Abstract
Aim
To investigate the effects of a comprehensive medication review intervention on health‐related quality of life (HRQoL) and clinical outcomes in geriatric outpatients exposed to polypharmacy.
Methods
Pragmatic, nonblinded, randomized clinical trial with follow‐up after 4 and 13 months. Participants were geriatric outpatients taking ≥9 medicines. The intervention was an additional consultation with a physician focusing on reviewing medication, informing patients about their medicines and increasing cross‐sectoral communication as supplement to and compared with usual care. The primary outcome was change in HRQoL after 4 months measured with the EuroQoL 5‐dimension 5‐level (EQ‐5D‐5L) questionnaire. Secondary outcomes were HRQoL after 13 months, mortality, admissions, falls and number of medicines after 4 and 13 months.
Results
Of 785 eligible patients, 408 were included (age: mean 80.6 [standard deviation 7.22] years; number of medicines: median 12 [interquartile range 10–14]; females 71%). After 4 months, the adjusted between‐group difference in EQ‐5D‐5L index score was 0.066 in favour of the medication consultation (95% confidence interval 0.01 to 0.12, P = .02). After 4 months, two (1%) participants had died in the medication‐consultation group and nine (4%) in the usual‐care group (log‐rank test, P = .045). The medication consultation reduced the number of medicines by 2.0 (15.8%) after 4 months and 1.3 (10.7%) after 13 months. There were no statistically significant differences in mortality or HRQoL after 13 months, and no differences in falls or admissions.
Conclusions
An additional consultation with medication review and increased communication as supplement to usual geriatric outpatient care improved HRQoL and reduced mortality after 4 months.
Keywords: geriatrics, health‐related quality of life, medication reviews, polypharmacy
What is already known about this subject
Medication reviews can reduce inappropriate polypharmacy, but the clinical impact is uncertain.
Possible reasons for low clinical impact include low implementation rates following medication reviews and deficient cross‐sectoral communication.
We investigated the effect of a consultation including medication review, patient information and increased cross‐sectoral communication as supplement to geriatric outpatient care.
What this study adds
Medication review, patient information and increased cross‐sectoral communication reduced the number of medicines and improved health‐related quality of life and survival after 4 months.
Physician resources to review medication, inform patients and increase cross‐sectoral communication can improve geriatric outpatients' health‐related quality of life and survival in the short term.
1. INTRODUCTION
The prevalence of polypharmacy is increasing, 1 , 2 , 3 and it is particularly prevalent in the multimorbid older population. 2 , 4 Polypharmacy is associated with inappropriate medicine use 5 and numerous adverse health outcomes, including adverse drug events and functional and cognitive decline. 6 Inappropriate medicine use occurs when the risk/benefit profile of the treatment is no longer favourable or when the treatment is no longer aligned with the patient's goal of care. 7 One tool to combat inappropriate polypharmacy is medication reviews, where a pharmacist or physician revises the patient's medicine to reduce/stop inappropriate medicines and initiate/increase treatment with appropriate medicines. 8 While medication reviews are often successful in reducing the use of inappropriate medicines, a beneficial effect on more important clinical outcomes has not been consistently demonstrated in clinical trials. 9 Reasons for the lack of clinical effects following medication reviews include a low implementation rate of proposed medicine changes 10 and numerous barriers towards lasting medicine changes, including deficient cross‐sectoral communication. 11 , 12 Other reasons for inconsistent and unfavourable results include qualitative differences in the medication reviews, the qualifications of the person reviewing the medication (eg, physician or pharmacist), the setting (eg, in‐hospital, outpatients, nursing homes) and the patient population (eg, number of medicines, comorbidities). While health‐related quality of life (HRQoL) is recognized as an important patient‐reported outcome, few trials of medication reviews have investigated changes in HRQoL 13 and most trials did not find an effect on HRQoL. 9 , 14 , 15 , 16 , 17 , 18
In this pragmatic trial, we investigated whether allocation of additional physician resources for medication reviews with direct implementation of the medicine changes based on patient wishes and prior contact to the primary care physician could improve HRQoL in geriatric outpatients exposed to polypharmacy.
2. METHODS
A pragmatic, nonblinded, single‐centre, randomized clinical trial with follow‐up at 4 months and 13 months after first visit. The trial registration is available at clinicaltrials.gov (NCT03911934).
2.1. Participants
Participants were included from June 2017 to December 2019 at the geriatric outpatient clinic, Copenhagen University Hospital, Frederiksberg, Denmark as part of routine outpatient care. Newly referred patients treated with at least nine different medicines according to the electronic prescription system were randomly allocated prior to their first visit to receive either usual geriatric care or usual geriatric care and an additional medication consultation with a physician focusing on reviewing medication, aligning treatment with the patient's wishes and ensuring cross‐sectoral communication. Medicines were defined as “different” based on the fifth level codes in the Anatomical Therapeutic Chemical (ATC) Classification System, 19 and the counted medicines included inhaled medicines, but excluded topical treatments such as eye drops, ear drops and lotions, antibiotics with limited treatment duration, multivitamins and protein drinks. The cut‐off of nine medicines was selected as a compromise between a steady patient flow and a high risk of inappropriate medicines. 5 Patients allocated to the additional medication consultation had their medication reviewed irrespective of their consent to participate in the follow‐up. Follow‐up was conducted for patients who, during their visit, agreed to have their administrative data collected and registered (by signing a written consent), and had sufficient cognitive and linguistic abilities to complete the EuroQoL 5‐dimension 5‐level (EQ‐5D‐5L) questionnaire. 20 The study was classified as a quality improvement study by the Regional Ethics Committee (correspondence number 17001679), as are similar studies in Denmark. 21 The project was approved by the Danish Data Protection Agency (BFH‐2017‐031).
