding or withdrawing life‐sustaining treatments outside ICU among the patients with severe frailty. 56 This could have influenced our results, but we would not expect this to mitigate an association between frailty and hospital survival. In addition, the National Institute for Health and Care Excellence (NICE triage guidelines) 61 could have influenced a lower priority for ICU admission to patients with severe frailty.
Frailty is an important predictive factor for adverse outcomes, including mortality, 62 hospitalisations, 63 and readmission. 64 In addition, older age (>60 years), presence of frailty, multiorgan failure and need for IMV were identified as clinical predictors of mortality in patients with COVID‐19. 65 A recent systematic review and meta‐analysis recommended that frailty screening should be performed early to stratify high‐risk groups. 16 Our absence of an independent association between frailty and short‐term mortality may be due to the limitations in the available data, but our findings suggest additional studies are needed before we can propose that frailty be an important predictor of outcome.
Frailty assessments in patients with COVID‐19 should not be used in isolation but might be considered as part of an integrated patient‐centred assessment along with other factors such as age, comorbidities, disease severity and the availability of medical interventions. 11 Despite vaccinations and public health measures to mitigate this pandemic, COVID‐19 might continue to impact severely frail older and vulnerable patients. Therefore, we must ensure that these frail older adults receive goal‐concordant care, which may avoid burdensome treatment. 66
With a plethora of tools available to measure frailty, there are significant variations amongst each measurement tool with feasibility, validity and predictive ability, 67 as different tools or scores identify different subsets of the population as frail. 68 In this systematic review, we included studies that measured frailty using four different tools. Although the most common frailty screening tool used was CFS, it is likely that the pooled data may have been skewed due to the large study that used the HFRS. While the concept of a single unified measurement tool that would enhance adaptability and ease of use seems logical or tempting, this may not be pragmatic. This is because it is unclear if one triage tool is superior to another in a particular setting and some authors advocate for different validated standardised tools for different clinical settings. 69 , 70 However, we demonstrated no differences in the outcomes based on the sensitivity analysis comparing CFS with other frailty measures grouped together. Although the comprehensive geriatric assessment is generally considered the gold standard, 71 it is impractical for quantifying frailty status in patients with COVID‐19. Furthermore, frailty is considered as a continuous measure; however, due to limitations in data and reporting, and because four different frailty tools were used, we had to resort to a dichotomous measure. This classification may have influenced the overall results. However, when we only analysed patients with the CFS score, we observed that the patients with the CFS score ≥4 had a higher risk of short‐term mortality.
Limitations
There are several limitations to this systematic review. First, a few included studies had very small numbers of patients. Second, multiple studies may have covered similar patient cohorts. However, each study's period, hospital, and location were considered in the final inclusion of studies to minimise overlap in patient cohorts. Third, the overall heterogeneity was high (I 2 >90%), which may limit the validity of the conclusion from pooled results. Although we performed a sensitivity analysis, the heterogeneity could not be minimised. This is most likely due to the case mix and the variable prevalence of older adults within included populations. Fourth, treatment limitations were not reported in many studies, and even where documented, there was no clear demarcation between frail and non‐frail patients. Fifth, a large proportion of patients (n = 18 234) were from one study, 31 that used the administrative HFRS that did not assess the frailty status just before the admission. However, we did sensitivity analysis on patients by including one the CFS demonstrated differences in patients admitted to ICU or those requiring IMV. Finally, limitations of the NOS in terms of inter‐rater reliability and external validation should be acknowledged. 72
Conclusion
This systematic review did not demonstrate an association between frailty status and short‐term mortality risk independent of patient age for patients with COVID‐19. Approximately 75% of patients with frailty were not admitted to ICU. Moreover, patients with frailty were less likely to receive IMV compared with non‐frail patients. Coupled together, these two findings might indicate that frailty was one of the factors used by intensivists to screen patients for ICU admission and/or appropriate limitations of treatment. These in turn might at least in part be related to the prudent selection of patients with frailty amidst the pandemic. There may be important unmeasured confounders, given the observational nature of included studies and that care provided in the context of the pandemic and the lack of data on advance care planning reported by most studies. Future studies should focus on using standardised frailty assessments with appropriate predictor variables including age, gender, and comorbidities. Our findings reinforce the need for an objective, reproducible measurement of frailty.
