Abstract
Aims
The aim of this study was to evaluate the comparative effects of low‐carbohydrate (LC), full‐strength (FS), and low‐alcohol (LA) beer on gastric emptying (GE), ethanol absorption, glycaemia and insulinaemia in health.
Methods
Eight subjects (four male, four female; age: 20.4 ± 0.4 years; BMI 22.7 ± 0.4 kg/m2) had concurrent measurements of GE, plasma ethanol, blood glucose and plasma insulin for 180 min on three separate occasions after ingesting 600 mL of (i) FS beer (5.0% w/v, 246 kcal, 19.2 g carbohydrate), (ii) LC beer (4.6% w/v, 180 kcal, 5.4 g carbohydrate) and (iii) LA beer (2.6% w/v, 162 kcal, 17.4 g carbohydrate) labelled with 20 MBq 99mTc‐calcium phytate, in random order.
Results
There was no difference in the gastric 50% emptying time (T50) (FS: 89.0 ± 13.5 min vs LC: 79.5 ± 12.9 min vs LA: 74.6 ± 12.4 min; P = .39). Plasma ethanol was less after LA than LC (P < .001) and FS (P < .001), with no difference between LC and FS (P = 1.0). There was an inverse relationship between plasma ethanol at 15 min and GE after LA (r = −0.87, P < .01) and a trend for inverse relationships after LC (r = −0.67, P = .07) and FS (r = −0.69, P = .06). The AUC 0–180 min for blood glucose was greater for LA than LC (P < .001), with no difference between LA and FS (P = .40) or LC and FS (P = 1.0).
Conclusion
In healthy young subjects, GE of FS, LC and LA beer is comparable and a determinant of the plasma ethanol response.
Keywords: absorption, beer, gastric emptying, glycaemia, insulinaemia
What is already known about this subject
Low‐carbohydrate beer is increasingly popular and there is a perception that it may represent a healthy alternative beer.
The rate of alcohol absorption is highly dependent on the rate of gastric emptying.
What this study adds
Gastric emptying of low‐carbohydrate, low‐alcohol and full‐strength beer are similar.
Gastric emptying is a determinant of the plasma ethanol response for all types of beer.
Compared with low‐carbohydrate beer, there is a greater glycaemic response to low‐alcohol, but not full‐strength, beer.
1. INTRODUCTION
Low‐carbohydrate (“low‐carb”) beer has become increasingly popular since its introduction. Consumption is popular, particularly amongst the tertiary‐educated, as well as men and women approaching middle age. 1 In the latter group, usage may reflect, in part, the presence of disorders including type 2 diabetes and cardiovascular disease, or as an attempt to “bust the beer gut”. 2 There is a frequent perception that low‐carb beer represents a “healthy” alternative to full‐strength beer because its caloric content is lower. 1
While low‐carb beers contain ~0.9 g carbohydrate per 100 mL (compared with ~3 g carbohydrate per 100 mL in full‐strength beer), there is little difference in the total caloric load, because, at least in most cases, there is no difference in alcohol content, which represents a major contributor to the total caloric load, compared with full‐strength beers. Low‐carb beers pose a potential health risk in those who ingest them in the belief that they contain less alcohol (and, hence, ingest more) or that they confer health benefits such as weight loss and/or lowered glycaemia, as in patients with type 2 diabetes. In contrast, “light” (low‐alcohol) beer represents a more appropriate alternative with respect to minimising both energy intake and the amount of alcohol ingested.