2.2. Outcomes, blinding and data collection
The primary outcome was the between‐group difference in change in HRQoL from baseline to 4‐month follow‐up measured with EQ‐5D‐5L index values. 20 We amended the protocol to use the recently published Danish utility weights 22 to convert the EQ‐5D‐5L health states to index values (ranges from −0.758 to 1 with 0 anchored at dead and 1 anchored at full health). Robustness to choice of value set was investigated in sensitivity analyses using the index values from the EQ‐5D Crosswalk Calculator. 23 The questionnaire was completed during the first visit in the outpatient clinic (baseline) and at follow‐up around 4 and 13 months after the first visit. Follow‐up was by telephone, letter with return envelope, email or visit in the patient's home depending on the patient's hearing and sight, and the patient's wishes. Blinding was not possible since the patients actively participated in the medication reviews. Secondary outcomes pertaining to HRQoL were (1) change in EQ‐5D‐5L index values from baseline to 4‐month follow‐up excluding participants who died, (2) change in EQ‐5D‐5L index values from baseline to 13‐month follow‐up including participants who died, (3) change in EQ‐5D‐5L index values from baseline to 13‐month follow‐up excluding participants who died and (4) change in EQ‐5D‐5L Visual Analog Scale (VAS) ranging from 0 to 100 at the same timepoints as EQ‐5D‐5L index values, both including and excluding participants who died. Other clinical secondary outcomes were (1) proportion of participants with at least one self‐reported fall within the last 3 months at 4‐ and 13‐month follow‐ups, (2) number of admissions and admission days from baseline to 4‐month follow‐up and from baseline to 13‐month follow‐up, (3) time to first admission and (4) mortality at 4‐month follow‐up and 13‐month follow‐up. To assess whether the additional medication consultation resulted in medicine changes compared with usual care, we also collected medicine data at baseline, after first visit and at 4‐ and 13‐month follow‐ups. As part of patient and public involvement in research, we conducted qualitative interviews to understand physicians' 11 and patients' opinions and interpretation of the intervention and the results.
2.3. Usual care and the intervention
Usual care depended on the reason for referral to the outpatient clinic. Patients referred due to falls were examined in an interdisciplinary team of nurses, geriatricians and physiotherapists, and routinely screened with biothesiometry, orthostatic blood pressure measurements and often Holter monitor or cardiac event recorder. Furthermore, the medication was by default reviewed, including optimizing/minimizing use of analgesics, psychotropics and other fall‐inducing medicines, and osteoporosis was treated as necessary. For patients referred to general geriatric assessment, the usual care depended on whether the patient was a follow‐up patient after admission or a new referral. Follow‐up after admissions were short consultations with a geriatrician to follow‐up on specific problems identified during admission. New referrals had a complete geriatric assessment by a geriatrician, including a standard array of blood tests and screening of weight, height, food intake and more by a nurse.
The intervention consisted of an additional medication consultation with a physician from the Department of Clinical Pharmacology focusing on reviewing the medication, ie ensuring the appropriateness of all prescriptions, informing the patient about their medicines, and ensuring enhanced cross‐sectoral communication and collaboration. The clinical medication review consisted of a thorough examination of the patient's medical chart to assess the risk/benefit profiles of all the patient's prescribed medicines, an interaction check using the Danish National Drug Interactions Database, 24 examination of prescription redemption patterns using data from the electronic prescription system and screening of inappropriate medicines using various tools 25 , 26 , 27 as per the physician's discretion. A physician from the Department of Clinical Pharmacology prepared and, when possible, discussed the medication review by telephone with the patient's primary care physician prior to the patient's first visit in the outpatient clinic. The potential changes in medication were consulted with the usual‐care geriatrician and implemented in agreement with the patient's wishes. After the consultation, the implemented changes and any medicine‐related considerations were sent to the patient's primary care physician using the routine electronic correspondence module.
2.4. Randomization
Randomization of patients was performed prior to obtaining consent for data registration and follow‐up. The medical secretaries at the outpatient clinic screened newly referred patients' medicine in the electronic prescription system and randomized eligible patients using REDCap. 28 The randomization list was generated in R 29 and concealed for the secretaries. Patients were randomized 1:1 in blocks of four stratified by age group (65‐70, 71‐80 or >80 years old), number of different medicines at randomization (9‐11, 12‐16 or >16 different medicines) and sex (male or female).