Supporting information
Figure S1 PRISMA flowchart of study inclusions and exclusions.
Figure S2 Standardised mean difference in age and age‐stratified raw outcomes between survivors and non‐survivors.
Figure S3 Age‐stratified gender difference amongst survivors and non‐survivors.
Figure S4 Frail versus non‐frail patients.
Figure S5 ICU Admission: survivor versus non‐survivor analysis.
Figure S6 Invasive Mechanical Ventilation (IMV).
Figure S7 Post Hoc Analysis CFS versus other frailty measures.
Figure S8 Post hoc sensitivity analysis using only CFS: Risk associated with increased frailty: CFS 1–3 (reference) with increasing CFS scores.
Table S1 Summary characteristics and descriptions for the included studies that investigated frailty and COVID‐19‐related mortality.
Acknowledgements
We thank Drs Marlon Juliano Romero Aliberti (Universidade de Sao Paulo, Sao Paulo, Brazil), Michael Laurent (Geriatrics Department, Imelda Hospital, Bonheiden, Belgium), Gouri Koduri (Rheumatology, Mid & South Essex NHS Foundation Trust, Southend University Hospital, Westcliff‐on‐Sea, Essex, UK), Alessandra Marengoni (Department of Clinical and Experimental Sciences, University of Brescia, Italy), John Prowle (Adult Critical Care Unit, The Royal London Hospital, Whitechapel London) and Carly Welch (Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham) for providing us with additional information to assist us with this meta‐analysis. Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Funding: None.
Disclosure: K. Shekar acknowledges research support from Metro North Hospital and Health Service. J. R. Curtis acknowledges research support from Cambia Health Foundation.
Conflict of interest: None
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He F, Deng Y, Li W. Coronavirus disease 2019: what we know? J Med Virol 2020; 92: 719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020: 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020: 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hagg S, Jylhava J, Wang Y, Xu H, Metzner C, Annetorp M et al. Age, frailty, and comorbidity as prognostic factors for short‐term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc 2020; 21: 1555–1559.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis P, Gibson R, Wright E, Bryan A, Ingram J, Lee RP et al. Atypical presentations in the hospitalised older adult testing positive for SARS‐CoV‐2: a retrospective observational study in Glasgow, Scotland. Scott Med J 2021; 66: (In eng): 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan D, Sze S, Minhas JS, Squire IB, Pareek M. Frailty and mortality in patients with COVID‐19. Lancet Public Health 2020; 5: e581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aw D, Woodrow L, Ogliari G, Harwood R. Association of frailty with mortality in older inpatients with Covid‐19: a cohort study. Age Ageing 2020; 49: 915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker KF, Hanrath AT, van der Loeff IS, Tee SA, Capstick R, Marchitelli G et al. COVID‐19 management in a UK NHS Foundation Trust with a high consequence infectious diseases centre: a retrospective analysis. Med Sci (Basel) 2021; 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hewitt J, Carter B, Vilches‐Moraga A, Quinn TJ, Braude P, Verduri A et al. The effect of frailty on survival in patients with COVID‐19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 2020; 5: e444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miles A, Webb TE, McLoughlin BC, Mannan I, Rather A, Knopp P et al. Outcomes from COVID‐19 across the range of frailty: excess mortality in fitter older people. Eur Geriatr Med 2020; 11: 851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owen RK, Conroy SP, Taub N, Jones W, Bryden D, Pareek M et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID‐19 infection: a retrospective observational study using electronic health records. Age Ageing 2021; 50: 307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dumitrascu F, Branje KE, Hladkowicz ES, Lalu M, McIsaac DI. Association of frailty with outcomes in individuals with COVID‐19: a living review and meta‐analysis. J Am Geriatr Soc 2021; 69: 