As with the majority of drugs, alcohol is absorbed predominantly from the small intestine rather than the stomach, in part reflecting the greater surface area for absorption provided by the small intestine. 3 , 4 , 5 Metabolism by gastric alcohol dehydrogenase is small. 6 We 7 , 8 , 9 and others 10 have demonstrated that the rate of alcohol absorption is highly dependent on the rate of gastric emptying. The latter is known to exhibit a substantial inter‐, but much lower intra‐individual variation in health. Gastric emptying of nutrients, including alcohol, is tightly regulated at an overall rate of ~1–4 kcal/min, primarily as a result of feedback inhibition generated by the interaction of nutrients with the small intestine. 11 , 12 The rate of gastric emptying is also a major determinant of the postprandial elevation in blood glucose in both health 13 and type 2 diabetes, 14 accounting for ~30% of the variance in peak plasma glucose after oral carbohydrate. 13 Accordingly, when gastric emptying is relatively faster, the initial rise in blood glucose is greater. 13 Not surprisingly, pharmacological 3 , 7 and dietary 8 , 15 interventions that modify (delay or accelerate) gastric emptying influence both alcohol absorption 5 and postprandial glycaemia. 16 Dietary and pharmacological strategies that slow gastric emptying are now used widely in both the prevention and management of type 2 diabetes. 17 Substitution of artificial sweeteners for regular mixers (containing sucrose) accelerates gastric emptying and increases peak blood alcohol concentrations in healthy males. 9 Surprisingly, the comparative effects of low‐carb, full‐strength and low‐alcohol beer on gastric emptying, alcohol absorption and glycaemia have not been evaluated, which was the purpose of the current study.
2. METHODS
2.1. Subjects
Eight healthy subjects (4 male, 4 female), mean age 20.4 ± 0.4 years (range 19 – 22 years), mean body mass index (BMI) 22.7 ± 0.4 kg/m2 (range 20.9 – 24.1 kg/m2), were recruited by advertisement. None had a history of significant disease, including diabetes, alcohol intake >20 g daily, drug use or smoking >10 cigarettes/day. No subject was pregnant, breastfeeding, or was taking any medication known to influence gastrointestinal function.
2.2. Protocol
Each subject was studied on three occasions in a randomised, double‐blind fashion, with each study day separated by a minimum of 1 week. On each study day, the subject attended the Department of Nuclear Medicine, Positron Emission Tomography and Bone Densitometry at the Royal Adelaide Hospital at 8:30 AM after fasting from solids for 14 hours, liquids for 12 hours, and abstaining from alcohol for at least 72 hours and tobacco for 12 hours. On arrival, the subject was seated in front of a gamma camera and an IV cannula inserted into an antecubital vein for blood sampling. They then consumed 600 mL of either (i) full‐strength beer (5.0% w/v alcohol, 246 kcal, 19.2 g carbohydrate); (ii) low‐carbohydrate beer (4.6% w/v alcohol, 180 kcal, 5.4 g carbohydrate); or (iii) low‐alcohol beer (2.6% w/v alcohol, 162 kcal, 17.4 g carbohydrate) (Hahn Brewing Company, Sydney, NSW, Australia) each radiolabelled with 20 MBq 99mTc‐calcium phytate (Radpharm Scientific, Belconnen, ACT, Australia), within 10 minutes. 99mTc‐calcium phytate is a colloid that has been used widely as a marker of liquid gastric emptying. 18 , 19 , 20 Another colloid (99mTc‐sulfur colloid) was tested to determine the stability of the radiolabel in the presence of acetic acid (to mimic the gastric environment) with and without alcohol. In vitro experiments utilising instant thin layer chromatography (ITLC) demonstrated at least 98% labelling over a 3 hour period, indicative of little, or no, degradation over time (data not shown). The beers were in their carbonated form when ingested and the time of drink completion was defined as t = 0 min. Blood samples and radioisotopic gastric emptying data were collected for 180 min following drink completion. At t = 180 min, the cannula was removed, and the subject was offered a meal prior to leaving the laboratory.
The protocol was approved by the Human Research Ethics Committees of the Royal Adelaide Hospital, the University of South Australia and the University of Adelaide, and each subject provided written, informed consent prior to their inclusion. All experiments were carried out in accordance with the Declaration of Helsinki.
2.3. Measurements
2.3.1. Gastric emptying
Radioisotopic data were acquired for 180 min following consumption of the drink (60 sec frames between t = 0–60 min, then 180 sec frames from t = 60–180 min). Data were corrected for subject movement, radionuclide decay and γ‐ray attenuation. 21 A region‐of‐interest was drawn around the total stomach and gastric emptying curves (expressed as percentage retention over time) derived. The amount of the drink remaining in the stomach at 15 min intervals between t = 0–180 min, as well as the 50% gastric emptying time (T50), 21 were calculated.