2.5. Sample size
Value sets for EQ‐5D‐5L differ between countries 30 and the minimal clinically important difference differs between patient populations. 31 Geriatric outpatients are a heterogeneous population and it is therefore difficult to establish an EQ‐5D‐5L minimal clinically important difference. Based on data from Walters and Brazier, 31 by including 208 patients in each group this study was powered to detect a treatment effect of 0.08 (SD = 0.29) with alpha = 0.05 and beta = 0.2, calculated using the formula for sample size for a two‐sample t‐test with the pwr 32 package in R. 29
2.6. Statistical methods
HRQoL outcomes were analysed with a baseline‐constrained linear mixed model 33 with group, time and the three stratification variables as covariates. Correlation of repeated measurements was modelled with an unstructured variance‐covariance matrix with correlation between measurements per participant and unequal variance per time point. The linear mixed model was fitted with the nlme package version 3.1‐150 34 in R version 3.6.3. 29
Differences in number of medicines were analysed with one model per time point (due to differences in missing data at different time points) using a generalized linear model with a quasi‐Poisson distribution with the logarithm of number of different medicines at baseline as offset and group as covariate. Differences in proportion of patients with falls were analysed using Fisher's exact test with one test per follow‐up. Differences in number of admissions and differences in number of days admitted were analysed with nonparametric methods using bootstrapping for calculation of confidence intervals and permutation testing for calculation of P values with one analysis per endpoint per time point. Mortality and time to first admission were analysed using Kaplan‐Meier curves with P values from log‐rank tests and with estimates and confidence intervals for the effect using Cox proportional hazards models adjusted for the same covariates as the primary endpoint. All secondary outcomes are hypothesis generating and no adjustments for multiple comparisons were performed.
Since the primary outcome required information from follow‐up, intention‐to‐treat analysis was not possible and available case analysis was performed instead (missingness could be intermittent). Sensitivity analyses were performed to explore the consequences of data missing not at random using worst/best single imputation (mean ± 2 × SD for the group for the time point).
3. RESULTS
A total of 408 patients were included, with 196 in the medication‐consultation group and 212 in the usual‐care group (see Figure 1 for patient flow and Supporting Information Table S1 for completeness of EQ‐5D‐5L index data). The baseline characteristics of the participants are available in Table 1. The baseline characteristics for participants with missing data are available in Supporting Information Table S2. The median (IQR) number of visits with the physician from the Department of Clinical Pharmacology was one (1 to 1) with no (0 to 0) telephone follow‐ups. The median (IQR) number of visits with the geriatrician was two (1 to 2) in the medication‐consultation group and two (1 to 3) in the usual‐care group with one (0 to 1) telephone follow‐up in the medication‐consultation group and one (0 to 1) in the usual‐care group.
FIGURE 1.
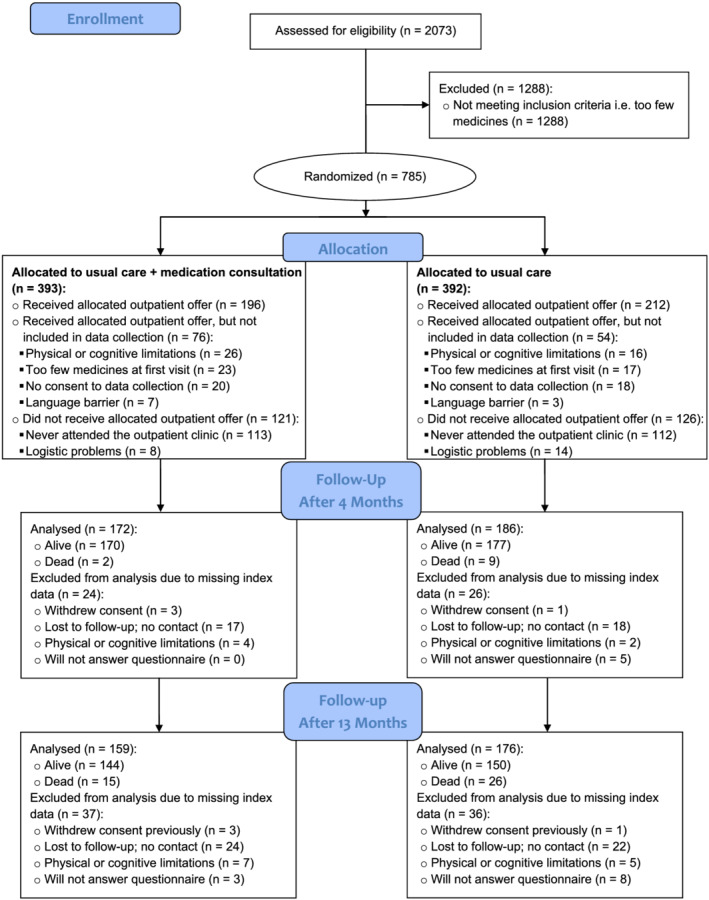
Patient flow through the study. Only data collection of the primary outcome measure (EQ‐5D‐5L index value) is depicted
TABLE 1.