2419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kastora S, Kounidas G, Perrott S, Carter B, Hewitt J, Myint PK. Clinical frailty scale as a point of care prognostic indicator of mortality in COVID‐19: a systematic review and meta‐analysis. EClinicalMedicine 2021; 36: 100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang XM, Jiao J, Cao J, Huo XP, Zhu C, Wu XJ et al. Frailty as a predictor of mortality among patients with COVID‐19: a systematic review and meta‐analysis. BMC Geriatr 2021; 21: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellelli G, Rebora P, Citerio G. The role of frailty in COVID‐19 patients. Intensive Care Med 2020; 46: 1958–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brill SE, Jarvis HC, Ozcan E, Burns TLP, Warraich RA, Amani LJ et al. COVID‐19: a retrospective cohort study with focus on the over‐80s and hospital‐onset disease. BMC Med 2020; 18: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chinnadurai R, Ogedengbe O, Agarwal P, Money‐Coomes S, Abdurrahman AZ, Mohammed S et al. Older age and frailty are the chief predictors of mortality in COVID‐19 patients admitted to an acute medical unit in a secondary care setting: a cohort study. BMC Geriatr 2020; 20: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Smet R, Mellaerts B, Vandewinckele H, Lybeert P, Frans E, Ombelet S et al. Frailty and mortality in hospitalized older adults with COVID‐19: retrospective observational study. J Am Med Dir Assoc 2020; 21: 928–932.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fagard K, Gielen E, Deschodt M, Devriendt E, Flamaing J. Risk factors for severe COVID‐19 disease and death in patients aged 70 and over: a retrospective observational cohort study. Acta Clin Belg 2021: 1–8. [DOI] [PubMed] [Google Scholar]
- 22. Hoek RAS, Manintveld OC, Betjes MGH, Hellemons ME, Seghers L, Van Kampen JAA et al. COVID‐19 in solid organ transplant recipients: a single‐center experience. Transpl Int 2020; 33: 1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knights H, Mayor N, Millar K, Cox M, Bunova E, Hughes M et al. Characteristics and outcomes of patients with COVID‐19 at a district general hospital in Surrey. UK Clin Med (Lond) 2020; 20: e148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koduri G, Gokaraju S, Darda M, Warrier V, Duta I, Hayes F et al. Clinical characteristics and outcomes of 500 patients with COVID pneumonia – results from a single center (Southend University Hospital). medRxiv 2020. 10.1101/2020.08.13.20163030. [DOI] [Google Scholar]
- 25. Kokoszka‐Bargieł I, Cyprys P, Rutkowska K, Madowicz J, Knapik P. Intensive care unit admissions during the first 3 months of the COVID‐19 pandemic in Poland: a single‐center, cross‐sectional study. Med Sci Monit 2020; 26: e926974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maguire D, Woods M, Richards C, Dolan R, Veitch JW, Sim WMJ et al. Prognostic factors in patients admitted to an urban teaching hospital with COVID‐19 infection. J Transl Med 2020; 18: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marengoni A, Zucchelli A, Grande G, Fratiglioni L, Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID‐19. Age Ageing 2020; 49: 923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steinmeyer Z, Vienne‐Noyes S, Bernard M, Steinmeyer A, Balardy L, Piau A et al. Acute care of older patients with COVID‐19: Clinical Characteristics and Outcomes. Geriatrics (Basel) 2020; 5: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tehrani S, Killander A, Astrand P, Jakobsson J, Gille‐Johnson P. Risk factors for death in adult COVID‐19 patients: frailty predicts fatal outcome in older patients. Int J Infect Dis 2021; 102: 415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geriatric Medicine Research Collaborative, Covid Collaborative, Welch C. Age and frailty are independently associated with increased COVID‐19 mortality: results of an international multi‐centre study. 