2.3.2. Plasma ethanol, blood glucose and serum insulin
Venous blood samples were obtained immediately prior to the ingestion of the drink (at t = −10 min) and then at t = 0, 15, 30, 45, 60, 75, 90, 105, 120, 150 and 180 minutes. Samples were separated by centrifugation at 3200 rpm (1032 g) for 15 minutes at 4 °C within 10 minutes of collection and stored at −70° for subsequent analysis.
Plasma ethanol was measured prior to the ingestion of the drink (at t = −10 min) and then at t = 15, 30, 60, 120 and 180 minutes by spectrophotometric enzymatic assay (ADVIA 2400; Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). The intra‐assay coefficient of variation (CV) of this technique is 0.9% with limits of determination ranging from 0.01 to 0.6 g/dL. 22
Blood glucose concentrations (mmol/L) were determined at all time points using the glucose oxidase method with a 2300 Stat Plus glucose analyser. 25 μL of whole blood was diluted in 600 μL of buffer solution and placed in direct contact with a glucose oxidase impregnated membrane for sampling. 23 The intra‐ and inter‐assay CV of this technique are 2% and 6.6%, respectively. 24
Serum insulin (mU/L) was measured prior to the ingestion of the drink (at t = −10 min) and then at t = 15, 30, 45, 60, 90, 120, 150 and 180 minutes by ELISA immunoassay (Diagnostics 10‐1113, Mercodia, Uppsala, Sweden). The sensitivity of the assay was 1.0 mU/L and intra‐ and inter‐assay CVs were 2.1% and 5.3%, respectively. 25
2.4. Statistical analysis
All variables were analysed as absolute values. Areas under the curve between t = 0 and 180 minutes were calculated for all variables using the trapezoidal rule. Changes in blood glucose, serum insulin and plasma ethanol over time (t = 0–180 min) were assessed with one‐way repeated measures analysis of variance (ANOVA). For all variables, differences between the conditions were assessed with two‐way repeated measures ANOVA with treatment and time as factors, and post hoc comparisons were adjusted using Bonferroni correction. Differences in the area under the curve (AUC) for all variables, as well as baseline blood glucose and serum insulin, were assessed with two‐way repeated measures ANOVA. Relationships between the variables were assessed with Pearson's correlation. A P‐value of <.05 was considered significant in all analyses. Data are presented as mean ± SEM (standard error of the mean).
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22.
3. RESULTS
The studies were all well tolerated.
3.1. Gastric emptying
GE of beer approximated a linear pattern for the first ~120 minutes. There was no treatment × time effect for total gastric emptying (P = .46), nor any difference in the AUCs for the three conditions (P = .29) (Figure 1). There was also no difference in T50 between the three conditions (low‐alcohol (LA) vs low‐carb (LC) vs full‐strength (FS): 74.6 ± 12.4 min vs 79.5 ± 12.9 min vs 89.0 ± 13.5 min; P = .39), although the mean T50 was higher for FS.
FIGURE 1.
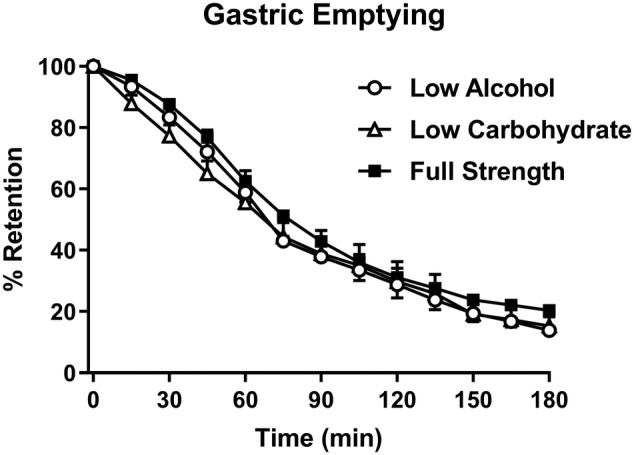
Gastric emptying (GE) of low‐alcohol (LA, open circles), low‐carbohydrate (LC, open triangles) and full‐strength (FS, closed squares) beers (mean ± SEM; n = 8)
3.2. Plasma ethanol
There was an increase in plasma ethanol during all three conditions (P < .001 for all) (Figure 2). There were treatment (P < .001), time (P < .001) and treatment × time (P < .001) effects (post‐hoc comparisons are shown in the figure). There was a difference (P < .001) between the AUC for all three conditions, so that the AUC for LA was lower than LC (P < .001) and FS (P < .001), with no difference between LC and FS (P = 1.0).