Baseline characteristics of the included patients
| Usual care (n = 212) | Usual care + medication consultation (n = 196) | |
|---|---|---|
| Age in years, mean (SD) | 80.8 (7.3) | 80.5 (7.2) |
| Females, n (%) | 149 (70) | 139 (71) |
| Number of medicines, median (range) | 12 (9, 24) | 12 (9, 27) |
| Number of diagnoses, median (range) | 6 (1, 16) | 6 (1, 15) |
| Charlson comorbidity index, median (IQR) | 5 (4, 6) | 5 (4, 6) |
| FRAIL score, median (IQR) | 2 (2, 3) | 3 (2, 3) |
| At least one fall in the last 3 months, n (%) | 128 (65) | 136 (64) |
| Number of admissions the last 3 months, median (IQR) | 1 (0, 2) | 1 (1, 2) |
| Not motivated for medicine changes, n (%) | 39 (18) | 39 (20) |
| Home care, n (%) | ||
| None | 58 (27) | 61 (31) |
| Daily | 52 (25) | 63 (32) |
| Less than daily | 77 (36) | 57 (29) |
| Nursing home resident | 25 (12) | 15 (7.7) |
| Medicine dispensed by, n (%) | ||
| The patient | 88 (42) | 91 (46) |
| Relative | 14 (6.6) | 14 (7.1) |
| Home nurse | 82 (39) | 73 (37) |
| Nursing home | 25 (12) | 15 (7.7) |
| Other | 3 (1.4) | 3 (1.5) |
| Referred from, n (%) | ||
| General practitioner | 73 (34) | 51 (26) |
| Geriatric department | 56 (26) | 59 (30) |
| Other departments | 83 (39) | 86 (44) |
| Referred to, n (%) | ||
| Geriatric assessment | 111 (52) | 108 (55) |
| Falls or hip fracture clinic | 101 (48) | 88 (45) |
Regarding HRQoL, at baseline the mean (SD) EQ‐5D‐5L index score was 0.615 (0.286) in the medication‐consultation group and 0.581 (0.309) in the usual‐care group (two‐sample t‐test for difference at baseline, P = .25). The analysis of the primary outcome showed an adjusted between‐group difference in EQ‐5D‐5L index value of 0.066 (95% confidence interval [CI] 0.01 to 0.12, P = .02) in favour of the medication consultation at 4‐month follow‐up (Figure 2A). The mean (SD) EQ‐5D‐5L index score was 0.631 (0.302) in the medication‐consultation group and 0.540 (0.337) in the usual‐care group at 4‐month follow‐up, and 0.571 (0.344) in the medication‐consultation group and 0.544 (0.340) in the usual‐care group at 13‐month follow‐up. The other analyses pertaining to changes in HRQoL are presented in Figure 2A and Table 2. At 4‐month follow‐up, 2 (1%) participants had died in the medication‐consultation group compared with 9 (4%) in the usual‐care group (log‐rank test, P = .045; Figures 2B and 2C). At 13‐month follow‐up, 14 (7.1%) participants had died in the medication‐consultation group compared with 26 (12.2%) in the usual‐care group (log‐rank test, P = .12; Figures 2B and 2C). Hazard ratios for the reduction in mortality are presented in Figure 2B. There were no statistically significant differences between groups at any time points regarding number of admissions, days admitted or time to first admission and no difference in proportion of patients with falls (Supporting Information Table S3 and Supporting Information Figure S1).
FIGURE 2.
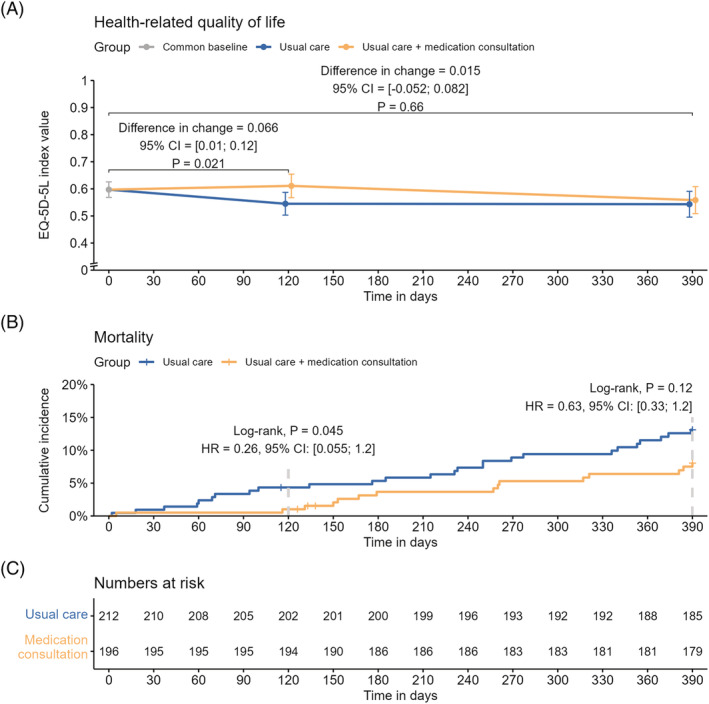
(A) Results from the analysis of the primary outcome measure showing the difference in change from baseline in health‐related quality of life between groups from baseline to follow‐up after 4 months, including subjects who died during follow‐up. The error bars are 95% confidence intervals for the estimated marginal means. The secondary outcome (change from baseline to follow‐up after 13 months) is also depicted since both outcomes were analysed in the same constrained linear mixed model adjusted for the stratification variables: number of medicines at baseline (three levels), age at baseline (three levels) and sex (two levels). CI, confidence interval; EQ‐5D‐5L, EuroQoL 5‐dimension 5‐level. (B) Cumulative incidence curves showing the incidence of death in the control and intervention groups. Hazard ratios were calculated using an adjusted Cox proportional hazards model. CI, confidence interval; HR, hazard ratio. (C) Numbers at risk of dying during the study. Only four patients withdrew consent and were censored prior to the end of the study
TABLE 2.