2020; 50: 617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kundi H, Cetin EHO, Canpolat U, Aras S, Celik O, Ata N et al. The role of frailty on adverse outcomes among older patients with COVID‐19. J Infect 2020; 81: 944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aliberti MJR, Szlejf C, Avelino‐Silva VI, Suemoto CK, Apolinario D, Dias MB et al. COVID‐19 is not over and age is not enough: using frailty for prognostication in hospitalized patients. J Am Geriatr Soc 2021; 69: 1116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apea VJ, Wan YI, Dhairyawan R, Puthucheary ZA, Pearse RM, Orkin CM et al. Ethnicity and outcomes in patients hospitalised with COVID‐19 infection in East London: an observational cohort study. BMJ Open 2021; 11: e042140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dres M, Hajage D, Lebbah S, Kimmoun A, Pham T, Beduneau G et al. Characteristics, management, and prognosis of elderly patients with COVID‐19 admitted in the ICU during the first wave: insights from the COVID‐ICU study: prognosis of COVID‐19 elderly critically ill patients in the ICU. Ann Intensive Care 2021; 11: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hendra H, Vajgel G, Antonelou M, Neradova A, Manson B, Clark SG et al. Identifying prognostic risk factors for poor outcome following COVID‐19 disease among in‐centre haemodialysis patients: role of inflammation and frailty. J Nephrol 2021; 34: 315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Osuafor CN, Davidson C, Mackett AJ, Goujon M, Van Der Poel L, Taylor V et al. Clinical features, inpatient trajectories and frailty in older inpatients with COVID‐19: a retrospective observational study. Geriatrics (Basel) 2021; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146‐56. [DOI] [PubMed] [Google Scholar]
- 39. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitnitski A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology 2013; 14: 709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Theou O, Walston J, Rockwood K. Operationalizing frailty using the frailty phenotype and deficit accumulation approaches. Interdiscip Top Gerontol Geriatr 2015; 41: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018; 391: 1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song X, Mitnitski A, Rockwood K. Prevalence and 10‐year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010; 58: 681–7. [DOI] [PubMed] [Google Scholar]
- 44. Cesari M, Demougeot L, Boccalon H, Guyonnet S, Kan GAV, Vellas B et al. A self‐reported screening tool for detecting community‐dwelling older persons with frailty syndrome in the absence of mobility disability: the FiND questionnaire. PLoS One 2014; 9: e101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. COVID‐19 Open Access Project (COAP) Living evidence on COVID‐19. COAP; 2020. Available from URL: https://ispmbern.github.io/covid-19/living-review/
- 46. Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E et al. Prediction models for diagnosis and prognosis of covid‐19: systematic review and critical appraisal. BMJ 2020; 369: m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bagshaw M, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care 2016; 20: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses; 2021. Available from URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 49. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 52. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fisher C, Karalapillai DK, Bailey M, Glassford NG, Bellomo R, Jones D. Predicting intensive care and hospital outcome with the Dalhousie Clinical Frailty Scale: a pilot assessment. Anaesth Intensive Care 2015; 43: 361–8. [DOI] [PubMed] [Google Scholar]
- 54. Redfern OC, Harford M, Gerry S, Prytherch D, Watkinson PJ. Frailty assessment in very old intensive care patients: the hospital frailty risk score answers another question. Intensive Care Med 2020; 46: 1516–17. [DOI] [PubMed] [Google Scholar]
- 55. Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta‐analysis. Intensive Care Med 2017; 43: 1105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Darvall JN, Bellomo R, Paul E, Subramaniam A, Santamaria JD, Bagshaw SM et al. Frailty in very old critically ill patients in Australia and New Zealand: a population‐based cohort study. Med J Aust 2019; 211: 318–23. [DOI] [PubMed] [Google Scholar]