FIGURE 2.
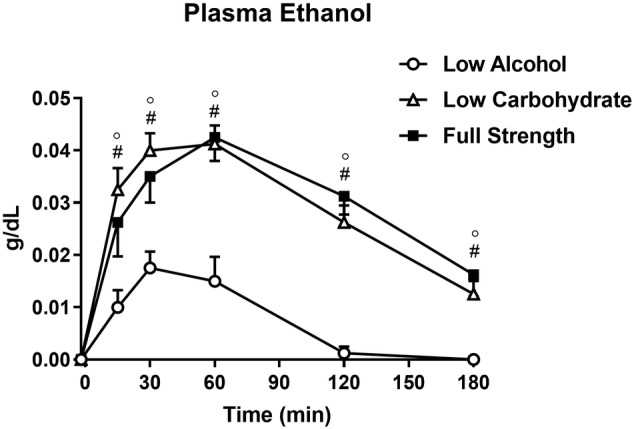
Plasma ethanol following low‐alcohol (LA, open circles), low‐carbohydrate (LC, open triangles) and full‐strength (FS, closed squares) beers. ° P < .05 LA vs LC, # P < .05 LA vs FS (mean ± SEM; n = 8)
3.3. Relationships between plasma ethanol and gastric emptying
Partial correlation coefficients (adjusted for sex, age and BMI) between plasma ethanol level and gastric emptying T50 were significant at 15 (r = −0.56, P = .01), 30 (r = −0.58, P < .01) and 60 minutes (r = −0.48, P = .03), but not 120 minutes (r = −0.46, P = NS). There was a strong, inverse relationship between the plasma ethanol response at t = 15 minutes during the LA condition and the T50 (r = −0.87, P < .01) and trends during the LC (r = −0.67, P = .07) and FS (r = −0.69, P = .06) conditions with an inverse relationship at t = 30 minutes (r = −0.74, P < .05) for FS but not for LA and LC.
3.4. Blood glucose
There was no difference in baseline blood glucose (P = .82) and an increase in blood glucose during all three conditions (P < .01 for all) (Figure 3A). There were treatment (P < .001), time (P < .001) and treatment × time (P < .001) effects (post‐hoc comparisons are shown on the figure). There was a difference (P < .001) between the AUCs for all three conditions, so that the AUC for low alcohol (LA) was greater than for low carbohydrate (LC) (P < .01) and for full strength (FS) (P < .05). While mean levels were lower for LC than FS, this difference was not significant (P = .11).
FIGURE 3.
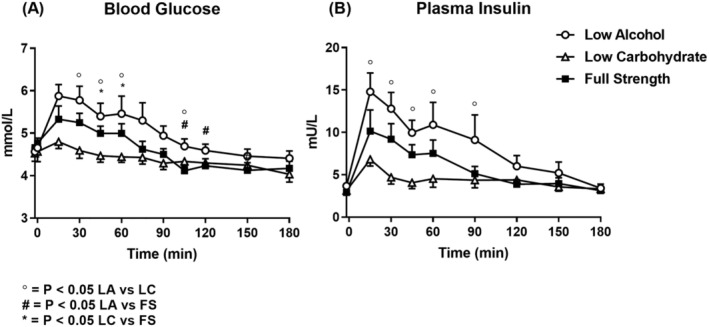
Blood glucose (A) and plasma insulin (B) responses following low‐alcohol (LA, open circles), low‐carbohydrate (LC, open triangles) and full‐strength (FS, closed squares) beers. ° P < .05 LA vs LC, # P < .05 LA vs FS, * LC vs FS (mean ± SEM; n = 8)
3.5. Insulin
There was no difference in baseline plasma insulin (P = .11) and an increase in insulin during all three conditions (P < .001 for all) (Figure 3B). There were treatment (P < .01), time (P < .001) and treatment × time (P < .001) effects (post‐hoc comparisons are shown in the figure). There was a difference (P < .05) between the AUCs (0–180 min) for all three conditions, so that for LA, the AUC was greater than for LC (P < .05), with no differences between LA and FS (P = .35) or LC and FS (P = .40).