Analyses of the secondary outcome measures pertaining to health‐related quality of life
| EQ‐5D‐5L measurement | Group | Unadjusted scores, mean (SD) | Adjusted mean difference in change from baseline (95% CI) a | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up 4 months | Follow‐up 13 months | Follow‐up 4 months | P value | Follow‐up 13 months | P value | ||
| Index value excluding those who died | Usual care + medication consultation | 0.621 (0.268) | 0.641 (0.288) | 0.635 (0.292) | 0.051 (−0.001 to 0.100) | .055 | −0.008 (−0.063 to 0.047) | .78 |
| Usual care | 0.592 (0.283) | 0.576 (0.303) | 0.639 (0.270) | |||||
| VAS value including those who died | Usual care + medication consultation | 57.3 (20.4) | 59.3 (19.9) | 51.1 (26.1) | 7.7 (3.3 to 12.1) | <.001 | 4.1 (−1.8 to 10) | .18 |
| Usual care | 53.5 (21.4) | 50.1 (24.3) | 46.4 (28.4) | |||||
| VAS value excluding those who died | Usual care + medication consultation | 57.3 (20.4) | 60.1 (18.9) | 55.3 (21.6) | 6.2 (2.1 to 10.2) | .003 | 0.6 (−4.1 to 5.3) | .80 |
| Usual care | 53.5 (21.4) | 52.7 (22.0) | 57.0 (20.5) | |||||
Abbreviations: CI, confidence interval; EQ‐5D‐5L, EuroQoL 5‐dimensions 5‐level; SD, standard deviation; VAS, visual analogue scale.
Baseline‐constrained linear mixed model with an unstructured variance‐covariance matrix for the residuals allowing for correlation between repeated measurements for participants and for unequal variance per time point. The model includes data from both follow‐ups. The model is adjusted for the stratification variables: number of medicines at baseline (three levels), age at baseline (three levels) and sex (two levels). Confidence limits and P values are not adjusted for multiple comparisons.
During the outpatient clinic visit, there were 1180 changes to the medicine in the medication‐consultation group compared with 456 changes in the usual‐care group. These changes were mostly discontinuations (53% of the changes in the medication‐consultation group and 49% in the usual‐care group), followed by reduced dosage (17% and 18%), new prescriptions (16% and 21%), increased dosage (4% and 6%) and other changes such as change from as‐needed to regular dosing (10% and 5%). These changes resulted in a statistically significantly reduced number of prescribed medicines in the medication‐consultation group compared with the usual‐care group at all time points after baseline (Table 3).
TABLE 3.
Analyses of the number of different medicines after the first visit in the outpatient clinic and during follow‐up
| Follow‐up time | Proportion of medicines compared with baseline, a mean number at follow‐up/mean number at baseline (%) | Comparison between groups b | ||
|---|---|---|---|---|
| Usual care + medication consultation | Usual care | Rate ratio (95% CI) | P value | |
| After first visit | 10.1/12.4 (81.7) | 11.6/12.2 (95.1) | 0.859 (0.829 to 0.890) | < .001 |
| After 4 months | 10.4/12.4 (84.2) | 11.6/12.2 (95.3) | 0.883 (0.848 to 0.921) | < .001 |
| After 13 months | 11.0/12.3 (89.3) | 11.8/12.1 (97.6) | 0.915 (0.873 to 0.960) | < .001 |
Abbreviations: ATC, Anatomical Therapeutic Chemical classification system; CI, confidence interval.
Only different medicines are counted, where different entails unique ATC codes at the fifth level. Only patients alive at follow‐up are included and therefore the number of baseline medicines may differ between time points.
Generalized linear model with a quasi‐Poisson distribution with the logarithm of the number of different medicines at baseline as offset, number of medicines at follow‐up as dependent variable and group as independent variable. Presented results are exponentiated. Confidence limits and P values are not adjusted for multiple comparisons.
The sensitivity analysis of the primary endpoint showed that results were robust to the choice of valuation set. Further sensitivity analyses adjusting the HRQoL and mortality data for the effect of nursing home status showed similar results as the primary analyses (Supporting Information Table S4). For missing data considered not missing at random, the worst/best sensitivity analysis showed a worst effect of the intervention of −0.057 and a best effect of 0.194.