- 57. Darvall JN, Bellomo R, Bailey M, Paul E, Young PJ, Rockwood K et al. Frailty and outcomes from pneumonia in critical illness: a population‐based cohort study. Br J Anaesth 2020; 125: 730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park CM, Kim W, Rhim HC, Lee ES, Kim JH, Cho KH et al. Frailty and hospitalization‐associated disability after pneumonia: a prospective cohort study. BMC Geriatr 2021; 21: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Asch DA. Opening hospitals to more patients during the COVID‐19 pandemic—making it safe and making it feel safe. JAMA Intern Med 2020; 180: 1048–9. [DOI] [PubMed] [Google Scholar]
- 60. Lim ZJ, Subramaniam A, Reddy MP, Blecher G, Kadam U, Afroz A et al. Case fatality rates for patients with COVID‐19 requiring invasive mechanical ventilation. A meta‐analysis. Am J Respir Crit Care Med 2021; 203: 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. National Institute for Health and Care Excellence (NICE) COVID‐19 rapid guideline: critical care in adults. London: NICE; 2020. Available from URL: https://www.nice.org.uk/guidance/ng159 [PubMed]
- 62. Zhang X, Dou Q, Zhang W, Wang C, Xie X, Yang Y et al. Frailty as a predictor of all‐cause mortality among older nursing home residents: a systematic review and meta‐analysis. J Am Med Dir Assoc 2019; 20: 657–663.e4. [DOI] [PubMed] [Google Scholar]
- 63. Chang SF, Lin HC, Cheng CL. The relationship of frailty and hospitalization among older people: evidence from a meta‐analysis. J Nurs Scholarsh 2018; 50: 383–91. [DOI] [PubMed] [Google Scholar]
- 64. Zhao F, Tang B, Hu C, Wang B, Wang Y, Zhang L. The impact of frailty on posttraumatic outcomes in older trauma patients: a systematic review and meta‐analysis. J Trauma Acute Care Surg 2020; 88: 546–54. [DOI] [PubMed] [Google Scholar]
- 65. Kurtz P, Bastos LSL, Dantas LF, Zampieri FG, Soares M, Hamacher S et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID‐19 over 8 months. Intensive Care Med 2021; 47: 538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. O’Mara L, Streiter S, Orkaby AR, Ouchi K, Bernacki R. A framework to triage older adults with Covid‐19 to provide patient‐centered care. NEJM Catalyst 2020: 1–11. [Google Scholar]
- 67. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all‐cause mortality. J Am Geriatr Soc 2013; 61: 1537–51. [DOI] [PubMed] [Google Scholar]
- 68. Aguayo GA, Donneau AF, Vaillant MT, Schritz A, Franco OH, Stranges S et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol 2017; 186: 420–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Checa‐López M, Oviedo‐Briones M, Pardo‐Gómez A, Gonzales‐Turin J, Guevara‐Guevara T, Carnicero JA et al. FRAILTOOLS study protocol: a comprehensive validation of frailty assessment tools to screen and diagnose frailty in different clinical and social settings and to provide instruments for integrated care in older adults. BMC Geriatr 2019; 19: 86. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ko FC. Preoperative frailty evaluation: a promising risk‐stratification tool in older adults undergoing general surgery. Clin Ther 2019; 41: 387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K et al. What is comprehensive geriatric assessment (CGA)? An umbrella review. Age Ageing 2018; 47: 149–55. [DOI] [PubMed] [Google Scholar]
- 72. Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A et al. Testing the Newcastle Ottawa scale showed low reliability between individual reviewers. J Clin Epidemiol 2013; 66: 982–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 PRISMA flowchart of study inclusions and exclusions.
Figure S2 Standardised mean difference in age and age‐stratified raw outcomes between survivors and non‐survivors.
Figure S3 Age‐stratified gender difference amongst survivors and non‐survivors.
Figure S4 Frail versus non‐frail patients.
Figure S5 ICU Admission: survivor versus non‐survivor analysis.
Figure S6 Invasive Mechanical Ventilation (IMV).
Figure S7 Post Hoc Analysis CFS versus other frailty measures.
Figure S8 Post hoc sensitivity analysis using only CFS: Risk associated with increased frailty: CFS 1–3 (reference) with increasing CFS scores.
Table S1 Summary characteristics and descriptions for the included studies that investigated frailty and COVID‐19‐related mortality.