4. DISCUSSION
We evaluated the effects of different types of beer on gastric emptying, plasma ethanol, glycaemia and plasma insulin response. Scintigraphy, which is recognised as the “gold standard” technique, was used to measure gastric emptying. 26 There was no difference in gastric emptying of low‐alcohol, low‐carb or full‐strength beer although the mean T50 with full‐strength, which had the most calories, was numerically greater. Predictably, the plasma alcohol response was least with low‐alcohol and there was no difference between full‐strength and low‐carb. An inverse relationship between the plasma ethanol response and the rate of gastric emptying was evident for low‐alcohol, with similar trends for low‐carb and full‐strength. The rise in blood glucose was modest, but was greater, with low‐alcohol than full‐strength and low‐carb.
The effect of alcohol and alcohol‐containing beverages on gastric emptying are contentious. Some studies report a relative delay in gastric emptying caused by beer or alcohol, 27 , 28 , 29 others observed accelerated gastric emptying of beer compared with an equivalent ethanol solution 30 , 31 and one showed no change in gastric emptying. 32 We did not observe any difference in gastric emptying between full‐strength, low‐carb and low‐alcohol beer, although the mean gastric emptying rate of full‐strength was (non‐significantly) slowest, which is likely to reflect its higher caloric density. 33 The beers were carbonated when ingested so as to mimic the “real‐world” setting as closely as possible. Carbonation of liquids has been reported to have little, or no, effect on gastric emptying. 34 , 35 Consistent with prior observations, 8 , 31 , 36 there was a relationship between the plasma ethanol response and the rate of gastric emptying. The substantial inter‐individual variation 37 of gastric emptying is not widely appreciated, nor the fact that the rise in blood alcohol occurs earlier when gastric emptying is relatively more rapid, both of which have potential medico‐legal implications. Gastric emptying is markedly accelerated after some forms of gastric surgery including Roux‐en‐Y gastric bypass that is used widely in the management of obesity, 38 which would affect the rate of alcohol absorption. Furthermore, addition of artificial sweeteners to “low‐carb” alcoholic beverages may have a marked effect on gastric emptying and, hence, ethanol absorption. 9 Consumption of low‐alcohol beer predictably resulted in reduced plasma ethanol concentrations compared with full‐strength and low‐carb.
The relationship between ethanol and glycaemia is complex given that acute ethanol ingestion may inhibit gluconeogenesis, 39 but also inhibits peripheral glucose uptake. 40 In our study, the consumption of low‐alcohol led to a greater increase in plasma glucose and insulin compared with full‐strength and low‐carb. This finding is unexpected given that low‐alcohol had the least calories and it has been reported that alcoholic beer results in a greater increase in plasma glucose and insulin compared to non‐alcoholic beer. 41 A possible explanation is that the effect of alcohol to inhibit gluconeogenesis is greater than the rise in glycaemia from absorption of the drink. The observed rises in blood glucose after the drinks were consumed were predictably modest—whether this effect is evident in individuals with insulin resistance and/or type 2 diabetes, where the rise in blood glucose would be anticipated to be greater, warrants evaluation.
Limitations of our study include the small sample size (this is likely to account for the observation that emptying of full‐strength beer was not significantly slower) and the evaluation of young participants only. Ageing is associated with a modest slowing of gastric emptying. 42 While only one volume of alcohol‐containing beverage was evaluated, there is no reason to expect a difference with high nutrient liquids. The alcohol‐containing beverages were also ingested without a meal—we have demonstrated that in this situation, liquids are still emptied before solids, but their emptying is slowed. 8
5. CONCLUSION
We conclude that in healthy, young individuals, gastric emptying of low‐carbohydrate, low‐alcohol and full‐strength beer is comparable and a determinant of the plasma ethanol response.