4. DISCUSSION
In this study, we investigated whether an allocation of additional physician resources could improve health outcomes for geriatric outpatients taking ≥9 medicines compared with usual care in a geriatric outpatient clinic. The additional physician focused on improving the care by performing a thorough medication review and carefully communicating with patients and primary care providers about the medication. We found that there were statistically significant improvements in HRQoL and mortality after 4 months, but that these effects were no longer statistically significant after 13 months.
The changes in HRQoL after 4 months were evident measured both with EQ‐5D‐5L index values and EQ‐5D‐5L VAS values. Part of the effect on HRQoL was due to a statistically significant difference in mortality, but there was still an effect on HRQoL when excluding participants who died (Table 2). After 13 months, there was no evidence of an effect on HRQoL both including and excluding participants who died during the study (Table 2), and the between group difference in mortality was also no longer statistically significant even though the effect estimate still favoured the intervention (Figures 2B and 2C). This study was not powered to detect a difference in mortality, but given the relatively large effect on mortality and that the survival curves for the groups were still clearly separated at the end of the study, a potential effect on mortality should be investigated in a new trial.
The effect of the intervention on the EQ‐5D‐5L index value of 0.066 was lower than the value used in our sample size calculation (0.080). This raises the question whether the statistically significant HRQoL difference is also clinically relevant. The clinical relevance of HRQoL changes is generally difficult to ascertain. Across various populations the minimal clinically important differences in EQ‐5D‐5L have been reported to range from 0.028 35 to 0.46 36 depending on patient population and calculation method. Another method to describe the minimal clinically important difference is to use Cohen's d effect sizes, where 0.20‐0.50 often is considered the minimal clinically important difference. 37 The Cohen's d effect in this study was 0.23 corresponding to a small effect. To involve patients and further develop the outpatient offer, we conducted interviews with five polypharmacy patients not included in the trial. All these patients would attend an outpatient clinic one or more times to achieve the observed effect. Also, the reduction in medicine was important for these patients irrespective of any gain in HRQoL especially if the reduction would lead to fewer daily administrations of medicine. Overall, we believe the change in HRQoL after 4 months constitutes a small but clinically relevant effect.
Our study resembles a recent trial by Romskaug et al, 38 where geriatric assessments with focus on medication reviews resulted in increased HRQoL measured with 15D 39 after 16 weeks in home‐dwelling older polypharmacy patients. In contrast to the trial by Romskaug et al, in this study both groups were treated by a geriatrician as part of usual care. Nevertheless, the Cohen's d effect size of 0.24 in the trial by Romskaug et al was nearly identical to the Cohen's d effect size of 0.23 in this study corresponding to a small clinically important difference. 37 As such, these studies complement each other and show that medication reviews can improve the HRQoL of geriatric patients exposed to polypharmacy.
The 13‐month follow‐up revealed that the HRQoL in this population is decreasing, and that more than two‐thirds of the patients were admitted at least once and approximately one in 10 died. The seemingly diminishing effects over time of the intervention on number of medicines, HRQoL and mortality suggest that perhaps follow‐up medication reviews are needed to ensure a continuous alignment of treatment goals and favourable risk/benefit profile.
We did not find any difference in admissions (including emergency department visits) between the groups, which has been reported in other trials of medication reviews performed in hospital. 10 , 21 The setting may have impacted this finding as the modifiable risk factors for admission and the baseline risk of admission may differ between outpatients and patients acutely admitted to a hospital.
4.1. Strengths and limitations
The major limitation to this trial is the unblinded design, which may bias the patient‐reported outcome measure in favour of the intervention. However, it is not possible to blind the participants to the additional medication consultation and this bias was likely limited as the additional consultation was provided as part of normal routine in the outpatient clinic. The trial was conducted in a single centre with the same geriatricians providing usual care for both groups, which may have introduced contamination bias and reduced the effect of the additional physician consultation. Likewise, there is a general focus in the geriatric outpatient clinic on medication reviews and the significant reductions of medicine in the usual care group are likely also to reduce the effect of the additional medication consultation. Due to the complex intervention, it is impossible to distinguish effects due to the medication review and the accompanying reduction in medicine from effects due to the increased communication with patient and primary care physicians.
The strengths of this trial include the long follow‐up period, the cross‐sectoral alliance with the primary care physician and the pragmatic nature, which means that, based on these results, a similar offer could easily be established within other geriatric outpatient clinics.
5. CONCLUSION
Allocation of additional physician resources focusing on the patient's medication with thorough medication review and enhanced communication with patients and primary care providers can persistently reduce medicine use for geriatric outpatients exposed to polypharmacy and increase HRQoL in the short term. Selecting appropriate patients for additional resource use seems important for combating the negative effects of polypharmacy.
COMPETING INTERESTS
No competing interests have been declared by the authors.
CONTRIBUTORS
J.K. conceptualization, methodology, formal analysis, investigation, data curation, writing – original draft, writing – review and editing, visualization, project administration. S.T.F. methodology, investigation, writing – review and editing. A.S.H. methodology, investigation, writing – review and editing, project administration. J.T.L. methodology, investigation, writing – review and editing, project administration. L.Ø.R. conceptualization, methodology, writing – review and editing. T.S.P. conceptualization, methodology, formal analysis, investigation, writing – review and editing, supervision. E.P. conceptualization, methodology, resources, writing – review and editing, supervision. M.B.C. conceptualization, methodology, investigation, writing – original draft, writing – review and editing, supervision, project administration, funding acquisition.
Supporting information
Supporting Information Table S1 Overview of data completeness for EQ‐5D‐5L index values
Supporting Information Table S2 Baseline characteristics of patients with missing follow‐up data
Supporting Information Table S3 Secondary outcome measures: falls and admissions
Supporting Information Table S4 Sensitivity analyses of health‐related quality of life and mortality adjusting for “nursing home status”
Supporting Information Figure S1 (A) Kaplan‐Meier curves showing the time to first admission. First admission includes emergency department visits while admittance for scheduled same‐day surgery (eg, cataract operation) or investigations (eg, colonoscopy) is excluded. Hazard ratios were calculated using an adjusted Cox proportional hazards model. CI, confidence interval; HR, hazard ratio. (B) Numbers at risk of admission during the study. In total, three patients were censored: one patient withdrew consent prior to the first admission and two patients died. Due to the small number of deaths before admission, a competing risk framework was not used for the analysis.
ACKNOWLEDGEMENTS
We would like to thank Ditte‐Marie Schärfe Kjærgaard and Linea Fugmann Thamdrup for assistance with data collection. We would like to thank all the physicians, nurses and medical secretaries at the geriatric outpatient clinic at Copenhagen University Hospital, Bispebjerg and Frederiksberg for assistance with the conduct of this study. This work was supported by the Velux Foundation [00025835] and the Capital Region of Denmark [E‐12825‐01‐12‐01]. The funders had no role in the design and conduct of the study, the collection, management, analysis and interpretation of the data, the preparation, review or approval of the manuscript or the decision to submit the manuscript for publication.
Kornholt J, Feizi ST, Hansen AS, et al. Effects of a comprehensive medication review intervention on health‐related quality of life and other clinical outcomes in geriatric outpatients with polypharmacy: A pragmatic randomized clinical trial. Br J Clin Pharmacol. 2022;88(7):3360-3369. doi: 10.1111/bcp.15287
The authors confirm that the Principal Investigators for this paper are Jonatan Kornholt and Mikkel Bring Christensen, and that they had direct clinical responsibility for patients.
Funding information The Capital Region of Denmark, Grant/Award Number: E‐12825‐01‐12‐01; Velux Foundation, Grant/Award Number: 00025835
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Hovstadius B, Hovstadius K, Astrand B, et al. Increasing polypharmacy – an individual‐based study of the Swedish population 2005‐2008. BMC Clin Pharmacol. 2010;10(1):10‐16. doi: 10.1186/1472-6904-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over‐the‐counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473‐482. doi: 10.1001/jamainternmed.2015.8581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Masnoon N, Shakib S, Kalisch‐Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kornholt J, Christensen MB. Prevalence of polypharmacy in Denmark. Dan Med J. 2020;67:A12190680. [PubMed] [Google Scholar]
- 5. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54(10):1516‐1523. doi: 10.1111/j.1532-5415.2006.00889.x [DOI] [PubMed] [Google Scholar]
- 6. Maher RL, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57‐65. doi: 10.1517/14740338.2013.827660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Medication Safety in Polypharmacy: Technical Report. Geneva: World Health Organization; 2019. [Google Scholar]
- 8. Blenkinsopp A, Bond C, Raynor DK. Medication reviews. Br J Clin Pharmacol. 2012;74(4):573‐580. doi: 10.1111/j.1365-2125.2012.04331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huiskes VJB, Burger DM, van den Ende CHM, van den Bemt BJF. Effectiveness of medication review: a systematic review and meta‐analysis of randomized controlled trials. BMC Fam Pract. 2017;18:5. doi: 10.1186/s12875-016-0577-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986. doi: 10.1002/14651858.CD008986.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laursen J, Kornholt J, Betzer C, Petersen TS, Christensen MB. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:1–7. doi: 10.1177/2333392818792169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bokhof B, Junius‐Walker U. Reducing polypharmacy from the perspectives of general practitioners and older patients: A synthesis of qualitative studies. Drugs Aging. 2016;33(4):249‐266. doi: 10.1007/s40266-016-0354-5 [DOI] [PubMed] [Google Scholar]
- 13. Beuscart J‐B, Pont LG, Thevelin S, et al. A systematic review of the outcomes reported in trials of medication review in older patients: the need for a core outcome set. Br J Clin Pharmacol. 2017;83(5):942‐952. doi: 10.1111/bcp.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Renaudin P, Boyer L, Esteve M, et al. Do pharmacist‐led medication reviews in hospitals help reduce hospital readmissions? A systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82(6):1660‐1673. doi: 10.1111/bcp.13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. Br J Clin Pharmacol. 2008;65(3):303‐316. doi: 10.1111/j.1365-2125.2007.03071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Almutairi H, Stafford A, Etherton‐Beer C, Flicker L. Optimisation of medications used in residential aged care facilities: a systematic review and meta‐analysis of randomised controlled trials. BMC Geriatr. 2020;20:236. doi: 10.1186/s12877-020-01634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alldred DP, Raynor DK, Hughes C, et al. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2013;CD009095. doi: 10.1002/14651858.CD009095.pub2 [DOI] [PubMed] [Google Scholar]