CONTRIBUTORS
J.E.S. designed and conducted research, provided essential materials, analysed data, performed statistical analysis, wrote the paper and has primary responsibility for final content. R.J.J. analysed data, performed statistical analysis and wrote the paper. L.G.T. conducted research, analysed data, performed statistical analysis and wrote the paper. C.S.M. analysed data, provided endocrinology expertise and wrote the paper. M.H. analysed data, provided endocrinology expertise and wrote the paper. K.L.J. designed and conducted research, provided essential materials, analysed data, performed statistical analysis, wrote the paper and provided nuclear medicine expertise.
COMPETING INTERESTS
The authors report no relevant conflicts of interest.
ACKNOWLEDGEMENTS
The authors thank Mr Raj Sardana for assisting with the conduct of the studies and the staff of the Department of Nuclear Medicine, PET and Bone Densitometry for the use of their gamma camera. This study was supported by a clinical project grant from the Royal Adelaide Hospital Research Foundation. The salary of K.L.J. was funded by the University of Adelaide William T. Southcott Fellowship and C.S.M. by an NHMRC Early Career Fellowship. Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Stevens JE, Jalleh RJ, Trahair LG, Marathe CS, Horowitz M, Jones KL. Comparative effects of low‐carbohydrate, full‐strength and low‐alcohol beer on gastric emptying, alcohol absorption, glycaemia and insulinaemia in health. Br J Clin Pharmacol. 2022;88(7):3421-3427. doi: 10.1111/bcp.15297
The authors confirm that the Principal Investigator for this paper is Julie E. Stevens and that she had direct clinical responsibility for participants.
[Correction added on 19 May 2022, after first online publication: CAUL funding statement has been added.]
Funding information Royal Adelaide Hospital Research Foundation
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available to maintain the participants' anonymity.
REFERENCES
- 1. Vichealth National Community Attitudes Survey: Awareness and behaviours of low carb beer drinkers. https://www.Vichealth.Vic.Gov.Au/~/media/resourcecentre/publicationsandresources/alcohol%20misuse/k‐013_low‐carb‐beer_factsheet_final.ashx. Published December 2010. Accessed September 14, 2021.
- 2. Miller PG, McKenzie SP, de Groot FP, Davoren SL, Leslie ER. The growing popularity of “low‐carb” beers: good marketing or community health risk? Med J Aust. 2010;192(4):235. doi: 10.5694/j.1326-5377.2010.tb03488.x [DOI] [PubMed] [Google Scholar]
- 3. Nimmo WS. Drugs, diseases and altered gastric emptying. Clin Pharmacokinet. 1976;1(3):189‐203. doi: 10.2165/00003088-197601030-00002 [DOI] [PubMed] [Google Scholar]
- 4. Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous n‐acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2(6198):1097‐1100. doi: 10.1136/bmj.2.6198.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holt S. Observations on the relation between alcohol absorption and the rate of gastric emptying. Can Med Assoc J. 1981;124(3):267‐277, 297. [PMC free article] [PubMed] [Google Scholar]
- 6. Oneta CM, Simanowski UA, Martinez M, et al. First pass metabolism of ethanol is strikingly influenced by the speed of gastric emptying. Gut. 1998;43(5):612‐619. doi: 10.1136/gut.43.5.612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaikomin R, Russo A, Rayner CK, et al. Effects of lipase inhibition on gastric emptying and alcohol absorption in healthy subjects. Br J Nutr. 2006;96(5):883‐887. doi: 10.1017/BJN20061922 [DOI] [PubMed] [Google Scholar]
- 8. Horowitz M, Maddox A, Bochner M, et al. Relationships between gastric emptying of solid and caloric liquid meals and alcohol absorption. Am J Physiol. 1989;257(2):291‐298. doi: 10.1152/ajpgi.1989.257.2.G291 [DOI] [PubMed] [Google Scholar]
- 9. Wu KL, Chaikomin R, Doran S, Jones KL, Horowitz M, Rayner CK. Artificially sweetened versus regular mixers increase gastric emptying and alcohol absorption. Am J Med. 2006;119(9):802‐804. doi: 10.1016/j.amjmed.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 10. Kechagias S, Jonsson KA, Jones AW. Impact of gastric emptying on the pharmacokinetics of ethanol as influenced by cisapride. Br J Clin Pharmacol. 1999;48(5):728‐732. doi: 10.1046/j.1365-2125.1999.00080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brener W, Hendrix TR, McHugh PR. Regulation of the gastric emptying of glucose. Gastroenterology. 1983;85(1):76‐82. doi: 10.1016/S0016-5085(83)80232-7 [DOI] [PubMed] [Google Scholar]
- 12. Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrient. Am J Physiol. 1989;256(2):404‐411. doi: 10.1152/ajpgi.1989.256.2.G404 [DOI] [PubMed] [Google Scholar]
- 13. Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36(9):857‐862. doi: 10.1007/BF00400362 [DOI] [PubMed] [Google Scholar]
- 14. Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin‐dependent diabetes mellitus. J Nucl Med. 1996;37(10):1643‐1648. [PubMed] [Google Scholar]
- 15. Russo A, Stevens JE, Wilson T, et al. Guar attenuates fall in postprandial blood pressure and slows gastric emptying of oral glucose in type 2 diabetes. Dig Dis Sci. 2003;48(7):1221‐1229. doi: 10.1023/A:1024182403984 [DOI] [PubMed] [Google Scholar]
- 16. Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36(5):1396‐1405. doi: 10.2337/dc12-1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma J, Stevens JE, Cukier K, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet‐controlled type 2 diabetes. Diabetes Care. 2009;32(9):1600‐1602. doi: 10.2337/dc09-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jalleh R, Pham H, Marathe CS, et al. Acute effects of lixisenatide on energy intake in healthy subjects and patients with type 2 diabetes: relationship to gastric emptying and intragastric distribution. Nutrients. 2020;12(7):1962. doi: 10.3390/nu12071962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rayner CK, Watson LE, Phillips LK, et al. Effects of sustained treatment with lixisenatide on gastric emptying and postprandial glucose metabolism in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2020;43(8):1813‐1821. doi: 10.2337/dc20-0190 [DOI] [PubMed] [Google Scholar]
- 20. Chapple LS, Summers MJ, Weinel LM, et al. Effects of standard vs energy‐dense formulae on gastric retention, energy delivery, and glycemia in critically ill patients. JPEN J Parenter Enteral Nutr. 2021;45(4):710‐719. doi: 10.1002/jpen.2065 [DOI] [PubMed] [Google Scholar]
- 21. Gentilcore D, Bryant B, Wishart JM, Morris HA, Horowitz M, Jones KL. Acarbose attenuates the hypotensive response to sucrose and slows gastric emptying in the elderly. Am J Med. 2005;118(11):1289. doi: 10.1016/j.amjmed.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 22.ADVIA chemistry systems. Instructions for use – Ethanol_2(ETOH_2); Siemens Healthcare Diagnostics Inc.; 10493987_en rev. B: 2011. [Google Scholar]
- 23. Nowotny B, Nowotny PJ, Strassburger K, Roden M. Precision and accuracy of blood glucose measurements using three different instruments. Diabet Med. 2012;29(2):260‐265. doi: 10.1111/j.1464-5491.2011.03406.x [DOI] [PubMed] [Google Scholar]
- 24. Astles JR, Sedor FA, Toffaletti JG. Evaluation of the YSI 2300 glucose analyzer: algorithm‐corrected results are accurate and specific. Clin Biochem. 1996;29(1):27‐31. doi: 10.1016/0009-9120(95)02010-1 [DOI] [PubMed] [Google Scholar]
- 25. Trahair LG, Horowitz M, Rayner CK, et al. Comparative effects of variations in duodenal glucose load on glycemic, insulinemic, and incretin responses in healthy young and older subjects. J Clin Endocrinol Metab. 2012;97(3):844‐851. doi: 10.1210/jc.2011-2583 [DOI] [PubMed] [Google Scholar]