- 18. Abbott RA, Moore DA, Rogers M, Bethel A, Stein K, Coon JT. Effectiveness of pharmacist home visits for individuals at risk of medication‐related problems: a systematic review and meta‐analysis of randomised controlled trials. BMC Health Serv Res. 2020;20:39. doi: 10.1186/s12913-019-4728-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO Collaborating Centre for Drug Statistics Methodology . ATC Classification Index with DDDs. Oslo, Norway: Oslo, Norway; 2020. [Google Scholar]
- 20. EuroQol Group . EuroQol – a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16(3):199‐208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 21. Ravn‐Nielsen LV, Duckert M‐L, Lund ML, et al. Effect of an in‐hospital multifaceted clinical pharmacist intervention on the risk of readmission. JAMA Intern Med. 2018;178(3):375‐382. doi: 10.1001/jamainternmed.2017.8274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen CE, Sørensen SS, Gudex C, Jensen MB, Pedersen KM, Ehlers LH. The Danish EQ‐5D‐5L Value Set: A hybrid model using cTTO and DCE data. Appl Health Econ Health Policy. 2021;19(4):579‐591. doi: 10.1007/s40258-021-00639-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Hout B, Janssen MF, Feng Y‐S, et al. Interim scoring for the EQ‐5D‐5L: mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value Health. 2012;15(5):708‐715. doi: 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 24. Aagaard L, Kristensen MB. The national drug interactions database. Ugeskr Laeger. 2005;167(35):3283‐3286. [PubMed] [Google Scholar]
- 25. O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213‐218. doi: 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sumukadas D, McMurdo MET, Mangoni AA, et al. Temporal trends in anticholinergic medication prescription in older people: repeated cross‐sectional analysis of population prescribing data. Age Ageing. 2014;43(4):515‐521. doi: 10.1093/ageing/aft199 [DOI] [PubMed] [Google Scholar]
- 27. Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug‐related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481‐1486. doi: 10.1177/0091270006292126 [DOI] [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria; 2019. [Google Scholar]
- 30. Gerlinger C, Bamber L, Leverkus F, et al. Comparing the EQ‐5D‐5L utility index based on value sets of different countries: impact on the interpretation of clinical study results. BMC Res Notes. 2019;12(1):18. doi: 10.1186/s13104-019-4067-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Qual Life Res. 2005;14(6):1523‐1532. doi: 10.1007/s11136-004-7713-0 [DOI] [PubMed] [Google Scholar]
- 32. Champely S. pwr: Basic Functions for Power Analysis. R package version 1.3‐0; 2020. https://CRAN.R‐project.org/package=pwr
- 33. Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med. 2009;28(20):2509‐2530. doi: 10.1002/sim.3639 [DOI] [PubMed] [Google Scholar]
- 34. Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team . nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1‐150; 2020. https://CRAN.R-project.org/package=nlme
- 35. Bae E, Choi S‐E, Lee H, Shin G, Kang D. Validity of EQ‐5D utility index and minimal clinically important difference estimation among patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2020;20:73. doi: 10.1186/s12890-020-1116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parker SL, Adogwa O, Paul AR, et al. Utility of minimum clinically important difference in assessing pain, disability, and health state after transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis: Clinical article. J Neurosurg Spine. 2011;14(5):598‐604. doi: 10.3171/2010.12.SPINE10472 [DOI] [PubMed] [Google Scholar]
- 37. Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures. Pharmacoeconomics. 1999;15(2):141‐155. doi: 10.2165/00019053-199915020-00003 [DOI] [PubMed] [Google Scholar]
- 38. Romskaug R, Skovlund E, Straand J, et al. Effect of clinical geriatric assessments and collaborative medication reviews by geriatrician and family physician for improving health‐related quality of life in home‐dwelling older patients receiving polypharmacy. JAMA Intern Med. 2020;180(2):181‐189. doi: 10.1001/jamainternmed.2019.5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sintonen H. The 15D instrument of health‐related quality of life: properties and applications. Ann Med. 2001;33(5):328‐336. doi: 10.3109/07853890109002086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1 Overview of data completeness for EQ‐5D‐5L index values
Supporting Information Table S2 Baseline characteristics of patients with missing follow‐up data
Supporting Information Table S3 Secondary outcome measures: falls and admissions
Supporting Information Table S4 Sensitivity analyses of health‐related quality of life and mortality adjusting for “nursing home status”
Supporting Information Figure S1 (A) Kaplan‐Meier curves showing the time to first admission. First admission includes emergency department visits while admittance for scheduled same‐day surgery (eg, cataract operation) or investigations (eg, colonoscopy) is excluded. Hazard ratios were calculated using an adjusted Cox proportional hazards model. CI, confidence interval; HR, hazard ratio. (B) Numbers at risk of admission during the study. In total, three patients were censored: one patient withdrew consent prior to the first admission and two patients died. Due to the small number of deaths before admission, a competing risk framework was not used for the analysis.
Data Availability Statement
Data available on request from the authors.