- 26. Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36(1):44‐54. doi: 10.2967/jnmt.107.048116 [DOI] [PubMed] [Google Scholar]
- 27. Franke A, Teyssen S, Harder H, Singer MV. Effect of ethanol and some alcoholic beverages on gastric emptying in humans. Scand J Gastroenterol. 2004;39(7):638‐644. doi: 10.1080/00365520410005009 [DOI] [PubMed] [Google Scholar]
- 28. Jian R, Cortot A, Ducrot F, Jobin G, Chayvialle JA, Modigliani R. Effect of ethanol ingestion on postprandial gastric emptying and secretion, biliopancreatic secretions, and duodenal absorption in man. Dig Dis Sci. 1986;31(6):604‐614. doi: 10.1007/BF01318691 [DOI] [PubMed] [Google Scholar]
- 29. Mushambi MC, Bailey SM, Trotter TN, Chadd GD, Rowbotham DJ. Effect of alcohol on gastric emptying in volunteers. Br J Anaesth. 1993;71(5):674‐676. doi: 10.1093/bja/71.5.674 [DOI] [PubMed] [Google Scholar]
- 30. Pfeiffer A, Hogl B, Kaess H. Effect of ethanol and commonly ingested alcoholic beverages on gastric emptying and gastrointestinal transit. Clin Investig. 1992;70(6):487‐491. doi: 10.1007/BF00210229 [DOI] [PubMed] [Google Scholar]
- 31. Schwartz JG, Salman UA, McMahan CA, Phillips WT. Gastric emptying of beer in Mexican‐Americans compared with non‐Hispanic whites. Metabolism. 1996;45(9):1174‐1178. doi: 10.1016/S0026-0495(96)90019-0 [DOI] [PubMed] [Google Scholar]
- 32. Charles F, Evans DF, Castillo FD, Wingate DL. Daytime ingestion of alcohol alters nighttime jejunal motility in man. Dig Dis Sci. 1994;39(1):51‐58. doi: 10.1007/BF02090060 [DOI] [PubMed] [Google Scholar]
- 33. Hunt JN, Stubbs DF. The volume and energy content of meals as determinants of gastric emptying. J Physiol. 1975;245(1):209‐225. doi: 10.1113/jphysiol.1975.sp010841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lau ERL, Henry CJ. No influence of carbonation on glycemic response, gastric emptying, and satiety of sweetened drinks. Nutrition. 2017;39‐40:1‐7. doi: 10.1016/j.nut.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 35. Zachwieja JJ, Costill DL, Widrick JJ, Anderson DE, McConell GK. Effects of drink carbonation on the gastric emptying characteristics of water and flavored water. Int J Sport Nutr. 1991;1(1):45‐51. doi: 10.1123/ijsn.1.1.45 [DOI] [PubMed] [Google Scholar]
- 36. Hebbard GS, Sun WM, Bochner F, Horowitz M. Pharmacokinetic considerations in gastrointestinal motor disorders. Clin Pharmacokinet. 1995;28(1):41‐66. doi: 10.2165/00003088-199528010-00005 [DOI] [PubMed] [Google Scholar]
- 37. Horowitz M, Dent J. Disordered gastric emptying: mechanical basis, assessment and treatment. Baillieres Clin Gastroenterol. 1991;5(2):371‐407. doi: 10.1016/0950-3528(91)90034-X [DOI] [PubMed] [Google Scholar]
- 38. Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003‐2009. doi: 10.1002/oby.20791 [DOI] [PubMed] [Google Scholar]
- 39. Krebs HA. The effects of ethanol on the metabolic activities of the liver. Adv Enzyme Regul. 1968;6:467‐480. doi: 10.1016/0065-2571(68)90029-0 [DOI] [PubMed] [Google Scholar]
- 40. Yki‐Jarvinen H, Nikkila EA. Ethanol decreases glucose utilization in healthy man. J Clin Endocrinol Metab. 1985;61(5):941‐945. doi: 10.1210/jcem-61-5-941 [DOI] [PubMed] [Google Scholar]
- 41. Hatonen KA, Virtamo J, Eriksson JG, et al. Modifying effects of alcohol on the postprandial glucose and insulin responses in healthy subjects. Am J Clin Nutr. 2012;96(1):44‐49. doi: 10.3945/ajcn.111.031682 [DOI] [PubMed] [Google Scholar]
- 42. Pham H, Phillips L, Trahair L, Hatzinikolas S, Horowitz M, Jones KL. Longitudinal changes in the blood pressure responses to, and gastric emptying of, an oral glucose load in healthy older subjects. J Gerontol A Biol Sci Med Sci. 2020;75(2):244‐248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available to maintain the participants' anonymity.


